Abstract
Jak2, the cognate tyrosine kinase for numerous cytokine receptors, undergoes multisite phosphorylation during cytokine stimulation. To understand the role of phosphorylation in Jak2 regulation, we used mass spectrometry to identify numerous Jak2 phosphorylation sites and characterize their significance for Jak2 function. Two sites outside of the tyrosine kinase domain, Tyr317 in the FERM domain and Tyr637 in the JH2 domain, exhibited strong regulation of Jak2 activity. Mutation of Tyr317 promotes increased Jak2 activity, and the phosphorylation of Tyr317 during cytokine signaling requires prior activation loop phosphorylation, which is consistent with a role for Tyr317 in the feedback inhibition of Jak2 kinase activity after receptor stimulation. Comparison to several previously identified regulatory phosphorylation sites on Jak2 revealed a dominant role for Tyr317 in the attenuation of Jak2 signaling. In contrast, mutation of Tyr637 decreased Jak2 signaling and activity and partially suppressed the activating JH2 V617F mutation, suggesting a role for Tyr637 phosphorylation in the release of JH2 domain-mediated suppression of Jak2 kinase activity during cytokine stimulation. The phosphorylation of Tyr317 and Tyr637 act in concert with other regulatory events to maintain appropriate control of Jak2 activity and cytokine signaling.
Type I cytokines act via cell surface receptors on target cells to mediate a plethora of physiologic processes, ranging from hematopoietic and immune functions (such as those controlled by erythropoietin and the interleukins), to growth and neuroendocrine responses (such as those modulated by growth hormone and leptin) (16, 19, 20, 29, 34). Cytokine receptors contain an extracellular ligand-binding domain, a single transmembrane domain, and an intracellular domain that, although devoid of enzymatic activity, transmits intracellular signals by means of an associated Jak family tyrosine kinase. Ligand binding activates the receptor-associated intracellular Jak kinase, resulting in Jak kinase autophosphorylation and activation, as well as the subsequent tyrosine phosphorylation of the intracellular domain of the cytokine receptor. These tyrosine phosphorylation events mediate downstream signaling by the cytokine receptor/Jak kinase complex (16, 20, 23, 29).
The Jak kinase family contains four members: Jak1 to Jak3 and Tyk2 (16, 20). Of these, Jak1-2 and Tyk2 are ubiquitously expressed, while Jak3 is found predominantly in immune and hematopoietic tissues. Jak kinases contain four conserved domains: the NH2-terminal FERM domain mediates cytokine receptor interactions (36, 39), while function of the adjacent (nonphosphotyrosine binding) SH2-like fold remains unclear. The COOH-terminal region of Jak kinases contains a kinase-like JH2 domain that is devoid of enzymatic activity but which regulates the activity of the COOH-terminal JH1 tyrosine kinase domain (11, 24, 32, 37, 38).
Our laboratory studies signaling by LepRb, which regulates energy balance, neuroendocrine homeostasis, and immune function in response to leptin, a hormonal signal of long-term energy stores (10, 12, 29, 34). Leptin binding to LepRb promotes the activation and tyrosine phosphorylation of the LepRb-associated Jak2, resulting in the phosphorylation of tyrosine residues on Jak2 and the intracellular tail of LepRb Jak2 (2, 22, 29). Tyrosine phosphorylation sites on LepRb recruit signal transducers and activators of transcription (STATs) and SHP-2 to mediate downstream signaling, as well as the suppressor of cytokine signaling-3 (SOCS3,) to attenuate LepRb signaling (2, 5, 29).
Several sites of Jak2 tyrosine phosphorylation have also been identified, and functions for some of these sites have been elucidated: within the FERM domain, phosphorylation of Tyr119 disrupts Jak2-cytokine receptor interactions (13). Within and adjacent to the JH2 domain, the phosphorylation of Ser523 and Tyr570 inhibits Jak2 kinase activity (1, 8). Within the kinase domain itself, phosphorylated Tyr813 mediates binding of SH2-B/SH2B1 to increase Jak2 signaling (23), phosphorylation of the activation loop residues Tyr1007 and Tyr1008 plays an essential role in kinase activation (9), and the phosphorylation of Tyr913 inhibits Jak2 signaling (15). Other sites of Jak2 phosphorylation also exist (some known, while others have remained undefined), although the function(s) for many of these remain unknown (1, 25). We report here the MS analysis of Jak2 protein, which revealed several novel sites of phosphorylation. We also report the in-depth analysis of two cytokine-regulated Jak2 phosphorylation sites outside of the kinase domain: Tyr317 and Tyr637. Phosphorylation of Tyr317 mediates negative-feedback regulation for Jak2, while phosphorylation of Tyr637 is necessary for maximal Jak2 kinase activity. We propose an integrated model for how these and other phosphorylation sites orchestrate the activity of Jak2.
MATERIALS AND METHODS
Antibodies, growth factors, and reagents.
Antibodies recognizing phosphorylated Tyr317 and phosphorylated Tyr637 were generated by raising rabbit polyclonal antibodies against these sites. Synthetic peptides corresponding to phosphorylated Tyr317 (CQDVQLY*CDFPD) and Tyr637 (CGSLDTY*LKKNK) motifs were generated in the MDRTC Peptide Core (University of Michigan), conjugated to keyhole limpet hemocyanin, and inoculated into rabbits. Antisera were affinity purified by using a 1:1 mixture of Affigel-10 and Affigel-15 coupled to phosphorylated synthetic peptide. The resulting antibody was further purified by incubating it with Affigel coupled to the nonphosphorylated variant of the peptide in combination with an irrelevant phosphorylated peptide. αJak2(476) antisera for immunoprecipitation were generated by injection into rabbits of keyhole limpet hemocyanin-couple synthetic peptide with the sequence DSQRKLQFYEDKHQLPAPK. Generation of αJak2(1139) for immunoblotting has been described previously [previously referred to as αJak2(NT)] (21). Antibodies to phosphorylated (activated) extracellular signal-regulated kinase (ERK) and phosphorylated (activated) STAT3 were purchased from Cell Signaling Technology (Danvers, MA). Antibody directed against the phosphorylated activation loop of Jak2 (phospho-Tyr1007/8) was purchased from Upstate Biotechnology (Lake Placid, NY). Antibody against the hemagglutinin (HA) epitope was purchased from Covance (Denver, PA). Antibody to the FLAG epitope was purchased from Sigma. Synthetic erythropoietin (Epogen) was obtained from Amgen (Thousand Oaks, CA).
Generation of Jak2 constructs.
The generation of ELR has been described previously (2). The generation of Jak2/pCDNA3 has been described previously (22). To make epitope-tagged Jak2, DNA encoding either a double HA epitope or a double FLAG epitope was added to the 3′ end of the Jak2 open reading frame by using a two-step strategy. In the first stage, PCR was conducted using an oligonucleotide that primed upstream of Jak2's internal XhoI site in conjunction with a long downstream oligonucleotide that contained the epitope tag, an NheI site, and a region homologues to the 3′ end of Jak2. The PCR product was inserted into TOPO (Invitrogen). An NheI/XhoI digest was used to move the epitope tag containing fragment back into the original Jak2 plasmid. QuikChange mutagenesis (Stratagene) was used to generate Jak2Y317F, Jak2Y637F, Jak2Y317E, Jak2Y637E, Jak2K882E, Jak2Y1007/8F, Jak2Y119E-HA, Jak2Y119E/Y317F-HA, Jak2Y119E/Y637F-HA, Jak2Y317F/S523A, Jak2Y317F/Y570F, Jak2S523A/Y570F, and Jak2Y317F/S523A/Y570F. The presence of the desired mutations and the absence of adventitious mutations were confirmed by DNA sequencing for all plasmids generated.
Cell lines and transfection.
HEK293 cells were grown at 37°C in humidified air with 5% CO2. Cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin. HEK293 cells were transfected by using Lipofectamine (Invitrogen). At 3 to 5 h after transfection, the cells were serum starved overnight in DMEM supplemented with 0.5% bovine serum albumin. 32D cells were grown in RPMI 1640 medium supplemented with 10% FBS and 5% interleukin-3 (IL-3) conditioned medium. IL-3 conditioned medium was generated by using WEHI cells grown in RPMI 1640 medium supplemented with 10% FBS. Cells were grown to confluence, and the medium was harvested and then filtered (0.2-μm pore size) and stored at 4°C.
Cytokine stimulation, lysis, and immunoprecipitation.
HEK293 cells were stimulated with Epogen at 12.5 U/ml. 32D cells were stimulated with undiluted IL-3 conditioned medium from WEHI cells. Cells were harvested with lysis buffer (20 mM Tris [pH 7.4], 150 mM NaCl, 1% Nonidet P-40, 2 mM phenylmethylsulfonyl fluoride, 2 mM sodium orthovanadate) and cleared by centrifugation. Lysates used for direct immunoblotting were mixed 1:1 with Laemmli buffer. Total Jak2 was immunoprecipitated using a polyclonal antibody to Jak2 and protein A-agarose (Invitrogen) overnight at 4°C. Immunoprecipitates were washed three times with lysis buffer before elution with Laemmli buffer. HA epitope-tagged Jak2 was immunoprecipitated with a monoclonal antibody to HA and protein G-Sepharose (GE Healthcare) overnight at 4°C. Immunoprecipitates were washed three times with lysis buffer before elution with Laemmli buffer.
MS.
For preparation of protein for MS analysis, material was immunoprecipitated from 5 to 10 15-cm dishes of HEK293 cells and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Jak2 protein was visualized by Coomassie brilliant blue G-250 stain. Jak2 protein was subject the mass spectrometry (MS) by using two different instruments and methods. In one method, gel slices containing Jak2 were digested with 5 ng of sequencing-grade modified trypsin (Promega)/μl in 25 mM ammonium bicarbonate containing 0.01% n-octylglucoside for 18 h at 37°C. Peptides were eluted from the gel slices with 80% acetonitrile-1% formic acid. Tryptic digests were separated by capillary high-pressure liquid chromatography (HPLC; C18; 75 μM [inner diameter] Picofrit column; New Objective) using a flow rate of 100 nl/min over a 3-h reversed-phase gradient and analyzed by using an LTQ two-dimensional linear ion trap mass spectrometer (ThermoFinnigan). Resultant tandem MS (MS/MS) spectra were matched against mouse Jak2 sequence by using TurboSequest (BioWorks 3.1) with a fragment ion tolerance of <0.5 and amino acid modification variables, including phosphorylation (80 Da) of Ser, Thr, and Tyr; oxidation (16 Da) of Met; and methylation (14 Da) of Lys.
For the second method, gel slices containing Jak2 were destained, reduced, and alkylated as described elsewhere (33). Proteolytic digestion was carried out with 13 ng of sequencing-grade trypsin (Promega)/μl in 25 mM ammonium bicarbonate for 18 h at 37°C. The resulting peptides were eluted with solution containing 60% methanol-5% acetic acid, and the peptide mixture was lyophilized (Labconco, Kansas City, MO). Esterification and immobilized metal affinity chromatography enrichment were performed with “optimized” buffers described previously (30). Phosphopeptide-enriched peptide mixture was loaded off-line onto precolumn packed with an 8-cm bed length of 5- to 15-μm spherical C18 beads (Applied Biosystems, Foster City, CA) in 75-μm (inner-diameter) fused silica capillary tubing. HPLC was performed with a high-performance analytical column (50 μm by 8 cm) packed with 5-μm-diameter C18 reversed-phase beads (YMC, Wilmington, NC) (30) at an estimated eluent flow rate of 20 to 50 nl/min. HPLC solvent A was 0.2 M acetic acid, and solvent B was 70% acetonitrile-0.2 M acetic acid. LC-MS analysis of phosphopeptide mixture was performed by using a solvent gradient of 0 to 5% solvent B for 5 min and 5 to 100% solvent B for 35 min. MS data acquisition was performed on QSTAR XL in data-dependent mode (MS scan, 300 ≤ m/z ≤ 2,000, top five most abundant MS/MS scans using low resolution for precursor isolation and using 1.5-s accumulation with enhance-all mode, 1.8-kV electron spray ionization voltage). The resulting spectra were compared against the NCBI mouse database using the search engine Mascot Daemon (Matrix Science, Inc., London, United Kingdom). The search parameters allowed for two missed cleavages for trypsin; a fixed modification of +14 for d0-methyl esters, for aspartic acid, glutamic acid, and peptide C terminus; and variable modifications of +80 for serine, threonine, and tyrosine phosphorylation and +16 for methionine oxidation. The mass tolerance was 1.0 Da for precursors and 0.35 Da for fragment ions. MS/MS spectra corresponding to phosphorylated peptides of Jak2 were manually verified.
Immunoblotting.
SDS-PAGE gels were transferred to nitrocellulose membranes (Whatman) in Towbin buffer containing 0.02% SDS and 20% methanol. Membranes were blocked for 1 h at room temperature or overnight at 4°C in buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, and 0.01% Tween 20 (wash buffer) supplemented with 3% bovine serum albumin (block buffer). Membranes were incubated in primary antibody in block buffer for 2 h at room temperature or overnight at 4°C. Membranes were rinsed three times with wash buffer and then incubated with a secondary antibody conjugated to either horseradish peroxidase (Santa Cruz) or a fluorescent tag (Molecular Probes or LI-COR). For horseradish peroxidase detection, membranes were rinsed three times in wash buffer before being treated with chemiluminescence reagent (Roche) and exposed to film (Kodak, Denville, NJ). For fluorescence detection, membranes were rinsed three times in wash buffer before detection using an Odyssey imaging system manufactured by LI-COR Biosciences (Lincoln, NE). Immunoreactive bands were identified and quantified using Odyssey application software version 2.1.
In vitro kinase assays.
HEK293 cells were transfected with plasmids encoding ELR and either mutant or wild-type (WT) Jak2. After transfection, the cells were serum starved overnight in DMEM supplemented with 0.5% bovine serum albumin. Prior to lysis, cells were treated with either vehicle or erythropoietin for 15 min. Cells were harvested with lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 2 mM EGTA, 0.2% Triton X-100, 0.5 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml) and cleared by centrifugation. Lysates were immunoprecipitated with an antibody to Jak2 or an antibody to the HA epitope. Immunoprecipitates were washed twice with lysis buffer and then twice with kinase buffer (50 mM HEPES [pH 7.5], 100 mM NaCl, 1 mM Na3VO4, 5 mM MnCl2, 0.5 mM dithiothreitol). Washed immunoprecipitates were split into two fractions. One fraction was used in the kinase assay. The kinase reaction was initiated by adding kinase buffer containing 500 mM STAT5 target peptide (AKAADGYVKPQIKQVV), 125 μM ATP, and 10 μCi of 32P-labeled γATP to each immunoprecipitate. Aliquots were taken from the kinase reaction at various time points and blotted on P81 papers. The P81 paper was washed in 75 mM H3PO4, scintillation fluid (Cytoscint [BP Biomedical]) was added, and bound 32P was counted by using a Packard scintillation counter. The second immunoprecipitate fraction was used to measure Jak2 content used to normalize kinase assay data. This fraction was resolved by SDS-PAGE, transferred to nitrocellulose, and then immunoblotted with a monoclonal antibody directed against the total Jak2 or a monoclonal antibody directed against the HA epitope. Fluorescent secondary antibody was used, and immunoreactive bands were imaged and quantified by using an Odyssey infrared imaging system (LI-COR Biosciences).
RESULTS
Identification of Jak2 phosphorylation sites.
We used MS analysis of Jak2 protein to identify phosphorylation sites on Jak2. We prepared Jak2 by overexpression in HEK293 cells. To enable cytokine-mediated activation, we cotransfected into HEK293 cells plasmids encoding Jak2 and ELR (a chimeric receptor containing the extracellular domain of the erythropoietin receptor and the intracellular domain of LepRb, which effectively places the LepRb intracellular domain under the control of erythropoietin). ELR was utilized because it is expressed more highly and allows more robust activation of Jak2 compared to native LepRb. HEK293 cells expressing Jak2 protein were stimulated with erythropoietin (Epo) for 15 min before lysis and immunoprecipitation to isolate Jak2. Immunoprecipitates were resolved by SDS-PAGE and stained with Coomassie blue to identify Jak2 protein. Jak2 bands were subjected to proteolytic cleavage and analyzed by two MS instruments, a linear ion trap mass spectrometer and a hybrid quadrupole time-of-flight mass spectrometer. Analysis of the MS/MS spectra revealed numerous precursor ions that represented phosphorylated Jak2 peptides, each of which was observed in multiple runs and conformed to strict standards of confidence by software analysis. Some of these had previously been noted in the literature or on scientific websites (1, 8, 9, 18, 21, 23, 25, 26; www.phosphosite.org), while others (three Tyr and two Ser/Thr residues) were novel; most (11 sites overall) had not been functionally characterized (Table 1).
TABLE 1.
Jak2 phosphorylation sites identified by MS/MS in the present study
| Residue(s) | Jak2 phosphorylation sitea | Previous identificationb | Functionc |
|---|---|---|---|
| Tyr | 134 | Novel | NS |
| 201 | 18 | SHP-2 binding? | |
| 206 | 18 | NS | |
| 221 | 1,8, 25 | Weakly activating? | |
| 317 | Novel | NS | |
| 372 | Novel | NS | |
| 423 | Psite | NS | |
| 435 | Psite | NS | |
| 570 | 1, 8, 25 | Inhibitory | |
| 637 | 25 | NS | |
| 813 | 23, 25 | Activating/SH2B binding | |
| 868 | 25* | NS | |
| 966 | 25* | NS | |
| 1007/8 | 9 | Activating | |
| Ser/Thr | 523 | 21, 26 | Inhibitory |
| 668 | Novel | NS | |
| 904 | Novel | NS |
That is, the sites identified with high confidence (each site has been observed in multiple runs, conforms to strict confidence standards, and was manually confirmed).
References for any previous description of the site are listed, or its novelty is indicated. PSite, reported but not otherwise studied at www.phosphosite.org. *, the subject of a separate study (L. S. Argetsinger and C. Carter-Su, unpublished data).
Any known or presumptive function for the site is indicated. NS, not previously studied.
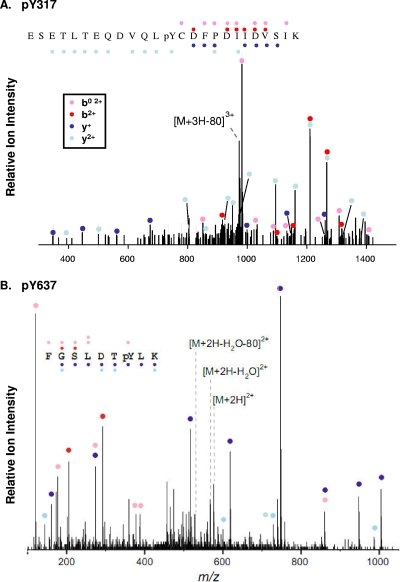
With respect to Tyr317 and Tyr637, which represent the focus of this report, analysis of MS/MS spectra from the linear trap instrument using TurboSequest revealed a 3+ charged species corresponding to a Tyr317-containing Jak2 tryptic peptide ESETLTEQDVQL(P)YCDFPDIIDVSIK with Xcorr 3.74. The spectra showed an ion consistent with the neutral loss of 80 Da resulting from the loss of HPO3 from a triply charged phosphotyrosine-containing peptide (Fig. 1A). Analysis of quadrupole time-of-flight MS/MS spectra using Mascot revealed a 2+ charged precursor corresponding to the Tyr637-containing Jak2 tryptic peptide FGSLDT(P)YLK with a score of 51. The spectrum also shows an ion consistent with neutral lost of 80 Da resulting from the loss of HPO3 from the doubly charged phosphotyrosine-containing peptide (Fig. 1B). Manual inspection and interpretation of fragmentation spectra confirmed peptide sequences in these and the other noted cases.
FIG. 1.
Representative MS/MS spectra (for pY317 and pY637). MS/MS spectra from Jak2 peptides containing pY317 (A) and pY637 (B) are shown. Sequence assignments of y+ and y+ ions are shown in blue and light blue, respectively. The assignments of b+ ions, and derivative b0+ and a+ ions are presented in red and pink, respectively. An ion corresponding to neutral loss of 80 Da from each precursor is shown.
Screening candidate phosphorylation sites for functional relevance.
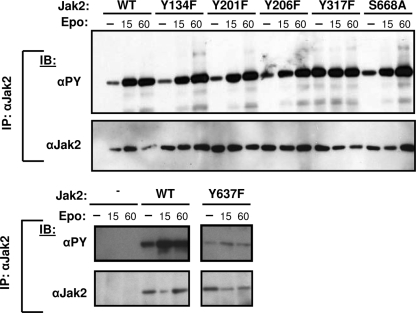
In order to determine which of the identified Jak2 phosphorylation sites merited in-depth study, we mutated each site to a nonphosphorylatable residue (using either a Tyr→Phe or Ser→Ala substitution) to create a panel of phosphorylation site-defective Jak2 molecules and examined the basal and hormone-stimulated tyrosine phosphorylation of each Jak2 mutant after coexpression with ELR in HEK293 cells (Fig. 2). While antiphosphotyrosine (αPY) immunoreactivity in immunoblotting represents an indirect measure of Jak2 function, multiple studies have revealed a correlation with Jak2 activity (7, 9), and this analysis should reveal any alterations in Jak2 activity resulting from the loss of a specific phosphorylation site. This analysis revealed two mutants for phosphorylation sites lying outside of the kinase domain that differed significantly from WT Jak2 with respect to αPY immunoreactivity. Jak2Y317F exhibited high αPY immunoreactivity in both stimulated and unstimulated states, suggesting that phosphorylation at Tyr317 (a novel site of Jak2 phosphorylation) inhibits Jak2 activity (Fig. 2). Jak2Y637F, conversely, exhibited reduced αPY immunoreactivity in both stimulated and unstimulated states, suggesting that phosphorylation at Tyr637 (a previously identified phosphorylation site that has not been functionally studied [25]) is required for maximal Jak2 activity (Fig. 2). Based on these screening data, Tyr317 and Tyr637 were chosen for further study. A number of sites within the kinase domain also modulate Jak2 tyrosine phosphorylation; the in-depth analysis of these sites will be reported elsewhere (C. Carter-Su and L. S. Argetsinger, unpublished data).
FIG. 2.
Representative examples of mutant screening data. HEK293 cells were transfected with the indicated Jak2 or Jak2 mutant plasmid. Cells were made quiescent overnight. Cells were either lysed without treatment (0) or incubated in the presence of Epo (12.5 U/ml) for either 15 or 60 min and then lysed. Lysates were immunoprecipitated (IP) with an antibody to the total Jak2. Immunoprecipitates were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted (IB) with the indicated antibodies. The results shown are typical of three independent experiments.
Phosphorylation of Jak2 Tyr317 and Tyr637 during cytokine stimulation.
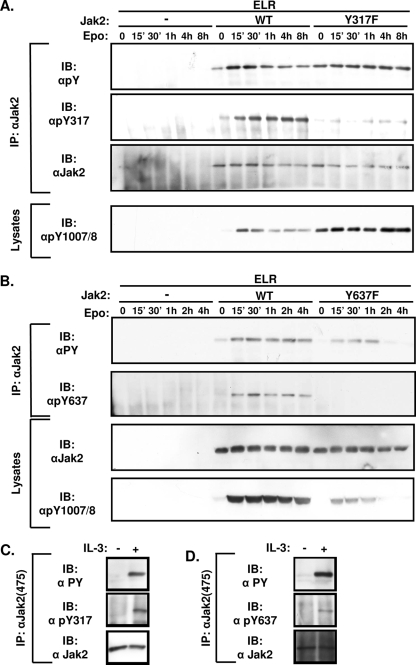
To examine the phosphorylation status of these sites under different conditions, we generated antibodies against phosphorylated Tyr317 and phosphorylated Tyr637 (αpY317 and αpY637, respectively). To examine the phosphorylation of Tyr317 and Tyr637 and the function of the Jak2 molecules mutated at these sites more thoroughly, we coexpressed ELR with either Jak2, Jak2-Y317For Jak2Y637F in HEK293 cells and stimulated them with Epo for various times before lysis. Cell lysates or Jak2 immunoprecipitates were immunoblotted with αPY, αpY1007/8 (which recognizes the phosphorylated activation loop of Jak2), αJak2, αPY, and αpY317 or αpY637 (Fig. 3).
FIG. 3.
Time course of phosphorylation of Tyr317 and Tyr637 after cytokine stimulation. (A and B) HEK293 cells were transfected with ELR and the indicated Jak2 isoform, made quiescent, and incubated with Epo (12.5 U/ml) for the indicated amounts of time before being lysed. Lysates were immunoprecipitated (IP) with the indicated antibodies. Lysates or immunoprecipitated proteins (as indicated) were resolved by SDS-PAGE and transferred to nitrocellulose for immunoblotting (IB) with the indicated antibodies. The figures shown are typical of multiple independent experiments. (C and D) 32D cells were made quiescent for 4 h and then incubated with either serum-free medium or undiluted IL-3 containing WEHI cell conditioned medium for 1 h (C) or 30 min (D) before lysis. Lysates were immunoprecipitated with an antibody to the total Jak2. Immunoprecipitates were resolved by SDS-PAGE, transferred to nitrocellulose, and blotted with the indicated antibodies. The results shown are representative of multiple independent experiments.
With respect to Jak2Y317F, we found high baseline αPY and αpY1007/8 immunoreactivity, which increased only slightly upon stimulation and remained elevated for the duration of the experiment (Fig. 3A). This contrasts with Jak2, in which αPY and αpY1007/8 immunoreactivity increased quickly and peaked in the first 30 min of stimulation before slowly decreasing over the course of several hours. The minimal regulation of the tyrosine phosphorylation of Jak2Y317F by Epo may suggests that this Jak2 mutant is close to maximal tyrosine phosphorylation at baseline, limiting further increases with cytokine stimulation. The failure of αpY317 to react with Jak2Y317F suggests the specificity of this antibody preparation for phosphorylated Tyr317. Phosphorylation at Tyr317 on Jak2, detected by αpY317 immunoblotting, peaked later than tyrosine phosphorylation or activation loop phosphorylation and remained elevated for at least 8 h after stimulation, suggesting a potential role for the phosphorylation of Tyr317 in the feedback inhibition of Jak2 activity following cytokine stimulation.
With respect to Jak2Y637F, overall αPY and αpY1007/8 immunoreactivity was decreased compared to Jak2 at baseline and over the entire time course of stimulation (Fig. 3B). The stimulation of αpY637 reactivity on Jak2 by cytokine treatment and the failure of αpY637 to react with Jak2Y637F revealed the specificity of this antibody for phosphorylated Tyr637. The phosphorylation of Tyr637 increased rapidly during the first 30 min of stimulation before declining (as for overall αPY and αpY1007/8 immunoreactivity), suggesting a potential role for the phosphorylation of Tyr637 in the activation of Jak2 during cytokine signaling. We also observed IL-3-stimulated phosphorylation of Tyr317 and Tyr637 on endogenous Jak2 purified from 32D myeloid progenitor cells (21, 35) (Fig. 3C and D), suggesting the phosphorylation of these sites in response to multiple cytokines.
Transphosphorylation of Tyr317 and Tyr637 during cytokine stimulation.
Protein autophosphorylation is often ordered and can occur by intramolecular (cis) or intermolecular (trans) mechanisms. To determine the potential the mechanisms underlying phosphorylation at Tyr317 and Tyr637 and their timing relative to the activating Tyr1007/1008 phosphorylation, we generated HA epitope-tagged Jak2 (Jak2-HA) and two different inactive variants of Jak2-HA. The mutation in Jak2-HAK882E blocks ATP binding to the kinase domain, while the alteration in Jak2-HAY1007/8F blocks the phosphorylation of the activation loop in the Jak2 kinase domain. We coexpressed these Jak2-HA constructs with ELR plus a FLAG epitope-tagged WT Jak2 (Jak2-FLAG) and used αHA to immunoprecipitate the Jak2-HA isoforms in order to assess their phosphorylation status with phospho-specific antibodies (Fig. 4). The finding of similarly insignificant amounts of αHA-precipitable αFLAG-reactive Jak2 under each condition (including without Jak2-HA isoforms [see overexposed αFLAG panel in Fig. 4]) revealed that the signal from each phospho-specific antibody is attributable to the precipitated Jak2-HA isoform. Although the phosphorylation of Jak2-HA on Tyr1007/8, Tyr317 and Tyr637 was consistently greater than for Jak2-HAK882E, each of these sites was phosphorylated on the ATP-binding mutant, a finding consistent with the transphosphorylation of these sites by Jak2-FLAG (Fig. 4). The decreased phosphorylation of Jak2-HAK882E relative to Jak2-HA could reflect a combination of absent cis autophosphorylation of Jak2-HAK882 (which might occur in Jak2-HA), decreased total kinase activity in Jak2-HAK882-expressing cells, and/or an altered conformation of the inactive Jak2-HAK882E. Interestingly, the decreased phosphorylation of Tyr317 and Tyr637 on Jak2-HAK882E was more pronounced than the decrease in total αPY reactivity: while αPY reactivity per unit of HA-immunoreactive protein on stimulated Jak2-HAK882E was decreased to ca. 40% of Jak2-HA levels, phosphorylation at Tyr317 and Tyr637 on stimulated Jak2-HAK882E was decreased to ca. 5 and 10% of Jak2-HA levels, respectively. This difference suggests a stricter requirement for Jak2 kinase activity in order to phosphorylate Tyr317 and Tyr637 compared to other major sites of tyrosine phosphorylation on Jak2. In addition, in Jak2-HAY1007/8F, which cannot undergo the conformational change associated with activation loop phosphorylation, the phosphorylation of both Tyr317 and Tyr637 was further decreased to ca. 1% compared to Jak2-HA (relative to HA-immunoreactive protein and relative to total αPY immunoreactivity). Thus, these data are consistent with the transphosphorylation of Tyr317 and Tyr637 and also with the requirement for Tyr1007/8- and activity-dependent conformational changes to enable the phosphorylation of Tyr317 and Tyr637 during Jak2 activation, placing the phosphorylation of these sites downstream of the initial phosphorylation event of kinase activation.
FIG. 4.
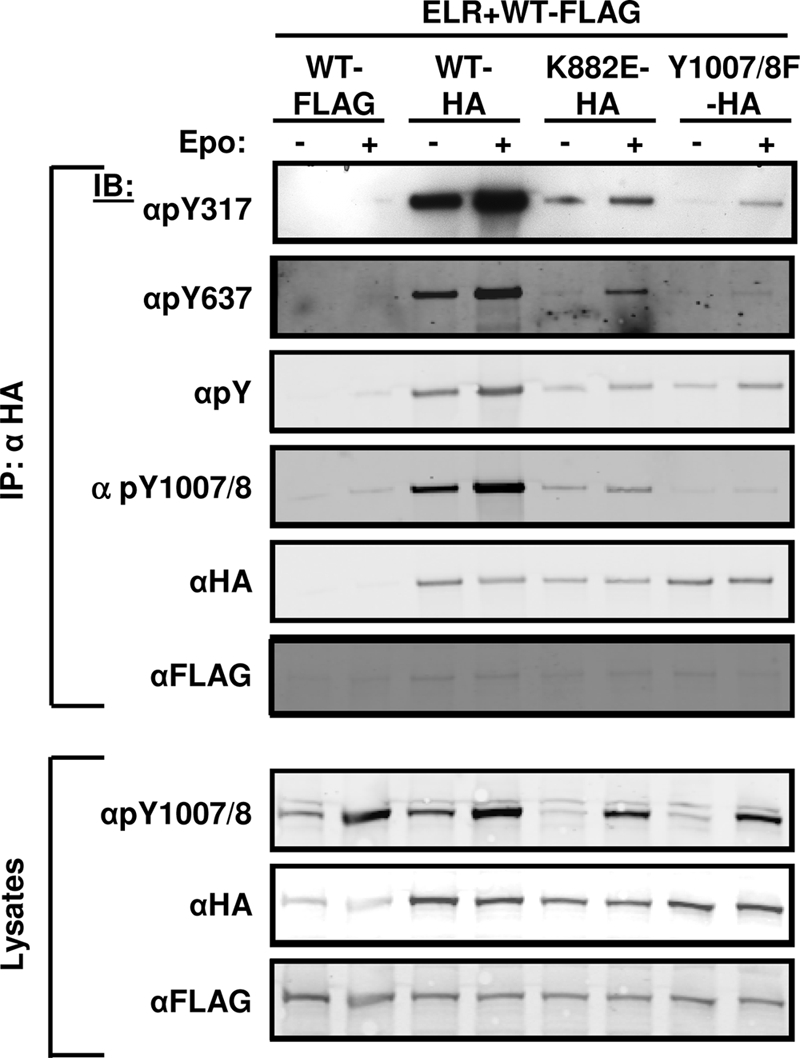
Transphosphorylation of Tyr317 and Tyr637 in response to cytokine stimulus. HEK293 cells were transfected with the indicated plasmids, made quiescent overnight, and incubated in the absence (−) or presence (+) of Epo (12.5 U/ml) for 15 min before lysis. Lysates were immunoprecipitated (IP) with αHA antibody. Immunoprecipitates or lysates (as indicated) were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted (IB) with the indicated antibodies. The results shown are typical of multiple independent experiments.
Effects of Tyr317 and Tyr637 on cytokine signaling.
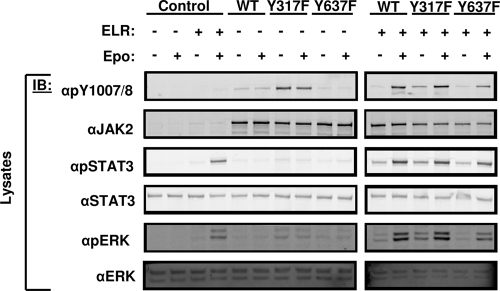
LepRb/Jak2 signaling promotes the activation of STAT3 via the phosphorylation of Tyr1138 on LepRb, and mediates the activation of ERK both via the phosphorylation of Tyr985 on LepRb and via Jak2-mediated signals that are independent of LepRb phosphorylation sites (3, 4). To determine the potential roles for Tyr317 or Tyr637 on these downstream pathways, we measured the activation of ERK and STAT3 by assessing their phosphorylation in cells expressing Jak2, Jak2Y317F, or Jak2Y637F in the presence or absence of ELR (Fig. 5). Consistent with its increased αpY1007/8 immunoreactivity, Jak2Y317F mediated increased downstream signaling to STAT3 and ERK compared to Jak2 in the presence of ELR. Across multiple experiments, Jak2Y317F mediated an ∼2-fold increase in phosphorylated STAT3 and phosphorylated ERK in unstimulated cells (n = 3, P < 0.05). The levels of these activated proteins were not significantly different in stimulated cells. In contrast to Jak2Y317F, Jak2Y637 exhibited less αpY1007/8 immunoreactivity and downstream STAT3 and ERK signaling than Jak2. Across multiple experiments, Jak2Y637F mediated an ∼40% decrease in phosphorylated STAT3 and phosphorylated ERK in stimulated cells (n = 3, P < 0.05). The levels of these activated proteins were not significantly different in unstimulated cells. Taken together, these data suggest that alterations in STAT3 and ERK activation are likely the result of changes in Jak2 kinase activity rather than any pathway-specific interactions that the phosphorylation at Tyr317 or Tyr637 might have on these pathways.
FIG. 5.
Effects of Tyr317 and Tyr637 on downstream signaling. HEK293 cells were transfected with the indicated plasmids, made quiescent overnight, and incubated in the absence (−) or presence (+) of Epo (12.5 U/ml) for 15 min before lysis. Lysates were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted (IB) with the indicated antibodies. The results shown are typical of multiple independent experiments.
Failure of negatively charged mutations of Tyr317 and Tyr637 to permit normal Jak2 activity.
Tyrosine phosphorylation may regulate protein function in many ways, including by the alteration of protein conformation by the addition of negative charge that accompanies the addition of a phosphate group. In order to determine whether this mechanism might operate for Tyr317 and/or Tyr637, we replaced these residues with the negatively charged amino acid Glu to generate Jak2Y317E and Jak2Y317E and studied their activity relative to Jak2 and their respective Tyr→Phe mutants (Fig. 6). We found that Jak2Y317E, like Jak2Y317F, was highly active in both stimulated and unstimulated states (Fig. 6A). Furthermore, Jak2Y637E was less active than Jak2 and exhibited similar or decreased activity compared to Jak2Y637F (Fig. 6B). Thus, the addition of a negative charge at these sites is not sufficient to mimic the effects of tyrosine phosphorylation. Neither are the altered activities of Jak2Y317F and Jak2Y637F likely due to nonspecific effects resulting from the substitution of hydrophobic Phe in place of Tyr, since both Glu and Phe produce the same phenotype at each of these sites.
FIG. 6.
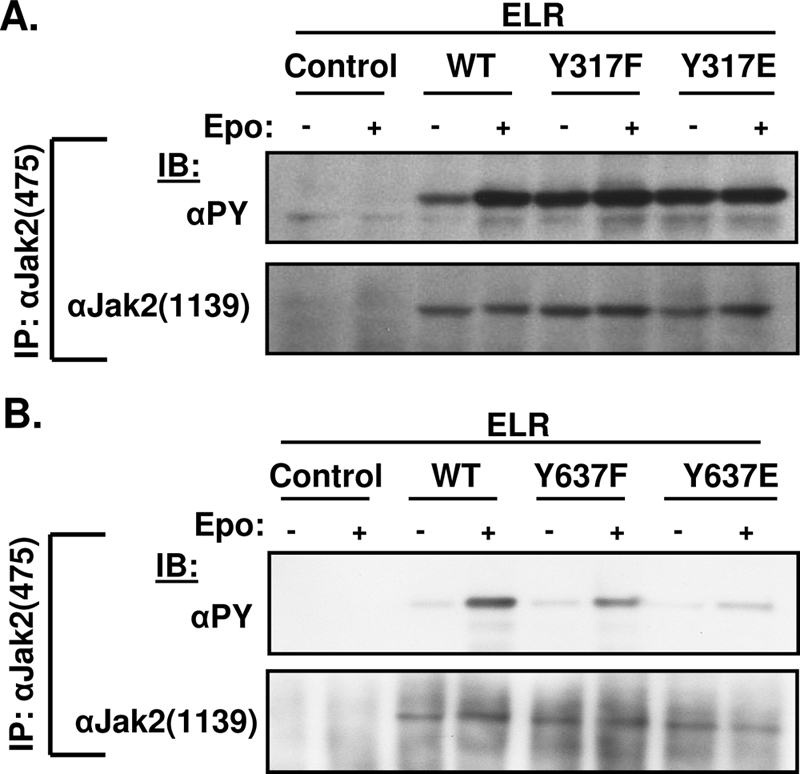
Similar phenotypes of Phe and Glu substitution mutants for Tyr317 and Tyr637. (A and B) HEK293 cells were transfected (IP) with the indicated plasmids, made quiescent overnight, and incubated in the presence or absence of Epo (12.5 U/ml) before lysis. Lysates were immunoprecipitated (IP) with the indicated antibody. Immunoprecipitates or lysates (as indicated) were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted (IB) with the indicated antibodies. The figures shown are typical of multiple independent experiments.
Regulation of Jak2 kinase activity by Tyr317 and Tyr637.
While αPY and αpY1007/8 immunoreactivity generally correlate well with Jak2 kinase activity, this relationship breaks down to some extent at high levels of overall Jak2 phosphorylation, where further increases in phosphorylation become difficult to detect. To gain a more accurate indication of enzymatic activity, we directly measured the in vitro kinase activity of immunoaffinity-purified Jak2 and Jak2Y317F toward a target peptide derived from STAT5B, a known substrate of Jak2 (1, 17) (Fig. 7A). These data demonstrated increased in vitro kinase activity for purified Jak2Y317F compared to Jak2. Importantly, these data also show that the Jak2Y317F tyrosine kinase, while highly active at baseline, remains responsive to Epo stimulation, suggesting that the mutation of this site does not uncouple kinase function from receptor-mediated regulation. Jak2Y637F displayed reduced kinase activity compared to Jak2 in the stimulated state (Fig. 7B). Jak2Y637F also undergoes activation upon ligand stimulation in the presence of ELR, however. These data confirm the ability of ELR stimulation to increase kinase activity and downstream signaling by Jak2Y317F and Jak2Y637F, suggesting that neither of these sites is required for functional regulation by cytokine receptors.
FIG. 7.
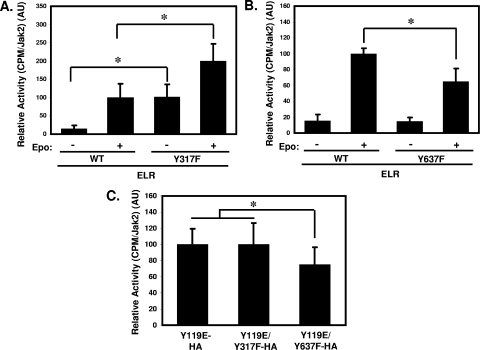
Modulation of Jak2 kinase activity by Tyr317 and Tyr637. (A and B) HEK293 cells were transfected with the indicated plasmids made quiescent overnight before treatment with Epo or vehicle and then lysed. Lysates were immunoprecipitated with an antibody to the total Jak2. Immunoprecipitates were subjected to in vitro kinase assays using a target peptide derived from STAT5B. Scintillation counts were normalized by Jak2 content (determined by immunoblotting aliquots of each sample in parallel with the kinase assay) and are plotted in arbitrary units (AU). Bars represent the mean value derived from two independent experiments with two replicates each (n = 4, total) ± the standard deviations. Values from each experiment were normalized by the mean value of the Jak2-stimulated samples, which was set at 100 for each experiment. *, P < 0.05 (Student unpaired t test). (C) HEK293 cells were transfected with the indicated plasmids made quiescent overnight and then lysed. Lysates were immunoprecipitated with αHA. Immunoprecipitates were subjected to in vitro kinase assays using a target peptide derived from STAT5B. Scintillation counts were normalized by HA content in each immunoprecipitate and are plotted in AU. Bars represent the mean value derived from three independent experiments with four replicates each (n = 12, total) ± the standard deviations. Values from each experiment were normalized by the mean value of the Jak2Y119E samples, which was set at 100 for each experiment. *, P < 0.05 (Student unpaired t test).
Regulation of Jak2 by Tyr317 and Tyr637: a role for cytokine receptor interaction.
In addition to the modulation of Jak2 activity by ligand binding to Jak2-associated receptors, the association of Jak2 with cytokine receptors promotes Jak2 activity (baseline Jak2 activity increases in the receptor-bound, relative to the unbound, state), presumably by modulating the conformation of Jak2 (13, 14). The phenotypes of Jak2Y317F and Jak2Y637F are independent of ELR expression in 293 cells (Fig. 5), but it is not clear whether endogenous cytokine receptors in HEK293 cells bind to Jak2 in the absence of ELR to permit the manifestation of potential receptor-dependent effects on signaling.
To determine whether the roles for Tyr317 and Tyr637 in the regulation of Jak2 function might be independent of cytokine receptor-FERM domain interactions, we examined Jak2Y317F and Jak2Y637F activity in the context of Jak2Y119E (Jak2Y119E/Y317F and Jak2Y119E/Y637F). Phosphorylation of Tyr119 disrupts Jak2-FERM domain interaction to most Jak2-binding cytokine receptors, and a Tyr→Glu substitution at Tyr119 mimics this effect (13, 14). We thus compared the kinase activity of Jak2Y119E, Jak2Y119E/Y317F, and Jak2Y119E/Y637F to determine whether FERM domain-cytokine receptor interactions were required for modulation of Jak2 activity by Tyr317 or Tyr637. We found that the activating effect of Tyr317 mutation was eliminated in the context of Jak2Y119E, while the inhibitory response to alteration of Tyr637 remained in Jak2Y119E/Y637F (Fig. 7C). This suggests that the FERM domain-mediated interaction between Jak2 and cytokine receptors is necessary for the negative regulation of Jak2 by phosphorylation at Tyr317 (presumably reflecting a requirement for FERM domain conformation), while the effect of Tyr637 on Jak2 kinase activity occurs independently of Tyr119. Since Jak2Y119E is still capable of binding gamma interferon receptors (13), it is possible that some residual receptor/Jak2Y119E binding could contribute to Tyr637 mutation-induced decrements in kinase activity, although the ability of the Tyr119→Glu substitution to abrogate the activating effect of Tyr317 mutation suggests the likely independence of Tyr637 function from FERM domain requirements.
Functional interaction of Tyr317 and Tyr637 with other regulatory sites on Jak2.
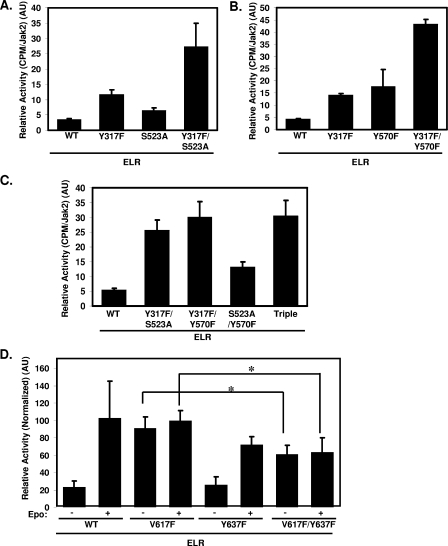
Tyr317 represents the third inhibitory phosphorylation site that we have identified on Jak2, since phosphorylation at Ser523 and Tyr570 also inhibits Jak2 (1, 8, 21, 26). To determine the relative contributions of these sites to Jak2 activity, we compared the kinase activity of Jak2Y317F, Jak2S523A, Jak2Y570F, and several Jak2 isoforms containing various combinations of mutations at these inhibitory sites (Fig. 8A to C). The cytokine-stimulated activity of each of these mutants was near maximal and relatively similar (data not shown), and we therefore focused on the kinase activity at baseline in the absence of cytokine stimulation. Jak2S523A, while more active than Jak2 (21, 26), was less active than Jak2Y317F, suggesting a stronger effect of Tyr317-mediated inhibition than that of Ser523-mediated inhibition (Fig. 8A). Mutation at both sites produced additive effects, however, suggesting the independent effects of these phosphorylation sites on Jak2 activity. The activities of Jak2Y317F and Jak2Y570F were increased relative to Jak2 and similar in magnitude; the additive effects of mutation at these sites also suggested independent effects on Jak2 activity (Fig. 8B). Comparing the kinase activity of each double mutant with the Jak2Y317F/S523A/Y570F triple mutant revealed that that the most active Jak2 species contain the Tyr317 mutation, and the triple mutant demonstrated no increased activity compared to Y317F-containing double mutants, underlining the importance of Tyr317 for the appropriate suppression of Jak2 activity (Fig. 8C).
FIG. 8.
Functional interaction of Tyr317 and Tyr637 with other regulatory sites on Jak2. (A to C) HEK293 cells were transfected with the indicated plasmids made quiescent overnight and then lysed. Lysates were immunoprecipitated with an antibody to the total Jak2. Immunoprecipitates were subjected to in vitro kinase assays using a target peptide derived from STAT5B. Scintillation counts were normalized by the Jak2 content in each immunoprecipitate and are plotted in AU. Bars indicate the mean of duplicate samples (n = 2). Error bars indicate the standard deviations. (D) HEK293 cells were transfected with the indicated plasmids, made quiescent overnight, and incubated in the presence or absence of Epo (12.5 U/ml) before lysis. Lysates were immunoprecipitated with an antibody to the total Jak2. Immunoprecipitates were subjected to in vitro kinase assays using a target peptide derived from STAT5B. Scintillation counts were normalized by Jak2 content in each immunoprecipitate and are reported in AU. Bars represent the mean values derived from two independent experiments with two replicates each (n = 4, total) ± the standard deviations. Values from each experiment were normalized by the mean value of the Jak2V617F-stimulated samples, which was set at 100. *, P < 0.05 (Student unpaired t test).
Somatic mutation that substitutes Phe in place Val617 of Jak2 (Jak2V617F) mediates the constitutive activation of Jak2 that underlies most cases of polycythemia vera and some portion of other myeloproliferative disorders (28). Val617, like Tyr637, lies in the JH2 pseudokinase domain of Jak2, and their close proximity suggested the potential for functional interactions between the two residues in the regulation of JH2-mediated control of kinase activity. To determine the ability of Tyr637 mutation to suppress the activity of Jak2V617F, we compared the kinase activities of Jak2, Jak2V617F, Jak2Y637F, and Jak2V617F/Y637F. Interestingly, Jak2V617F/Y637F is less active than Jak2V617F, demonstrating that Tyr637 influences kinase activity in Jak2V617F (Fig. 8D). Although Jak2V617F/Y637F is less active than Jak2Y617F, it remains more active than Jak2 at baseline, suggesting that Tyr637 contributes to only a portion of Val617-mediated Jak2 activation.
DISCUSSION
Although many sites of Jak2 phosphorylation have been identified, previous analyses have suggested the presence of others; the function of most Jak2 phosphorylation sites has also remained unclear. Since the analysis of in vitro autophosphorylated Jak2 may not reveal the full complement of physiologically important phosphorylation sites (especially Ser/Thr phosphorylation sites), we utilized MS analysis of Jak2 protein prepared from mammalian cells following cytokine receptor activation in order to identify phosphorylation sites on Jak2. This analysis revealed 18 phosphorylation sites on Jak2, including 15 Tyr phosphorylation sites and 3 Ser/Thr phosphorylation sites. Of these, 12 sites had been previously published by ourselves or others (1, 8, 9, 18, 21, 23, 25, 26), and two had been presumptively identified by MS analysis reported (www.phosphosite.org). Thus, our present analysis identified three novel sites of Tyr phosphorylation and two novel sites of Ser/Thr phosphorylation on Jak2. The MS data for each of the sites we report here possessed high confidence scores by initial software analysis, and their MS/MS spectra were manually verified and generally showed specific evidence of phosphorylation, such as fragment ions consistent with neutral phosphate loss. Our analysis did not identify some previously reported sites of phosphorylation, including Tyr119 in the FERM domain and Tyr972 (as well as a number of other presumptive sites) in the kinase domain: although spectra potentially consistent with many of these sites were noted, they did not pass our rigorous criteria for inclusion, presumably due to the behavior of the peptides on MS/MS analysis.
We initially assayed the potential importance of these sites by analyzing the basal and ligand-stimulated tyrosine phosphorylation of Jak2 molecules mutant for each identified site and focused our subsequent functional analysis on the two sites outside of the kinase domain that altered Jak2 activity when mutated: Tyr317 and Tyr637. Additional sites within the kinase domain also regulate Jak2 kinase activity (including Tyr868, Tyr966, and Tyr972); these represent the topic of another study (Carter-Su and Argetsinger, unpublished). Since our screening assay most sensitively detects large changes in Jak2 activity, we cannot rule out more modest roles in Jak2 regulation for additional sites. Since this screen focused on Jak2 phosphorylation, it is also possible that some of the identified sites may play other, unknown, roles in downstream signal transmission for which no assays currently exist.
While cytokine receptor stimulation mediates the phosphorylation of both Tyr317 and Tyr637, these residues oppositely regulate Jak2-dependent signaling: the mutation of Tyr317 enhances Jak2 function, suggesting a role for the phosphorylation of Tyr317 in the inhibition of Jak2. Conversely, mutation of Tyr637 reduces Jak2 signaling, suggesting a role for the phosphorylation of this residue in the activation of Jak2. Tyr637 lies in the highly conserved pseudokinase domain of Jak2, and a corresponding residue is also present in Jak3, suggesting potential partial conservation of the function of this site across the Jak kinase family. In contrast, Tyr317 is not conserved with any other member of the Jak family of kinases, suggesting the mechanism by which Tyr317 affects protein function is Jak2 specific.
With respect to both Tyr317 and Tyr637, the functional equivalence of Glu substitution of either of these sites compared to Phe indicates that the negatively charged phosphate in the context of Tyr is required for the function of both Tyr317 and Tyr637, suggesting that criteria stricter than a simple negative charge must be met to mediate the presumptive intramolecular interactions that underlie the function of these sites. The finding that Glu and Phe substitutions mediate similar affects and the mutants are regulated by cytokine stimulation in a manner proportional to their baseline activity also suggests that nonspecific disruption of Jak2 domain structure does not underlie the phenotype of these mutants.
Our data suggest that the interaction of Jak2 with cytokine receptors and/or the conformation of the FERM domain is required for the modulation of Jak2 activity by Tyr317, which is consistent with the location of Tyr317 in the FERM domain. The decreased activity of free Jak2 (e.g., Jak2Y119E) compared to receptor-associated Jak2 (6, 13, 31), the ability of receptor stimulation to promote the increased activity of Jak2Y317F, and the placement of Tyr317 in the extreme COOH terminus of the FERM domain (beyond the presumptive receptor interaction motifs) suggest that Tyr317 is unlikely to act by altering the stability of the receptor/Jak2 complex. It is more likely that receptor interaction and/or the conformation of the FERM domain regulates the phosphorylation or function of Tyr317; this is consistent with the dependence of Tyr317 phosphorylation on the prior phosphorylation of Tyr1007/1008 in the activation loop of the kinase domain, which suggests the requirement for a specific, activated, conformation of Jak2 for the phosphorylation of Tyr317. Indeed, we did not detect Tyr317 phosphorylation on Jak2Y119E (data not shown), although we cannot rule out the possibility that any Tyr317 phosphorylation on this poorly active protein was below our detection threshold.
Our data are thus consistent with a role for Tyr317 in the attenuation of Jak2 activity following receptor activation, rather than in suppressing inactive Jak2. Indeed, the phosphorylation of Tyr317 is very low in unstimulated Jak2, increases significantly with stimulation, and is highest after full activation of Jak2. Furthermore, the phosphorylation of Tyr1007/1008 and the adoption of an activated conformation are required even for the transphosphorylation of Tyr317 by an activated Jak2 molecule. Hence, the increased baseline activity of Jak2Y317F in cells prior to cytokine stimulation may result from the inability to inactivate the normally small portion of Jak2 that becomes activated in quiescent HEK293 cells; the failure to return Jak2Y317F to the pool of inactive Jak2 would eventually lead to the accumulation of Jak2 protein that is locked in the active state.
Mutation of Tyr637 inhibits kinase activity under most of the conditions we tested: the activity of Jak2Y637F was decreased in the absence or presence of receptor and/or ligand activation and in the context of a Jak2 molecule that fails to bind most cytokine receptors. This is consistent with its position in the JH2 domain of Jak2, which constitutes a major regulator of Jak2 kinase activity (11, 24, 32, 37, 38). The phosphorylation of Tyr637 during cytokine stimulation may thus act to alleviate JH2-mediated inhibition to promote the appropriate robust activation of Jak2 kinase activity. Indeed, while the phosphorylation of Tyr637 requires the prior phosphorylation of Tyr1007/1008 (which promotes kinase activity by altering the conformation of the kinase domain), the phosphorylation and dephosphorylation of Tyr637 progresses with a time course very similar to that of Tyr1007/1008. That mutation of Tyr637 partially suppresses the activating V617F JH2 mutation is also consistent with a role for Tyr637 in reducing JH2-mediated inhibition of kinase function.
The activated phenotype of Jak2 molecules mutated at Ser523, Tyr317, and Tyr570 suggests that the phosphorylation of each residue acts to inhibit the activity of Jak2. Ser523 exhibits several differences compared to Tyr317 and Tyr570, however. Not only is the Ser523-mediated inhibition weaker than that mediated by these other two sites (as shown by the relatively modest activation exhibited by its cognate Jak2 mutant), but Ser523 is also highly (stoichiometrically) phosphorylated in the baseline state and not further phosphorylated during cytokine stimulation, at least in rapidly proliferating cells such as HEK293 and 32D cells (21). In contrast, Tyr317 and Tyr570 are phosphorylated upon cytokine receptor/Jak2 activation and more strongly inhibit Jak2 kinase activity. Interestingly, the phosphorylation of Tyr317 is delayed compared to that of activating sites such as Tyr637 and Tyr1007/1008, depends upon prior phosphorylation of Tyr1007/1008 and an active conformation of Jak2, and remains highly phosphorylated even after these activating sites become relatively dephosphorylated.
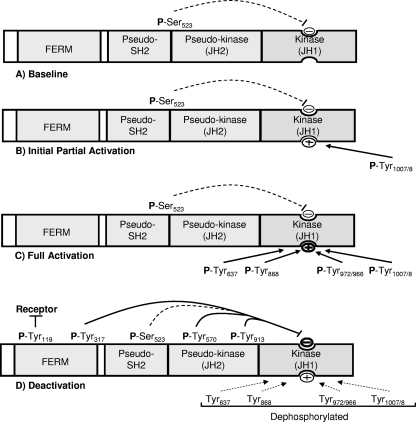
Together, these observations suggest a model of phosphorylation-dependent regulation of Jak2 (Fig. 9) in which the baseline phosphorylation of Ser523 may serve to preserve the relative inactivity of Jak2 in the absence of cytokine stimulation. Since few forces promote the activation of Jak2 in the absence of stimulation, the relatively modest Ser523-mediated inhibition suffices to suppress Jak2 activation in the absence of ligand binding, while the strong cytokine-mediated stimulus can overcome the weak inhibition by Ser523. Upon cytokine stimulation, the rapid phosphorylation of Tyr1007/1008 alters conformation of the JH1 domain activation loop and partially activates the kinase, permitting the phosphorylation of Tyr637, which promotes further Jak2 activation. Other sites in the kinase domain, such as Tyr868, Tyr966, and Tyr972, may also be involved in fully activating Jak2 (27; Carter-Su and Argetsinger, unpublished).
FIG. 9.
Model of phosphorylation-dependent regulation of Jak2 activity. (A) Without stimulation, Jak2 is essentially unphosphorylated on most sites, although Tyr523 is constitutively phosphorylated, providing a baseline of negative feedback to block kinase activity in the absence of stimulation. (B) Upon cytokine stimulation, Tyr1007/8 (the activation loop sites) are rapidly phosphorylated to promote partial activation of the kinase domain. (C) The phosphorylation of Tyr1007/1008 permits the phosphorylation of additional activating sites, including Tyr637, as well as other sites in kinase domain, leading to full activation of Jak2. (D) After acute activation, strongly inhibitory sites, including Tyr317, Tyr570, Tyr913, and Tyr119, are phosphorylated to limit the extent of Jak2 activity, concomitant with the dephosphorylation of activating sites.
The subsequent dephosphorylation of these stimulatory sites coincides with the relatively slower phosphorylation of strong inhibitory sites, such as Tyr317 (and Tyr570) to mediate feedback inhibition to limit the duration of the signal. Prolonged phosphorylation of the strong inhibitory sites also protects against continued high activity. The phosphorylation of Tyr119 (which promotes receptor/Jak2 dissociation) and the inhibitory Tyr913 in the kinase domain is also slightly delayed and more sustained compared to Tyr1007/1008 (13, 15), and the phosphorylation of Tyr119 and Tyr913 likely functions together with Tyr317 and Tyr570 to limit the amplitude and duration of Jak2-dependent cytokine signaling.
Thus, a substantial subset of phosphorylation sites on Jak2 function to regulate Jak2 kinase activity. Each of these phosphorylation sites plays a specific role in the choreography of Jak2 activation and deactivation and, together, they ensure appropriate levels of Jak2 activity under a variety of circumstances. The functions of many other phosphorylation sites on Jak2 remain unknown. Although certain of these may possess relatively minor functions, others presumably contribute substantially to the regulation of Jak2 kinase activity and/or mediate as-yet-undiscovered downstream signals.
Acknowledgments
This study was supported by NIH DK57631 and NIH D57768 (to M.G.M.), NIH DK34171 (to C.C.-S.), predoctoral fellowships from the American Diabetes Association and the American Heart Association (to S.A.R.), and support from the Dana Farber Cancer Institute (to J.A.M.). Support for peptide synthesis and DNA sequencing was provided by the Biomedical Research Core facilities at the University of Michigan, which are supported in part by the Michigan Diabetes Research and Training Center (NIH P60 DK20572) and the University of Michigan Comprehensive Cancer Center (NIH P30 CA46592).
Footnotes
Published ahead of print on 13 April 2009.
REFERENCES
- 1.Argetsinger, L. S., J. L. Kouadio, H. Steen, A. Stensballe, O. N. Jensen, and C. Carter-Su. 2004. Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity. Mol. Cell. Biol. 244955-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks, A. S., S. M. Davis, S. H. Bates, and M. G. Myers, Jr. 2000. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 275:14563-14572. [DOI] [PubMed] [Google Scholar]
- 3.Baumann, H., K. K. Morella, D. W. White, M. Dembski, P. S. Bailon, H. Kim, C. F. Lai, and L. A. Tartaglia. 1996. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc. Natl. Acad. Sci. USA 938374-8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorbaek, C., R. M. Buchholz, S. M. Davis, S. H. Bates, D. D. Pierroz, H. Gu, B. G. Neel, M. G. Myers, Jr., and J. S. Flier. 2001. Divergent roles of SHP-2 in ERK activation by leptin receptors. J. Biol. Chem. 276:4747-4755. [DOI] [PubMed] [Google Scholar]
- 5.Bjorbaek, C., H. J. Lavery, S. H. Bates, R. K. Olson, S. M. Davis, J. S. Flier, and M. G. Myers, Jr. 2000. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 275:40649-40657. [DOI] [PubMed] [Google Scholar]
- 6.Coppari, R., M. Ichinose, C. E. Lee, A. E. Pullen, C. D. Kenny, R. A. McGovern, V. Tang, S. M. Liu, T. Ludwig, S. C. Chua, Jr., B. B. Lowell, and J. K. Elmquist. 2005. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 163-72. [DOI] [PubMed] [Google Scholar]
- 7.Dusa, A., J. Staerk, J. Elliott, C. Pecquet, H. A. Poirel, J. A. Johnston, and S. N. Constantinescu. 2008. Substitution of pseudokinase domain residue Val-617 by large non-polar amino acids causes activation of JAK2. J. Biol. Chem. 28312941-12948. [DOI] [PubMed] [Google Scholar]
- 8.Feener, E. P., F. Rosario, S. L. Dunn, Z. Stancheva, and M. G. Myers, Jr. 2004. Tyrosine phosphorylation of Jak2 in the JH2 domain inhibits cytokine signaling. Mol. Cell. Biol. 24:4968-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng, J., B. A. Witthuhn, T. Matsuda, F. Kohlhuber, I. M. Kerr, and J. N. Ihle. 1997. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 172497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flier, J. S., and E. Maratos-Flier. 1998. Obesity and the Hypothalamus: novel peptides for new pathways. Cell 92437-440. [DOI] [PubMed] [Google Scholar]
- 11.Frank, S. J., W. Yi, Y. Zhao, J. F. Goldsmith, G. Gilliland, J. Jiang, I. Sakai, and A. S. Kraft. 1995. Regions of the Jak2 tyrosine kinase required for coupling to the growth hormone receptor. J. Biol. Chem. 27014776-14785. [DOI] [PubMed] [Google Scholar]
- 12.Friedman, J. M. 2002. The function of leptin in nutrition, weight, and physiology. Nutr. Rev. 60S1-S14. [DOI] [PubMed] [Google Scholar]
- 13.Funakoshi-Tago, M., S. Pelletier, T. Matsuda, E. Parganas, and J. N. Ihle. 2006. Receptor specific downregulation of cytokine signaling by autophosphorylation in the FERM domain of Jak2. EMBO J. 254763-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funakoshi-Tago, M., S. Pelletier, H. Moritake, E. Parganas, and J. N. Ihle. 2008. Jak2 FERM domain interaction with the erythropoietin receptor regulates Jak2 kinase activity. Mol. Cell. Biol. 281792-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funakoshi-Tago, M., K. Tago, T. Kasahara, E. Parganas, and J. N. Ihle. 2008. Negative regulation of Jak2 by its auto-phosphorylation at tyrosine 913 via the Epo signaling pathway. Cell. Signal. 201995-2001. [DOI] [PubMed] [Google Scholar]
- 16.Gadina, M., D. Hilton, J. A. Johnston, A. Morinobu, A. Lighvani, Y. J. Zhou, R. Visconti, and J. J. O'Shea. 2001. Signaling by type I and II cytokine receptors: ten years after. Curr. Opin. Immunol. 13:363-373. [DOI] [PubMed] [Google Scholar]
- 17.Ghilardi, N., S. Ziegler, A. Wiestner, R. Stoffel, and R. C. Skoda. 1996. Defective STAT signaling by the leptin receptor in diabetic mice. Proc. Natl. Acad. Sci. USA 936231-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godeny, M. D., J. Sayyah, D. Vonderlinden, M. Johns, D. A. Ostrov, J. Caldwell-Busby, and P. P. Sayeski. 2006. The N-terminal SH2 domain of the tyrosine phosphatase, SHP-2, is essential for Jak2-dependent signaling via the angiotensin II type AT(1) receptor. Cell. Signal. 19600-609. [DOI] [PubMed] [Google Scholar]
- 19.Herrington, J., and C. Carter-Su. 2001. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol. Metab. 12252-257. [DOI] [PubMed] [Google Scholar]
- 20.Ihle, J. N., W. Thierfelder, S. Teglund, D. Stravapodis, D. Wang, J. Feng, and E. Parganas. 1998. Signaling by the cytokine receptor superfamily. Ann. N. Y. Acad. Sci. 8651-9. [DOI] [PubMed] [Google Scholar]
- 21.Ishida-Takahashi, R., F. Rosario, Y. Gong, K. Kopp, Z. Stancheva, X. Chen, E. P. Feener, and M. G. Myers, Jr. 2006. Phosphorylation of Jak2 on Ser(523) inhibits Jak2-dependent leptin receptor signaling. Mol. Cell. Biol. 26:4063-4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kloek, C., A. K. Haq, S. L. Dunn, H. J. Lavery, A. S. Banks, and M. G. Myers, Jr. 2002. Regulation of Jak kinases by intracellular leptin receptor sequences. J. Biol. Chem. 277:41547-41555. [DOI] [PubMed] [Google Scholar]
- 23.Kurzer, J. H., L. S. Argetsinger, Y. J. Zhou, J. L. Kouadio, J. J. O'Shea, and C. Carter-Su. 2004. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B beta. Mol. Cell. Biol. 24:4557-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo, H., P. Rose, D. Barber, W. P. Hanratty, S. Lee, T. M. Roberts, A. D. D'Andrea, and C. R. Dearolf. 1997. Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol. Cell. Biol. 17:1562-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuda, T., J. Feng, B. A. Witthuhn, Y. Sekine, and J. N. Ihle. 2004. Determination of the transphosphorylation sites of Jak2 kinase. Biochem. Biophys. Res. Commun. 325586-594. [DOI] [PubMed] [Google Scholar]
- 26.Mazurkiewicz-Munoz, A. M., L. S. Argetsinger, J. L. Kouadio, A. Stensballe, O. N. Jensen, J. M. Cline, and C. Carter-Su. 2006. Phosphorylation of JAK2 at serine 523: a negative regulator of JAK2 that is stimulated by growth hormone and epidermal growth factor. Mol. Cell. Biol. 264052-4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDoom, I., X. Ma, A. Kirabo, K. Y. Lee, D. A. Ostrov, and P. P. Sayeski. 2008. Identification of tyrosine 972 as a novel site of Jak2 tyrosine kinase phosphorylation and its role in Jak2 activation. Biochemistry 478326-8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan, K. J., and D. G. Gilliland. 2008. A role for JAK2 mutations in myeloproliferative diseases. Annu. Rev. Med. 59213-222. [DOI] [PubMed] [Google Scholar]
- 29.Myers, M. G., Jr. 2004. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog. Horm. Res. 59287-304. [DOI] [PubMed] [Google Scholar]
- 30.Ndassa, Y. M., C. Orsi, J. A. Marto, S. Chen, and M. M. Ross. 2006. Improved immobilized metal affinity chromatography for large-scale phosphoproteomics applications. J. Proteome Res. 52789-2799. [DOI] [PubMed] [Google Scholar]
- 31.Ragimbeau, J., E. Dondi, A. Alcover, P. Eid, G. Uze, and S. Pellegrini. 2003. The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO J. 22537-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saharinen, P., and O. Silvennoinen. 2002. The pseudokinase domain is required for suppression of basal activity of Jak2 and Jak3 tyrosine kinases and for cytokine-inducible activation of signal transduction. J. Biol. Chem. 27747954-47963. [DOI] [PubMed] [Google Scholar]
- 33.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68850-858. [DOI] [PubMed] [Google Scholar]
- 34.Tartaglia, L. A. 1997. The leptin receptor. J. Biol. Chem. 2726093-6096. [DOI] [PubMed] [Google Scholar]
- 35.Ungureanu, D., P. Saharinen, I. Junttila, D. J. Hilton, and O. Silvennoinen. 2002. Regulation of Jak2 through the ubiquitin-proteasome pathway involves phosphorylation of Jak2 on Y1007 and interaction with SOCS-1. Mol. Cell. Biol. 223316-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velazquez, L., K. E. Mogensen, G. Barbieri, M. Fellous, G. Uze, and S. Pellegrini. 1995. Distinct domains of the protein tyrosine kinase tyk2 required for binding of interferon-α/β and for signal transduction. J. Biol. Chem. 2703327-3334. [DOI] [PubMed] [Google Scholar]
- 37.Yeh, T. C., E. Dondi, G. Uze, and S. Pellegrini. 2000. A dual role for the kinase-like domain of the tyrosine kinase Tyk2 in interferon-alpha signaling. Proc. Natl. Acad. Sci. USA 978991-8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao, Y., F. Wagner, S. J. Frank, and A. S. Kraft. 1995. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor beta c chain. J. Biol. Chem. 27013814-13818. [DOI] [PubMed] [Google Scholar]
- 39.Zhou, Y. J., M. Chen, N. A. Cusack, L. H. Kimmel, K. S. Magnuson, J. G. Boyd, W. Lin, J. L. Roberts, A. Lengi, R. H. Buckley, R. L. Geahlen, F. Candotti, M. Gadina, P. S. Changelian, and J. J. O'Shea. 2001. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Mol. Cell 8:959-969. [DOI] [PubMed] [Google Scholar]