Abstract
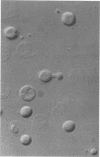
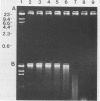
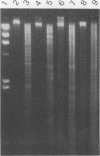
A rapid and easy method for the extraction of total cellular DNA from Cryptococcus neoformans is described. This procedure modifies and considerably simplifies previously reported methods. Numerous steps were either eliminated or replaced, including preincubations with cell wall permeability agents such as beta-mercaptoethanol and dithiothreitol. The commercially available enzyme preparation Novozyme 234 was found to contain a potent concentration of DNases which actively degrade DNA. Degradation and loss of DNA was prevented by maintaining a high concentration of EDTA in the lysing solution. This procedure resulted in high yields (150 to 200 micrograms of DNA from 100 ml of culture) of good-quality (undegraded), high-molecular-weight DNA which was readily digested by restriction endonucleases, making it suitable for use in various molecular applications.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. On the structure of the capsular polysaccharide from Cryptococcus neoformans serotype C. Immunochemistry. 1978 Sep;15(9):673–679. doi: 10.2196/40846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. Structural studies on the major, capsular polysaccharide from Cryptococcus bacillisporus serotype B. Carbohydr Res. 1980 Jun;82(1):103–111. doi: 10.1016/s0008-6215(00)85524-x. [DOI] [PubMed] [Google Scholar]
- Cazin J., Jr, Kozel T. R., Lupan D. M., Burt W. R. Extracellular deoxyribonuclease production by yeasts. J Bacteriol. 1969 Nov;100(2):760–762. doi: 10.1128/jb.100.2.760-762.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman J. C., Kwon-Chung K. J. Isolation of the URA5 gene from Cryptococcus neoformans var. neoformans and its use as a selective marker for transformation. Mol Cell Biol. 1990 Sep;10(9):4538–4544. doi: 10.1128/mcb.10.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo B. I., Barbour A. G. Cloning of 18S and 25S rDNAs from the pathogenic fungus Cryptococcus neoformans. J Bacteriol. 1989 Oct;171(10):5596–5600. doi: 10.1128/jb.171.10.5596-5600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. C., Kwon-Chung K. J. Production and regeneration of protoplasts from Cryptococcus. Sabouraudia. 1985 Feb;23(1):77–80. [PubMed] [Google Scholar]
- Stephen E. R., Nasim A. Production of protoplasts in different yeasts by mutanase. Can J Microbiol. 1981 May;27(5):550–553. doi: 10.1139/m81-082. [DOI] [PubMed] [Google Scholar]
- Varma A., Kwon-Chung K. J. Restriction fragment polymorphism in mitochondrial DNA of Cryptococcus neoformans. J Gen Microbiol. 1989 Dec;135(12):3353–3362. doi: 10.1099/00221287-135-12-3353. [DOI] [PubMed] [Google Scholar]