Abstract
Human DNA polymerase η (Pol η) modulates susceptibility to skin cancer by promoting translesion DNA synthesis (TLS) past sunlight-induced cyclobutane pyrimidine dimers. Despite its well-established role in TLS synthesis, the role of Pol η in maintaining genome stability in the absence of external DNA damage has not been well explored. We show here that short hairpin RNA-mediated depletion of Pol η from undamaged human cells affects cell cycle progression and the rate of cell proliferation and results in increased spontaneous chromosome breaks and common fragile site expression with the activation of ATM-mediated DNA damage checkpoint signaling. These phenotypes were also observed in association with modified replication factory dynamics during S phase. In contrast to that seen in Pol η-depleted cells, none of these cellular or karyotypic defects were observed in cells depleted for Pol ι, the closest relative of Pol η. Our results identify a new role for Pol η in maintaining genomic stability during unperturbed S phase and challenge the idea that the sole functional role of Pol η in human cells is in TLS DNA damage tolerance and/or repair pathways following exogenous DNA damage.
Mutations in the POLH gene that encodes DNA polymerase η (Pol η) are responsible for the variant form of xeroderma pigmentosum (XP-V). XP-V is a rare autosomal recessive disorder characterized by extreme sensitivity to sunlight and a very high incidence of sunlight-induced skin cancer, as are the other forms of “classical” XP (17, 27). However, in contrast to the other nucleotide excision repair (NER)-defective XP complementation groups (XP-A to XP-G), XP-V cells have normal NER but cannot support translesion synthesis (TLS) past DNA-containing cyclobutane pyrimidine dimers (CPDs) (27). Purified Pol η, the TLS polymerase that is mutated in XP-V, is able to synthesize past this lesion with a high level of efficiency (28), and in a majority of cases it inserts the correct nucleotide, adenine, opposite the two thymines contained in the cyclobutane pyrimidine dimer ring (26).
The ability to replicate efficiently past UV pyrimidine dimers has been the principal—or sole—function assigned thus far to Pol η. In the absence of Pol η, cells display an increased rate of UV-induced mutagenesis and carcinogenesis (23) that may reflect inefficient or error-prone synthesis by another polymerase. In mouse cells, this back-up polymerase may be Pol ι (12). Despite its ability to replicate past cyclobutane pyrimidine dimers, Pol η does not appear to be able to carry out TLS past the other major UV photoproduct, the pyrimidine (6-4) pyrimidone photoproduct [(6-4)PP] in vitro or in vivo. It can, however, replicate past a limited number of other types of DNA damage in vitro, albeit with a lower level of efficiency than past CPDs (21). Whether the bypass of these lesions is performed in vivo by Pol η is less clear. For example, XP-V cells are sensitive to cisplatin, suggesting that bypass of cisplatin lesions may depend on Pol η (1). Combined NER- and Pol η-mediated lesion bypass has also been suggested as the likely mechanism for repairing DNA interstrand cross-links formed by mitomycin C (46) and psoralen (32). In contrast, Pol η does not appear to play a role in replication past endogenous lesions such as 8-oxoguanine (3) or abasic sites (2).
It has been difficult to visualize or identify sites of action of Pol η or any of the other TLS polymerases by immunofluorescence due to their low levels of expression. However, in cells that mildly overexpress Pol η, it has been possible to localize the polymerase to nuclear replication factories during S phase. This localization depends on several motifs located close to the C terminus of Pol η, including an NLS and a ubiquitin-binding zinc finger domain (7, 18). Localization of Pol η in replication factories may concentrate the polymerase near sites of replication to facilitate recruitment to carry out TLS. If cells cannot remove or synthesize through a lesion blocking the replication fork, then homology-dependent recombinational repair (HRR) may be used to restart the replication fork (11, 34). RAD51-mediated HRR has been shown to be important for the repair of DNA damage during replication in all organisms (20, 31, 42). Recent evidence has suggested that Pol η, in addition to its role in TLS, may participate in HRR. This has been suggested by analyses of gene conversion in chicken DT40 cells during immunoglobulin gene diversification (19), as well as by in vitro experiments showing that Pol η is capable of promoting extension of the invading strand in D-loop structures to facilitate RAD52-mediated second-end capture during recombination-mediated repair (29, 30). The functional importance of this observation is less clear. Recent evidence from yeast argues that the bulk of heteroduplex DNA strand extension during HRR is mediated by the preferential recruitment of a replicative DNA polymerase, Pol δ (25). Moreover, there is no obvious recombination deficit in XP-V patients or in XP-V cells beyond a modest elevation in the frequency of UV-induced sister chromatid exchanges (10).
In order to better understand the functional roles and importance of Pol η in human cells, we used short hairpin RNAs (shRNAs) to selectively deplete Pol η from cells and then determined how the loss of Pol η affected cell cycle progression, DNA replication dynamics, and cell proliferation in otherwise unperturbed cells. These experiments revealed an unexpected role for Pol η in maintaining chromosomal stability and preventing common fragile site (CFS) breakage during unperturbed S phase. Our results thus broaden the functional role of Pol η in human cells to include the maintenance of genomic stability during unperturbed DNA replication in S phase.
MATERIALS AND METHODS
Pol η cloning and mutagenesis.
The human Pol η coding sequence was cloned as a NheI-XhoI fragment from the pGBKT7-hpoleta vector (44) into pET28 (Novagen). A catalytically inactive mutant of human Pol η (Dead Pol η) was constructed by using QuikChange (Stratagene) according to the manufacturer's instructions to incorporate mutations (D115A and E116A) as well as a silent SacII site used for the mutant selection. The resulting products were sequence verified prior to transfection into Escherichia coli host strain BL21-CodonPlus(DE3)-RIL (Stratagene) for protein production. The open reading frames of native and Dead Pol η were also subcloned into the cytomegalovirus-driven expression vector pcDNA.3.1/Hygro (Invitrogen) to allow transient ectopic expression of Pol η. A new multiple cloning site (Table 1) was introduced into the pcDNA3.1/Hygro vector (Invitrogen) as a NheI-XhoI fragment. A double FLAG sequence (Table 1) has been inserted as a HindIII-BamHI fragment. Finally, the Pol η PCR product obtained from pET28 plasmid using Eta Fw and Eta Rev primers (Table 1) was cloned into the modified pCDNA3.1/Hygro vector as a BamHI-NotI fragment.
TABLE 1.
Oligonucleotides and sequences used in this study
| Oligonucleotide | Oligonucleotide sequence |
|---|---|
| shη1 | 5′-GTGTTGAAGTGATGGAGAT-3′ |
| shη2 = siη (3′ UTR) | 5′-GCAATGAGGGCCTTGAACA-3′ |
| shι1 | 5′-CTCAGTCCTTTAGTGAAGA-3′ |
| shι2 (3′ UTR) | 5′-GAAGTAAATTCTGGCACAA-3′ |
| FLAG | 5′-AGCTTTTAATTAAGCCGCCACCATGGATTATAAAGATCATGACATCGATTACAAGGATGACGATGACAAGGCTAGCG-3′ |
| MCS | 5′-CTAGTAAGCTTGGGTTAATTAAGGATAGCTAGCGCAGGCGGATCCGGCGGAAAAGCGGCCGCTTAC-3′ |
| Eta Fw | 5′-CGCGGATCCCTTATGGCTACTGGACAGGATCG-3′ |
| Eta Rev | 5′-TTTTCCTTTTGCGGCCGCCTAATGTGTTAATGGCTTAAAAAATG-3′ |
RheoSwitch-inducible system (New England Biolabs) for the regulated expression of native and catalytically inactive ectopic Pol η.
A double FLAG tag (Table 1) was introduced in the pNEBR-X1 plasmid as a BamHI-HindIII fragment. The open reading frames were cloned in the pNEBR-X1-FLAG expression vector as a BamHI-NotI fragment. The pNEBR-R1 vector was transfected into U2OS cells, and clones were selected with neomycin (Invitrogen) at 50 μg/ml for 15 days. To select the best repressor clone, we have transiently transfected the pNEBR-X1 vector expressing green fluorescent protein into these clones and we have analyzed green fluorescent protein expression by flow cytometry 24 h after the addition of the RSL1 ligand (500 nM; New England Biolabs). The best clone was then cotransfected with the pNEBR-X1-FLAG vector expressing either the wild-type (WT) Pol η or the Dead Pol η together with the pcDNA3.1/Hygro vector (Invitrogen), so selection was achieved by addition of hygromycin (Invitrogen) at 250 μg/ml for 15 days. Regulated expression of proteins was assayed after RSL1 induction, followed by Western blotting. Clones presenting the expression level of ectopic Pol η similar to the endogenous Pol η were used in this study.
Cell culture.
U2OS cells, MRC5 fibroblasts, and XP-V XP30RO cells (kindly provided by Patricia Kannouche, Villejuif, France) were grown as described previously (18, 38, 43). Control or depletion replicative vectors (pEBVsiRNA) (6) were calcium phosphate transfected into U2OS cells. Individual stable clones were selected and maintained in 200 μg/ml hygromycin-B (Invitrogen). For transient depletion experiments, 100 nM of control small interfering RNA (siRNA) (Eurogentec) or a Pol η-specific siRNA (Sigma) (Table 1) was transfected 24 h after seeding by using the Lipofectamine 2000 protocol.
Cell growth and survival assays.
Growth curves were determined by seeding 75,000 cells in duplicate into six-well plates, counting duplicate wells each day over the subsequent 4 days of growth. Doubling time [Td = ln(2)/slope], means, and standard deviations were calculated from three independent experiments. For cell survival after UVC radiation (2.5 and 5 J/m2) or caffeine (100, 500, and 1,000 μM) exposure, 500 to 1,000 cells were seeded into six-well plates and treated 24 h after seeding, and 8 days later cells were stained with crystal violet. Mean survivals and standard deviations were determined from three independent experiments.
Antibodies for Western blot analysis.
We used primary antibodies against Pol η (50 μg/ml; Abcam ab17725), Pol ι (a gift from R. Woodgate [NIH, Bethesda, MD]), phosphorylated Ser345-Chk1 (1:1,000; 133D3; Cell Signaling), total Chk1 (1:1,000; G-4, Cell Signaling), Ku70 (1:10,000; N3H10; Interchim), and actin (1:10,000; C4; Chemicon).
Immunofluorescence.
Experiments were performed as described in reference 18. Different primary antibodies directed against PCNA (1:500; Abcam ab-18197), Pol η (1:100; FLAG M2 F-3165; Sigma), γ-H2AX (1:500; JBW 301; Upstate Biotechnology), and pChk2 (1:100; pThr68-Chk2; Cell Signaling) were used. Alexa Fluor 488 and 594 (Fisher) were used as secondary antibodies at a dilution of 1:1,000. All antibody incubations were performed for 1 h at 37°C. Images were collected through a 12-filter set on a Leica microscope (×100 objective), using a CoolSNAP HQ charge-coupled-device camera (Photometrics, Roper Scientific) driven by MetaMorph software (Roper Scientifics).
Cell cycle.
To determine the cell cycle phase distribution, cells were fixed in 70% ethanol and then labeled with propidium iodide as described previously (35). S-phase progression analysis was performed as described in reference 37, except that cells were blocked in G2/M phase by the addition of colcemid (0.1 μg/ml; Gibco). Cells were then incubated for 15 min at 37°C in phosphate-buffered saline (PBS)-5 mM EDTA containing 10 μg/ml propidium iodide (Sigma) and 1 mg/ml RNase A (Sigma). Fluorescence-activated cell sorter (FACS) analysis of the doubly labeled cells was performed on a FACScan (Becton Dickinson). Results were analyzed using ModFIT or WinMDI 1.8 software. Means and standard deviations were calculated from three independent experiments.
DNA fiber stretching.
DNA was stretched on silanized glass and loaded into channels by capillary tension as described in reference 41. Immunodetection with confocal microscopy of stretched DNAs was performed on the Zeiss Axiovert microscope with a ×63 objective. Up to 70 merged two-color digital images of tracks were collected and saved. Lengths of tracks were subsequently measured in these images using the attached Zeiss AxioVision software.
Metaphase.
Cells (500,000) were seeded into 10-cm dishes in growth medium for 24 h, at which point 100 nM aphidicolin (Sigma) was added or not for an additional 24 h. Colcemid (0.1 μg/ml; Gibco) was added for the last 3 h to accumulate mitotic cells prior to centrifugation, resuspension in PBS, and hypotonic swelling in 0.075 M KCl for 6 min at 37°C. Cells were then centrifuged, rinsed, and fixed in methanol-acetic acid (3:1). Spread chromosomes were stained for 5 min with 70 μg/ml acridine orange (Sigma), washed with several changes of PBS, and then mounted on slides in mounting medium (DakoCytomation) prior to microscopy (see “Immunofluorescence”).
FISH analysis.
A total of 500 ng of FRA7H probe (BAC 36B6 RP-11) was labeled with Chromatid Alexa Fluor 488 dUTP (Invitrogen) using the BioPrime DNA labeling system (Invitrogen), purified on Probe Quant microcolumns (GE Healthcare), and then ethanol precipitated with human Cot-1 DNA (Invitrogen; 0.1 μg/μl) overnight at −20°C. Precipitated DNA was collected by centrifugation (30 min at 10,000 × g) and incubated for 2 h at 37°C in hybridization mix (45) prior to use. A human chromosome 7-specific centromeric probe labeled with Cy3 was also used according to the supplier's recommendations (Cambio). A fluorescence in situ hybridization (FISH) experiment was carried out as described in reference 45. Analyses were performed on a Zeiss microscope Imager.Z1 (×100 objective) coupled with a SenSys camera using the CytoVision software measuring module (Applied Image Corp.).
RESULTS
Generation of Pol η- and Pol ι-depleted human cells.
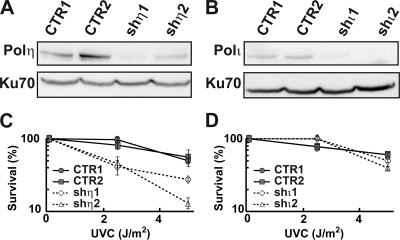
We constructed independent clonal derivatives of human p53-proficient osteosarcoma U2OS cells in which the expression of POLH or POLI had been silenced by using replicative vectors (pEBVsiRNA) (6) expressing independent shRNA sequences specific for the POLH (shη1 and shη2) or POLI (shι1 and shι2) mRNAs (see Materials and Methods) (Table 1). The control shRNA sequence was previously validated in several studies (5). This vector system produced stable gene silencing even after several months in culture, with substantial depletion of Pol η (90% and 70% for shη1 and shη2, respectively) or Pol ι (75% and 80% for shι1 and shι2, respectively) as assessed by Western blot analysis (Fig. 1A and B). We verified both the extent and specificity of depletions by using real-time PCR as well and used the same method to demonstrate that expression of none of the other replicative or Y family DNA polymerases that were examined was altered in cells depleted of Pol η or Pol ι (data not shown). The phenotype of Pol η-depleted cells was further verified by demonstrating increased UV sensitivity compared to cell clones expressing control shRNAs (Fig. 1C and D).
FIG. 1.
Pol η depletion from U2OS cells affects UV survival. (A and B) Western blot analyses of whole-cell extracts prepared from U2OS cell clones that are expressing either a control shRNA (CTR1, CTR2), Pol η shRNAs shη1 or shη2, or Pol ι shRNAs shι1 or shι2 revealed near-complete depletion of both polymerases by each polymerase-specific shRNA. Ku70 was used as a loading control. (C and D) Colony-forming assay results of Pol η- and Pol ι-depleted U2OS cells described above were determined as a function of UV dose. Cells depleted of Pol η (shη1 and shη2) (dotted lines) displayed a significant dose-dependent decrease in survival after UVC radiation, in contrast to Pol ι-depleted or control cells. Experiments were done twice, with triplicate samples for each UV dose, in order to determine mean colony-forming assay results, and the standard deviations are indicated by error bars.
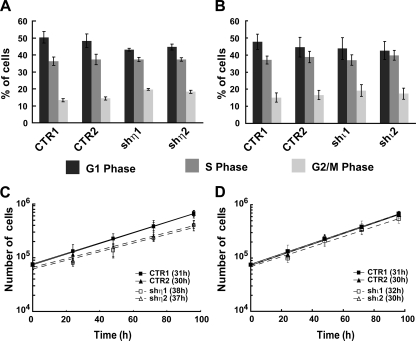
Pol η-depleted cells have G2/M and proliferative defects.
We used quantitative FACS analysis to determine whether Pol η- or Pol ι-depleted cells displayed a cell cycle progression defect in the absence of DNA damage. These analyses were performed using asynchronous cultures of shη1- and shη2-depleted and CTR1 and CTR2 clones in order to avoid potential artifacts introduced by synchronization. Flow analyses revealed a modest but significant (P values of 0.002 for shη1 and 0.01 for shη2) accumulation of Pol η-depleted cells in G2/M with a correspondingly reduced G1 fraction compared with that in control cells (Fig. 2A). In agreement with these results, Pol η-depleted cells proliferated more slowly in exponential culture (Fig. 2C) with a doubling time ∼7 h longer than that of control cells (37 and 38 h for shη1 and shη2, respectively, versus 30 and 31 h for CTR1 and CTR2, respectively). In contrast, we observed no difference in cell cycle phase distribution or doubling time in cells depleted of the Y family Pol ι (Fig. 2B and D).
FIG. 2.
Pol η-deficient cells present a defect of cell cycle progression. (A and B) Cell cycle progression was investigated by flow cytometry analysis after propidium iodide incorporation on controls (CTR1, CTR2) and Pol η-deficient cells (shη1, shη2) (A) and on controls (CTR1, CTR2) and Pol ι-deficient cells (shι1, shι2) (B). Experiments were done three times, and standard deviations are indicated by error bars. P values were calculated using the Student t test (P values of 0.002 for shη1 and 0.01 for shη2). (C and D) Growth curve analysis was done over 96 h on control cells (CTR1 and CTR2) (full line) and on Pol η-deficient cells (shη1 and shη2) (dotted line), shown in panel C, and on control cells (CTR1, CTR2) and Pol ι-deficient cells (shι1 and shι2) (dotted line), shown in panel D. Experiments were done three times in triplicate, and standard deviations are indicated by error bars. The doubling time was calculated from this curve.
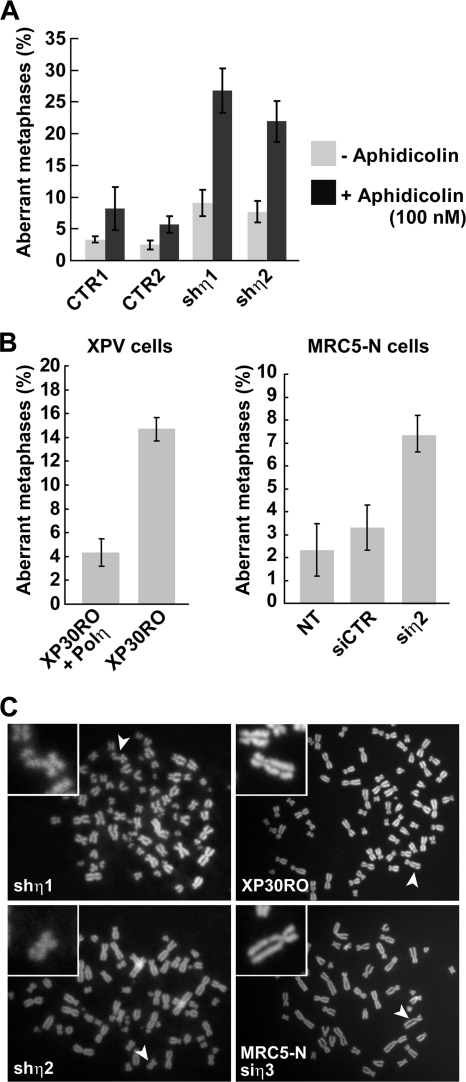
Spontaneous chromosome abnormalities in Pol η-depleted cells.
We analyzed metaphase spreads prepared from Pol η (shη1 and shη2)-depleted and control (CTR1 and CTR2) cells to further investigate the consequences of loss of Pol η for chromosomal stability. Pol η depletion from U2OS cells resulted in a significant increase in the frequency of cells displaying chromatid breaks in the absence of exogenous DNA damage or S-phase perturbation (7 to 8% of metaphases in depleted cells versus 2 to 3% of metaphases in control cells) (Fig. 3A). Two additional experiments were performed to demonstrate the specificity of this effect for Pol η loss of function. First, we depleted Pol η from normal diploid human MRC5 fibroblasts using siη2 (Table 1) and confirmed the karyotypic instability first observed in Pol η-depleted U2OS cells (Fig. 3B). Second, we confirmed an elevated level of spontaneous chromatid breaks in XP30RO cells from an XP-V patient who carries a POLH gene deletion that leads to truncation of Pol η at residue 35 (Ala35) (17) and demonstrated that breakage was suppressed when these cells were complemented with a POLH cDNA encoding full-length Pol η (Fig. 3B). Chromatid breaks and gaps were the most common abnormalities in all of the Pol η-deficient cell lines analyzed (Fig. 3C). We also observed a substantially higher frequency of aberrant metaphases with more than two breaks or gaps per metaphase in Pol η-depleted cells treated with the replication inhibitor aphidicolin (Fig. 3A). This observation, together with the preponderance of chromatid-type lesions, indicates that most or all chromosomal abnormalities in Pol η-depleted cells are likely to be replication dependent (Fig. 3A). These data are, to the best of our knowledge, the first demonstration that spontaneous chromosome instability occurs in XP-V/Pol η-deficient cells in the absence of exogenous stress.
FIG. 3.
Loss of Pol η affects chromosomal stability. The frequency of spontaneous chromatid breaks in three independent experiments (>50 metaphases for each) was quantified from spread metaphase analysis by fluorescence microscopy from U2OS control (CTR1 and CTR2) and Pol η-deficient U2OS cells (shη1 and shη2) treated or not treated with 100 nM of aphidicolin (A) and XP30RO/XP30RO-Pol η cells and MRC5 cells (untransfected [NT], siCTR and siη2) (B). Standard deviations are indicated by error bars. (C) Illustration of chromatid breaks (noted with arrows) in all Pol η-deficient cell lines.
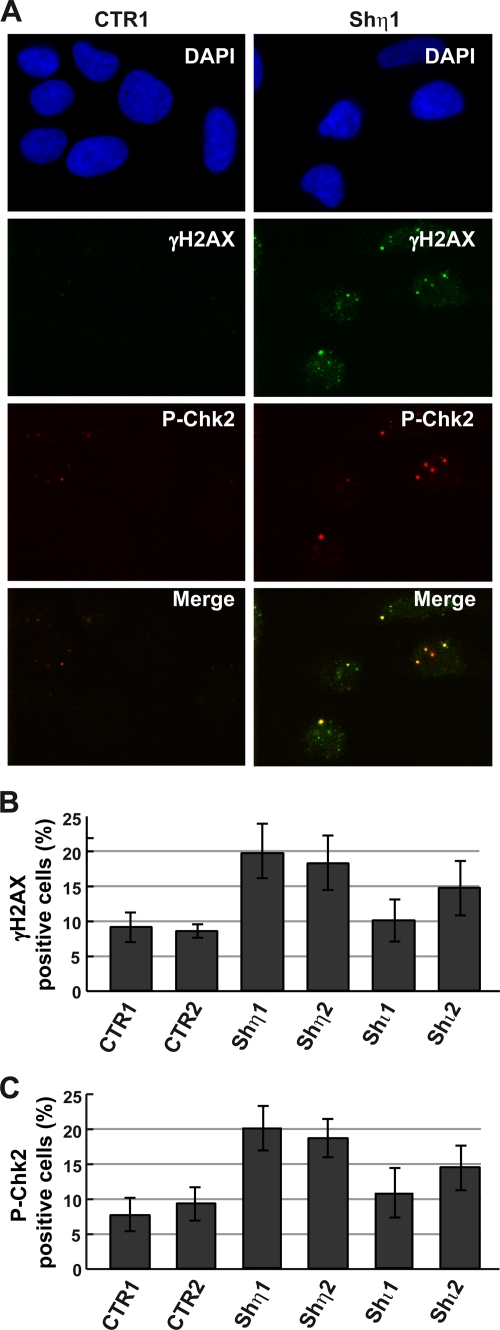
Pol η depletion leads to DNA break-mediated damage signaling.
We noticed that a subset of Pol η-deficient cells showed elevated levels of γ-H2AX focus formation, an indicator of DNA strand breakage, in the absence of exogenous DNA damage (Fig. 4A and B). Although the intensity of the γ-H2AX response in Pol η-depleted cells was weaker than that after exposure to exogenous DNA damage, it was reproducible and significantly elevated over the background compared with that in Pol η-proficient cells. We also observed, although at a lesser extent and at a lesser intensity, elevated γ-H2AX focus formation in Pol ι-depleted cells (Fig. 4B), probably due to the recently identified novel role of Pol ι in protecting cells from endogenous oxidative damage (36).
FIG. 4.
Increase of DNA breaks and activation of cell cycle checkpoint Chk2 in the absence of Pol η. (A) γH2AX and P-Chk2 focus formations were analyzed by immunofluorescence in control cells (CTR1) and Pol η-deficient cells (shη1) 48 h after seeding. DNA content was visualized by 4′,6-diamidino-2-phenylindole (DAPI) coloration. Merged images show the partial colocalization of each protein. The number of cells with γH2AX or P-Chk2 foci (called positive cells) was determined in control cells (CTR1 and CTR2), in Pol η-deficient cells (shη1 and shη2), and in Pol ι-deficient cells (shι1 and shι2). The quantification in three experiments (>100 cells) is presented in panels B and C, and standard deviations are indicated by error bars.
These focus-forming data indicated that Pol η might be localizing to sites of DNA double-strand breaks (DSBs) marked by γ-H2AX in Pol η-depleted cells.
In order to further investigate this possibility, we determined the level of expression and the phosphorylation status of two central transducer kinases in DNA damage response signaling, Chk1 and Chk2. These kinases are phosphorylated by the apical DNA damage response kinases ATR and ATM in response to replication stress (33). The level of phosphorylated Chk1, assessed by Western blotting with a Chk1 anti-phosphoserine 345-specific antibody, did not change when either Pol η or Pol ι was depleted from U2OS cells (data not presented). In contrast, there was a twofold induction of pThr68-Chk2 formation in cells depleted of Pol η (Fig. 4C). This response was not observed in mock-depleted cells and was observed to a lesser extent in cells depleted of Pol ι (Fig. 4A and C). Double immunostaining revealed that pThr68-Chk2 colocalized in nuclear foci with γ-H2AX in cells depleted of Pol η (Fig. 4A). These results indicate that depleting Pol η from human cells causes increased genomic instability, DSB accumulation, and damage-induced focus formation in the absence of added DNA damage and suggest that these events occur late in S phase or in G2 phase.
Increased CFS expression following Pol η depletion.
The karyotypic and cytologic results reported above raised the question of whether specific chromosomal regions might be preferentially affected or destabilized in Pol η-depleted cells. We focused first on CFSs, specific regions of the human karyotype that preferentially exhibit metaphase gaps or breaks following partial inhibition of DNA synthesis. These sites are also frequently “expressed” in human tumors, meaning rearranged or lost as a consequence of chromosomal breakage, and may be sensitive indicators of replication stress in premalignant cells (4, 14). The most highly expressed CFSs in human cells include 3p14.2 (FRA3B), 16q23 (FRA16D), 6q26 (FRA6E), 7q32.3 (FRA7H), and Xp22.3 (FRAXB). Fragile site sensitivity is in part cell line dependent, and among the sites listed above, the typical expression of the only 7q32.3 FRA7H CFS can be observed in U2OS cells, probably due to rearrangements in other CFSs in this tumor cell line (9). In order to determine whether depletion of Pol η from U20S cells promoted instability at the 7q32.3 (FRA7H) fragile site, we used a FISH-based assay to quantify the fraction of chromosomal breaks that localized to 7q32.3 (FRA7H) in Pol η-depleted U2OS cells (Fig. 5).
FIG. 5.
Role of Pol η in CFS stability. (A) Localization of FRA7H probe (in green) was analyzed by using FISH in control cells (CTR1) and checked using a centromeric probe (in red) specific to chromosome 7. (B and C) Expression of the CFS FRA7H (translocations, amplifications, deletions) was analyzed by using FISH in Pol η-deficient cells (shη1 and shη2). The quantification of FRA7H expression in cells treated or not treated with aphidicolin (100 nM) for 24 h is presented in panel D (three experiments; >50 metaphases), and standard deviations are indicated by error bars. (E) Colony-forming assay of Pol η-depleted U2OS cells was determined as a function of caffeine concentration. Experiments were done three times, with triplicate samples for each caffeine dose, in order to determine mean numbers of CFUs, and standard deviations are indicated by error bars.
We first verified the specificity of our FRA7H probe by hybridizing it along with a chromosome 7-specific centromeric probe to metaphase spreads from diploid cells. FRA7H probe was then used to assess expression of the FRA7H fragile site in Pol η-depleted cells. We observed an eightfold increase in FRA7H expression (translocations, amplifications, deletions) under conditions of replication stress in Pol η-depleted cells (Fig. 5D). When we analyzed the same cells under unperturbed growth conditions, there was a threefold increase of FRA7H expression in Pol η-depleted cells compared with that in control cells (Fig. 5A to D). FRA7H expression was not elevated in either mock-depleted or Pol ι-depleted U2OS cells (not shown). These results indicate that Pol η may contribute to CFS stability under conditions of unperturbed growth as well as under conditions of replicative stress.
ATR was found to play a major role in maintaining the stability of CFSs by directing the cellular checkpoint response to stalled replication at these sites (9). Cells lacking ATR showed a dramatic increase in CFS expression following treatment with low doses of aphidicolin (50 and 100 nM). Furthermore, ATR deficiency alone induced a low frequency of spontaneous gaps and breaks at CFSs, showing that ATR is required for CFS stability even during unperturbed replication, as we show here for Pol η. One prediction from these observations was that Pol η-depleted U2OS cells should have enhanced sensitivity to the ATR inhibitor caffeine compared to cells expressing control shRNAs. The data demonstrate that this is the case: the absence of Pol η in conjunction with ATR deficiency further suppresses cell viability (Fig. 5E). This result further supports a role for Pol η in maintaining CFS stability.
Analysis of replication dynamics in Pol η-depleted cells.
Our fragile site results led us to examine replication dynamics in Pol η-depleted cells. CFSs are known to be induced by inhibitors of DNA synthesis, are late replicating, and are dependent upon a functional intra-S checkpoint for expression (14). These observations have led to the hypothesis that CFSs may result from the delayed or incomplete replication of genomic regions that are inherently difficult to replicate, with the production of stalled or broken replication forks or DNA single-strand gaps. In order to better understand how Pol η depletion was contributing to CFS expression in the absence of added DNA damage, we determined whether Pol η-deficient cells had altered S-phase progression, replication fork rates, or S-phase nuclear replication factory dynamics.
We analyzed the duration of S phase in Pol η-depleted and control cells by performing a flow cytometric analysis of cells that had been pulse-labeled for 15 min in early S phase with bromodeoxyuridine followed by a thymidine “chase” and were then analyzed over a 10-h time course (Fig. 6A). Pol η-depleted cells and control cells did not differ significantly in progression through S phase until 6 h after labeling, when a slight delay in S phase was observed in Pol η-depleted cells (Fig. 6A).
FIG. 6.
Analysis of S-phase duration and fork progression in Pol η-deficient cells. (A) Percentage of cells in S phase for control (CTR1, CTR2) and Pol η shRNA clones (shη1, shη2) as determined by bromodeoxyuridine incorporation is presented. Experiments were done three times, and standard deviations are indicated by error bars. (B) Analysis of replication fork progression at a single-molecule level in Pol η-depleted cells as described in Materials and Methods. Replication tracks that represented individual ongoing forks, for example, labeled with both IdU (green) and CldU (red) as shown in the image (marked by green and red arrows), were identified and the lengths of their IdU and CldU segments (on average, 100) were measured for each sample. (C and D) Fork rates in micrometers/10 min were determined by dividing the length of IdU or CldU segments by the labeling time coefficient (1.5 and 3, respectively). (C) Cumulative plot of typical fork rate distributions derived from CldU segments in one of the experiments. (D) Summary of the rates obtained by measuring lengths of IdU or CldU segments in ongoing forks in all three independent experiments. In this case, median fork rates were calculated and then averaged between experiments. Error bars indicate standard deviations. Of note, fork rates estimated on the basis of IdU or CldU segment lengths were very similar, as anticipated.
We next asked whether Pol η-depleted cells exhibited alterations in the rate of replication fork progression on undamaged DNA. For this analysis, we used a newly developed method in which replicated DNA is labeled in vivo with the halogenated nucleosides IdU and CldU and then isolated and stretched by passive flow through microchannels prior to capture on silanized glass coverslips (see Materials and Methods). Newly replicated regions (“replication tracks”) in the stretched DNA molecules could then be visualized and measured by immunostaining with antibodies that are specific to CldU or IdU (41). This method enabled us to monitor replication fork progression at the level of individual replicating DNA molecules (Fig. 6B) and to show that, as it was recently observed in avian cell lines (15), fork progression rates did not differ between Pol η-depleted cells and control cells in the absence of DNA damage (Fig. 6C and D).
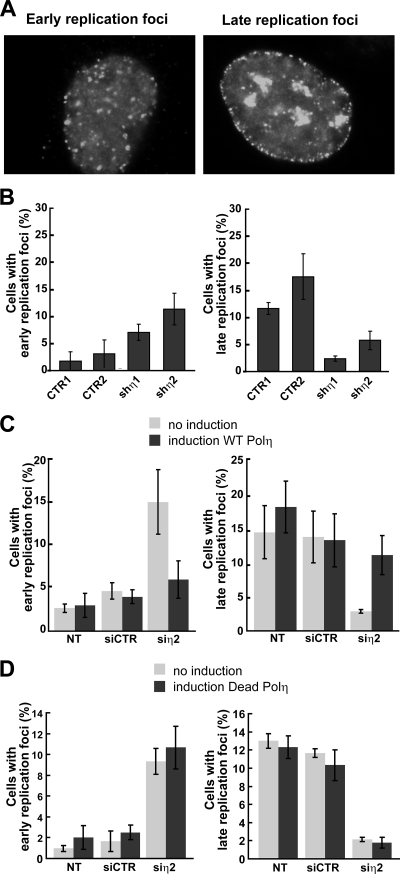
We also determined whether Pol η depletion affected replication factory dynamics over the duration of S phase in otherwise unperturbed cells (Fig. 7). DNA replication in human cells takes place at discrete sites in the nucleus, termed replication factories, where DNA is colocalized with large protein complexes that contain DNA polymerases and accessory replication proteins such as PCNA. These replication foci undergo reproducible changes in number, size, and location during S-phase progression (22). During early S phase, hundreds of small factories are distributed throughout the nucleoplasm, with the exception of the nucleoli (Fig. 7A). In late S phase, replication foci decrease in number but increase in size and often form characteristic ringlike structures in the nucleoplasm together with factories distributed along the nuclear membrane (Fig. 7A). We found that Pol η-depleted U2OS cells contained predominantly early replication factories with fewer cells displaying late replication factory morphologies (Fig. 7B).
FIG. 7.
Depletion of Pol η affects the spatial organization of active replication foci in U2OS cells. (A) An image of early and late replication PCNA foci in U2OS cells analyzed by immunofluorescence. (B) Quantification of cells with early and late replication foci was performed in three experiments (>100 cells) for control cells (CTR1, CTR2) and Pol η-deficient cells (shη1, shη2). (C and D) Normal replication focus pattern is restored after expression of a WT form, but not a catalytically inactive form, of Pol η in U2OS cells containing a RheoSwitch-inducible system. Cells with early and late replication foci were quantified in untransfected cells (NT), cells transfected by control siRNA, or cells transfected by Pol η siRNA after induction or not of ectopic Pol η forms by the RSL1 ligand (500 nM) (see Materials and Methods). Standard deviations are indicated by error bars.
In order to determine whether these changes in replication factory dynamics depend on Pol η catalytic activity, we used a RheoSwitch-regulated, inducible gene expression system (see Materials and Methods) to reexpress at comparable levels (as evidenced by the intensity of the green flag-stained cells) catalytically active or inactive Pol η in depleted cells (Fig. 8) prior to analyzing replication factory morphology by PCNA immunostaining (Fig. 7C and D). The catalytically inactive mutant of Pol η (Dead Pol η) contains two mutations (D115A and E116A) in the two critical active site residues. This mutant Pol η open reading frame was also tagged with a double FLAG epitope tag to facilitate cytologic analyses (see Fig. S1A in the supplemental material). The absence of catalytic activity of this double mutant form of Pol η was verified by purifying Dead Pol η and showing that it was not able to replicate past CPDs in vitro (see Fig. S1B in the supplemental material). We demonstrated that replication factory abnormalities observed in Pol η-depleted cells could be suppressed by native, though not catalytically inactive, Pol η (Fig. 7C and D). We observed similar perturbed replication factory dynamics in Pol η-depleted normal human diploid fibroblasts and in XP-V cells prior to complementation with catalytically active Pol η (see Fig. S2A and B in the supplemental material). These results collectively indicate that Pol η activity is required for normal replication factory formation and maturation during S phase.
FIG. 8.
Rescue experiments by the RheoSwitch-inducible system. (A and C) Western blots of whole-cell extracts prepared from U2OS cells containing a RheoSwitch-inducible system, cells not transfected (lane 1 and 4), and cells transfected by control siRNA (lane 2 and 5) or Pol η siRNA (lane 3 and 6). Blots were incubated with DNA Pol η antibody and Ku70 antibody, with the latter used as a normalization control. The Pol η siRNA (siη2) used in this experiment corresponds to the sequence of the shRNA used to construct shη2 stable clones. To induce the expression of the tagged WT form of Pol η (FLAG-WT Pol η) (A, lanes 4 to 6) or the tagged catalytically inactive mutant of Pol η (FLAG-Dead Pol η) (C, lanes 4 to 6), cells were exposed to the ligand RSL1 (500 nM) (see Materials and Methods). (B and D) Induction of the expression of tagged FLAG-WT Pol η and FLAG-Dead Pol η was analyzed by immunofluorescence in control cells (siCTR) and in Pol η-deficient cells (shη1). The DNA content was visualized by staining with 4′,6-diamidino-2-phenylindole (DAPI).
Our results provide the first documentation of an altered DNA replication program in XP-V or Pol η-depleted cells in the absence of exogenous DNA damage that is associated with and may be mechanistically linked to fragile site expression. Interestingly, we observed similar changes in replication factories in cells partially depleted for Rad51 by siRNA (40; data not shown), a key component in HRR. This could reflect the presence or persistence of DSBs occurring during replication of CFSs, or that Pol η and Rad51 act in the same postreplication repair pathway during S phase.
DISCUSSION
Pol η is best known for its role in responding to genome damage that arises when a cell is exposed to an extrinsic genotoxic stress, such as UV irradiation. Individuals with mutations in the human POLH gene are affected by the variant form XP-V, a rare, autosomal recessive human genetic syndrome with sun hypersensitivity associated with UV hypermutability, numerous skin abnormalities, and a high level of early and multiple skin cancers on sun-exposed sites of the body, which is consistent with the ability of Pol η to support efficient and error-prone TLS across one of the sites of major UV damage, the cyclobutane thymine dimer.
We used shRNA-mediated Pol η depletion from human cells to explore and better understand physiologic roles of Pol η in both unperturbed S phase and in cells following the addition of replication inhibitors. Pol η-depleted cells demonstrated two consistent abnormalities that may be mechanistically linked: altered replication factory dynamics and elevated instability of a CFS. Of importance, these abnormalities were demonstrated in unperturbed replicating cells and in several different cell types, including primary fibroblasts, and appeared to depend on Pol η catalytic activity as demonstrated by a combination of depletion and complementation experiments. Moreover, these effects are specific to Pol η since none of these cellular or karyotypic defects was observed in cells depleted for Pol ι, the closest relative of Pol η. These observations provide new information on the role of Pol η during S phase in unperturbed normal cells and extend the function of Pol η to normal DNA replication in cycling cells.
This newly identified role of Pol η in suppressing CFS instability is of particular interest as the regulation of CFS stability is still poorly understood. CFSs are typically relatively large (several-hundred-kilobase) regions of chromosomal DNA of unremarkable sequence that are replicated in late S phase. Delay or inhibition of replication has been shown to induce chromosomal breakage at these sites. CFS instability is enhanced in ATR-deficient cells (9) or when Chk1 activity is compromised (13). These results have led to the proposal that the ATR pathway stabilizes replication forks stalled within fragile site sequences to ensure replication and the completion of S phase while avoiding DNA breakage (14). We found that cells lacking Pol η were hypersensitive to the ATR inhibitor caffeine. One mechanistic explanation for this observation is that fork stalling in the absence of Pol η cannot be stabilized in cells where ATR is inhibited by caffeine. The resulting collapsed or broken forks, at CFS and other genomic sites undergoing replication, would be predicted to lead to chromosomal instability and cell death. This model would explain the observation made by several groups that XP-V cells are hypersensitive to killing by UV irradiation in the presence of caffeine (8, 24, 43). Thus, combined treatment with UV and caffeine may target functional roles for Pol η in human cells in the TLS of UV damage and in ensuring CFS stability.
We also analyzed the replication program of Pol η-depleted cells by examining replication fork rates and replication factory dynamics. Using replication track analysis (RTA) on stretched DNA, we detected no change in global fork progression rates in cells depleted of Pol η compared to those in controls. However, if replication in Pol η-depleted cells is impaired only at a specific subset of loci, such as CFSs, our assay may not reveal it. In the future, it should be possible to apply a variant of RTA, RTA-FISH with a FRA7H probe, to determine whether fork rates in FRA7H itself are altered in Pol η-deficient cells prior to and after DNA damage. Our analyses of replication factory dynamics, in contrast, revealed an unexpected decrease in the proportion of cells with a late-replication foci pattern and a corresponding increase in cells with early patterns in Pol η-depleted cultures. This may be an indication of delayed completion of bulk replication occurring in early S and/or a delayed or impaired onset of late replication. One potentially interesting experiment would be to determine whether the timing of FRA7H replication is delayed or otherwise altered in Pol η-depleted cells, as little is known about mechanisms that regulate replication timing or switching between early and late replication timing.
One of the most intriguing ideas suggested by our results is that Pol η may have two fundamentally different functional roles in human cells, one in the context of the replisome, where Pol η is recruited to allow the replication of UV dimers and related types of DNA damage, and a second role in the context of DNA synthetic events that do not involve the full replisome. Two roles of the latter type may be envisioned. Pol η may extend invading strands in D loops as it has been recently proposed (19, 29, 30), and in addition, Pol η may be recruited at the termination of replication when DNA between two converging forks needs to be completely replicated prior to topological resolution of the two replicated sister chromatids. Alternatively, Pol η may be required for the replication per se of naturally occurring DNA structures capable of forming unusual secondary structures, as many breakage hot spots in the human genome are mapped inside or near sequences that have the potential to adopt non-B DNA structures. A failure of any of these postulated roles for Pol η would be expected to produce the phenotype that we have observed, namely, chromatid breaks with the accompanying damage response activation, CFS expression, and potentially altered maturation of replication factories as seen with replication focus patterns.
The XP-V syndrome has been estimated to comprise 20% of XP patients with mild or severe clinical features, including late-onset skin cancers (16). Most mutations in the POLH gene in XP-V patients are heavily biased toward the N-terminal region and encode a Pol η protein with either missense mutations or severe truncations that abolish both DNA polymerase and bypass activities. Our results make several predictions about the pathogenesis of XP-V and suggest additional analyses of tumors arising in XP-V patients as well as in acquired Pol η deficiencies and in Polh mutant mice.
First, CFS instability should be elevated in otherwise normal XP-V cells and may lead to a higher percentage of cell death or senescence in vivo in the absence of exogenous DNA damage. A prediction is that the frequency of senescent cells should be higher in tissue from XP-V patients or Polh mutant mice. It may also be possible to address this issue by determining the mitotic history of cells from XP-V patients and controls (39), where the prediction is that cells from XP-V patients or Polh mutant mice would have undergone more mitotic divisions at any given chronological age due to higher cell loss during and after development.
A second prediction is that CFS instability in Pol η-deficient cells may be further accentuated by exposure of UV or other forms of DNA damage, such as cisplatin, that may depend on Pol η for accurate replication or repair. This type of analysis could be performed initially in cell lines or strains and could be readily extended to primary cells from XP-V patients or from Polh mutant mice. A related, third prediction is that there should be a higher frequency of CFS-associated loss of heterozygosity in tumors arising in sun-exposed skin and perhaps at other sites of the body exposed to high levels of exogenous or endogenous DNA damage (e.g., the liver and kidney) and that instability or loss of heterozygosity would be more pronounced in the absence of Pol η.
The tumor spectrum in XP-V patients and in Polh mutant mice argues that Pol η deficiency alone may lead to instability or mutagenesis but is not sufficient to cause tumors in mice or humans. The absence of Pol η becomes a powerful cocarcinogen only in conjunction with a strong “driver” that could be either chemical (in the form of DNA damage) or genetic. This requirement likely reflects the relatively highly specialized functional roles that Pol η appears to fill in mammalian cells. It should be possible to further explore the mechanistic and molecular aspects of these roles using human cells that are either mutant for or depleted of Pol η, as well as in Polh-deficient mice with biochemical and cellular defects that closely resemble the Pol η-deficient/XP-V human phenotype. Our results provide an important contribution to these analyses by identifying an unexpected new function of Pol η during S phase in unperturbed normal human cells that is in addition to the well-established role of Pol η in TLS.
Supplementary Material
Acknowledgments
This work was supported by an INCa award (Checkpol Projet Libre 2007) and an Association pour la Recherche sur le Cancer (no. 4887) to J.-S.H., by a Canceropole Grand Sud Ouest 2004-2009 award to C.C., by an NIH PO1 award (PO1 CA77852) to R.J.M., and by an EDF (Electricité de France) award to D.S.F.B. L.R. received a fellowship from La Région Midi-Pyrénées-CNRS.
We thank M. Yerle and F. Mompart (INRA-Toulouse) for helpful advice for FISH analysis and A. Tissier (Marseille) for the Pol η cDNA.
J.-S.H. and C.C. jointly direct the Genetic Instability and Cancer Group at IPBS.
Footnotes
Published ahead of print on 20 April 2009.
REFERENCES
- 1.Albertella, M. R., C. M. Green, A. R. Lehmann, and M. J. O'Connor. 2005. A role for polymerase eta in the cellular tolerance to cisplatin-induced damage. Cancer Res. 659799-9806. [DOI] [PubMed] [Google Scholar]
- 2.Avkin, S., S. Adar, G. Blander, and Z. Livneh. 2002. Quantitative measurement of translesion replication in human cells: evidence for bypass of abasic sites by a replicative DNA polymerase. Proc. Natl. Acad. Sci. USA 993764-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avkin, S., and Z. Livneh. 2002. Efficiency, specificity and DNA polymerase-dependence of translesion replication across the oxidative DNA lesion 8-oxoguanine in human cells. Mutat. Res. 51081-90. [DOI] [PubMed] [Google Scholar]
- 4.Bartkova, J., Z. Horejsi, K. Koed, A. Kramer, F. Tort, K. Zieger, P. Guldberg, M. Sehested, J. M. Nesland, C. Lukas, T. Orntoft, J. Lukas, and J. Bartek. 2005. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434864-870. [DOI] [PubMed] [Google Scholar]
- 5.Biard, D. S. 2007. Untangling the relationships between DNA repair pathways by silencing more than 20 DNA repair genes in human stable clones. Nucleic Acids Res. 353535-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biard, D. S., E. Despras, A. Sarasin, and J. F. Angulo. 2005. Development of new EBV-based vectors for stable expression of small interfering RNA to mimick human syndromes: application to NER gene silencing. Mol. Cancer Res. 3519-529. [DOI] [PubMed] [Google Scholar]
- 7.Bomar, M. G., M. T. Pai, S. R. Tzeng, S. S. Li, and P. Zhou. 2007. Structure of the ubiquitin-binding zinc finger domain of human DNA Y-polymerase eta. EMBO Rep. 8247-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broughton, B. C., A. Cordonnier, W. J. Kleijer, N. G. Jaspers, H. Fawcett, A. Raams, V. H. Garritsen, A. Stary, M. F. Avril, F. Boudsocq, C. Masutani, F. Hanaoka, R. P. Fuchs, A. Sarasin, and A. R. Lehmann. 2002. Molecular analysis of mutations in DNA polymerase eta in xeroderma pigmentosum-variant patients. Proc. Natl. Acad. Sci. USA 99815-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casper, A. M., P. Nghiem, M. F. Arlt, and T. W. Glover. 2002. ATR regulates fragile site stability. Cell 111779-789. [DOI] [PubMed] [Google Scholar]
- 10.Cleaver, J. E., V. Afzal, L. Feeney, M. McDowell, W. Sadinski, J. P. Volpe, D. B. Busch, D. M. Coleman, D. W. Ziffer, Y. Yu, H. Nagasawa, and J. B. Little. 1999. Increased ultraviolet sensitivity and chromosomal instability related to P53 function in the xeroderma pigmentosum variant. Cancer Res. 591102-1108. [PubMed] [Google Scholar]
- 11.Cox, M. M. 2001. Recombinational DNA repair of damaged replication forks in Escherichia coli: questions. Annu. Rev. Genet. 3553-82. [DOI] [PubMed] [Google Scholar]
- 12.Dumstorf, C. A., A. B. Clark, Q. Lin, G. E. Kissling, T. Yuan, R. Kucherlapati, W. G. McGregor, and T. A. Kunkel. 2006. Participation of mouse DNA polymerase iota in strand-biased mutagenic bypass of UV photoproducts and suppression of skin cancer. Proc. Natl. Acad. Sci. USA 10318083-18088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durkin, S. G., M. F. Arlt, N. G. Howlett, and T. W. Glover. 2006. Depletion of CHK1, but not CHK2, induces chromosomal instability and breaks at common fragile sites. Oncogene 254381-4388. [DOI] [PubMed] [Google Scholar]
- 14.Durkin, S. G., and T. W. Glover. 2007. Chromosome fragile sites. Annu. Rev. Genet. 41169-192. [DOI] [PubMed] [Google Scholar]
- 15.Edmunds, C. E., L. J. Simpson, and J. E. Sale. 2008. PCNA ubiquitination and REV1 define temporally distinct mechanisms for controlling translesion synthesis in the avian cell line DT40. Mol. Cell 30519-529. [DOI] [PubMed] [Google Scholar]
- 16.Inui, H., K. S. Oh, C. Nadem, T. Ueda, S. G. Khan, A. Metin, E. Gozukara, S. Emmert, H. Slor, D. B. Busch, C. C. Baker, J. J. DiGiovanna, D. Tamura, C. S. Seitz, A. Gratchev, W. H. Wu, K. Y. Chung, H. J. Chung, E. Azizi, R. Woodgate, T. D. Schneider, and K. H. Kraemer. 2008. Xeroderma pigmentosum-variant patients from America, Europe, and Asia. J. Investig. Dermatol. 1282055-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, R. E., C. M. Kondratick, S. Prakash, and L. Prakash. 1999. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science 285263-265. [DOI] [PubMed] [Google Scholar]
- 18.Kannouche, P., B. C. Broughton, M. Volker, F. Hanaoka, L. H. Mullenders, and A. R. Lehmann. 2001. Domain structure, localization, and function of DNA polymerase eta, defective in xeroderma pigmentosum variant cells. Genes Dev. 15158-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawamoto, T., K. Araki, E. Sonoda, Y. M. Yamashita, K. Harada, K. Kikuchi, C. Masutani, F. Hanaoka, K. Nozaki, N. Hashimoto, and S. Takeda. 2005. Dual roles for DNA polymerase eta in homologous DNA recombination and translesion DNA synthesis. Mol. Cell 20793-799. [DOI] [PubMed] [Google Scholar]
- 20.Kraus, E., W. Y. Leung, and J. E. Haber. 2001. Break-induced replication: a review and an example in budding yeast. Proc. Natl. Acad. Sci. USA 988255-8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehmann, A. R. 2002. Replication of damaged DNA in mammalian cells: new solutions to an old problem. Mutat. Res. 50923-34. [DOI] [PubMed] [Google Scholar]
- 22.Leonhardt, H., H. P. Rahn, P. Weinzierl, A. Sporbert, T. Cremer, D. Zink, and M. C. Cardoso. 2000. Dynamics of DNA replication factories in living cells. J. Cell Biol. 149271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, Q., A. B. Clark, S. D. McCulloch, T. Yuan, R. T. Bronson, T. A. Kunkel, and R. Kucherlapati. 2006. Increased susceptibility to UV-induced skin carcinogenesis in polymerase eta-deficient mice. Cancer Res. 6687-94. [DOI] [PubMed] [Google Scholar]
- 24.Maher, V. M., L. M. Ouellette, R. D. Curren, and J. J. McCormick. 1976. Caffeine enhancement of the cytotoxic and mutagenic effect of ultraviolet irradiation in a xeroderma pigmentosum variant strain of human cells. Biochem. Biophys. Res. Commun. 71228-234. [DOI] [PubMed] [Google Scholar]
- 25.Maloisel, L., F. Fabre, and S. Gangloff. 2008. DNA polymerase δ is preferentially recruited during homologous recombination to promote heteroduplex DNA extension. Mol. Cell. Biol. 281373-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masutani, C., R. Kusumoto, S. Iwai, and F. Hanaoka. 2000. Mechanisms of accurate translesion synthesis by human DNA polymerase eta. EMBO J. 193100-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masutani, C., R. Kusumoto, A. Yamada, N. Dohmae, M. Yokoi, M. Yuasa, M. Araki, S. Iwai, K. Takio, and F. Hanaoka. 1999. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature 399700-704. [DOI] [PubMed] [Google Scholar]
- 28.McCulloch, S. D., R. J. Kokoska, C. Masutani, S. Iwai, F. Hanaoka, and T. A. Kunkel. 2004. Preferential cis-syn thymine dimer bypass by DNA polymerase eta occurs with biased fidelity. Nature 42897-100. [DOI] [PubMed] [Google Scholar]
- 29.McIlwraith, M. J., A. Vaisman, Y. Liu, E. Fanning, R. Woodgate, and S. C. West. 2005. Human DNA polymerase eta promotes DNA synthesis from strand invasion intermediates of homologous recombination. Mol. Cell 20783-792. [DOI] [PubMed] [Google Scholar]
- 30.McIlwraith, M. J., and S. C. West. 2008. DNA repair synthesis facilitates RAD52-mediated second-end capture during DSB repair. Mol. Cell 29510-516. [DOI] [PubMed] [Google Scholar]
- 31.Michel, B., M. J. Flores, E. Viguera, G. Grompone, M. Seigneur, and V. Bidnenko. 2001. Rescue of arrested replication forks by homologous recombination. Proc. Natl. Acad. Sci. USA 988181-8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mogi, S., C. E. Butcher, and D. H. Oh. 2008. DNA polymerase eta reduces the gamma-H2AX response to psoralen interstrand crosslinks in human cells. Exp. Cell Res. 314887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyberg, K. A., R. J. Michelson, C. W. Putnam, and T. A. Weinert. 2002. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36617-656. [DOI] [PubMed] [Google Scholar]
- 34.Pâques, F., and J. E. Haber. 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63349-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng, M., R. Litman, J. Xie, S. Sharma, R. M. Brosh, Jr., and S. B. Cantor. 2007. The FANCJ/MutLalpha interaction is required for correction of the cross-link response in FA-J. cells. EMBO J. 263238-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petta, T. B., S. Nakajima, A. Zlatanou, E. Despras, S. Couve-Privat, A. Ishchenko, A. Sarasin, A. Yasui, and P. Kannouche. 2008. Human DNA polymerase iota protects cells against oxidative stress. EMBO J. 272883-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pillaire, M. J., R. Betous, C. Conti, J. Czaplicki, P. Pasero, A. Bensimon, C. Cazaux, and J. S. Hoffmann. 2007. Upregulation of error-prone DNA polymerases beta and kappa slows down fork progression without activating the replication checkpoint. Cell Cycle 6471-477. [DOI] [PubMed] [Google Scholar]
- 38.Puget, N., M. Knowlton, and R. Scully. 2005. Molecular analysis of sister chromatid recombination in mammalian cells. DNA Repair (Amsterdam) 4149-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salipante, S. J., and M. S. Horwitz. 2007. A phylogenetic approach to mapping cell fate. Curr. Top. Dev. Biol. 79157-184. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz, M., E. Zlotorynski, M. Goldberg, E. Ozeri, A. Rahat, C. le Sage, B. P. Chen, D. J. Chen, R. Agami, and B. Kerem. 2005. Homologous recombination and nonhomologous end-joining repair pathways regulate fragile site stability. Genes Dev. 192715-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sidorova, J. M., N. Li, A. Folch, and R. J. Monnat, Jr. 2008. The RecQ helicase WRN is required for normal replication fork progression after DNA damage or replication fork arrest. Cell Cycle 7796-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sonoda, E., M. S. Sasaki, J. M. Buerstedde, O. Bezzubova, A. Shinohara, H. Ogawa, M. Takata, Y. Yamaguchi-Iwai, and S. Takeda. 1998. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 17598-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stary, A., P. Kannouche, A. R. Lehmann, and A. Sarasin. 2003. Role of DNA polymerase eta in the UV mutation spectrum in human cells. J. Biol. Chem. 27818767-18775. [DOI] [PubMed] [Google Scholar]
- 44.Tissier, A., P. Kannouche, M. P. Reck, A. R. Lehmann, R. P. Fuchs, and A. Cordonnier. 2004. Co-localization in replication foci and interaction of human Y-family members, DNA polymerase pol eta and REVl protein. DNA Repair (Amsterdam) 31503-1514. [DOI] [PubMed] [Google Scholar]
- 45.Yerle, M., A. Goureau, J. Gellin, P. Le Tissier, and C. Moran. 1994. Rapid mapping of cosmid clones on pig chromosomes by fluorescence in situ hybridization. Mamm. Genome 534-37. [DOI] [PubMed] [Google Scholar]
- 46.Zheng, H., X. Wang, A. J. Warren, R. J. Legerski, R. S. Nairn, J. W. Hamilton, and L. Li. 2003. Nucleotide excision repair- and polymerase η-mediated error-prone removal of mitomycin C interstrand cross-links. Mol. Cell. Biol. 23754-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.