Abstract
In a genomewide anoikis suppression screen for metastasis genes, we previously identified the neurotrophic receptor tyrosine kinase TrkB. In mouse xenografts, activated TrkB caused highly invasive and metastatic tumors. Here, we describe that TrkB also induces a strong morphological transformation, resembling epithelial-mesenchymal transition (EMT). This required TrkB kinase activity, a functional mitogen-activated protein kinase pathway, suppression of E-cadherin, and induction of Twist, a transcription factor contributing to EMT and metastasis. RNA interference (RNAi)-mediated Twist depletion blocked TrkB-induced EMT-like transformation, anoikis suppression, and growth of tumor xenografts. By searching for essential effectors of TrkB-Twist signaling, we found that Twist induces Snail, another EMT regulator associated with poor cancer prognosis. Snail depletion impaired EMT-like transformation and anoikis suppression induced by TrkB, but in contrast to Twist depletion, it failed to inhibit tumor growth. Instead, Snail RNAi specifically impaired the formation of lung metastases. Epistasis experiments suggested that Twist acts upstream from Snail. Our results demonstrate that TrkB signaling activates a Twist-Snail axis that is critically involved in EMT-like transformation, tumorigenesis, and metastasis. Moreover, our data shed more light on the epistatic relationship between Twist and Snail, two key transcriptional regulators of EMT and metastasis.
The successful treatment of cancer patients is inversely correlated with the occurrence of secondary tumors, or metastases. A better understanding of the molecular mechanisms underlying metastasis will conceivably help to improve cancer treatment in the future. Metastasis is a multistep process in which tumor cells have to overcome several barriers to form a secondary tumor at a distant anatomical site (21, 34). One such barrier is imposed by the epithelium (the origin of most solid tumors), a highly organized structure with strong cell-cell adhesions and lined by a basement membrane composed of a dense extracellular matrix. To disseminate from the primary tumor and to invade neighboring tissue or vessels, epithelial tumor cells must acquire a more flexible and migratory phenotype, like that of mesenchymal cells (16, 73). This can be achieved by an epithelial-mesenchymal transition (EMT), a process that was initially described for embryogenesis (74, 79). EMT is characterized by the loss of polarized organization and a downregulation of epithelial proteins, including E-cadherin, γ-catenin/plakoglobin, α-catenin, and β-catenin (33). At the same time, mesenchymal proteins are often induced, including smooth muscle actin (47), fibronectin, N-cadherin, or vimentin (6, 41). This is mediated (either directly or indirectly) by transcription factors like Twist, E12/E47, and members of the Snail and ZEB protein families (38). In vitro, EMT can be induced by activated oncogenes, like RASV12, or by several receptor tyrosine kinases, such as MET or the epidermal growth factor receptor, often in cooperation with transforming growth factor β (TGF-β) (33). However, the extent of up- and downregulation of the epithelial markers and, in particular, of the mesenchymal markers varies among different cell lines and stimuli. For this reason and also because intermediate forms of EMT with only a partial phenotype have been described, a precise definition of EMT is still under debate (33). In vivo, in the course of tumor cell invasion and metastasis, EMT is thought to occur mainly in a transient and reversible way, under the influence of the tumor stroma (46, 79).
Once tumor cells have left their original site and encounter new microenvironments during invasion, they are challenged by another barrier against metastasis: anoikis (apoptosis induced by inappropriate, or the lack of, cell adhesion) (28, 49). Apart from its role in tissue homeostasis (11, 36), anoikis conceivably also restricts the spread of tumor cells through tissues and via circulation (44). In an attempt to identify new mediators of metastasis, we have previously used anoikis suppression as the basis for a genomewide functional screen. In this way, we identified the neurotrophic tyrosine kinase receptor 2 (Ntrk2/TrkB) as a potent anoikis suppressor (20). TrkB and its ligand brain-derived neurotrophic factor (BDNF) play a crucial role in the development and function of the nervous system, including the promotion of neuronal survival (25, 42, 43). Consistent with the premise that anoikis forms a barrier to metastasis, TrkB-expressing epithelial cells form metastatic tumors in vivo (20). We recently showed that the ability of TrkB to suppress anoikis and induce metastasis requires its kinase function to be intact (29). Supporting the notion that TrkB may also play an important role in human cancer, it is found overexpressed in several human malignancies, including neuroblastoma (12, 53), prostate cancer (19), and pancreatic cancer (50; reviewed in reference 30). In line with this, TrkB-interfering agents are currently being developed and tested for anticancer activity (18, 68). However, the molecular mechanisms as to how TrkB signaling induces metastasis remain largely unknown.
Because improved understanding of the fundamental mechanistic aspects of TrkB signaling in metastasis is likely to be of preclinical relevance, we aimed here to reveal factors that are critically required for TrkB-induced anoikis suppression and metastasis, with a focus on EMT.
MATERIALS AND METHODS
Vector constructs.
Mouse pBabe-Hygro-BDNF (pBH-BDNF) was described before (20). Mouse TrkB (GenBank accession number X17647) in the pBabe-Puro (pBP) vector was subcloned into pMSCV-blast using the EcoRI restriction site. Human BDNF, human wild-type TrkB, and the human kinase inactive point mutant TrkBK588M were described before (29). Mouse E-cadherin (X06115) in the pBP-IRES-green fluorescent protein (GFP) vector was a gift from C. Niessen (32). 3′ hemagglutinin (HA)-tagged mouse Snail (NM011427) in the pRV-IRES-GFP vector was a gift from A. Munoz (62). Mouse Twist1 (M63649) was a gift from R. Weinberg (78) and was subcloned by PCR into the pLZRS-MS-IRES-EGFP (pLSIE) vector and pBP vector, adding a 5′ HA tag and a Kozak sequence. Forward primer used was 5′-CGGGATCCGCCGCCATGGCTTACCCATACGATGTTCCAGATTACGCTATGCACGTGTCCAGC-3′, and the reverse primer used was 5′-GGAATTCCTAGTGGGACGCGGACATGG-3′. Short hairpin RNAs (shRNAs) were expressed from pRetroSuper (13), with the following targeting sequences: EGFP, 5′-GCTGACCCTGAAGTTCATC-3′ (sh-EGFP); rat Twist1 #1, 5′-AGACCAAATTTCACAAGAA-3′ (sh-Twist #1); rat and human Twist1 #2, 5′-GGATCAAACTGGCCTGCAA-3′ (sh-Twist #2); human Twist1 #3, 5′-GAACACCTTTAGAAATAAA-3′ (sh-Twist #3); rat Snail #1, 5′-GAATGTCCTTGCTCCACAA-3′ (sh-Snail #1); and rat Snail #2, 5′-ACAGCTGCTTCGAGCCATA-3′ (sh-Snail #2).
Cell culture and retroviral transduction.
Rat intestinal epithelial 1 (RIE-1) cells (a kind gift from R. D. Beauchamp and K. D. Brown [9]) and E1A-immortalized rat kidney RK3E cells (ATCC) were cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 9% fetal calf serum (FCS; PAA Laboratories GmbH) and penicillin-streptomycin (Gibco). Human breast epithelial MCF10A cells expressing the ecotropic receptor were cultured in Dulbecco's modified Eagle's medium-F12 (1:1 dilution) (Gibco) supplemented with 15 mM HEPES buffer, 5% equine serum, 1% penicillin-streptomycin, 0.5 μg/ml amphotericin B, 10 μg/ml insulin, 20 ng/ml epidermal growth factor, 100 ng/ml cholera toxin, and 0.5 μg/ml hydrocortisone.
Ecotropic retrovirus was produced in Phoenix packaging cells (http://www.stanford.edu/group/nolan/retroviral_systems/phx.html). We transduced RIE-1, RK3E, and MCF10A cells first with pBH-BDNF viral supernatant in the presence of 3.5 μg/ml Polybrene (Sigma). Cells were selected (112.5 μg/ml for RIE-1 cells and 75 μg/ml for RK3E and MCF10A cells) with hygromycin B and subsequently infected with wild-type or mutant TrkB viral supernatant. Stable cell lines were established by selection with blasticidin (5 μg/ml for RIE-1 cells and 2.5 μg/ml for RK3E cells and MCF10A cells) or with puromycin (1.0 μg/ml for MCF10A cells). RK3E cells expressing TrkB and BDNF were subsequently transduced with pBP-HA-Twist or pRV-IRES-GFP-Snail-HA viral supernatant. To generate cell lines expressing shRNAs, pBH-BDNF-expressing RK3E, RIE-1, and MCF10A cell pools were infected with pRS-Twist, pRS-Snail, or pRS-EGFP. Cells were selected with 1.0 μg/ml puromycin (1.5 μg/ml for RIE-1 cells) and subsequently infected with pMSCV-blast-TrkB. To generate stable cell clones, cells were seeded at clonal densities and selected in medium containing 2.5 μg/ml blasticidin. One week later, single clones were picked and expanded. For reintroduction of E-cadherin, RK3E cells expressing MSCV-blast-TrkB plus pBH-BDNF (RK3ETB cells) were transduced with pBP-GFP-E-cadherin and seeded at low density in medium containing 1.0 μg/ml puromycin to obtain independent cell clones. For functional rescue experiments, RK3ETB cell clones expressing shRNAs against Twist or Snail were infected with pRV-IRES-GFP-Snail-HA or pLSIE-HA-Twist and subjected to fluorescence-activated cell sorting. For cells expressing dominant-negative RACN17 (66), RK3E cells were transduced first with pBH-BDNF (selection with 75 μg/ml blasticidin), then with LZRS-mycRACN17-IRES-ZEO (selection with 100 μg/ml zeocin), and subsequently with MSCV-TrkB (selection with 2.5 μg/ml blasticidin).
Pharmacological inhibition of TrkB, mitogen-activated protein kinase (MAPK), PI3 kinase (PI3K), and RAC1 pathways.
Cells expressing the empty vector control or TrkB and BDNF were treated with 20 μM U0126 (Cell Signaling Technology), 1 μM CI-1040 (Axon Medchem), 500 nM PI-103 (Echelon), or 75 μM NSC23766 (Calbiochem) for the indicated time points. Cells were harvested in the presence of phosphatase inhibitors (1 mM sodium pyrophosphate, 2 mM sodium fluoride, 10 mM beta-glycophosphate, and 2 mM sodium orthovanadate). For BDNF stimulation, cells were serum starved for 4 h, pretreated with the inhibitor for 30 min, and stimulated with 50 ng/ml recombinant human BDNF (Peprotech) for 5 min. Cells were harvested in the presence of phosphatase inhibitors.
RK3E cells expressing the vector control, TrkB only, TrkB and BDNF, or RASV12 were treated with 300 nM K252a (Calbiochem) or 20 μM GW 441756 (Tocris Bioscience) overnight. Cells were trypsinized and resuspended in trypan blue-phosphate-buffered saline (PBS), with a 1:1 dilution. Percentages of dead cells were determined using trypan blue exclusion. For Western blot analysis, cells were harvested in the presence of phosphatase inhibitors.
For transient TrkB activation, RK3E cells expressing TrkB were treated with 50 ng/ml recombinant human BDNF (PeproTech) for 2 days (medium was changed daily) and cultured for another 3 days without BDNF. Alternatively, TrkB-expressing RK3E cells were cultured in 1% FCS, treated with 50 ng/ml recombinant BDNF for 2 days (medium was changed daily), and harvested 1 day after the removal of BDNF.
Anoikis, soft agar, migration, and invasion assays.
To induce anoikis, we seeded 4 × 105 freshly trypsinized cells into ultra-low cluster (ULC) six-well cell culture dishes (Costar). Plates were scanned with an Epson Perfection 4990 photo scanner 4 days later. For quantification of anoikis suppression, total protein amounts in each well were measured by cell lysis in RIPA buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) and subsequent Bio-Rad protein assay.
For soft agar assays, 1,000 trypsinized cells were seeded in 0.4% low-melting-point agarose (Sigma) on top of a 1% agarose layer, and scans were taken 11 days later. The numbers of macroscopic colonies were determined using ImageJ software (http://rsb.info.nih.gov/ij/index.html).
For migration and invasion assays, 250,000 freshly trypsinized cells were seeded on control inserts (for migration) or Matrigel (for invasion) in medium without FCS. The lower compartment contained 9% FCS. After 24 h, noninvaded cells were removed, and invaded cell were stained with crystal violet. Quantification was performed by counting invaded cells on five independent pictures of the well. All pictures of adherent cells were taken at a magnification of ×50.
RAC1 activity assay.
To determine RAC1 activity levels, cells were harvested in lysis buffer (0.5% Nonidet P-40, 50 mM Tris [pH 7.5], 150 mM NaCl, 5 mM MgCl2, 10% glycerol and protease inhibitors) and sheared three times through a G25 syringe. Total protein amounts were determined using a Bio-Rad protein assay. Samples (900 μg protein) were incubated for 30 min with biotin-Pak-CRIB peptide and streptavidin magnetic beads (Invitrogen). The Pak-CRIB peptide binds RAC in its active conformation only (65). Samples were washed twice with lysis buffer and analyzed for RAC1 by Western blotting.
Immunoblotting and antibodies.
Cell pellets were lysed in RIPA buffer, and protein concentration was determined using the Bio-Rad protein assay. Immunoblot analysis was performed using standard techniques, either on 7% sodium dodecyl sulfate-polyacrylamide gels or on 4 to 12% bis-Tris precast gels (NuPAGE) for Twist and Snail. The antibodies used were pan-Trk (C14; Santa Cruz); BDNF (N20; Santa Cruz); Twist hybridoma supernatant (31); Snail (H130 [Santa Cruz] for the detection of overexpressed mouse Snail, hybridoma supernatant Sn9H2 from K. Becker [67] for endogenous human Snail); E-cadherin, P-cadherin, N-cadherin, α-catenin, β-catenin, γ-catenin, fibronectin, vimentin, and RAS (all from BD); smooth muscle actin (1A4; Sigma); CDK4 (C22; Santa Cruz); β-actin (AC74; Sigma); α-tubulin (DM1A; Sigma); and RAC (Upstate). All antibodies were diluted 1:2,000 in 4% Protifar Plus (Nutricia), except for the antibody for TrkB that was diluted 1:1000, RAC diluted 1:1,000, BDNF diluted 1:500, Snail H130 diluted 1:1,000, Twist diluted 1:3 in PBS-0.2% Tween, and Snail diluted 1:10 in PBS-0.2% Tween. Antibodies for pERK and extracellular signal-regulated kinase (ERK) (p44/42 MAPK, Cell Signaling Technology) were diluted 1:1,000 in 4% bovine serum albumin, and antibodies against pAKT (Cell Signaling Technology) and AKT (Santa Cruz) were diluted 1:1,000 in 4% bovine serum albumin and 1:50 in Western blot blocking reagent (Roche). Protein detection for Western blotting was done with ECL reagent (Amersham), and exposed films were scanned with an Epson Perfection 4990 photo scanner.
Immunofluorescence.
Cells were grown on glass coverslips, fixed in 4% formaldehyde in PBS (in 70% ethanol for cells expressing pBP-IRES-GFP and pBP-IRES-GFP-E-cadherin to inactivate the GFP signal), permeabilized with 0.2% Triton X-100, and blocked in 5% normal goat serum in PBS-0.2% Tween for 30 min. Coverslips were incubated with E-cadherin antibody (1:200 dilution) in blocking solution for 1 h at room temperature. After washing the cells with PBS-0.2% Tween, we incubated the cells for 1 h with Alexa Fluor 488 goat anti-mouse secondary antibody (diluted 1:1,000; Molecular Probes) and for 15 min with TO-PRO (diluted 1:500; Molecular Probes). Coverslips were mounted with Aqua-Poly/Mount (Polysciences, Inc) and analyzed by confocal microscopy on a Leica TCS NT confocal system (Leica Microsystems, Heidelberg, Germany), equipped with an Ar/Kr laser. Images were taken using a 100×, 1.32-numerical-aperture objective lens, with standard filter combination(s) and Kalman averaging.
qRT-PCR.
Total RNA was isolated using Trizol (Invitrogen) and treated with DNase for 1 h at 37°C (Promega). Quantitative reverse transcriptase PCR (qRT-PCR) was performed using the reverse transcriptase kit from Invitrogen. Primers were designed using Primer Express software. The primers used were as follows: Twist-forward, 5′-CGCTGAACGAGGCATTTGC-3′; Twist-reverse, 5′-CCAGTTTGAGGGTCTGAATC-3′; Snail-forward, 5′-CCACACTGGTGAGAAGCCTTTC-3′; Snail-reverse, 5′-GTCTGGAGGTGGGCACGTA-3′; TBP-forward, 5′-GATGTGAAGTTCCCCATAAGGC-3′; TBP-reverse, 5′-TCTGGCTCATAGCTACTGAACTGC-3′; N-cadherin-forward, 5′-AGGGCCTTAAAGCTGCTGACA-3′ N-cadherin-reverse, 5′-TCATAGTCGAAGACTAAAAGGGAGTCATAT-3′; E-cadherin-forward, 5′-TGAGCATGCCCCAGTATCG-3′; E-cadherin-reverse, 5′-CTGCCTTCAGGTTTTCATCGA-3′; Slug-forward, 5-CCAACTACAGCGAACTGGACAC-3′; Slug-reverse, 5′-TCTCACAGAGGTATGGAGAAATGATC-3′; CTGF-forward, 5′-GTCTCTTCTGCGACTTCGGCT-3′; CTGF-reverse, 5′-CATCTTTGGCAGTGCACACG-3′; BMP-4-forward, 5′-CTCAAGGGAGTGGAAATTGGG-3′; BMP-4-reverse, 5′-CATCGTGGCCAAAAGTGACC-3′. Detection was done with SYBR green master mix (Applied Biosystems) on an ABI Prism 700 thermal cycler (Applied Biosystems). RNA levels were normalized against rat TATA box binding protein (TBP).
In vivo assays.
Eight- to 14-week-old female BALB/c nude mice were subcutaneously injected with 1 × 105 cells into both flanks. Mice were inspected twice a week and euthanized by CO2 when tumors reached a volume of 1 cm3 or started to ulcerate, according to a protocol approved by the Institutional Animal Experiment Ethics Committee. Tumor size was measured with a caliper, and tumor volume was calculated by the formula (a × b2)/2, with a being the longest diameter and b the respective perpendicular diameter of the tumor. Metastatic lesions in the lungs were counted by visual inspection of hematoxylin and eosin-stained histological tissue sections. Sections were analyzed with an Axiovert S100 microscope system, with a color charge-coupled-device camera and AxioVision software (Zeiss). The sizes of metastases and the area of lung tissue per section were determined with ImageJ software. The total lung area analyzed was 19.5 cm2 for sh-EGFP #1, 17.3 cm2 for sh-EGFP #2, 11.6 cm2 for sh-Snail #1, and 19.1 cm2 for sh-Snail #2. For experimental metastasis, 1 × 106 cells were injected into the tail veins of 8- to 14-week-old female BALB/c nude mice. Mice were inspected daily and euthanized by CO2 when clinical symptoms became apparent. Kaplan-Meier survival curves were generated using SPSS 14.0.
RESULTS
TrkB induces EMT-like transformation in epithelial cells.
Consistent with our previous observations (20, 29), overexpression of TrkB and BDNF in several nonmalignant epithelial cell lines induced a striking morphological transformation, characterized by a spindle-shaped morphology and the loss of cell-cell contacts (Fig. 1A). This effect was observed in the following three different nonmalignant epithelial cell lines, originating from different tissues and species: RIE-1 cells, E1A-transformed rat kidney epithelial cells (RK3E cells), and to a somewhat lesser extent, human breast epithelial cells (MCF10A cells). To determine whether this morphological transformation represents EMT, we analyzed the levels of several epithelial and mesenchymal proteins (see the introduction). We observed a downregulation by activated TrkB of E-cadherin, α-catenin, β-catenin, γ-catenin, and P-cadherin in both rat epithelial cell lines, whereas in MCF10A cells, α-catenin and P-cadherin were slightly suppressed (Fig. 1B). In the latter cell type but not in the rodent cells, induction of fibronectin and vimentin was prominent (Fig. 1B). EMT markers are often differentially regulated across cell lines (33). To investigate a larger number of EMT-associated genes, we compared the gene expression profile of TrkB- and BDNF-expressing RK3E cells (referred to as RK3ETB cells) (C. Desmet and D. Peeper, submitted for publication) to the reported profile of RASV12-expressing and TGF-β1-treated EpH4 mouse mammary epithelial cells (EpH4RasTgf-β) (41), a classical system for studying EMT (33, 56). This analysis showed that 67% of the genes significantly deregulated in both RK3ETB cells, and EpH4RasTgf-β cells changed the expression level in the same direction.
FIG. 1.
TrkB induces EMT-like transformation in epithelial cells. (A) Mesenchymal morphology induced by overexpression of TrkB and BDNF in epithelial RIE-1, RK3E, and MCF10A cells. (B) Effect on expression of epithelial and mesenchymal markers by TrkB and BDNF, as analyzed by Western blotting. V, vector; TB, TrkB and BDNF; sm-actin, smooth muscle actin. β-actin serves as the loading control. (C) TrkB and BDNF induce downregulation of E-cadherin mRNA levels and upregulation of N-cadherin mRNA levels in RIE-1 and RK3E cells (measured by qRT-PCR; n = 3; error bars represent standard deviations). Rel., relative. (D) Effect of TrkB-BDNF on the transcription factors Twist, Snail, and Slug in RIE-1 and RK3E cells, as measured by qRT-PCR (n = 3; error bars represent standard deviations). (E) Downregulation of BMP-4 and CTGF mRNA levels by TrkB and BDNF, as measured by qRT-PCR (n = 3; error bars represents standard deviations).
The EMT-associated “cadherin switch” from E-cadherin to N-cadherin (16) was present in both rodent cell lines upon expression of TrkB and BDNF (Fig. 1B and C). Several transcription factors, including Twist and members of the Snail protein family, are known to repress E-cadherin, via E boxes in the E-cadherin promoter (38, 54). When we assessed the mRNA levels of Twist (Twist1), Snail (Snai1), and Slug (Snai2), we observed a marked induction of Twist and a small but reproducible induction of Snail in TrkB- and BDNF-expressing RIE-1 (RIE-1TB) and RK3ETB cells (Fig. 1D). Slug was not induced in this setting. Furthermore, two genes, BMP-4 and CTGF, which are downregulated in the EMT profile of EpH4RasTgf-β cells (41), were also downregulated at the mRNA level by activated TrkB (Fig. 1E). Together, our analyses demonstrate that in epithelial cells, TrkB induces a strong morphological transformation resembling EMT. As EMT is a complex and dynamic process involving a plethora of factors, we will refer to this process here conservatively as EMT-like transformation.
Continuous TrkB signaling is required for EMT-like transformation and survival of TrkB-expressing RK3E cells.
Previously, we have shown that TrkB kinase activity is required for anoikis suppression and tumor formation in rat epithelial cells (29). Therefore, we speculated that kinase activity is also required for TrkB-induced EMT-like transformation. Indeed, a kinase-inactive point mutant, TrkBK588M (23, 35, 29) was unable to morphologically transform RK3E cells and to downregulate E-cadherin (Fig. 2A and B). In line with this observation, stimulation of TrkB-expressing cells with recombinant BDNF for 2 days induced a spindle-shaped morphology and the loss of E-cadherin, which was reverted upon withdrawal of BDNF for another 3 days (Fig. 2C and D). We then repeated this experiment in a more clinically relevant setting, with pharmacological inhibition of TrkB using the K252a alkaloid (72). RK3ETB cells treated with K252a induced a strong apoptotic response, as revealed by trypan blue exclusion (Fig. 2E) and accumulation of cleaved caspase 3 (Fig. 2F). This was not due to the unspecific toxicity of the inhibitor, because RASV12-transformed RK3E cells did not die upon treatment with K252a (Fig. 2E and F). Furthermore, we obtained similar results using another Trk inhibitor, GW 441756 (77) (see Fig. S1 in the supplemental material). K252a and GW 441756 each blocked BDNF-induced autophosphorylation of TrkB and subsequent phosphorylation of ERK but not RASV12-induced ERK phosphorylation (Fig. 2F; see also Fig. S1 in the supplemental material). Notably, parental RK3E cells were largely insensitive to the Trk inhibitors and so were cells in which TrkB was not activated by BDNF stimulation. Lastly, when we treated serum-starved, TrkB-expressing RK3E cells with BDNF for 2 days and subsequently removed it from the medium, we observed massive cell death (Fig. 2G). These results demonstrate that continuous TrkB signaling is required for EMT-like transformation. Furthermore, they show that RK3E cells become addicted to activated TrkB and that they do so within a short period of time.
FIG. 2.
Continuous TrkB signaling is required for the EMT-like transformation and survival of RK3ETB cells. (A) Kinase-inactive TrkBK588M does not change the epithelial morphology of RK3E cells. Wild-type TrkB, TrkBwt. (B) Kinase-inactive TrkBK588M does not affect E-cadherin protein levels, as shown by Western blot analysis. (C) Stimulation of TrkB-expressing RK3E cells with recombinant BDNF induces morphological transformation within 2 days, which is reverted back to an epithelial morphology after removal of BDNF for 3 days. (D) Western blot analysis of the cells described in the legend to panel C. (E) RK3ETB cells treated overnight with the Trk inhibitor K252a show increased cell death, as measured by trypan blue exclusion. The average of three independent experiments is shown, and error bars indicate standard deviations. TB, TrkB and BDNF. (F) RK3ETB cells treated overnight with the Trk inhibitor K252a show cleaved caspase 3, and K252a inhibits autophosphorylation of TrkB and BDNF-induced phosphorylation of ERK, all determined by Western blot analysis. β-actin serves as the loading control for all Western blots. TB, TrkB and BDNF. (G) Cells growing in serum-reduced medium (1% FCS) were treated with BDNF for 2 days. Cells were harvested 1 day after BDNF was removed from the medium. Apoptotic cells in the supernatant were included in the analysis for panels E, F, and G.
Loss of E-cadherin is an essential feature of TrkB function.
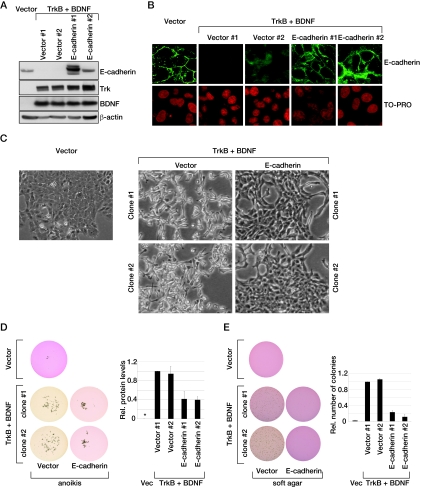
E-cadherin is regarded as a major player in EMT (5). Therefore, we next investigated whether E-cadherin corresponds to a critical target for TrkB function by reintroducing E-cadherin into spindle-shaped RK3ETB cells. E-cadherin was expressed to relatively high levels in two independent cell clones (Fig. 3A) and correctly localized to the cell membrane (Fig. 3B). Importantly, restoration of E-cadherin levels reverted the cell morphology to an epithelial phenotype (Fig. 3C). Like our previous observations in RIE-1 and MCF10A cells (20, 29), active TrkB also suppressed anoikis in RK3E cells (Fig. 3D). E-cadherin restoration impaired anoikis suppression by TrkB (Fig. 3D) as well as anchorage-independent growth in soft agar (Fig. 3E). These findings demonstrate that the loss of E-cadherin is an essential feature of the mechanism by which TrkB activates an EMT-like program and suppresses anoikis.
FIG. 3.
Loss of E-cadherin is an essential feature of TrkB function. (A) Western blot analysis of E-cadherin, TrkB, and BDNF from independent cell clones expressing indicated cDNAs. β-actin serves as the loading control. (B) Overexpressed E-cadherin localizes at the cell membrane, as shown by indirect immunofluorescence and confocal microscopy. TO-PRO stains DNA. (C) Epithelial morphology induced by overexpression of E-cadherin in RK3ETB cells. (D) E-cadherin restoration impairs TrkB-mediated anoikis suppression. Vector or RK3ETB cells and derived cell clones overexpressing E-cadherin or vector control were cultured on ULC plates for 4 days and scanned at a magnification of ×1 (left) or quantified by measuring protein levels (right) (n = 3; error bars represent standard deviations). An asterisk indicates a measured value of 0. Rel., relative; Vec, vector. (E) E-cadherin restoration impairs TrkB-mediated anchorage-independent growth. A total of 1,000 cells expressing the indicated cDNAs were grown in 0.4% agarose for 11 days; a scan with a magnification of ×1 (left) and its quantification (right) are shown (error bars represent the standard deviations of an experiment done in triplicate).
Twist is required for TrkB-induced EMT-like transformation, anoikis suppression, and tumorigenesis.
In view of the important role of E-cadherin in TrkB-induced EMT and anoikis resistance, we next addressed how TrkB downregulates E-cadherin. As Twist can induce EMT and plays a critical role in metastasis (78), we first focused on this basic helix-loop-helix transcription factor, investigating whether it corresponds to a critical TrkB target in E-cadherin repression. In support of a role for Twist in this setting, activated TrkB induced the expression of Twist both at the mRNA (Fig. 1D) and protein levels (Fig. 4A; see also Fig. S2A in the supplemental material). As expected, kinase-inactive TrkB failed to induce Twist (see Fig. S2B in the supplemental material). To assess the requirement of Twist for TrkB function in this regard, we generated stable cell lines expressing TrkB, BDNF, and shRNAs against Twist (or against EGFP, as a control). To rule out off-target effects (22), we used two independent, nonoverlapping shRNAs against Twist. Upon expression of sh-Twist in polyclonal RK3ETB cell pools, EMT was partially reverted (data not shown). To enhance this effect, we first knocked down Twist in RK3E cells expressing BDNF, subsequently transduced the cells with TrkB-encoding retrovirus, and then established independent stable clonal cell lines. Indeed, this led to a robust block to TrkB-induced EMT-like transformation (Fig. 4B). As anticipated, this correlated well with restoration and correct subcellular localization of E-cadherin (Fig. 4C and D). This was seen also for RIE-1TB cells (see Fig. S2C in the supplemental material). Consistently, Twist depletion caused a marked reduction in anoikis suppression (see Fig. S3A in the supplemental material), cell migration, invasion (see Fig. S3B in the supplemental material), and anchorage-independent growth in soft agar (see Fig. S3C in the supplemental material) induced by TrkB, which was not due to inhibition of cell proliferation (see Fig. S3D in the supplemental material).
FIG. 4.
Twist is required for TrkB-induced EMT and tumorigenesis. (A) Induction of Twist protein levels in TrkB and BDNF-expressing RK3E cells, analyzed by Western blotting. V, vector; TB, TrkB and BDNF. CDK4 serves as the loading control. (B) sh-Twist prevents morphological transformation of RK3E cells by TrkB and BDNF. (C) sh-Twist prevents downregulation of E-cadherin by TrkB and BDNF in RK3E cells, as shown by Western blot analysis for the indicated proteins. β-actin serves as the loading control. (D) E-cadherin localizes at the cell membrane of RK3ETB cells upon Twist depletion, as shown by immunofluorescence (TO-PRO stains DNA). (E) sh-Twist impairs TrkB-mediated tumorigenesis. BALB/c nude mice were subcutaneously injected with 1 × 105 RK3ETB cells plus the indicated shRNAs into each flank. Growth curves for average tumor volumes are shown, with 8 tumors for sh-EGFP #1, sh-EGFP #2, and sh-Twist #1 and 11 tumors for sh-Twist #2. Error bars represent the standard errors of the mean. An asterisk indicates a P value of <0.01 in a two-sided Student t test. (F) Effect of sh-Twist on experimental metastasis. Mice were intravenously injected with 1 × 106 RK3ETB cells plus the indicated shRNAs. Mice were euthanized when clinical symptoms became apparent, with four mice from each cell line. Significance values were obtained by first combining the data from both shRNAs against the same gene (EGFP or Twist) and subsequently performing a log rank test.
Like our previous findings in RIE-1 cells (20, 29), RK3ETB cells (but not parental RK3E cells, which are devoid of tumorigenic potential [69; data not shown]) were highly tumorigenic in nude mice (Fig. 4E). In contrast, subcutaneous injection of RK3ETB cells in which Twist had been depleted resulted in tumor growth that was significantly, albeit moderately, reduced (Fig. 4E; see also Fig. S3E in the supplemental material). In a previous study, using a different cell system, knockdown of Twist did not affect tumor growth but inhibited only metastasis (78), which probably reflects differences in the cellular context or origin. In view of the observed difference in primary tumor growth as a function of Twist expression, we considered this setting not suitable to study the requirement of Twist in TrkB-induced metastasis. Therefore, we injected cells intravenously into nude mice. Although this experimental metastasis assay precludes an assessment of the early steps of tumor progression (tumor growth, invasion, and intravasation into vessels), it does allow measurement of the capacity of tumor cells to colonize the lungs. Silencing of Twist in RK3ETB cells prolonged the survival of the mice (Fig. 4F), which is consistent with previous findings (78). These results suggest that Twist plays an important role in both TrkB-induced oncogenicity and metastasis.
TrkB-induced EMT-like transformation and induction of Twist is mediated via the MAPK pathway.
To shed more light on how TrkB upregulates Twist and triggers EMT-like transformation, we assessed which pathways mediate these TrkB-dependent signals. Several canonical signal transduction pathways are stimulated by TrkB, including the MAPK pathway, PI3K pathway, and RAC1 GTPase signaling (37, 51). Therefore, we used pharmacological inhibitors of MEK (U0126) (27) and PI3K (PI-103) (26). To inhibit RAC1, we used NSC23766 (70) and a dominant-negative variant, RACN17 (66). Inhibition of the MAPK pathway with U0126 was confirmed by measuring phospho-ERK levels (Fig. 5A). U0126 treatment abolished the induction of Twist by TrkB (Fig. 5B) and restored E-cadherin levels in RK3ETB cells (Fig. 5C), which was accompanied by a reversion of the spindle-shaped cell morphology toward an epithelial appearance (Fig. 5D). To rule out off-target effects of the U0126 inhibitor, we used a second MEK inhibitor, CI-1040 (1), which gave identical results (see Fig. S4 in the supplemental material). The effects of MEK inhibition on E-cadherin levels and cell morphology could be reverted significantly by overexpression of the presumptive downstream effector Twist (Fig. 5E and F) (the remainder of these panels will be discussed below). The partial effect suggests that activation of Twist occurs at multiple levels. Inhibition of the PI3K pathway failed to have this effect on Twist, E-cadherin, or cell morphology (Fig. 5H to J), despite the fact that pAKT levels could be effectively downregulated (Fig. 5G). Likewise, a dominant-negative RAC1N17 mutant failed to significantly affect cell morphology, E-cadherin, or Twist levels. Nor did it impede the migratory or invasive properties of RK3ETB cells (see Fig. S5B to D in the supplemental material). This was in spite of the observation that RAC1N17 suppressed RAC1 activity below that of the control RK3E cells, as shown by the RAC1 pulldown assay (see Fig. S5A in the supplemental material). In line with this, treatment with the RAC1 inhibitor NSC23766 altered neither cell morphology nor E-cadherin levels (see Fig. S5E and F in the supplemental material). These results demonstrate that TrkB-induced EMT-like transformation and induction of Twist is mediated mainly via the MAPK pathway.
FIG. 5.
TrkB-induced EMT-like transformation is mediated via the MAPK pathway. (A) Downregulation of phospho-ERK (pERK) upon treatment with U0126. Cells were serum starved for 4 h, pretreated with 20 μM U0126 for 30 min, and stimulated with 50 ng/ml BDNF for 5 min. Cells were harvested in the presence of phosphatase inhibitors. (B) Induction of Twist in RK3ETB cells requires an intact MAPK pathway, as shown by Western blot analysis. Cells were treated overnight with 20 μM U0126 and analyzed by Western blot analysis. Two parts derived from the same gel are shown. TB, TrkB and BDNF. (C) TrkB-induced EMT-like transformation is dependent on the MAPK pathway. RK3ETB cells were treated with 20 μM U0126 for 2 days and analyzed by Western blotting. (D) Morphology of the cells described in the legend to panel C. (E) HA-Twist or Snail-HA overexpression in RK3ETB cells partially prevented the reversion to an epithelial morphology induced by U0126 treatment for 2 days. (F) Western blot analysis of cells described in the legend to panel E. (G) Downregulation of pAKT upon treatment with PI-103. Cells were serum starved for 4 h, pretreated with 500 nM PI-103 for 30 min, and stimulated with 50 ng/ml BDNF for 5 min. Cells were harvested in the presence of phosphatase inhibitors. (H) Induction of Twist in RK3ETB cells is not dependent on the PI3K pathway. Cells were treated overnight with 500 nM PI-103 and analyzed by Western blotting. (I) TrkB-induced EMT-like transformation is not dependent on the PI3K pathway. Cells were treated with 500 nM PI-103 for 2 days and analyzed by Western blotting. (J) Morphology of cells described in the legend to panel I. β-actin serves as the loading control for all Western blots.
Snail is required for TrkB-induced EMT-like transformation, anoikis resistance, migration, invasion, and anchorage-independent growth.
In view of these results, we set out to dissect the different functions of Twist, aiming to identify its downstream target(s) that is specifically required for metastasis in this system. The zinc finger transcription factor Snail is a direct repressor of E-cadherin (2, 14). Studies of Drosophila have shown that Twist can induce Snail (39). Consistent with this, ectopic expression of Twist led to a induction by fivefold of Snail mRNA levels in RK3E cells (see Fig. 8A). Furthermore, Snail was induced by overexpression of TrkB and BDNF in MCF10A cells (Fig. 6A). We were unable to assess endogenous Snail protein levels in RK3E and RIE-1 cells by any of the available Snail antibodies. To investigate the contribution of Snail to the prooncogenic and prometastatic functions of TrkB, we generated stable cell clones of RK3ETB cells and two independent shRNAs against Snail (Fig. 6B). Like what was observed for Twist, silencing of Snail in RK3E cells prevented TrkB-induced EMT-like transformation (Fig. 6C). Again, this correlated well with restoration of E-cadherin levels and its correct localization at the cell membrane (Fig. 6D and E). Furthermore, Snail was required for TrkB-induced anoikis resistance (Fig. 6F), cell migration and invasion (Fig. 6G), and anchorage-independent growth (Fig. 6H). Of note, as we had observed for Twist, Snail overexpression also rescued the effects of MEK inhibition on cell morphology and E-cadherin levels (Fig. 5E and F; see also S4D and E in the supplemental material).
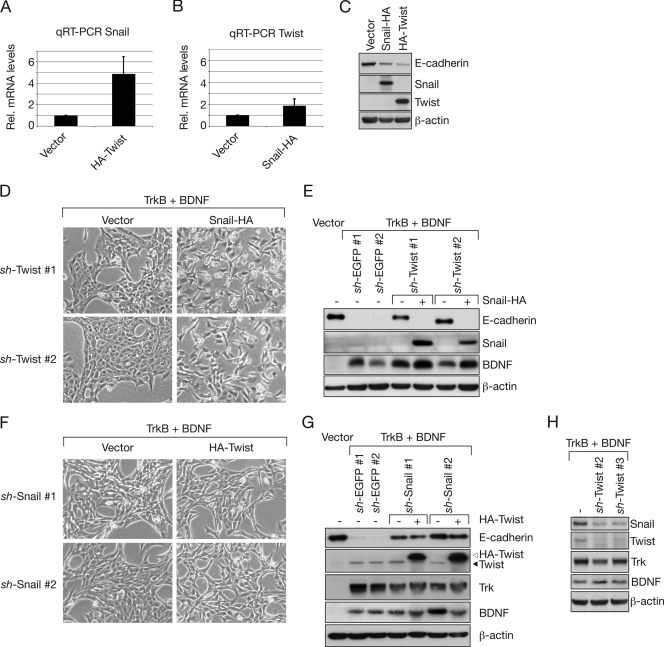
FIG. 8.
Snail acts downstream from Twist. (A) Induction of Snail mRNA by overexpression of HA-Twist in RK3E cells, as measured by qRT-PCR (n = 3; error bars represent standard deviations). Rel., relative. (B) mRNA levels of Twist in RK3E cells overexpressing Snail-HA, measured by qRT-PCR (n = 3; error bars represent standard deviations). (C) Snail-HA and HA-Twist are both expressed at high levels and downregulate E-cadherin, as showed by Western blot analysis. (D) Functional rescue of cell morphology by overexpression of Snail-HA in RK3ETB cells expressing sh-Twist. (E) Western blot analysis for E-cadherin, Snail, and BDNF of the cells used for panel D. (F) No morphological change by overexpression of HA-Twist in RK3ETB cells plus sh-Snail. (G) Western blot analysis for E-cadherin, Twist, and other proteins, as indicated by the cells used for panel F. The open arrowhead indicates ectopic HA-Twist, and the filled arrowhead indicates endogenous Twist. (H) Snail protein levels are downregulated upon Twist knockdown in TrkB- and BDNF-expressing MCF10A cells, as measured by Western blot analysis. β-actin serves as the loading control for all Western blots.
FIG. 6.
Snail is required for TrkB-induced EMT-like transformation, anoikis resistance, and anchorage-independent growth. (A) Increased protein levels of Snail in TrkB and BDNF-expressing MCF10A cells, as judged by Western blotting of the indicated proteins. (B) Snail knockdown in RK3ETB cells, as measured by qRT-PCR (n = 3; error bars represent standard deviations). Rel., relative. (C) sh-Snail prevents morphological transformation of RK3E cells by TrkB and BDNF. Photographs shown here and in Fig. 4B are derived from the same experiment. (D) sh-Snail prevents downregulation of E-cadherin by TrkB and BDNF in RK3E cells, as judged by Western blotting of the indicated proteins. (E) E-cadherin localizes at the cell membrane of RK3ETB cells upon Snail depletion, as shown by immunofluorescence (TO-PRO stains DNA). Pictures shown here and in Fig. 4D are derived from the same experiment. (F) sh-Snail impairs TrkB-mediated anoikis suppression. RK3E cells expressing the indicated cDNAs were cultured on ULC plates and scanned at a magnification of ×1 after 4 days (left) or quantified by determining the total protein levels (right) (n = 3; error bars represent standard deviations). Pictures shown here and in Fig. S2A in the supplemental material are derived from the same experiment. (G) Snail is involved in TrkB-induced migration and invasion, as determined by migration and invasion assays. A total of 250,000 freshly trypsinized cells were seeded on control inserts (for migration) or Matrigel (for invasion), and cells that translocated toward a serum gradient were counted 24 h later. Error bars represent the standard deviations of three independent experiments. Graphs shown here and in Fig. S2B in the supplemental material were derived from the same experiment. (H) sh-Snail impairs TrkB-mediated anchorage-independent growth. A total of 1,000 RK3E cells expressing the indicated cDNAs were grown in 0.4% agarose for 11 days; magnification of ×1 (left) and quantification of the number of colonies (right) are shown. β-actin serves as the loading control for all Western blots.
Snail is required for TrkB-induced metastasis.
In contrast to sh-Twist, however, subcutaneous injection of sh-Snail-expressing RK3ETB cells into nude mice resulted in the formation of primary tumors, with kinetics indistinguishable from those of tumor cells expressing control shRNA (Fig. 7A; see also Fig. S6 in the supplemental material). This allowed us to specifically address the role of Snail in TrkB-induced metastasis, using an experimental system comprising all steps of the metastatic cascade. Almost 100% of the subcutaneous RK3ETB tumors metastasized to the lungs, which was strongly suppressed upon Snail depletion. For each cell line (RK3ETB cells plus indicated shRNAs), the incidence of metastasis, measured by the number of mice that developed pulmonary metastases (>0.1 mm in size) out of the total number of mice with subcutaneous tumors, observed was as follows: sh-EGFP #1, 15/15; sh-EGFP #2, 14/15; sh-Snail #1, 6/14; and sh-Snail #2, 6/15. This was accompanied by, on average, a >fivefold drop in the number of metastatic pulmonary lesions (Fig. 7B and C). Consistent with and extending these findings, Snail silencing also strongly delayed the outgrowth of intravenously injected RK3ETB cells in the lungs (Fig. 7D). Taken together, these results show that Snail is dispensable for TrkB-expressing cells to produce a primary tumor but strongly contributes to their capacity to metastasize.
FIG. 7.
Snail is required for TrkB-induced metastasis. (A) sh-Snail does not affect TrkB-induced tumorigenesis. BALB/c nude mice were subcutaneously injected with 1 × 105 RK3ETB cells plus the indicated shRNAs into each flank. Growth curves for average tumor volumes are shown; n = 8 tumors for all cell lines. Error bars represent standard errors of the mean. Curves shown here and in Fig. 4E were derived from the same experiment. (B) Hematoxylin- and eosin-stained histological sections of lungs from the mice described in the legend to panel A, showing metastatic tumor lesions. (C) Quantification of lung metastases from mice described in the legend to panel A. Lesions of ≥0.1 mm were counted from 17 to 23 slides per cell line. Bar diagram shows the average values from three experiments, and error bars represent standard deviations. (D) Effect of sh-Snail on experimental metastasis. Mice were intravenously injected with 1 × 106 RK3ETB cells expressing the indicated shRNAs. Mice were euthanized when clinical symptoms became apparent; n = 4 mice for each cell line. Significance values were obtained by first combining the data from both shRNAs against the same gene (EGFP or Snail) and subsequently performing a log rank test. Curves shown here and in Fig. 4F were derived from the same experiment.
Snail acts downstream from Twist.
Since it is unknown whether the Twist-Snail axis present in Drosophila (39) is also operational in mammalian cells, we determined whether Twist induces Snail in rat epithelial cells. In support of this possibility, we observed that overexpression of HA-Twist in RK3E cells increased Snail mRNA levels by a factor of 5 (Fig. 8A). Conversely, Snail-HA overexpression hardly affected Twist mRNA levels (Fig. 8B), although HA-Twist and Snail-HA were both expressed to high levels, which were sufficient to downregulate E-cadherin (Fig. 8C). The model in which Twist acts upstream from Snail predicts that the latter protein should be able to antagonize a phenotype that is altered as a function of the first one. Thus, we examined whether overexpression of Snail-HA rescues the reversion of TrkB-expressing cells to an epithelial morphology, owing to Twist depletion. Indeed, ectopic expression of Snail-HA in RK3ETB-sh-Twist cells induced a dramatic EMT-like morphological transformation, changing the epithelial morphology back to a spindle-shaped appearance (Fig. 8D). Consistent with this observation, E-cadherin levels, which initially were high after Twist depletion, were suppressed strongly by overexpression of Snail-HA (Fig. 8E). These results suggest an epistatic relationship between Twist and Snail, with Twist acting upstream from Snail, at least in the context of this experimental setting. This functional interaction was confirmed by the reverse experiment, in which HA-Twist overexpression in RK3ETB-sh-Snail cells failed to affect cell morphology (Fig. 8F). In line with this, E-cadherin levels remained unchanged upon HA-Twist overexpression in Snail-depleted cells (Fig. 8G). Furthermore, in TrkB- and BDNF-expressing MCF10A cells, Snail levels were downregulated upon knockdown of Twist (Fig. 8H). This demonstrates that Twist is required for the induction of Snail by TrkB.
DISCUSSION
The results presented here show that TrkB induces an EMT-like transformation in epithelial cells and that it does so through a Twist-Snail signaling axis, which is dependent on the MAPK pathway. Furthermore, we demonstrate that Snail plays a critical and specific role in TrkB-mediated metastasis (Fig. 9).
FIG. 9.
Model depicting a critical TrkB effector pathway contributing to EMT, anoikis suppression, and metastasis. TrkB activation leads to induction of both Twist and Snail. These transcription factors repress E-cadherin, thereby inducing EMT and anoikis suppression and facilitating metastasis. Twist, acting upstream from Snail, conceivably has additional targets, including those that are primarily required for tumor growth. For simplicity, only the functional and epistatic relationships that are addressed in this paper are indicated. We do not exclude other upstream and downstream factors and feedback loops that may be present.
The term EMT has been used quite broadly to describe processes that enable epithelial cells to acquire fibroblastoid properties (33). In line with a prototypic EMT, activated TrkB in rat epithelial cells induced a switch from E-cadherin to N-cadherin, the downregulation of several catenin proteins, and a spindle-shaped morphology with reduced cell-cell adhesion. However, in this system, TrkB failed to induce the mesenchymal proteins vimentin, smooth-muscle actin, and fibronectin. In human mammary epithelial MCF10A cells, most epithelial markers were not changed by TrkB expression, but the mesenchymal proteins were induced, which is consistent with the increasing notion that cellular context has to be taken into consideration when studying EMT. By comparing the gene expression profile of RK3ETB cells to that of EpH4RasTgf-β cells, we observed that 67% of the significantly deregulated EMT-associated genes changed in the expected direction (Desmet and Peeper, submitted; data not shown). Consistently, by assessing the mRNA expression levels of several known EMT mediators, we found an induction by TrkB of Twist and, to a smaller extent, of Snail. We show that both transcription factors are critically required for the EMT-like changes induced by TrkB, while two other mediators of EMT, Slug (Fig. 1D) and E12/E47 (data not shown), were not induced by TrkB. We infer from these results that TrkB activation changes the phenotype of epithelial cells in a way that strongly resembles EMT, which depends on the presence of both Twist and Snail.
We show that the EMT-like changes induced by TrkB via upregulation of Twist rely on a functional MAPK pathway. Like these findings, RASV12-TGF-β-induced EMT in EpH4 cells is also mediated mainly via MAPK pathway (40). The signaling pathways required for EMT and anoikis suppression are likely to be different across cell types, as TrkB-induced anoikis suppression in RIE-1 cells seems to be more dependent on PI3K signaling (20). Interestingly, whereas MEK inhibition did not affect the viability of RK3ETB cells, treatment with the Trk inhibitors K252a or GW 441756 induced a strong apoptotic response. The viability of RK3E cells expressing no or inactive TrkB hardly depended on Trk signaling, as both inhibitors induced little death in that setting. Remarkably, this was also seen for cells expressing activated RAS, implying some specificity for these inhibitors. This result also suggests that RK3E cells with sustained TrkB activation undergo “oncogene addiction” (76). Only 2 days of activation by BDNF was sufficient to induce this dependency. Our findings raise the possibility that certain tumors may critically rely on TrkB signaling, offering an opportunity for a TrkB-based anticancer therapy.
Previous studies performed in Drosophila showed that Twist can bind to the Snail promoter and induce its expression (39). However, to date, it is unclear whether this epistatic relationship is conserved in mammalian cells. As Twist is known to induce EMT and to play a critical role in breast cancer metastasis (78), we determined whether a Twist-Snail signaling pathway is conserved in the rat epithelial cells. As depletion of either Twist or Snail each fully restored E-cadherin levels to those seen in parental RK3E cells (Fig. 4C and 6D), this suggests that these two factors act in one and the same pathway rather than in parallel pathways. Furthermore, our results suggest that Twist acts upstream from Snail both in regulating E-cadherin and in mediating EMT. Others have shown recently that in human breast tumor cells, Twist can also directly bind to the E-cadherin promoter (75); a possible contribution of Snail was not addressed in that study. It thus appears that E-cadherin is subject to the following two modes of Twist-dependent regulation: a direct one involving Twist binding to the promoter and an indirect one involving Snail upregulation.
Whereas it has been proven difficult to provide histopathological evidence for EMT in human carcinomas (79), several reports have shown a correlation between Snail expression levels and the propensity to metastasize (reviewed in reference 63). For breast cancer, particularly, Snail overexpression has been associated with lymph node metastasis (8) and shorter overall survival (24). Besides metastasis, Snail overexpression also predicts tumor relapse, which has functionally been confirmed in a breast cancer mouse model (52). Snail has also been associated with aggressive disease in other cancer types (for a review, see reference 3), including hepatocellular carcinoma (71), ovarian cancer (10), and head and neck squamous cell carcinoma (80). Although these observations imply that Snail is functionally involved in metastasis, the experimental evidence for this is still incomplete. Overexpression of Snail has been shown to induce EMT, cellular migration (14), and increased levels metastasis after orthotopic injection into nude mice (81). Conversely, RNA interference (RNAi)-mediated inhibition of Snail impairs metastasis of subcutaneously injected HaCa4 cells (59). However, a limitation of these as well as other tumor cell lines is that downregulation of Snail not only decreased metastasis but also dramatically impaired the growth of the primary tumor (58-60). A similar phenomenon, albeit less dramatic, is shown in this paper for Twist. Therefore, it has not been straightforward to conclude whether delayed metastasis is due to the metastasis-specific functions of Snail (e.g., cell invasion and anoikis suppression) or due to its more general function with in vivo tumor cell proliferation. The cell system used in our study is genetically better defined than tumor cell lines, because its oncogenic and metastatic potentials strictly depend on activated TrkB. Here, RNAi against Snail did not affect growth of the primary subcutaneous tumors, although knockdown of Snail partially impaired TrkB-mediated anoikis suppression and growth in soft agar. It thus appears that the microenvironment in the subcutaneous compartment in the mouse allows for compensation of the loss of Snail, something the soft agar conditions in vitro cannot allow. We can only speculate what the reason for this is. Conceivably, this involves ECM components as well as growth and/or survival factors provided by blood vessels. In contrast to primary tumor growth and consistent with the strong negative effect that a Snail knockdown had on cell migration and invasion, the formation of lung metastases was strongly impaired, thereby unmasking the specific requirement for Snail in spontaneous TrkB-driven metastasis. Our results, together with those discussed above, therefore raise the possibility that functional inhibition of Snail in cancer may impact tumor growth, tumor cell survival, and/or tumor cell metastasis. As transcription factors are not the favorite class of targets for pharmacological inhibition, upstream regulators of Snail, like GSK3β (82, 83), might provide better targets for therapeutic intervention. However, our knowledge about the (de)regulation and activation of Snail is still incomplete and should be further investigated, as is also warranted by this work.
This model leads to the question as to which of the targets of Snail are most relevant for its metastatic function. One of the best-studied Snail targets is E-cadherin (2), playing an essential role in EMT (4), tumor progression (17, 64), and metastasis (48, 57, 61). The loss of E-cadherin-mediated cell-cell adhesion is thought to promote cell migration and invasion (7). Furthermore, E-cadherin is part of a complex that regulates β-catenin signaling (15, 32, 61) and the activity of Rho GTPases (55), further impinging on cell migration and invasion. Others have demonstrated that RNAi-mediated downregulation of E-cadherin (but not overexpression of a dominant-negative mutant) is sufficient to induce EMT and metastasis (61). Furthermore, the loss of E-cadherin induces Twist (61), suggesting that a feed-forward loop could strengthen the maintenance of EMT. We show that restoration of E-cadherin expression in TrkB-expressing cells that have undergone EMT not only restored the epithelial morphology but also interfered with anoikis suppression (Fig. 3). This observation is consistent with the findings from a conditional E-cadherin knockout mouse model for breast carcinoma, in which the loss of E-cadherin resulted in anoikis suppression (17). Therefore, Twist, Snail, and E-cadherin conceivably provide a link between EMT and anoikis suppression, both of which contribute to metastasis.
A recent report showed that Twist and Snail also induce stem cell-like properties in nontumorigenic, as well as transformed, human mammary epithelial cells (45). Taken together with the role of Twist and Snail in tumor expansion and metastasis, this raises the interesting possibility that genes from within the Twist/Snail transcriptome are involved in regulating both stem cell phenotype and oncogenic transformation. While further work should shed light on such a possible connection, here, starting from the observation that TrkB suppresses anoikis and promotes metastasis, we established that a Twist-Snail axis mediating EMT is critically required for these important cancer biological processes. Further elucidation of the factors and pathways involved in this program may reveal targets for therapeutic intervention.
Supplementary Material
Acknowledgments
We thank M. Voetel, S. Greven, H. Grimminck, and all animal caretakers for their excellent technical help with the in vivo experiments; L. Brocks and L. Oomen for advice with image analysis; and the experimental animal pathology department and J. Zevenhoven for help with the pathological analysis. We thank M. Heimerikx, M. Nieuwland, J. de Ronde, D. Sie, and R. Kerkhoven for performing microarray analysis; C. Desmet for communication of results prior to publication; N. Armstrong for statistical advice; A. Pfauth and F. van Diepen for help with fluorescence-activated cell sorting; and S. Ellenbroek and S. Mertens for advice on RAC activity assays. We thank all members of the Peeper laboratory for their valuable input and A. Prieur and C. Hömig-Hölzel for critical reading of the manuscript. We thank K. Becker for the Snail antibody, C. Niessen for the E-cadherin plasmid, A. Munoz for the Snail-HA plasmid, and R. Weinberg for the Twist plasmid.
This work was supported by the Dutch Cancer Society (KWF) grant to M.A.S. and D.S.P. and a European Union FP6 grant to T.R.G. and D.S.P. D.S.P. is also supported by the European Molecular Biology Organization (EMBO) Young Investigator program and by a KWF Queen Wilhelmina program grant.
Footnotes
Published ahead of print on 4 May 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Barrett, S. D., A. J. Bridges, D. T. Dudley, A. R. Saltiel, J. H. Fergus, C. M. Flamme, A. M. Delaney, M. Kaufman, S. LePage, W. R. Leopold, S. A. Przybranowski, J. Sebolt-Leopold, K. Van Becelaere, A. M. Doherty, R. M. Kennedy, D. Marston, W. A. Howard, Jr., Y. Smith, J. S. Warmus, and H. Tecle. 2008. The discovery of the benzhydroxamate MEK inhibitors CI-1040 and PD 0325901. Bioorg. Med. Chem. Lett. 186501-6504. [DOI] [PubMed] [Google Scholar]
- 2.Batlle, E., E. Sancho, C. Franci, D. Dominguez, M. Monfar, J. Baulida, and A. Garcia De Herreros. 2000. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 284-89. [DOI] [PubMed] [Google Scholar]
- 3.Becker, K. F., E. Rosivatz, K. Blechschmidt, E. Kremmer, M. Sarbia, and H. Hofler. 2007. Analysis of the E-cadherin repressor Snail in primary human cancers. Cells Tissues Organs 185204-212. [DOI] [PubMed] [Google Scholar]
- 4.Behrens, J., M. M. Mareel, F. M. Van Roy, and W. Birchmeier. 1989. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J. Cell Biol. 1082435-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrens, J., K. M. Weidner, U. H. Frixen, J. H. Schipper, M. Sachs, N. Arakaki, Y. Daikuhara, and W. Birchmeier. 1991. The role of E-cadherin and scatter factor in tumor invasion and cell motility. EXS 59109-126. [DOI] [PubMed] [Google Scholar]
- 6.Berx, G., E. Raspe, G. Christofori, J. P. Thiery, and J. P. Sleeman. 2007. Pre-EMTing metastasis? Recapitulation of morphogenetic processes in cancer. Clin. Exp. Metastasis 24587-597. [DOI] [PubMed] [Google Scholar]
- 7.Birchmeier, W., and J. Behrens. 1994. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim. Biophys. Acta 119811-26. [DOI] [PubMed] [Google Scholar]
- 8.Blanco, M. J., G. Moreno-Bueno, D. Sarrio, A. Locascio, A. Cano, J. Palacios, and M. A. Nieto. 2002. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 213241-3246. [DOI] [PubMed] [Google Scholar]
- 9.Blay, J., and K. D. Brown. 1984. Characterization of an epithelioid cell line derived from rat small intestine: demonstration of cytokeratin filaments. Cell Biol. Int. Rep. 8551-560. [DOI] [PubMed] [Google Scholar]
- 10.Blechschmidt, K., S. Sassen, B. Schmalfeldt, T. Schuster, H. Hofler, and K. F. Becker. 2008. The E-cadherin repressor Snail is associated with lower overall survival of ovarian cancer patients. Br. J. Cancer 98489-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudreau, N., C. J. Sympson, Z. Werb, and M. J. Bissell. 1995. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 267891-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodeur, G. M. 2003. Neuroblastoma: biological insights into a clinical enigma. Nat. Rev. Cancer 3203-216. [DOI] [PubMed] [Google Scholar]
- 13.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2243-247. [DOI] [PubMed] [Google Scholar]
- 14.Cano, A., M. A. Perez-Moreno, I. Rodrigo, A. Locascio, M. J. Blanco, M. G. del Barrio, F. Portillo, and M. A. Nieto. 2000. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 276-83. [DOI] [PubMed] [Google Scholar]
- 15.Cavallaro, U., and G. Christofori. 2001. Cell adhesion in tumor invasion and metastasis: loss of the glue is not enough. Biochim. Biophys. Acta 155239-45. [DOI] [PubMed] [Google Scholar]
- 16.Christofori, G. 2006. New signals from the invasive front. Nature 441444-450. [DOI] [PubMed] [Google Scholar]
- 17.Derksen, P. W., X. Liu, F. Saridin, H. van der Gulden, J. Zevenhoven, B. Evers, J. R. van Beijnum, A. W. Griffioen, J. Vink, P. Krimpenfort, J. L. Peterse, R. D. Cardiff, A. Berns, and J. Jonkers. 2006. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 10437-449. [DOI] [PubMed] [Google Scholar]
- 18.Desmet, C. J., and D. S. Peeper. 2006. The neurotrophic receptor TrkB: a drug target in anti-cancer therapy? Cell. Mol. Life Sci. 63755-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dionne, C. A., A. M. Camoratto, J. P. Jani, E. Emerson, N. Neff, J. L. Vaught, C. Murakata, D. Djakiew, J. Lamb, S. Bova, D. George, and J. T. Isaacs. 1998. Cell cycle-independent death of prostate adenocarcinoma is induced by the trk tyrosine kinase inhibitor CEP-751 (KT6587). Clin. Cancer Res. 41887-1898. [PubMed] [Google Scholar]
- 20.Douma, S., T. Van Laar, J. Zevenhoven, R. Meuwissen, E. Van Garderen, and D. S. Peeper. 2004. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 4301034-1039. [DOI] [PubMed] [Google Scholar]
- 21.Eccles, S. A., and D. R. Welch. 2007. Metastasis: recent discoveries and novel treatment strategies. Lancet 3691742-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Echeverri, C. J., P. A. Beachy, B. Baum, M. Boutros, F. Buchholz, S. K. Chanda, J. Downward, J. Ellenberg, A. G. Fraser, N. Hacohen, W. C. Hahn, A. L. Jackson, A. Kiger, P. S. Linsley, L. Lum, Y. Ma, B. Mathey-Prevot, D. E. Root, D. M. Sabatini, J. Taipale, N. Perrimon, and R. Bernards. 2006. Minimizing the risk of reporting false positives in large-scale RNAi screens. Nat. Methods 3777-779. [DOI] [PubMed] [Google Scholar]
- 23.Eide, F. F., E. R. Vining, B. L. Eide, K. Zang, X. Y. Wang, and L. F. Reichardt. 1996. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J. Neurosci. 163123-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elloul, S., M. B. Elstrand, J. M. Nesland, C. G. Trope, G. Kvalheim, I. Goldberg, R. Reich, and B. Davidson. 2005. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer 1031631-1643. [DOI] [PubMed] [Google Scholar]
- 25.Ernfors, P., K. F. Lee, and R. Jaenisch. 1994. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature 368147-150. [DOI] [PubMed] [Google Scholar]
- 26.Fan, Q. W., Z. A. Knight, D. D. Goldenberg, W. Yu, K. E. Mostov, D. Stokoe, K. M. Shokat, and W. A. Weiss. 2006. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell 9341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Favata, M. F., K. Y. Horiuchi, E. J. Manos, A. J. Daulerio, D. A. Stradley, W. S. Feeser, D. E. Van Dyk, W. J. Pitts, R. A. Earl, F. Hobbs, R. A. Copeland, R. L. Magolda, P. A. Scherle, and J. M. Trzaskos. 1998. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 27318623-18632. [DOI] [PubMed] [Google Scholar]
- 28.Frisch, S. M., and H. Francis. 1994. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geiger, T. R., and D. S. Peeper. 2007. Critical role for TrkB kinase function in anoikis suppression, tumorigenesis, and metastasis. Cancer Res. 676221-6229. [DOI] [PubMed] [Google Scholar]
- 30.Geiger, T. R., and D. S. Peeper. 2005. The neurotrophic receptor TrkB in anoikis resistance and metastasis: a perspective. Cancer Res. 657033-7036. [DOI] [PubMed] [Google Scholar]
- 31.Gitelman, I. 1997. Twist protein in mouse embryogenesis. Dev. Biol. 189205-214. [DOI] [PubMed] [Google Scholar]
- 32.Gottardi, C. J., E. Wong, and B. M. Gumbiner. 2001. E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J. Cell Biol. 1531049-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grunert, S., M. Jechlinger, and H. Beug. 2003. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat. Rev. Mol. Cell Biol. 4657-665. [DOI] [PubMed] [Google Scholar]
- 34.Gupta, G. P., and J. Massague. 2006. Cancer metastasis: building a framework. Cell 127679-695. [DOI] [PubMed] [Google Scholar]
- 35.Haapasalo, A., I. Sipola, K. Larsson, K. E. Akerman, P. Stoilov, S. Stamm, G. Wong, and E. Castren. 2002. Regulation of TRKB surface expression by brain-derived neurotrophic factor and truncated TRKB isoforms. J. Biol. Chem. 27743160-43167. [DOI] [PubMed] [Google Scholar]
- 36.Hall, P. A., P. J. Coates, B. Ansari, and D. Hopwood. 1994. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J. Cell Sci. 1073569-3577. [DOI] [PubMed] [Google Scholar]
- 37.Huang, E. J., and L. F. Reichardt. 2003. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72609-642. [DOI] [PubMed] [Google Scholar]
- 38.Huber, M. A., N. Kraut, and H. Beug. 2005. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17548-558. [DOI] [PubMed] [Google Scholar]
- 39.Ip, Y. T., R. E. Park, D. Kosman, K. Yazdanbakhsh, and M. Levine. 1992. Dorsal-twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes Dev. 61518-1530. [DOI] [PubMed] [Google Scholar]
- 40.Janda, E., K. Lehmann, I. Killisch, M. Jechlinger, M. Herzig, J. Downward, H. Beug, and S. Grunert. 2002. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 156299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jechlinger, M., S. Grunert, I. H. Tamir, E. Janda, S. Ludemann, T. Waerner, P. Seither, A. Weith, H. Beug, and N. Kraut. 2003. Expression profiling of epithelial plasticity in tumor progression. Oncogene 227155-7169. [DOI] [PubMed] [Google Scholar]
- 42.Jones, K. R., I. Farinas, C. Backus, and L. F. Reichardt. 1994. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell 76989-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein, R., R. J. Smeyne, W. Wurst, L. K. Long, B. A. Auerbach, A. L. Joyner, and M. Barbacid. 1993. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell 75113-122. [PubMed] [Google Scholar]
- 44.Liotta, L. A., and E. Kohn. 2004. Anoikis: cancer and the homeless cell. Nature 430973-974. [DOI] [PubMed] [Google Scholar]
- 45.Mani, S. A., W. Guo, M. J. Liao, E. N. Eaton, A. Ayyanan, A. Y. Zhou, M. Brooks, F. Reinhard, C. C. Zhang, M. Shipitsin, L. L. Campbell, K. Polyak, C. Brisken, J. Yang, and R. A. Weinberg. 2008. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133704-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massague, J. 2008. TGFbeta in cancer. Cell 134215-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masszi, A., C. Di Ciano, G. Sirokmany, W. T. Arthur, O. D. Rotstein, J. Wang, C. A. McCulloch, L. Rosivall, I. Mucsi, and A. Kapus. 2003. Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am. J. Physiol. Renal Physiol. 284F911-F924. [DOI] [PubMed] [Google Scholar]
- 48.Mbalaviele, G., C. R. Dunstan, A. Sasaki, P. J. Williams, G. R. Mundy, and T. Yoneda. 1996. E-cadherin expression in human breast cancer cells suppresses the development of osteolytic bone metastases in an experimental metastasis model. Cancer Res. 564063-4070. [PubMed] [Google Scholar]
- 49.Meredith, J. E., Jr., B. Fazeli, and M. A. Schwartz. 1993. The extracellular matrix as a cell survival factor. Mol. Biol. Cell 4953-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miknyoczki, S. J., D. Lang, L. Huang, A. J. Klein-Szanto, C. A. Dionne, and B. A. Ruggeri. 1999. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. Int. J. Cancer 81417-427. [DOI] [PubMed] [Google Scholar]
- 51.Miyamoto, Y., J. Yamauchi, A. Tanoue, C. Wu, and W. C. Mobley. 2006. TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc. Natl. Acad. Sci. USA 10310444-10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moody, S. E., D. Perez, T. C. Pan, C. J. Sarkisian, C. P. Portocarrero, C. J. Sterner, K. L. Notorfrancesco, R. D. Cardiff, and L. A. Chodosh. 2005. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 8197-209. [DOI] [PubMed] [Google Scholar]
- 53.Nakagawara, A., C. G. Azar, N. J. Scavarda, and G. M. Brodeur. 1994. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol. Cell. Biol. 14759-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nieto, M. A. 2002. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 3155-166. [DOI] [PubMed] [Google Scholar]
- 55.Noren, N. K., B. P. Liu, K. Burridge, and B. Kreft. 2000. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 150567-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oft, M., J. Peli, C. Rudaz, H. Schwarz, H. Beug, and E. Reichmann. 1996. TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 102462-2477. [DOI] [PubMed] [Google Scholar]
- 57.Oka, H., H. Shiozaki, K. Kobayashi, M. Inoue, H. Tahara, T. Kobayashi, Y. Takatsuka, N. Matsuyoshi, S. Hirano, M. Takeichi, et al. 1993. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 531696-1701. [PubMed] [Google Scholar]
- 58.Olmeda, D., M. Jorda, H. Peinado, A. Fabra, and A. Cano. 2007. Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene 261862-1874. [DOI] [PubMed] [Google Scholar]
- 59.Olmeda, D., A. Montes, G. Moreno-Bueno, J. M. Flores, F. Portillo, and A. Cano. 2008. Snai1 and Snai2 collaborate on tumor growth and metastasis properties of mouse skin carcinoma cell lines. Oncogene 274690-4701. [DOI] [PubMed] [Google Scholar]
- 60.Olmeda, D., G. Moreno-Bueno, J. M. Flores, A. Fabra, F. Portillo, and A. Cano. 2007. SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDA-MB-231 cells. Cancer Res. 6711721-11731. [DOI] [PubMed] [Google Scholar]
- 61.Onder, T. T., P. B. Gupta, S. A. Mani, J. Yang, E. S. Lander, and R. A. Weinberg. 2008. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 683645-3654. [DOI] [PubMed] [Google Scholar]
- 62.Palmer, H. G., M. J. Larriba, J. M. Garcia, P. Ordonez-Moran, C. Pena, S. Peiro, I. Puig, R. Rodriguez, R. de la Fuente, A. Bernad, M. Pollan, F. Bonilla, C. Gamallo, A. G. de Herreros, and A. Munoz. 2004. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat. Med. 10917-919. [DOI] [PubMed] [Google Scholar]
- 63.Peinado, H., D. Olmeda, and A. Cano. 2007. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer 7415-428. [DOI] [PubMed] [Google Scholar]
- 64.Perl, A. K., P. Wilgenbus, U. Dahl, H. Semb, and G. Christofori. 1998. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 392190-193. [DOI] [PubMed] [Google Scholar]
- 65.Price, L. S., M. Langeslag, J. P. ten Klooster, P. L. Hordijk, K. Jalink, and J. G. Collard. 2003. Calcium signaling regulates translocation and activation of Rac. J. Biol. Chem. 27839413-39421. [DOI] [PubMed] [Google Scholar]
- 66.Ridley, A. J., H. F. Paterson, C. L. Johnston, D. Diekmann, and A. Hall. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70401-410. [DOI] [PubMed] [Google Scholar]
- 67.Rosivatz, E., K. F. Becker, E. Kremmer, C. Schott, K. Blechschmidt, H. Hofler, and M. Sarbia. 2006. Expression and nuclear localization of Snail, an E-cadherin repressor, in adenocarcinomas of the upper gastrointestinal tract. Virchows Arch. 448277-287. [DOI] [PubMed] [Google Scholar]
- 68.Ruggeri, B. A., S. J. Miknyoczki, J. Singh, and R. L. Hudkins. 1999. Role of neurotrophin-trk interactions in oncology: the anti-tumor efficacy of potent and selective trk tyrosine kinase inhibitors in pre-clinical tumor models. Curr. Med. Chem. 6845-857. [PubMed] [Google Scholar]
- 69.Ruppert, J. M., B. Vogelstein, and K. W. Kinzler. 1991. The zinc finger protein GLI transforms primary cells in cooperation with adenovirus E1A. Mol. Cell. Biol. 111724-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanz-Moreno, V., G. Gadea, J. Ahn, H. Paterson, P. Marra, S. Pinner, E. Sahai, and C. J. Marshall. 2008. Rac activation and inactivation control plasticity of tumor cell movement. Cell 135510-523. [DOI] [PubMed] [Google Scholar]
- 71.Sugimachi, K., S. Tanaka, T. Kameyama, K. Taguchi, S. Aishima, M. Shimada, K. Sugimachi, and M. Tsuneyoshi. 2003. Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clin. Cancer Res. 92657-2664. [PubMed] [Google Scholar]
- 72.Tapley, P., F. Lamballe, and M. Barbacid. 1992. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene 7371-381. [PubMed] [Google Scholar]
- 73.Thiery, J. P. 2002. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2442-454. [DOI] [PubMed] [Google Scholar]
- 74.Thiery, J. P., and J. P. Sleeman. 2006. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7131-142. [DOI] [PubMed] [Google Scholar]
- 75.Vesuna, F., P. van Diest, J. H. Chen, and V. Raman. 2008. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem. Biophys. Res. Commun. 367235-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weinstein, I. B., and A. K. Joe. 2006. Mechanisms of disease: oncogene addiction—a rationale for molecular targeting in cancer therapy. Nat. Clin. Pract. Oncol. 3448-457. [DOI] [PubMed] [Google Scholar]
- 77.Wood, E. R., L. Kuyper, K. G. Petrov, R. N. Hunter III, P. A. Harris, and K. Lackey. 2004. Discovery and in vitro evaluation of potent TrkA kinase inhibitors: oxindole and aza-oxindoles. Bioorg. Med. Chem. Lett. 14953-957. [DOI] [PubMed] [Google Scholar]
- 78.Yang, J., S. A. Mani, J. L. Donaher, S. Ramaswamy, R. A. Itzykson, C. Come, P. Savagner, I. Gitelman, A. Richardson, and R. A. Weinberg. 2004. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117927-939. [DOI] [PubMed] [Google Scholar]
- 79.Yang, J., and R. A. Weinberg. 2008. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14818-829. [DOI] [PubMed] [Google Scholar]
- 80.Yang, M. H., S. Y. Chang, S. H. Chiou, C. J. Liu, C. W. Chi, P. M. Chen, S. C. Teng, and K. J. Wu. 2007. Overexpression of NBS1 induces epithelial-mesenchymal transition and co-expression of NBS1 and Snail predicts metastasis of head and neck cancer. Oncogene 261459-1467. [DOI] [PubMed] [Google Scholar]
- 81.Yin, T., C. Wang, T. Liu, G. Zhao, Y. Zha, and M. Yang. 2007. Expression of snail in pancreatic cancer promotes metastasis and chemoresistance. J. Surg. Res. 141196-203. [DOI] [PubMed] [Google Scholar]
- 82.Yook, J. I., X. Y. Li, I. Ota, C. Hu, H. S. Kim, N. H. Kim, S. Y. Cha, J. K. Ryu, Y. J. Choi, J. Kim, E. R. Fearon, and S. J. Weiss. 2006. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat. Cell Biol. 81398-1406. [DOI] [PubMed] [Google Scholar]
- 83.Zhou, B. P., J. Deng, W. Xia, J. Xu, Y. M. Li, M. Gunduz, and M. C. Hung. 2004. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 6931-940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.