Abstract
Contact inhibition is a fundamental process in multicellular organisms aimed at inhibiting proliferation at high cellular densities through poorly characterized intracellular signals, despite availability of growth factors. We have previously identified the protein kinase p38α as a novel regulator of contact inhibition, as p38α is activated upon cell-cell contacts and p38α-deficient cells are impaired in both confluence-induced proliferation arrest and p27Kip1 accumulation. Here, we establish that p27Kip1 plays a key role downstream of p38α to arrest proliferation at high cellular densities. Surprisingly, p38α does not directly regulate p27Kip1 expression levels but leads indirectly to confluent upregulation of p27Kip1 and cell cycle arrest via the inhibition of mitogenic signals originating from the epidermal growth factor receptor (EGFR). Hence, confluent activation of p38α uncouples cell proliferation from mitogenic stimulation by inducing EGFR degradation through downregulation of the EGFR-stabilizing protein Sprouty2 (Spry2). Accordingly, confluent p38α-deficient cells fail to downregulate Spry2, providing them in turn with sustained EGFR signaling that facilitates cell overgrowth and oncogenic transformation. Our results provide novel mechanistic insight into the role of p38α as a sensor of cell density, which induces confluent cell cycle arrest via the Spry2-EGFR-p27Kip1 network.
Contact inhibition is a term that was coined half a century ago to refer to the process by which cells stop proliferating when they reach confluence, despite availability of extracellular nutrients and growth factors (26). This process ensures tissue homeostasis in multicellular organisms by uncoupling cell proliferation from mitogenic stimulation (9). Accordingly, deregulation of contact inhibition leads to hyperplasia in vivo and facilitates tumor progression by providing the incipient malignant cell with unrestrained proliferative capabilities (1). Not surprisingly, the majority of cancer cells are refractory to confluence-induced proliferation arrest (20), and the loss of contact inhibition is actually used as an in vivo prognostic factor in human cancer (16). However, in spite of its biological significance and potential diagnostic value, the molecular mechanisms underlying this process have been elusive. It is believed that membrane proteins act as sensors of cell-cell contacts, whereas at the other end, there is evidence indicating that cyclin-dependent kinase inhibitors play a key role in the proliferation arrest. Namely, p16Ink4a (41) and p27Kip1 (33) are known to mediate the contact inhibition response, although the signaling pathways leading to their upregulation, as well as their relative contributions to the process, are not well understood.
Recent work has started to shed some light on the intracellular signals that regulate this process. For instance, the mammalian Hippo pathway, which was known to control organ size in Drosophila melanogaster, has been proposed as an important regulator of contact inhibition in mammals (44). In fact, the Hippo kinase (known as Mst1/2 in mammals) inhibits the expression of proliferative and prosurvival proteins in confluence by inactivating the YAP transcription factor, which results in confluent cell cycle arrest (44, 45). Conversely, interference with the inhibition of YAP by Hippo/Mst facilitates cell transformation in vitro and results in organ hyperplasia in vivo (44, 45). In addition, there is evidence that p38α, the most abundant and widely expressed member of the p38 mitogen-activated protein kinase (MAPK) family, is involved in the regulation of contact inhibition as well. We have previously shown that p38α activation correlates with confluence-induced proliferation arrest in human and mouse fibroblasts (14). Accordingly, genetic inactivation of p38α facilitates oncogene-induced malignant transformation of fibroblasts in vitro, as well as tumorigenesis in nude mice (13), and also results in tissue hyperplasia in vivo (38).
We provide herein novel mechanistic insight into the role of p38α in contact inhibition. We show that p38α indirectly upregulates p27Kip1 in the nucleus of confluent cells by inhibiting mitogenic signals from the membrane-anchored epidermal growth factor receptor (EGFR) which destabilize p27Kip1. The inhibitory effect of p38α on EGFR signaling is mediated by the ubiquitin ligase cCbl, which induces degradation of the receptor and consequent signal termination. Mechanistically, p38α negatively regulates the expression levels of the cCbl inhibitor Sprouty2 (Spry2) in confluent cells, which leads to enhanced activity of cCbl toward EGFR and subsequent receptor ubiquitination and degradation. Taken together, our results indicate that upregulation of p27Kip1 by the p38α-Spry2-EGFR pathway is an important mechanism regulating cell density, which in turn protects the cells against oncogene-induced transformation.
MATERIALS AND METHODS
Reagents.
Cycloheximide (30 μg/ml; Sigma) and MG132 (25 μM; Calbiochem) were used to evaluate protein stability and to inhibit the proteasome, respectively. Recombinant murine EGF (50 ng/ml) was purchased from REALIATech GMBH. Where indicated, cultured cells were treated for 6 to 12 h with the following chemical inhibitors: p38α and p38β inhibitor SB203580 (10 μM; Calbiochem), Src inhibitor PP2 (10 μM; Calbiochem), EGFR inhibitor tyrphostin AG 1478 (10 μM; Sigma), MEK1 and MEK2 (MEK1/2) inhibitor PD98059 (15 μM; Calbiochem), phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 (30 μM; Calbiochem), and glycogen synthase kinase 3β inhibitor SB216763 (40 μM; Calbiochem).
Expression constructs and cell culture.
Human p27Kip1 cDNA cloned into pBluescript-KS was obtained from the CNIO DNA collection (GenBank accession no. NM_004064) and subcloned using BamHI-EcoRI into pcDNA3.1, pGEX-KG, and pBabe-puro. The pBabe-puro-p27Kip1-3 M vector was obtained from M. Barbacid (CNIO, Madrid) (29). The constructs for the expression of wild-type (WT) human Spry2 and the human Spry2-Y55F mutant, both in the pCEFL-KZ plasmid and the pLPCX retroviral vector, were provided by N. Martinez (Centro Nacional de Microbiologia, Madrid, Spain) (30). The pcDNA3.1-based constructs encoding WT Siah2 and the ring finger H99A/C102A mutant (Siah2-RM) were a kind gift from Z. Ronai (Burnham Institute for Medical Research, La Jolla, CA) (23). The pRK5-HA-cCbl (22) and the pcDNA3.1-HA-Ubiquitin constructs were kind gifts from J. Bravo (CNIO, Madrid, Spain) and I. Dikic (Goethe University, Frankfurt, Germany), respectively. The pcDNA3.1-based constructs to express WT human EGFR and the Y1045F mutant (46) were kindly provided by Y. Yarden (The Weizman Institute of Science, Rehobot, Israel). MSCV-p38α (4) was used to add p38α back into p38α-deficient mouse embryo fibroblasts (MEFs). The pEFmlink-MKK6-DD expression construct was previously described (3), as was the pBabe-puro-H-RasG12V retroviral vector (13). pEGFP was purchased from Clontech.
Control and H-RasG12V-expressing, immortalized WT and p38α−/− MEFs were generated as described previously (13). Primary WT and p27Kip1−/− MEFs (24) were kindly provided by A. Vidal (Univ. Santiago de Compostela, Spain) and subsequently immortalized by using the 3T3 protocol. 293/Ampho cells and the MDA-MB-231 breast cancer cell line were obtained from the ATCC. Human kidney 293T cells were provided by M. Serrano (CNIO, Madrid, Spain). Human HEK293 cells and mouse NIH 3T3 fibroblasts were used for transient transfections. All cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum, 1% l-glutamine, and 1% penicillin-streptomycin (all from GIBCO-Invitrogen).
Cell density assays.
Cell density was normally determined during the course of 7 days by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (Roche Diagnostics) after seeding 1,500 cells/well in triplicates in 96-well plates. Alternatively, cells were counted under a microscope with a hemacytometer. Briefly, cells were seeded in triplicates in 60-mm plates and grown to saturation (1 to 2 days after 100% confluence), followed by washing with phosphate-buffered saline (PBS), trypsinization, Trypan Blue staining, and counting with a Neubauer chamber.
Immunoblotting, immunoprecipitation, and ubiquitination assays.
The following primary antibodies (product identification is in parentheses) were purchased from Santa Cruz Biotechnology: p27Kip1 (C-19), p38α (C20-G), EGFR (SC-03), p21Cip1 (C19-G), p16Ink4a (M-156), phospho-Thr187-p27Kip1 (SC-16324), phospho-Y1173-EGFR (SC-12351), cCbl (C-15), ubiquitin (P4D1), Siah2 (N-14), ERK (C-16), Akt (C-20), and c-Myc (9E10). Antibodies against phospho-p38 MAPK (9211), phospho-ERK (9101), phospho-Akt (9271), phospho-MAPK-activated protein kinase 2 (3041), phospho-Src (2101), and phospho-Y1045-EGFR (2237) were from Cell Signaling Technology. Anti-green fluorescent protein, antitubulin (DM1A), and anti-Sprouty2 (S1444) were from Sigma; anti-phospho-Ser/Thr (612543) and anti-Ras (clone 18) were from BD Transduction Laboratories; and antiphosphotyrosine (clone 4G10) and anti-EGFR (06-129) were from Upstate. Cetuximab was from ImClone Systems, Inc.
Cell lysates were prepared, quantified, and processed for immunoblotting as previously reported (13). Samples labeled as “subconfluent” or “confluent” refer to cell cultures harvested at around 70 to 80% confluence or 1 to 2 days after reaching 100% confluence, respectively.
For immunoprecipitations, different cell lysis buffers were used, depending on the target protein. All buffers contained the following protease and phosphatase inhibitors: 20 mM NaF, 0.1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 2.5 mM benzamidine, 2 μM microcystin, and 10 μg/ml of leupeptin and aprotinin. Triton X-100 buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA) was used for p27Kip1, Siah2, and cCbl immunoprecipitations with antibodies from Santa Cruz Biotechnology. Modified radioimmunoprecipitation assay buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM EGTA, 1% Triton X-100, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 3 mM dithiothreitol) was used for EGFR immunoprecipitation with either cetuximab or Upstate antibodies, for the human or murine EGFR, respectively. Cell lysates (0.5 to 1 mg) were first precleared with 20 μl of protein G PLUS-agarose beads (Santa Cruz Biotechnology) for 1 h at 4°C and then incubated with 2 μg of antibody for 14 h at 4°C. Immunoprecipitates were subsequently recovered by incubation with protein G PLUS-agarose beads for 1 h at 4°C, washed three times with lysis buffer, and then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by immunoblotting.
For EGFR ubiquitination assays, HEK293 cells or MEFs were serum starved (0.5% fetal bovine serum) during 12 to 48 h before EGF stimulation (50 ng/ml for 15 min). EGFR immunoprecipitates were immunoblotted with antiubiquitin (P4D1) and anti-EGFR (SC-03) antibodies from Santa Cruz Biotechnology.
Transfection, retroviral infection, and focus formation assays.
Transient transfection of NIH 3T3 and HEK293 cells was performed with Fugene6 reagent (Roche Applied Science), following the manufacturer's instructions, and cell lysates were normally analyzed 48 h posttransfection. Retrovirus production in 293T cells, along with MEF transduction and selection to generate stable cell lines or to perform rescue experiments, was performed as described previously (13). Focus formation assays with MEFs were done following transient infection with 293/Ampho-packaged retroviruses as previously reported (13). Foci were allowed to grow for 1 to 2 weeks.
Knockdown of Spry2 by shRNA.
Mission short hairpin RNA (shRNA) lentiviral particles against mouse Spry2 (GenBank accession number NM_011897) or scrambled negative control shRNA (Sigma) was used to knock down Spry2 expression in p38α−/− MEFs. Transduction was performed in triplicates in 96-well plates according to the manufacturer's instructions. At 48 h after transduction, cells expressing control or Spry2 shRNAs were selected with puromycin (1.5 μg/ml) for 1 week, and clones with good levels of Spry2 downregulation were further cultured in antibiotic-containing medium.
Immunofluorescence and confocal microscopy.
Coverslips were rinsed in PBS, fixed in 4% paraformaldehyde for 30 min at room temperature, and washed again with PBS. Nonspecific sites were blocked by incubation in PBS containing 1% BSA and 0.5% Triton X-100 for 1 h at room temperature. Cells were then washed four times in PBS and incubated with the following primary antibodies (1/100): EGFR (06-129; Upstate), p27Kip1 (C-19; Santa Cruz Biotechnology, Inc.), and CD63/LAMP-3 and transferrin receptor, provided by J. J. Bravo and M. Montoya (CNIO, Madrid, Spain), respectively. After four washes with PBS, cells were incubated with Alexa Fluor 488 and 594 (1:500) secondary antibodies for 30 min (Molecular Probes), stained with 4′,6′-diamidino-2-phenylindole (DAPI) (0.1 μg/ml) for 2 min to visualize cell nuclei, and mounted in Mowiol. Samples were examined by using confocal microscopy (Leica Microsystems).
Flow cytometry.
Cell cycle profiles were obtained by flow cytometry analysis. Briefly, sparse and confluent cell cultures were trypsinized, washed twice with chilled PBS, and fixed in ice-cold 70% ethanol while vortexing. Cell pellets were subsequently washed once more with chilled PBS and then treated with RNase (1 mg/ml; Qiagen) and propidium iodide (25 μg/ml; Sigma) at 37°C for 20 min. Cell cycle distributions were determined by using 488-nm excitation and collecting fluorescence above 620 nm.
mRNA extraction and quantitative real-time PCR.
Total RNA from sparse and confluent MEFs was isolated with an RNeasy kit (Qiagen), and cDNA was synthesized by SuperScript-II reverse transcription using random hexamer primers (Invitrogen) as indicated by the manufacturer. An Applied Biosystems 7900HT fast real-time PCR system was used to determine the mRNA levels of p27Kip1 using the following primers: Fwd, 5′-GAGGTGGAGAGGGGCAGC-3′, and Rev, 5′-TTCGGGGAACCGTCTGAAAC-3′. Data analysis was done by normalizing to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels.
Statistical analysis.
All results are expressed as the mean ± standard deviation for at least three independent experiments. Statistical analysis was performed using Student's t test with a statistically significant P value (P < 0.01).
RESULTS
p38α regulates cell density in confluence, but this function is impaired in transformed cells.
We have previously reported that p38α-deficient MEFs are apparently highly susceptible to the loss of contact inhibition induced by oncogenic insults (13), which led us to investigate the connection between p38α and the establishment of the contact inhibition response. Supporting the idea that p38α triggers contact inhibition, we could modify cell density in confluence by modulating p38α activity, either by treating WT MEFs with the p38α and p38β chemical inhibitor SB203580 or by stably reconstituting p38α into p38α−/− MEFs (Fig. 1A). Furthermore, confluent cultures of p38α−/− MEFs contained fewer cells in the G0/G1 phases of the cell cycle, along with more cells in the S and G2/M phases, than cultures of WT MEFs (Fig. 1B), indicating that the higher saturation densities observed in the absence of p38α (Fig. 1A) are likely due to a defect in confluence-induced proliferation arrest. Of note, WT and p38α−/− MEFs proliferated similarly in sparse conditions of growth and also had similar cell morphologies and sizes (2, 13), which supports a specific function for p38α in triggering cell cycle arrest induced by high cellular density. Interestingly, p38α activity strongly increased in MEFs early upon achievement of confluence and was sustained over time, correlating with upregulation of the cell cycle inhibitor p27Kip1 (Fig. 1C).
FIG. 1.
p38α is required for confluent cell cycle arrest and p27Kip1 accumulation, but its function is impaired in transformed cells. (A) WT and p38α−/− MEFs and p38α−/− MEFs with p38α added back were grown to confluence, and cells were counted. SB203580 (SB) was added to confluent WT cultures 12 h before counting. Values are numbers of cells in 10,000s. (B) Percentages of confluent WT and p38α−/− MEFs in the different phases of the cell cycle were determined by flow cytometry. Error bars indicate standard deviations of results for biological replicates. P values were determined by using Student's t test and indicate 99% error-free (P < 0.01) statistical significance. (C) WT and p38α−/− MEFs grown to sparse (SC) or confluent (C) conditions, as well as 1 day (C+1) or 2 days (C+2) postconfluence, were analyzed by immunoblotting. (D and E) Total lysates from subconfluent (SubC) and confluent (Conf) empty vector- or H-RasG12V-transduced MEFs (D) and from human MDA-MB-231 breast carcinoma cells (E) were analyzed by immunoblotting. (F) NIH 3T3 fibroblasts were transiently transfected with H-RasG12V alone or together with MKK6-DD and analyzed by immunoblotting. +, present; −, absent; P-p38α, phospho-p38α.
As impaired contact inhibition is considered a hallmark of cell transformation (1, 20), we next investigated whether the more relaxed contact inhibition response of p38α−/− cells would make them more susceptible to malignant transformation. Indeed, we observed that transient transduction with oncogenic H-RasG12V resulted in more efficient multilayered-foci formation in confluent p38α−/− than in WT MEFs (data not shown). Of note, this could not be accounted for by proliferation or apoptosis differences, as H-RasG12V-transformed WT and p38α−/− cells proliferated similarly in overconfluence and also displayed similar levels of basal apoptosis (data not shown). Consistent with the idea that regulation of contact inhibition contributes to the tumor suppressor activity of p38α, we found that WT cells stably transformed by H-RasG12V lost the ability both to activate p38α and to upregulate p27Kip1 in confluence (Fig. 1D). Similar results were observed in a human mammary carcinoma cell line (Fig. 1E). Thus, at high cellular densities, transformed cells most likely interfere with p38α activation to prevent the induction of p27Kip1 accumulation and cell cycle arrest. This idea was supported by the strong upregulation of p27Kip1 protein levels observed in H-RasG12V-transformed cells by forced, MKK6-DD-induced activation of p38α (Fig. 1F), suggesting that although transformation uncouples the confluence state from p38α activation, this pathway is still potentially functional.
p38α and p27Kip1 are both required for contact inhibition.
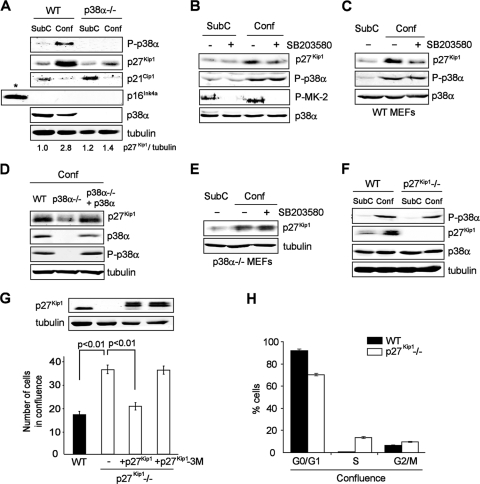
To investigate the mechanism by which p38α induces confluent proliferation arrest in immortalized cells, we analyzed WT and p38α−/− MEFs for the expression of cell cycle regulators previously associated with contact inhibition (10, 41). We failed to detect p16Ink4a expression, whereas p21Cip1 was downregulated in confluence in both cell lines and inversely correlated with p27Kip1 levels (Fig. 2A), in agreement with previous reports (43). Moreover, we found that p38α also regulated p27Kip1 levels in NIH 3T3 fibroblasts lacking p16Ink4a (Fig. 2B), suggesting that p27Kip1 is a major effector of p38α for the regulation of contact inhibition in premalignant cells.
FIG. 2.
p27Kip1 mediates the antiproliferative effect of p38α in contact inhibition. (A) Immunoblot analysis of subconfluent (SubC) and confluent (Conf) WT and p38α−/− MEFs. Primary MEFs were used as a control for p16Ink4a expression (indicated by an asterisk). p27Kip1 protein levels were quantified by densitometry and normalized to tubulin. (B) Mouse NIH 3T3 fibroblasts were grown to subconfluence (SubC) or confluence (Conf), treated overnight with the p38α and p38β inhibitor SB203580 where indicated (+, present; −, absent), and analyzed by immunoblotting. P-MK-2, phospho-MAPK-activated protein kinase 2. (C to E) WT and p38α−/− MEFs and p38α−/− MEFs with p38α added back were grown to subconfluence (SubC) or confluence (Conf), treated overnight with SB203580 as indicated (+, present; −, absent), and analyzed by immunoblotting. (F) Sparse (SubC) and confluent (Conf) WT and p27Kip1−/− MEFs were analyzed by immunoblotting. (G) WT and p27Kip1−/− MEFs stably transduced with WT p27Kip1, mutant p27Kip1-3M, or empty vector (−) were grown to confluence and counted. Total lysates were analyzed by immunoblotting. Values are numbers of cells in 10,000s. (H) Percentages of confluent WT and p27Kip1−/− MEFs in the different phases of the cell cycle were determined by flow cytometry. Error bars indicate standard deviations of results for biological replicates. P values were determined by Student's t test and indicate 99% error-free (P < 0.01) statistical significance.
The functional connection between p38α and p27Kip1 was further supported by the observation that treatment with the p38α and p38β inhibitor SB203580 impaired p27Kip1 accumulation in confluent WT cells (Fig. 2C). Moreover, retrovirus-mediated reconstitution of p38α into p38α−/− MEFs restored p27Kip1 levels to those observed in WT cells (Fig. 2D), indicating that p38α is the main p38 family member regulating p27Kip1 in confluence (14). The lack of effect of SB203580 on p27Kip1 accumulation in confluent p38α−/− MEFs (Fig. 2E) also supported the idea that p38β does not significantly contribute to this process. In line with the results described above, p27Kip1-deficient MEFs reached higher cellular densities than WT MEFs (Fig. 2G), phenocopying the effect of p38α downregulation. Notably, whereas p38α deficiency impaired both p27Kip1 accumulation and contact inhibition (Fig. 1A and C), the absence of p27Kip1 resulted in impaired contact inhibition response without significantly affecting p38α activation (Fig. 2F), indicating that p27Kip1 lies downstream of p38α. Accordingly, treatment with SB203580 did not affect the saturation density of p27Kip1−/− MEFs (data not shown).
We then investigated the mechanism by which p27Kip1 regulates contact inhibition and observed that reconstitution of p27Kip1−/− MEFs with p27Kip1 rescued their confluence defect, whereas expression of the cyclin-dependent kinase-binding-defective mutant p27Kip1-3M with mutations L32H, P35A, and F64A (29) did not (Fig. 2G). This demonstrates that the cell cycle-inhibitory function of p27Kip1 is required to implement the confluence-induced proliferation arrest downstream of p38α. In agreement with this idea, cultures of p27Kip1−/− MEFs contained fewer G0/G1 cells at high cellular densities than WT MEFs (Fig. 2H).
p38α indirectly regulates p27Kip1 protein stability in confluence.
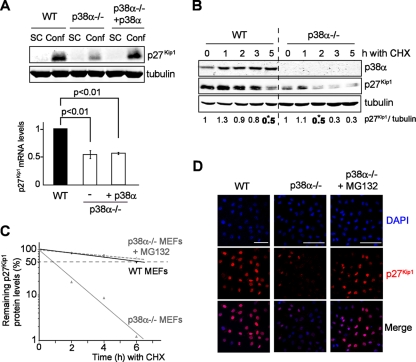
Next, we addressed how p38α induces p27Kip1 upregulation in confluence. Quantitative reverse transcription-PCR analysis revealed that p27Kip1 mRNA levels were lower in confluent p38α−/− than in WT MEFs, which correlated well with the differences observed in p27Kip1 protein accumulation (Fig. 3A). However, adding p38α back into p38α−/− MEFs rescued p27Kip1 protein accumulation without affecting its mRNA levels (Fig. 3A), arguing that regulation of p27Kip1 by p38α in confluence is not transcriptionally mediated. Of note, this also indicates that the observed differences in p27Kip1 mRNA levels of WT and p38α−/− MEFs are not related to the absence of p38α but are likely due to the immortalization process. Neither did we observe significant differences in p27Kip1 mRNA stability between confluent WT and p38α−/− MEFs (data not shown).
FIG. 3.
p38α indirectly regulates p27Kip1 protein stability in confluent cells. (A) Confluent (Conf) WT and p38α−/− MEFs and p38α−/− MEFs with p38α added back were analyzed by immunoblotting (top) and quantitative reverse transcription-PCR (bottom). SC, subconfluent; −, no p38α. (B) Confluent WT and p38α−/− MEFs were treated with cycloheximide (CHX) for the indicated times and analyzed by immunoblotting. p27Kip1 levels were quantified by densitometry and normalized to the tubulin level. Asterisks indicate p27Kip1 half-lives. (C) Confluent WT and p38α−/− MEFs were treated with cycloheximide (CHX) for the indicated times, in the presence of MG132 where indicated. Total cell lysates were subsequently analyzed by immunoblotting and quantified by densitometry. Tubulin-normalized p27Kip1 protein levels are shown as a function of time in a log10 plot. (D) Confluent WT and p38α−/− MEFs were treated with MG132 or dimethyl sulfoxide for 6 h, formalin fixed, stained with anti-p27Kip1 antibody, and visualized by confocal microscopy. p27Kip1 staining is shown in red, and nuclear staining (DAPI) in blue. Bars = 100 μm.
As p27Kip1 expression is known to be regulated by phosphorylation (6, 7), we also investigated whether p27Kip1 accumulation could be due to direct phosphorylation by p38α. We observed that Ser10, a residue that has been previously associated with p27Kip1 protein stabilization (21), was a major site phosphorylated by p38α (data not shown). However, we failed to observe any functional effect of p27Kip1 phosphorylation on Ser10 in contact inhibition, as both WT p27Kip1 and the S10A mutant protein accumulated similarly in confluence and rescued the contact inhibition defect of p27Kip1−/− MEFs (data not shown). This indicated that direct p27Kip1 phosphorylation by p38α was unlikely to mediate the accumulation of p27Kip1 in confluent cells. In contrast, treatment with cycloheximide showed that p27Kip1 was more stable in confluent WT than in p38α−/− MEFs, with half-lives of about 5 h and 2 h, respectively (Fig. 3B). Treatment of p38α−/− MEFs with the proteasome inhibitor MG132 further supported the idea that p38α regulates p27Kip1 protein stability (Fig. 3C). Taken together, our results suggest that the reduced accumulation of p27Kip1 in confluent p38α−/− cells is probably due to enhanced proteasome-mediated degradation. Of note, confluent p38α−/− MEFs treated with MG132 contained large amounts of nuclear p27Kip1 without any obvious accumulation of p27Kip1 in the cytoplasm (Fig. 3D), arguing against a role for p38α in the regulation of p27Kip1 nucleocytoplasmic shuttling.
p38α stabilizes p27Kip1 in confluent cells by inhibiting EGFR signaling.
The enhanced stability of p27Kip1 in confluent WT MEFs led us then to investigate the nature of the p38α-opposed signals that were responsible for p27Kip1 degradation in p38α-deficient cells. A suggestive observation was that the levels of Thr187-phosphorylated p27Kip1 were higher in confluent p38α−/− than in WT MEFs (Fig. 4A), as this phosphorylation is known to induce p27Kip1 degradation (37) and can be stimulated by mitogenic signals downstream of EGFR (11, 17). Treatment of confluent p38α−/− MEFs with chemical inhibitors of various signaling proteins revealed that EGFR and its downstream effectors Src and MEK1/2, but not PI3K/Akt or GSK3β, were responsible for p27Kip1 instability in the absence of p38α (Fig. 4B). In agreement with the idea that deregulated EGFR signaling was the cause of reduced p27Kip1 accumulation in p38α-deficient cells, we found higher levels of active EGFR and its downstream targets Akt, ERK, and Src in confluent p38α−/− than in WT cells (Fig. 4C). Of note, the rescue of p27Kip1 protein levels following reintroduction of p38α into p38α−/− MEFs (Fig. 2D) correlated with the downregulation of all of these signaling pathways (Fig. 4C). Accordingly, EGF treatment induced more-sustained EGFR activation in p38α−/− than in WT MEFs, as indicated by the higher levels of phosphorylated EGFR and ERK1/2 at later time points in the absence of p38α (Fig. 4D). Importantly, no differences in EGFR activation were observed between WT and p38α−/− MEFs when sparse cultures were stimulated with EGF (data not shown), supporting the idea that confluence-induced activation of p38α is required to inhibit EGFR signaling. Along this line, forced activation of p38α by MKK6-DD inhibited the EGFR-induced degradation of p27Kip1 in NIH 3T3 fibroblasts (Fig. 4E), whereas treatment of confluent p38α−/− MEFs with the EGFR inhibitor AG 1478 not only upregulated p27Kip1 to WT levels (Fig. 4F) but also rescued their enhanced susceptibility to oncogene-induced transformation (Fig. 4G). Altogether, these results delineate a novel regulatory mechanism in contact inhibition which involves p38α-mediated downregulation of EGFR signaling and subsequent p27Kip1 accumulation. Importantly, whereas SB203580 treatment interfered with the accumulation of p27Kip1 in confluent WT MEFs (Fig. 2C), this p38α and p38β inhibitor had no effect on the p27Kip1 levels in confluent EGFR−/− MEFs (data not shown), further pointing to EGFR as a key target of p38α in the confluence-induced upregulation of p27Kip1.
FIG. 4.
p38α stabilizes p27Kip1 by negatively regulating EGFR signaling in confluent cells. (A) p27Kip1 immunoprecipitates from sparse (SubC) or confluent (Conf) WT and p38α−/− MEFs were analyzed by immunoblotting. IgG, immunoglobulin G; IP, immunoprecipitated. (B) Confluent (Conf) p38α−/− MEFs were incubated for 8 h with either dimethyl sulfoxide (−) or chemical inhibitors against Src (PP2), Gsk3β (SB216763), MEK1/2 (PD98059), PI3K (LY294002), or EGFR (AG 1478) and then analyzed by immunoblotting. (C) Sparse (SC) and confluent (Conf) WT and p38α−/− MEFs and p38α−/− MEFs with p38α added back were analyzed by immunoblotting. (D) Confluent WT and p38α−/− MEFs were serum starved and subsequently treated with EGF for the indicated times. Total cell lysates were analyzed by immunoblotting. −, not treated. (E) NIH 3T3 cells were transiently transfected with EGFR, p27Kip1, and the p38 MAPK activator MKK6-DD, as indicated. MKK6-DD was detected by using an anti-Myc antibody. Cell lysates were analyzed by immunoblotting, with green fluorescent protein (GFP) as a transfection control. +, present; −, absent. (F) Subconfluent (SC) and confluent (Conf) WT and p38α−/− MEFs were incubated for 8 h with the EGFR inhibitor AG 1478 or with dimethyl sulfoxide (DMSO) and analyzed by immunoblotting. (G) Focus formation assay using WT and p38α−/− MEFs transiently transduced with H-RasG12V. Plates were treated daily with the EGFR inhibitor AG 1478 where indicated. +, present; −, absent; P, phospho.
Confluent activation of p38α attenuates mitogenic signals by inducing EGFR degradation.
Our results indicated that enhanced EGFR signaling was responsible for impaired p27Kip1 accumulation and contact inhibition in p38α−/− cells. Thus, we investigated whether p38α could regulate EGFR degradation in confluent cells, since EGFR mutants resistant to degradation are known to display sustained mitogenic signaling (35). We found higher levels of ubiquitinated EGFR, a prerequisite for EGFR degradation (18, 27), in confluent WT than in p38α−/− MEFs (Fig. 5A). Furthermore, ligand-induced internalization of EGFR in confluent WT MEFs resulted in rapid colocalization with the lysosomal marker CD63/LAMP-3 (Fig. 5B, upper middle), which was impaired in p38α−/− MEFs (Fig. 5B, lower middle). Conversely, stimulated EGFR in p38α−/− cells was mostly found in transferrin receptor-containing recycling endosomes (Fig. 5B, lower right), which are known to serve as platforms for sustained EGFR signaling (35), but this hardly occurred in WT cells (Fig. 5B, upper right). These results suggested that p38α regulates the confluence-induced degradation of EGFR by modulating its ubiquitination, which in turn affects the duration of EGFR downstream signals and, as a consequence, p27Kip1 protein stability. Accordingly, p38α activation enhanced, whereas SB203580 treatment inhibited, the EGF-induced ubiquitination of EGFR in HEK293 cells as well (Fig. 5C). However, p38α did not seem to affect the ligand-induced internalization of EGFR, as confluent p38α−/− and WT MEFs showed similar colocalization of the internalized EGFR with clathrin-coated pits following EGF stimulation (data not shown).
FIG. 5.
p38α regulates EGFR degradation in confluent cells. (A) Confluent (Conf) WT and p38α−/− MEFs were serum starved, treated with EGF, and analyzed for EGFR ubiquitination by immunoprecipitation of the endogenous EGFR followed by immunoblotting. The position of the ubiquitinated EGFR (Ub-EGFR) is indicated with a black arrowhead. Molecular mass markers in kDa are indicated. (B) Confluent WT and p38α−/− MEFs were incubated with 0.5% serum for 48 h and then left nonstimulated or treated with EGF (50 ng/ml) for 40 min. Cells were fixed, stained with antibodies against EGFR (green, all) and CD63 (red, middle) or the transferrin receptor (TFR; red, right) and visualized by confocal microscopy. Arrows (left) indicate EGFR localization, and arrowheads the colocalization of EGFR with either CD63 (middle) or transferrin receptor (right). Insets show magnifications. Bars = 2 μm. (C) HEK293 cells were transiently transfected with plasmids expressing EGFR, cCbl, or MKK6-DD; serum-starved; and treated with EGF and SB203580 as indicated. Cell lysates were subjected to EGFR immunoprecipitation at 48 h posttransfection, and the immunocomplexes were analyzed by immunoblotting. Ubiquitinated EGFR was quantified by densitometry and normalized to the total EGFR levels of each sample. (D) HEK293 cells were transiently transfected with the indicated plasmids, treated as described for panel C, and subjected to EGFR (upper) or cCbl (lower) immunoprecipitation followed by immunoblotting. EGFR phosphorylated on Tyr1045 was quantified by densitometry and normalized to the total EGFR levels. (E) HEK293 cells were transiently transfected with EGFR and the p38 activator MKK6-DD, as indicated, treated as described for panel C, and subjected to EGFR immunoprecipitation followed by immunoblotting. (F) HEK293 cells were transiently transfected with WT EGFR or the Y1045F mutant together with cCbl and MKK6-DD, as indicated. EGFR immunoprecipitates were prepared and analyzed to quantify the ubiquitinated EGFR as described for panel C. +, present; −, absent; IP, immunoprecipitated; Ub, ubiquitinated; IgG, immunoglobulin G; P, phospho.
We then evaluated whether p38α might impinge on EGFR ubiquitination by regulating the extent of receptor phosphorylation on Tyr1045 and/or the subsequent recruitment of the E3 ubiquitin ligase cCbl to phospho-Tyr1045 (27). We found that, whereas p38α did not seem to stimulate EGFR phosphorylation on Tyr1045 (Fig. 5D, top, and E), it efficiently enhanced cCbl binding to the receptor, as indicated by the high levels of Tyr-phosphorylated cCbl (Fig. 5D, bottom) (27). Therefore, we hypothesized that p38α mainly regulated the loading of cCbl onto the Tyr1045-phosphorylated receptor. In agreement with this, p38α activation stimulated EGFR ubiquitination and cCbl binding, which were both impaired after mutation of Tyr1045 (Fig. 5F), supporting the idea that p38α most likely regulates EGFR ubiquitination through the recruitment of cCbl.
p38α induces EGFR signal termination in confluence by downregulating Spry2.
While investigating how p38α could regulate the association between cCbl and EGFR in confluent cells, we found that Spry2, an inhibitor of cCbl-mediated EGFR ubiquitination (34, 42), was highly upregulated in p38α-deficient MEFs and was rescued to WT levels by p38α reintroduction (Fig. 6A). This suggested that Spry2 could be functionally implicated in the enhanced EGFR signaling and impaired contact inhibition response of p38α−/− MEFs. In agreement with this idea, Spry2 downregulation reduced EGFR phosphorylation, as well as its downstream mitogenic signaling, and also increased p27Kip1 accumulation to the levels in confluent WT cells (Fig. 6B). Downregulation of Spry2 in p38α−/− MEFs also resulted in a more robust contact inhibition response, as indicated by the dramatic decrease in confluent cell numbers (Fig. 6C), as well as resulting in enhanced EGFR ubiquitination (Fig. 6D), demonstrating that Spry2 acts downstream of p38α to inhibit EGFR ubiquitination. Furthermore, Spry2 downregulation increased the resistance of p38α−/− MEFs to oncogene-induced foci formation to the level in WT cells (Fig. 6E), functionally phenocopying the effect of inhibiting EGFR in p38α−/− MEFs (Fig. 4G).
FIG. 6.
Spry2 mediates the inhibitory effect of p38α on EGFR signaling in confluent cells. (A) Subconfluent (SubC) and confluent (Conf) WT and p38α−/− MEFs and p38α−/− MEFs with p38α added back were analyzed by immunoblotting. (B) Subconfluent (SC) and confluent (Conf) p38α−/− MEFs were stably transduced with scrambled (C) or Sprouty2-directed (Spry2) shRNAs, and total lysates were analyzed by immunoblotting. (C) Overconfluent cell numbers of p38α−/− MEFs expressing shRNAs as described for panel B were determined by the MTT assay, reading the absorbance (optical density) at 595 nm (OD595). Error bars indicate standard deviation of biological replicates. (D) Confluent WT, p38α−/−, and Spry2 shRNA-expressing p38α−/− MEFs were serum starved, treated with EGF, and analyzed for EGFR ubiquitination by immunoprecipitation of the endogenous EGFR followed by immunoblotting. The position of the ubiquitinated EGFR (Ub-EGFR) is indicated with a black arrowhead. Molecular mass markers in kDa are indicated. (E) H-RasG12V-induced focus formation assay using WT and p38α−/− MEFs expressing scrambled (−) or Spry2 shRNA. (F) Cell lysates from confluent (Conf) WT and p38α−/− MEFs were subjected to cCbl immunoprecipitation and then analyzed by immunoblotting. The immunoprecipitation control was performed with an anti-p27Kip1 antibody. (G) Subconfluent (SubC) or confluent (Conf) WT MEFs were incubated for 12 h with SB203580 or dimethyl sulfoxide (−) and then analyzed by immunoblotting. (H) NIH 3T3 cells (90% confluence) were transiently transfected with MKK6-DD or empty vector (−), harvested when they were confluent (Conf), and analyzed by immunoblotting. (I) Confluent WT and p38α−/− MEFs were treated with cycloheximide (CHX) for the indicated times and analyzed by immunoblotting. +, present; −, absent; IP, immunoprecipitated; Ub, ubiquitinated; IgG, immunoglobulin G; P, phospho.
Our results indicated that high Spry2 levels were the likely cause of enhanced EGFR signaling. This was further supported by the increased binding of Spry2 to immunoprecipitated cCbl in confluent p38α−/− MEFs (Fig. 6F), which correlated with reduced cCbl-mediated ubiquitination and degradation of EGFR in these cells (Fig. 5A and B). Moreover, inhibition of p38α in confluent cells resulted in Spry2 accumulation and reduced levels of p27Kip1 (Fig. 6G), whereas MKK6-DD-induced activation of p38α had the opposite effect (Fig. 6H), demonstrating an inverse correlation between p38α activity and Spry2 protein levels. Accordingly, the Spry2 protein was more stable in p38α−/−-deficient MEFs (Fig. 6I). Altogether, Spry2 appears to be a key target for p38α to induce p27Kip1 accumulation via cCbl-dependent EGFR degradation and subsequent cell cycle arrest.
p38α downregulates Spry2 in confluence through the ubiquitin ligase Siah2.
The difference in Spry2 protein stability in confluent WT and p38α−/− MEFs prompted us to explore whether p38α could regulate cCbl or Siah2, two ubiquitin ligases that regulate Spry2 (19, 32). Although Spry2 interacted with cCbl in confluent p38α−/− MEFs (Fig. 6F), we found that cotransfection with cCbl decreased Spry2 protein levels only slightly (Fig. 7A), suggesting that cCbl was a poor Spry2 ubiquitin ligase. In contrast, cotransfection with Siah2 caused a strong downregulation of Spry2 (Fig. 7A). In line with Siah2 but not cCbl being responsible for the p38α-induced downregulation of Spry2 in confluence, we observed that the Spry2-Y55F mutant, which is impaired in cCbl binding (31), was downregulated as efficiently as WT Spry2 in confluent cells (Fig. 7B).
FIG. 7.
p38α downregulates Spry2 through the ubiquitin ligase Siah2. (A) NIH 3T3 cells were transiently transfected with plasmids encoding Spry2, Siah2, and cCbl, as indicated, and were analyzed by immunoblotting. (B) WT MEFs stably expressing WT Spry2 or the Y55F mutant, as well as WT and p38α−/− MEFs transduced with empty vector (−), were grown to sparse (SC) or confluent (Conf) conditions and analyzed by immunoblotting. (C) Cell lysates (150 μg) from sparse (SC) and confluent (Conf) WT and p38α−/− MEFs were analyzed by immunoblotting. (D) NIH 3T3 fibroblasts were transiently transfected with Spry2, Siah2, and MKK6-DD, and 36 h later were treated with either SB203580 or dimethyl sulfoxide (−) for another 12 h, as indicated. Cell lysates were analyzed by immunoblotting. (E) Siah2 was immunoprecipitated from lysates of HEK293 cells transfected with plasmids encoding WT Siah2 or Siah2-RM, and the immunocomplexes were analyzed by immunoblotting. (F) HEK293 cells were transiently transfected with Siah2 and MKK6-DD, as indicated, incubated for 30 h, and then treated with MG132 for 6 h more. Total lysates were immunoprecipitated with Siah2 antibody and analyzed by immunoblotting. +, present; −, absent; IgG, immunoglobulin G; IP, immunoprecipitated; P, phospho.
As p38α has been recently reported to regulate Siah2 activity in the context of hypoxia signaling (23), we investigated whether Siah2 could function downstream of p38α in contact inhibition by regulating Spry2 stability in confluent MEFs. Indeed, high cellular density increased Siah2 expression in WT but not in p38α−/− MEFs (Fig. 7C), which correlated inversely with Spry2 expression in these cells (Fig. 6A). To evaluate the causality of this correlation in the regulation of p27Kip1 by p38α, we coexpressed Spry2 together with Siah2 in the presence of MKK6-DD or SB203580 (Fig. 7D). Activation of p38α by MKK6-DD increased Siah2 levels, which correlated with both Spry2 downregulation and p27Kip1 upregulation, pointing to Siah2 as a p38α target that mediates the regulation of p27Kip1 protein levels through Spry2. This was confirmed by treatment with the inhibitor SB203580, which reversed the effect of p38α activation on the Siah2-Spry2-p27Kip1 axis (Fig. 7D). Furthermore, we were able to coimmunoprecipitate Spry2 together with endogenous p38α in a complex with the catalytic inactive mutant Siah2-RM (Fig. 7E), suggesting that p38α can modulate Spry2 stability by directly binding to and regulating Siah2. Of note, despite Spry2 not being present in the complex, we could also see an interaction between endogenous p38α and WT Siah2 (Fig. 7E), most likely due to the strong ubiquitin ligase activity of Siah2 on Spry2 (Fig. 7A), which indicates that p38α binds Siah2 independently of Spry2. Interestingly, the stabilization of Siah2 upon p38α activation (Fig. 7C and D) correlated with increased Ser/Thr phosphorylation of Siah2 in cells (Fig. 7F), suggesting that p38α likely stabilizes Siah2 protein levels in confluence by phosphorylation. In support of the posttranslational regulation of Siah2 by p38α, we found that treatment of p38α−/− MEFs with the proteasome inhibitor MG132 rescued Siah2 protein to the levels in WT MEFs, whereas we did not observe any differences in Siah2 mRNA levels of WT and p38α−/− MEFs (data not shown). Accordingly, we found that Thr24 and Ser29, two sites that have been previously reported to regulate Siah2 stability and its ubiquitin ligase activity in hypoxia signaling (23), were the main residues targeted by p38α on Siah2 (data not shown).
DISCUSSION
Contact inhibition is an important process for the regulation of tissue homeostasis for which proper understanding of the underlying molecular events is still lacking. Here, we provide evidence for a novel, multistep mechanism engaged by p38α at high cellular densities which involves the inhibition of EGFR-induced mitogenic signals that destabilize p27Kip1 (Fig. 8). We found that confluent activation of p38α stimulates the cCbl-mediated ubiquitination and degradation of EGFR, which in turn shuts down mitogenic signals, such as those relied on by the Src nonreceptor tyrosine kinase and the ERK1 and ERK2 MAPKs (5, 11, 17, 40), indirectly resulting in p27Kip1 upregulation and cell cycle arrest. Our data indicate that p38α does not regulate cCbl protein levels per se but does so through the phosphorylation and stabilization of the ubiquitin ligase Siah2, which induces degradation of the cCbl inhibitor Spry2 and facilitates the loading of the ubiquitin ligase cCbl onto EGFR.
FIG. 8.
Proposed model for the regulation of contact inhibition by p38α. Confluent cell-to-cell contacts activate p38α, which induces Siah2-mediated downregulation of Spry2 and subsequent dissociation of cCbl, triggering EGFR ubiquitination and degradation. Termination of EGFR-induced mitogenic signaling results in p27Kip1 accumulation and cell cycle arrest.
An intriguing question is how p38α is activated when cells reach confluence. Two possible membrane initiators of the intracellular signals engaged by direct cell-to-cell contacts are the mammalian N-cadherin (25) and the Drosophila protocadherin Fat (44). Nevertheless, solid evidence linking these proteins to contact inhibition is missing and no ortholog for Drosophila's Fat has yet been characterized in mammals. Interestingly, several members of the Ste20 family of kinases that link membrane receptors with MAPK pathways have been shown to activate p38α in response to various stimuli. Thus, it would be interesting to investigate whether the Ste20 kinase Hippo/Mst, which has been implicated in contact inhibition through the inhibition of YAP-mediated transcription (45), also regulates p38α activation in confluence. Of note, we have observed that p38α affects neither the cytoplasmic accumulation of YAP nor the expression of the YAP target cyclin E in confluent MEFs (data not shown), suggesting that the Hippo-YAP and p38α signaling pathways are likely to function as parallel regulators of contact inhibition in mammalian cells. This might explain why contact inhibition is impaired but not fully abolished in p38α−/− cells, as the Hippo pathway may partially compensate for p38α deficiency in confluent cells.
EGFR as a new p38α target in contact inhibition.
Previous work suggested p27Kip1 as a possible p38α target in the regulation of contact inhibition (14). However, we show here that p27Kip1 is not a direct target of p38α, although it indeed lies downstream from p38α in confluence-induced proliferation arrest. For instance, we found that p38α phosphorylates p27Kip1 on Ser10, a residue whose phosphorylation has been previously associated with p27Kip1 protein stabilization (21), but that this phosphorylation does not regulate the p38α-mediated accumulation of p27Kip1 in confluent cells. Furthermore, confluent WT and p38α−/− MEFs show similar levels of p27Kip1 Ser10 phosphorylation (data not shown), and inhibition of p38α in confluent EGFR−/− MEFs does not affect p27Kip1 levels, indicating that p38α regulates p27Kip1 indirectly through EGFR.
How, then, does p38α regulate EGFR signaling upon cell-to-cell contact? Recent studies have shown that, upon stress or cytotoxic stimuli, p38α directly phosphorylates and induces the internalization of EGFR in the absence of growth factors, which leads to reduced cell survival and could have implications for cancer cell chemotherapy (39, 46). In contrast, p38α does not seem to be required for growth factor-induced EGFR internalization (39, 46), although it can regulate the EGFR degradation pathway in epithelial cells to coordinate proliferation-to-migration cell responses in the presence of mitogens (15). In agreement with the idea that p38α can induce EGFR degradation in the presence of growth factors, we report here that p38α inhibits EGFR signaling in confluent cells (grown in the presence of serum) by promoting receptor ubiquitination and degradation. Our results indicate that this is unlikely to be modulated by direct p38α phosphorylation of EGFR but involves downregulation of the cCbl inhibitor Spry2 through the ubiquitin ligase Siah2, which results in enhanced loading of cCbl onto EGFR and subsequent receptor degradation.
This leads to the question of why p38α should regulate contact inhibition through such a complex mechanism, impinging on membrane-bound EGFR, instead of by directly phosphorylating and stabilizing p27Kip1 in the nucleus. A plausible explanation may rely on the nature of the contact inhibition response, which is not so much a matter of upregulating cell cycle inhibitors in confluence as of shutting down the intracellular mitogenic signals that fuel proliferation by shielding the cells against extracellular growth factors (12, 26). This implies that confluence-induced upregulation of cell cycle inhibitors might be an effect that is secondary to the downregulation of mitogenic signaling. In agreement with this, our results show that p38α is activated at high cellular densities and in turn downregulates mitogenic signals relayed from EGFR via receptor removal from the plasma membrane. As a consequence, mitogenic signaling is uncoupled from extracellularly available EGFR ligands, resulting in p27Kip1 accumulation and cell cycle arrest. The idea that EGFR is a key antiproliferative target for p38α is also supported by in vivo evidence. For example, p38α downregulation in mice produces hyperproliferative and hyperplasic lung epithelia with higher levels of EGFR signaling, which are required for the enhanced proliferation ex vivo of the lung cells derived from these mice (38).
EGFR-dependent regulation of cell transformation by p38α.
Over the past 10 years, p38α has been established as an efficient inhibitor of the process of cell transformation (8), although different traits of the transformed cell seem to be regulated by p38α through partially independent mechanisms. Thus, the effect of p38α on the proliferation, focus formation, or anchorage-independent growth capabilities of transformed cells depends on the particular oncogene expressed (13). For instance, the inhibition of anchorage-independent growth relies on the proapoptotic activity of p38α in response to the intracellular accumulation of reactive oxygen species (ROS) induced by oncogenes, such as H- and N-Ras (13). In contrast, there is no mechanistic basis for the inhibitory effect of p38α on foci formation induced by oncogenes that do not signal primarily through ROS, such as Raf (13).
Our results indicate that the regulation of contact inhibition through the Spry2-EGFR-p27Kip1 network may be an important mechanism for p38α to specifically inhibit oncogene-induced foci formation independently of ROS sensing. In particular, enhanced levels of EGFR signaling, due to Spry2 upregulation, sensitize p38α−/− cells to malignant transformation by H-Ras, and high Spry2 levels are also required for Raf to efficiently induce foci formation in p38α-deficient MEFs (data not shown). Accordingly, efficient transformation by H-Ras has been shown to require EGFR signaling through a positive feedback loop involving Spry2 (28), in agreement with previous reports highlighting the importance of EGFR for Ras-induced tumorigenesis (36). Of note, in a recent microarray analysis comparing transformed WT and p38α−/− cells, we found a large number of genes coding for positive regulators of EGFR signaling that are downregulated by p38α (our unpublished data), further supporting the functional importance of the p38α-EGFR link in the regulation of cell transformation.
In summary, it appears that downregulation of EGFR signaling by p38α negatively regulates the initiation of tumorigenesis by inhibiting proliferation at high cellular densities. Accordingly, early transformed cells uncouple confluent growth from p38α activation, suggesting that the role of p38α in contact inhibition is probably under negative selective pressure in transformation in order to evade antiproliferative constraints and facilitate progression toward malignancy.
Acknowledgments
We thank A. Vidal for p27Kip1−/− MEFs; M. Sibilia for EGFR−/− MEFs; Y. Yarden, Z. Ronai, N. Martinez, M. Barbacid, I. Dikic, and J. Bravo for providing expression constructs; J. J. Bravo, D. Megias, and M. Montoya for antibodies and advice on confocal microscopy; J. J. Ventura, J. Arribas, A. Cuadrado, and M. Sibilia for useful discussions; and E. Seco for technical support.
A.S. and I. D. were supported by FPU fellowships from the Spanish Ministerio de Educacion y Ciencia (MEC). This work was funded by grants from MICINN (BFU2007-60575 and SAF2006-04247), ISCIII-RETIC RD06/0020/0083 and RD06/0020/0003, Fundación Científica de la AECC, and Fundación La Caixa.
Footnotes
Published ahead of print on 13 April 2009.
REFERENCES
- 1.Abercrombie, M. 1979. Contact inhibition and malignancy. Nature 281259-262. [DOI] [PubMed] [Google Scholar]
- 2.Alfonso, P., I. Dolado, A. Swat, A. Nunez, A. Cuadrado, A. R. Nebreda, and J. I. Casal. 2006. Proteomic analysis of p38alpha mitogen-activated protein kinase-regulated changes in membrane fractions of RAS-transformed fibroblasts. Proteomics 6(Suppl. 1)S262-S271. [DOI] [PubMed] [Google Scholar]
- 3.Alonso, G., C. Ambrosino, M. Jones, and A. R. Nebreda. 2000. Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. J. Biol. Chem. 27540641-40648. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosino, C., G. Mace, S. Galban, C. Fritsch, K. Vintersten, E. Black, M. Gorospe, and A. R. Nebreda. 2003. Negative feedback regulation of MKK6 mRNA stability by p38α mitogen-activated protein kinase. Mol. Cell. Biol. 23370-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balmanno, K., and S. J. Cook. 1999. Sustained MAP kinase activation is required for the expression of cyclin D1, p21Cip1 and a subset of AP-1 proteins in CCL39 cells. Oncogene 183085-3097. [DOI] [PubMed] [Google Scholar]
- 6.Besson, A., S. F. Dowdy, and J. M. Roberts. 2008. CDK inhibitors: cell cycle regulators and beyond. Dev. Cell 14159-169. [DOI] [PubMed] [Google Scholar]
- 7.Bloom, J., and M. Pagano. 2003. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin. Cancer Biol. 1341-47. [DOI] [PubMed] [Google Scholar]
- 8.Bulavin, D. V., and A. J. Fornace, Jr. 2004. p38 MAP kinase's emerging role as a tumor suppressor. Adv. Cancer Res. 9295-118. [DOI] [PubMed] [Google Scholar]
- 9.Carter, S. B. 1968. Tissue homeostasis and the biological basis of cancer. Nature 220970-974. [DOI] [PubMed] [Google Scholar]
- 10.Cho, Y. S., J. M. Bae, Y. S. Chun, J. H. Chung, Y. K. Jeon, I. S. Kim, M. S. Kim, and J. W. Park. 2008. HIF-1alpha controls keratinocyte proliferation by up-regulating p21(WAF1/Cip1). Biochim. Biophys. Acta 1783323-333. [DOI] [PubMed] [Google Scholar]
- 11.Chu, I., J. Sun, A. Arnaout, H. Kahn, W. Hanna, S. Narod, P. Sun, C. K. Tan, L. Hengst, and J. Slingerland. 2007. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 128281-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curto, M., and A. I. McClatchey. 2008. Nf2/Merlin: a coordinator of receptor signalling and intercellular contact. Br J. Cancer 98256-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolado, I., A. Swat, N. Ajenjo, G. De Vita, A. Cuadrado, and A. R. Nebreda. 2007. p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell 11191-205. [DOI] [PubMed] [Google Scholar]
- 14.Faust, D., I. Dolado, A. Cuadrado, F. Oesch, C. Weiss, A. R. Nebreda, and C. Dietrich. 2005. p38alpha MAPK is required for contact inhibition. Oncogene 247941-7945. [DOI] [PubMed] [Google Scholar]
- 15.Frey, M. R., R. S. Dise, K. L. Edelblum, and D. B. Polk. 2006. p38 kinase regulates epidermal growth factor receptor downregulation and cellular migration. EMBO J. 255683-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuse, T., M. Tanikawa, M. Nakanishi, K. Ikeda, T. Tada, H. Inagaki, K. Asai, T. Kato, and K. Yamada. 2000. p27Kip1 expression by contact inhibition as a prognostic index of human glioma. J. Neurochem. 741393-1399. [DOI] [PubMed] [Google Scholar]
- 17.Grimmler, M., Y. Wang, T. Mund, Z. Cilensek, E. M. Keidel, M. B. Waddell, H. Jakel, M. Kullmann, R. W. Kriwacki, and L. Hengst. 2007. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128269-280. [DOI] [PubMed] [Google Scholar]
- 18.Grovdal, L. M., E. Stang, A. Sorkin, and I. H. Madshus. 2004. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp. Cell Res. 300388-395. [DOI] [PubMed] [Google Scholar]
- 19.Hall, A. B., N. Jura, J. DaSilva, Y. J. Jang, D. Gong, and D. Bar-Sagi. 2003. hSpry2 is targeted to the ubiquitin-dependent proteasome pathway by c-Cbl. Curr. Biol. 13308-314. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 10057-70. [DOI] [PubMed] [Google Scholar]
- 21.Ishida, N., M. Kitagawa, S. Hatakeyama, and K. Nakayama. 2000. Phosphorylation at serine 10, a major phosphorylation site of p27(Kip1), increases its protein stability. J. Biol. Chem. 27525146-25154. [DOI] [PubMed] [Google Scholar]
- 22.Jozic, D., N. Cardenes, Y. L. Deribe, G. Moncalian, D. Hoeller, Y. Groemping, I. Dikic, K. Rittinger, and J. Bravo. 2005. Cbl promotes clustering of endocytic adaptor proteins. Nat. Struct. Mol. Biol. 12972-979. [DOI] [PubMed] [Google Scholar]
- 23.Khurana, A., K. Nakayama, S. Williams, R. J. Davis, T. Mustelin, and Z. Ronai. 2006. Regulation of the ring finger E3 ligase Siah2 by p38 MAPK. J. Biol. Chem. 28135316-35326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiyokawa, H., R. D. Kineman, K. O. Manova-Todorova, V. C. Soares, E. S. Hoffman, M. Ono, D. Khanam, A. C. Hayday, L. A. Frohman, and A. Koff. 1996. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85721-732. [DOI] [PubMed] [Google Scholar]
- 25.Levenberg, S., A. Yarden, Z. Kam, and B. Geiger. 1999. p27 is involved in N-cadherin-mediated contact inhibition of cell growth and S-phase entry. Oncogene 18869-876. [DOI] [PubMed] [Google Scholar]
- 26.Levine, E. M., Y. Becker, C. W. Boone, and H. Eagle. 1965. Contact inhibition, macromolecular synthesis, and polyribosomes in cultured human diploid fibroblasts. Proc. Natl. Acad. Sci. USA 53350-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levkowitz, G., H. Waterman, E. Zamir, Z. Kam, S. Oved, W. Y. Langdon, L. Beguinot, B. Geiger, and Y. Yarden. 1998. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 123663-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lito, P., B. D. Mets, S. Kleff, S. O'Reilly, V. M. Maher, and J. J. McCormick. 2008. Evidence that sprouty 2 is necessary for sarcoma formation by H-Ras oncogene-transformed human fibroblasts. J. Biol. Chem. 2832002-2009. [DOI] [PubMed] [Google Scholar]
- 29.Martin, A., J. Odajima, S. L. Hunt, P. Dubus, S. Ortega, M. Malumbres, and M. Barbacid. 2005. Cdk2 is dispensable for cell cycle inhibition and tumor suppression mediated by p27(Kip1) and p21(Cip1). Cancer Cell 7591-598. [DOI] [PubMed] [Google Scholar]
- 30.Martinez, N., C. A. Garcia-Dominguez, B. Domingo, J. L. Oliva, N. Zarich, A. Sanchez, S. Gutierrez-Eisman, J. Llopis, and J. M. Rojas. 2007. Sprouty2 binds Grb2 at two different proline-rich regions, and the mechanism of ERK inhibition is independent of this interaction. Cell Signal. 192277-2285. [DOI] [PubMed] [Google Scholar]
- 31.Mason, J. M., D. J. Morrison, B. Bassit, M. Dimri, H. Band, J. D. Licht, and I. Gross. 2004. Tyrosine phosphorylation of Sprouty proteins regulates their ability to inhibit growth factor signaling: a dual feedback loop. Mol. Biol. Cell 152176-2188. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Nadeau, R. J., J. L. Toher, X. Yang, D. Kovalenko, and R. Friesel. 2007. Regulation of Sprouty2 stability by mammalian Seven-in-Absentia homolog 2. J. Cell Biochem. 100151-160. [DOI] [PubMed] [Google Scholar]
- 33.Polyak, K., J. Y. Kato, M. J. Solomon, C. J. Sherr, J. Massague, J. M. Roberts, and A. Koff. 1994. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 89-22. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt, M. H., and I. Dikic. 2005. The Cbl interactome and its functions. Nat. Rev. Mol. Cell Biol. 6907-918. [DOI] [PubMed] [Google Scholar]
- 35.Shtiegman, K., B. S. Kochupurakkal, Y. Zwang, G. Pines, A. Starr, A. Vexler, A. Citri, M. Katz, S. Lavi, Y. Ben-Basat, S. Benjamin, S. Corso, J. Gan, R. B. Yosef, S. Giordano, and Y. Yarden. 2007. Defective ubiquitinylation of EGFR mutants of lung cancer confers prolonged signaling. Oncogene 266968-6978. [DOI] [PubMed] [Google Scholar]
- 36.Sibilia, M., A. Fleischmann, A. Behrens, L. Stingl, J. Carroll, F. M. Watt, J. Schlessinger, and E. F. Wagner. 2000. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell 102211-220. [DOI] [PubMed] [Google Scholar]
- 37.Tsvetkov, L. M., K. H. Yeh, S. J. Lee, H. Sun, and H. Zhang. 1999. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr. Biol. 9661-664. [DOI] [PubMed] [Google Scholar]
- 38.Ventura, J. J., S. Tenbaum, E. Perdiguero, M. Huth, C. Guerra, M. Barbacid, M. Pasparakis, and A. R. Nebreda. 2007. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat. Genet. 39750-758. [DOI] [PubMed] [Google Scholar]
- 39.Vergarajauregui, S., A. San Miguel, and R. Puertollano. 2006. Activation of p38 mitogen-activated protein kinase promotes epidermal growth factor receptor internalization. Traffic 7686-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber, J. D., D. M. Raben, P. J. Phillips, and J. J. Baldassare. 1997. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem. J. 326(Pt. 1)61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wieser, R. J., D. Faust, C. Dietrich, and F. Oesch. 1999. p16INK4 mediates contact-inhibition of growth. Oncogene 18277-281. [DOI] [PubMed] [Google Scholar]
- 42.Wong, E. S., C. W. Fong, J. Lim, P. Yusoff, B. C. Low, W. Y. Langdon, and G. R. Guy. 2002. Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. EMBO J. 214796-4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yanagisawa, K., A. Kosaka, H. Iwahana, M. Nakanishi, and S. Tominaga. 1999. Opposite regulation of the expression of cyclin-dependent kinase inhibitors during contact inhibition. J. Biochem. 12536-40. [DOI] [PubMed] [Google Scholar]
- 44.Zeng, Q., and W. Hong. 2008. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 13188-192. [DOI] [PubMed] [Google Scholar]
- 45.Zhao, B., X. Wei, W. Li, R. S. Udan, Q. Yang, J. Kim, J. Xie, T. Ikenoue, J. Yu, L. Li, P. Zheng, K. Ye, A. Chinnaiyan, G. Halder, Z. C. Lai, and K. L. Guan. 2007. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 212747-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zwang, Y., and Y. Yarden. 2006. p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. EMBO J. 254195-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]