Abstract
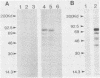
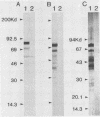
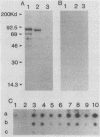
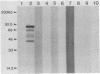
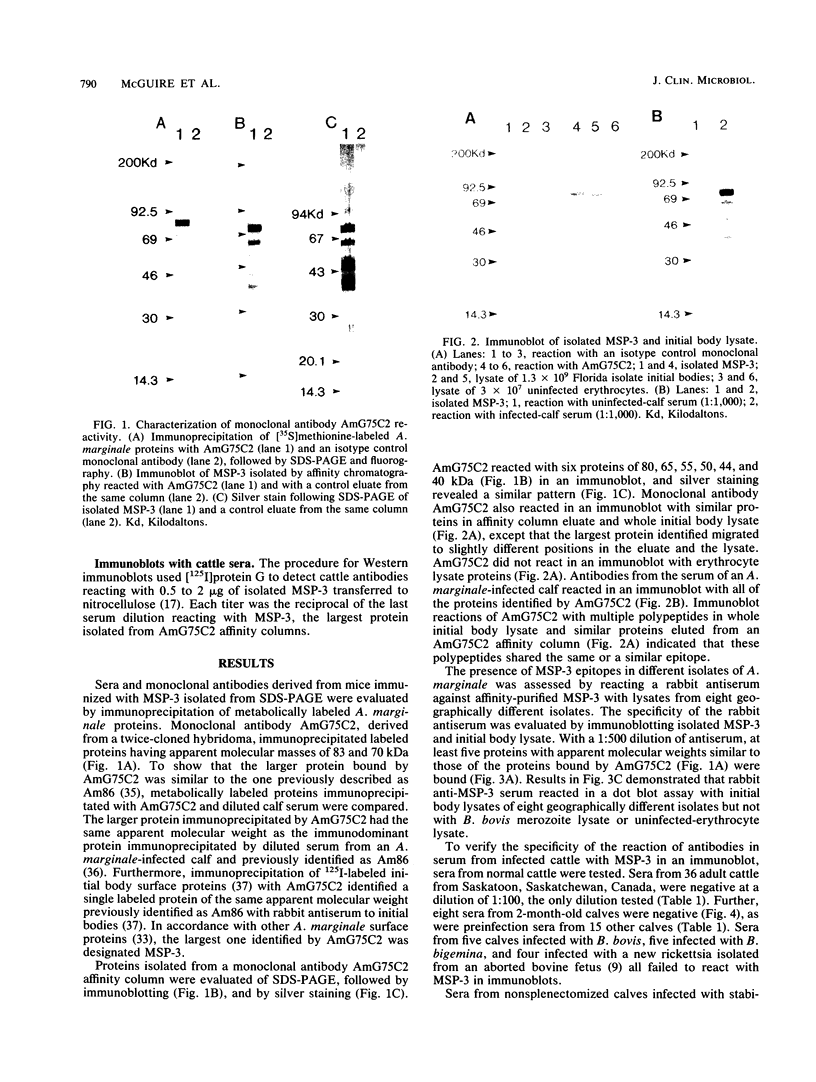
Rapid and accurate detection of Anaplasma marginale-infected cattle would enhance anaplasmosis control procedures and evaluation of vaccines. Current tests based on detection of antibodies in serum are not widely used for several reasons, including the occurrence of either false-positive or false-negative results. We evaluated binding of antibodies in serum to a subunit antigen isolated from A. marginale initial bodies--major surface protein 3 (MSP-3). MSP-3 was detected in lysates of eight geographically different isolates of A. marginale and purified by affinity chromatography with monoclonal antibody AmG75C2. Antibodies from cattle infected with any of five geographically different isolates of A. marginale reacted in immunoblots with MSP-3. Sera from uninfected cattle and cattle infected with another rickettsial organism and two hemoprotozoal organisms failed to react with MSP-3. Six carrier cattle infected with the Florida isolate of A. marginale had antibody titers to MSP-3 ranging from 10(3) to 10(6) during a 5-year evaluation period. Since specific antibodies to isolated MSP-3 persist in high titers in long-term carrier cattle sera and MSP-3 is common among A. marginale isolates, it is recommended as a subunit antigen for an anaplasmosis test.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. H., Smith R. D., Kuhlenschmidt M. S. Identification of antigens of two isolates of Anaplasma marginale, using a western blot technique. Am J Vet Res. 1986 Mar;47(3):501–506. [PubMed] [Google Scholar]
- Amerault T. E., Roby T. O. A rapid card agglutination test for bovine anaplasmosis. J Am Vet Med Assoc. 1968 Dec 15;153(12):1828–1834. [PubMed] [Google Scholar]
- Barbet A. F., Anderson L. W., Palmer G. H., McGuire T. C. Comparison of proteins synthesized by two different isolates of Anaplasma marginale. Infect Immun. 1983 Jun;40(3):1068–1074. doi: 10.1128/iai.40.3.1068-1074.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry D. N., Parker R. J., De Vos A. J., Dunster P., Rodwell B. J. A microplate enzyme-linked immunosorbent assay for measuring antibody to Anaplasma marginale in cattle serum. Aust Vet J. 1986 Mar;63(3):76–79. doi: 10.1111/j.1751-0813.1986.tb02934.x. [DOI] [PubMed] [Google Scholar]
- Boulanger P., Ruckerbauer G. M., Bannister G. L., MacKay R. R., Peter N. H. Anaplasmosis: control of the first outbreak in Canada by serological identification and slaughter. Can J Comp Med. 1971 Jul;35(3):249–257. [PMC free article] [PubMed] [Google Scholar]
- Carson C. A., Adams L. G., Todorovic R. A. An antigenic and serologic comparison of two virulent strains and an attenuated strain of Anaplasma marginale. Am J Vet Res. 1970 Jun;31(6):1071–1078. [PubMed] [Google Scholar]
- Correa W. M., Correa C. N., Gottschalk A. F. Bovine abortion associated with Anaplasma marginale. Can J Comp Med. 1978 Apr;42(2):227–228. [PMC free article] [PubMed] [Google Scholar]
- Dilbeck P. M., Evermann J. F., Crawford T. B., Ward A. C., Leathers C. W., Holland C. J., Mebus C. A., Logan L. L., Rurangirwa F. R., McGuire T. C. Isolation of a previously undescribed rickettsia from an aborted bovine fetus. J Clin Microbiol. 1990 Apr;28(4):814–816. doi: 10.1128/jcm.28.4.814-816.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzgun A., Schuntner C. A., Wright I. G., Leatch G., Waltisbuhl D. J. A sensitive ELISA technique for the diagnosis of Anaplasma marginale infections. Vet Parasitol. 1988 Jul;29(1):1–7. doi: 10.1016/0304-4017(88)90002-7. [DOI] [PubMed] [Google Scholar]
- Eriks I. S., Palmer G. H., McGuire T. C., Allred D. R., Barbet A. F. Detection and quantitation of Anaplasma marginale in carrier cattle by using a nucleic acid probe. J Clin Microbiol. 1989 Feb;27(2):279–284. doi: 10.1128/jcm.27.2.279-284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff W. L., Johnson W. C., Kuttler K. L. Development of an indirect fluorescent antibody test, using microfluorometry as a diagnostic test for bovine anaplasmosis. Am J Vet Res. 1985 May;46(5):1080–1084. [PubMed] [Google Scholar]
- Goff W. L., Winward L. D. Detection of geographic isolates of Anaplasma marginale, using polyclonal bovine antisera and microfluorometry. Am J Vet Res. 1985 Nov;46(11):2399–2400. [PubMed] [Google Scholar]
- Goff W., Barbet A., Stiller D., Palmer G., Knowles D., Kocan K., Gorham J., McGuire T. Detection of Anaplasma-marginale-infected tick vectors by using a cloned DNA probe. Proc Natl Acad Sci U S A. 1988 Feb;85(3):919–923. doi: 10.1073/pnas.85.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E. F., Long R. F., Todorovic R. A. Comparisons of the complement-fixation, indirect fluorescent antibody, and card agglutination tests for the diagnosis of bovine anaplasmosis. Am J Vet Res. 1978 Sep;39(9):1538–1541. [PubMed] [Google Scholar]
- Hines S. A., McElwain T. F., Buening G. M., Palmer G. H. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol Biochem Parasitol. 1989 Nov;37(1):1–9. doi: 10.1016/0166-6851(89)90096-0. [DOI] [PubMed] [Google Scholar]
- Johnson G. C., Barbet A. F., Klevjer-Anderson P., McGuire T. C. Preferential immune response to virion surface glycoproteins by caprine arthritis-encephalitis virus-infected goats. Infect Immun. 1983 Aug;41(2):657–665. doi: 10.1128/iai.41.2.657-665.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. W., Norman B. B., Kliewer I. O., Brock W. E. Anaplasma marginale infection in splenectomized calves. Am J Vet Res. 1968 Mar;29(3):523–533. [PubMed] [Google Scholar]
- KREIER J. P., RISTIC M. Anaplasmosis. X. Morphologic characteristics of the parasites present in the blood of calves infected with the Oregon strain of Anaplasma marginale. Am J Vet Res. 1963 Jul;24:676–687. [PubMed] [Google Scholar]
- KREIER J. P., RISTIC M. Anaplasmosis. XI. Immunoserologic characteristics of the parasites present in the blood of calves infected with the Oregon strain of Anaplasma marginale. Am J Vet Res. 1963 Jul;24:688–696. [PubMed] [Google Scholar]
- Kieser S. T., Eriks I. S., Palmer G. H. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect Immun. 1990 Apr;58(4):1117–1119. doi: 10.1128/iai.58.4.1117-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocan K. M., Venable J. H., Brock W. E. Ultrastructure of anaplasmal inclusions (Pawhuska isolate) and their appendages in intact and hemolyzed erythrocytes and in complement-fixation antigen. Am J Vet Res. 1978 Jul;39(7):1123–1130. [PubMed] [Google Scholar]
- Kuttler K. L. Serological relationship of Anaplasma marginale and Anaplasma centrale as measured by the complement-fixation and capillary tube agglutination tests. Res Vet Sci. 1967 Apr;8(2):207–211. [PubMed] [Google Scholar]
- Kuttler K. L., Zaugg J. L., Johnson L. W. Serologic and clinical responses of premunized, vaccinated, and previously infected cattle to challenge exposure by two different Anaplasma marginale isolates. Am J Vet Res. 1984 Nov;45(11):2223–2226. [PubMed] [Google Scholar]
- Luther D. G., Cox H. U., Nelson W. O. Comparisons of serotests with calf inoculations for detection of carriers in anaplasmosis- vaccinated cattle. Am J Vet Res. 1980 Dec;41(12):2085–2086. [PubMed] [Google Scholar]
- McGuire T. C., Musoke A. J., Kurtti T. Functional properties of bovine IgG1 and IgG2: interaction with complement, macrophages, neutrophils and skin. Immunology. 1979 Oct;38(2):249–256. [PMC free article] [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire T. C., Perryman L. E., Davis W. C. Analysis of serum and lymphocyte surface IgM of healthy and immunodeficient horses with monoclonal antibodies. Am J Vet Res. 1983 Jul;44(7):1284–1288. [PubMed] [Google Scholar]
- Montenegro-James S., James M. A., Ristic M. Modified indirect fluorescent antibody test for the serodiagnosis of Anaplasma marginale infections in cattle. Am J Vet Res. 1985 Nov;46(11):2401–2403. [PubMed] [Google Scholar]
- Norton J. H., Parker R. J., Forbes-Faulkner J. C. Neonatal anaplasmosis in a calf. Aust Vet J. 1983 Nov;60(11):348–348. doi: 10.1111/j.1751-0813.1983.tb02844.x. [DOI] [PubMed] [Google Scholar]
- Oberle S. M., Palmer G. H., Barbet A. F., McGuire T. C. Molecular size variations in an immunoprotective protein complex among isolates of Anaplasma marginale. Infect Immun. 1988 Jun;56(6):1567–1573. doi: 10.1128/iai.56.6.1567-1573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Cantor G. H., McGuire T. C. Immunization of cattle with the MSP-1 surface protein complex induces protection against a structurally variant Anaplasma marginale isolate. Infect Immun. 1989 Nov;57(11):3666–3669. doi: 10.1128/iai.57.11.3666-3669.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Davis W. C., McGuire T. C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science. 1986 Mar 14;231(4743):1299–1302. doi: 10.1126/science.3945825. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Kuttler K. L., McGuire T. C. Detection of an Anaplasma marginale common surface protein present in all stages of infection. J Clin Microbiol. 1986 Jun;23(6):1078–1083. doi: 10.1128/jcm.23.6.1078-1083.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., McGuire T. C. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984 Aug;133(2):1010–1015. [PubMed] [Google Scholar]
- RISTIC M. A capillary tube-agglutination test for anaplasmosis--a preliminary report. J Am Vet Med Assoc. 1962 Sep 1;141:588–594. [PubMed] [Google Scholar]
- Rees C. W. THE EXPERIMENTAL TRANSMISSION OF ANAPLASMOSIS BY DERMACENTOR VARIABILIS. Science. 1932 Mar 18;75(1942):318–320. doi: 10.1126/science.75.1942.318. [DOI] [PubMed] [Google Scholar]
- Ristic M., Carson C. A. Methods of immunoprophylaxis against bovine anaplasmosis with emphasis on use of the attenuated Anaplasma marginale vaccine. Adv Exp Med Biol. 1977;93:151–188. doi: 10.1007/978-1-4615-8855-9_10. [DOI] [PubMed] [Google Scholar]
- Schuntner C. A., Leatch G. Radioimmunoassay for Anaplasma marginale antibodies in cattle. Am J Vet Res. 1988 Apr;49(4):504–507. [PubMed] [Google Scholar]
- Swift B. L., Thomas G. M. Bovine anaplasmosis: elimination of the carrier state with injectable long-acting oxytetracycline. J Am Vet Med Assoc. 1983 Jul 1;183(1):63–65. [PubMed] [Google Scholar]
- Thoen C. O., Blackburn B., Mills K., Lomme J., Hopkins M. P. Enzyme-linked immunosorbent assay for detecting antibodies in cattle in a herd in which anaplasmosis was diagnosed. J Clin Microbiol. 1980 May;11(5):499–502. doi: 10.1128/jcm.11.5.499-502.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler G. C., Brown G. M., Lutz H. Detection of antibodies to Anaplasma marginale by an improved enzyme-linked immunosorbent assay with sodium dodecyl sulfate-disrupted antigen. J Clin Microbiol. 1987 Apr;25(4):633–636. doi: 10.1128/jcm.25.4.633-636.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg J. L., Stiller D., Coan M. E., Lincoln S. D. Transmission of Anaplasma marginale Theiler by males of Dermacentor andersoni Stiles fed on an Idaho field-infected, chronic carrier cow. Am J Vet Res. 1986 Oct;47(10):2269–2271. [PubMed] [Google Scholar]