Abstract
The nonsense-mediated mRNA decay (NMD) pathway promotes rapid degradation of mRNAs containing premature translation termination codons (PTCs or nonsense codons), preventing accumulation of potentially detrimental truncated proteins. In metazoa, seven genes (upf1, upf2, upf3, smg1, smg5, smg6, and smg7) have been identified as essential for NMD; here we show that the zebrafish genome encodes orthologs of upf1, upf2, smg1, and smg5 to smg7 and two upf3 paralogs. We also show that Upf1 is required for degradation of PTC-containing mRNAs in zebrafish embryos. Moreover, its depletion has a severe impact on embryonic development, early patterning, and viability. Similar phenotypes are observed in Upf2-, Smg5-, or Smg6-depleted embryos, suggesting that zebrafish embryogenesis requires an active NMD pathway. Using cultured cells, we demonstrate that the ability of a PTC to trigger NMD is strongly stimulated by downstream exon-exon boundaries. Thus, as in mammals and plants but in contrast to invertebrates and fungi, NMD is coupled to splicing in zebrafish. Our results together with previous studies show that NMD effectors are essential for vertebrate embryogenesis and suggest that the coupling of splicing and NMD has been maintained in vertebrates but lost in fungi and invertebrates.
The nonsense-mediated mRNA decay (NMD) pathway is a conserved mRNA quality control mechanism that ensures the fidelity of gene expression by detecting and degrading mRNAs containing premature translation termination codons (PTCs or nonsense codons). In doing so, the NMD pathway protects eukaryotic cells from potentially dominant-negative effects resulting from the accumulation of truncated proteins (3, 12, 33). In addition to this protective function, NMD regulates 1 to 10% of wild-type transcripts, thereby influencing a broad range of biological processes including development, signal transduction, and cell cycle progression (25, 44).
NMD is triggered when ribosomes terminate translation prematurely. This event leads to the assembly of what is known as the surveillance complex, which links a premature translation termination event to degradation of a PTC-containing mRNA. To achieve this function, the surveillance complex interacts both with eukaryotic translation termination factors (i.e., eukaryotic release factors 1 and 3) and with the general cellular mRNA decay machinery, thereby accelerating the degradation of mRNAs harboring nonsense codons (8).
The components of the surveillance complex (or NMD effectors) were originally identified in genetic screens in Saccharomyces cerevisiae and Caenorhabditis elegans and subsequently by homology searches in other organisms (3, 12, 33). The surveillance complex components include the Upf1, Upf2, and Upf3 proteins (also known as suppressors with morphogenetic effects on genitalia [Smg-2, Smg-3, and Smg-4] in C. elegans), which are conserved in all eukaryotes, and the Smg1, Smg5, Smg6, and Smg7 proteins, which are conserved in multicellular organisms (3, 12, 33).
Upf1 is an RNA helicase whose activity is regulated by cycles of phosphorylation and dephosphorylation, a process that requires the additional NMD effectors. Phosphorylation of Upf1 requires Upf2 and Upf3 and is catalyzed by Smg1, a phosphatidylinositol 3-kinase-related kinase (13, 20, 26, 41, 42, 53). Dephosphorylation of Upf1 is mediated by Smg5, Smg6, and Smg7, three related proteins that bind phosphorylated Upf1 and recruit protein phosphatase 2A (4, 11, 16, 40). Smg5 and Smg7 also provide a molecular link between the core of the surveillance complex and general cellular mRNA decay enzymes (50). In addition, Smg6 has a C-terminal PIN domain with endonuclease activity, which contributes to degradation of nonsense mRNAs both in invertebrates and in mammals (14, 19, 24). Upf1 phosphorylation occurs in cis on mRNAs that terminate translation prematurely, leading to the recruitment of Smg5 to Smg7 and ultimately to mRNA degradation.
Studies of the NMD pathway in plants, fungi, invertebrates, and mammals have shown that although the NMD effectors are highly conserved, the detailed molecular mechanisms of NMD vary among different organisms (44). In mammals, PTCs trigger efficient NMD when located at least 50 to 55 nucleotides (nt) upstream of an exon-exon boundary, whereas in S. cerevisiae and invertebrates exon-exon boundaries do not play an essential role in NMD (2, 7, 17, 36, 39). These observations suggested that during evolution the mechanism by which nonsense codons are defined switched from the ancestral intron-independent mode still used by invertebrates to a predominantly intron-dependent mode in vertebrates. However, recent studies revealed that the intron-dependent and intron-independent mechanisms coexist in plants and therefore were most likely already present in stem eukaryotes (22, 27, 28, 48, 52). Consistently, intron-independent NMD was also observed in human cells (15, 49).
Across species, the importance of NMD effectors also varies (44). Indeed, NMD effectors are not essential in S. cerevisiae or C. elegans (3, 21). In contrast, upf1 is an essential gene in Drosophila melanogaster (38). In Arabidopsis thaliana, the UPF1 and SMG7 genes are essential for embryonic viability, and loss-of-function mutations in the UPF3 gene result in strong phenotypes (5, 23, 45, 54). upf1 and upf2 are also essential for early embryonic development in the mouse (37, 51). Remarkably, phenotypes associated with the depletion of Smg1 and Smg5 to Smg7 have not been described in vertebrates. The lack of information on the importance of these additional NMD effectors in the context of a vertebrate organism raises the question of whether the phenotypes seen in upf1 and upf2 knockouts can be ascribed to the inhibition of the NMD pathway or to the inhibition of an unknown function that Upf1 or Upf2 acquired over the course of evolution.
To gain further insight on the evolution and physiological relevance of NMD, we investigated this pathway in a basal vertebrate, the zebrafish Danio rerio. We show that mRNAs encoding orthologs of all known NMD effectors are provided maternally and are also ubiquitously expressed throughout early zebrafish embryogenesis. We also show that Upf1, Upf2, Smg5, Smg6, and Smg7 are required for embryonic development and survival. Using cultured cells, we demonstrate that the intron-dependent NMD mechanism operates in zebrafish. These results together with previous studies reveal that the link between splicing and NMD has been maintained in vertebrates. Finally, we show that overexpressing an SMG6 protein mutated at catalytic residues inhibits NMD in a dominant-negative manner, suggesting that SMG6 contributes to the degradation of NMD targets also in zebrafish.
MATERIALS AND METHODS
Fish husbandry and strains.
Fish were maintained, raised, and staged as described before (29). Unless stated otherwise, in all experiments the Tuebingen strain was used. The presence of the golb1 allele in the golden strain (31) was confirmed by sequencing.
Fish in situ hybridization and image acquisition.
All cDNAs were cloned into pCRII TOPO (Invitrogen) and sequenced. To synthesize digoxigenin-labeled antisense and sense RNA probes, the plasmids were linearized with either NotI or SpeI and transcribed using either SP6 or T7 RNA polymerase and the digoxigenin RNA labeling kit (Roche Diagnostics). Labeled RNA probes were purified using Micro Bio-Spin P30 Tris columns (Bio-Rad). Equal amounts of sense and antisense probes were used for adjacent in situ hybridization (20 to 100 ng).
Whole-mount in situ hybridizations were performed as described before (47). Prehybridization and hybridization were carried out at 64°C. Alkaline phosphatase-conjugated antidigoxigenin Fab fragments (Roche) were diluted 1:2,000 in phosphate-buffered saline (PBS) supplemented with 5% fetal calf serum and 0.1% Tween 20 and incubated overnight at 4°C.
Stained embryos were mounted in 100% glycerol. Living embryos were anesthetized with 0.04% buffered 3-aminobenzoic acid methyl ester (Sigma), dechorionated, and embedded in 5% methylcellulose in E3 medium. Images were obtained using an Axiophot2 fluorescence microscope (Zeiss).
Morpholino and capped mRNA injections in zebrafish.
Morpholino-modified oligonucleotides (MOs) were designed and synthesized by Gene-Tools LLC, Philomath, OR. MOs and the corresponding 5-bp mismatch control were injected in parallel into the yolk of one-cell-stage embryos. Morpholino sequences and injected concentrations are provided in Table S4 in the supplemental material. Capped human Upf1 was synthesized using the mMessage mMachine SP6 kit (Ambion) following the manufacturer's instructions with the exception that reaction mixtures were supplemented with 0.5 μl 20 mM GTP.
RNA isolation and cDNA preparation.
Embryos were dechorionated using 40 μl pronase (30 mg/ml) per petri dish for 1 h. After repeated washes in E3, 100 to 150 embryos were homogenized in 1 ml TriFast (PeqLab) and heated for 15 min at 37°C. One microliter of glycogen (10 mg/ml) was added per ml of TriFast to facilitate RNA precipitation.
Total RNA was reverse transcribed using oligo(dT) and Superscript II reverse transcriptase (Invitrogen). PCRs were performed using the Expand High Fidelity enzyme mix (Roche). Quantitative PCRs were carried out using target-specific primers and the iTaq Sybr green Supermix (Bio-Rad) in the DNA Engine Opticon 2 continuous fluorescence detection system (Bio-Rad). Data analysis was performed as described previously (35). Oligonucleotide sequences are provided in Table S4 in the supplemental material.
Western blotting.
For Western blotting, 150 embryos (1 day) were dechorionated and homogenized in 200 μl of 2× sample buffer (41.6 mM Tris-HCl, pH 6.8, 12% sodium dodecyl sulfate, 0.3% glycerol, 0.1 M dithiothreitol, bromophenol blue). Samples were incubated for 10 min at 90°C and centrifuged for 15 min at 10,000 rpm. Four microliters of the supernatant was loaded on a 6% sodium dodecyl sulfate-polyacrylamide gel. Proteins were transferred into a nitrocellulose membrane (Protran; Whatman). The membranes were incubated overnight at 4°C in PBS supplemented with 5% bovine serum albumin and 0.3% Tween 20. A goat anti-Rent1 (Upf1) antibody (Bethyl Laboratories; A300-036A) was diluted 1:5,000 in PBS containing 5% bovine serum albumin and 0.3% Tween 20. Mouse anti-α-tubulin antibody (Sigma T6199, clone DM1A) was diluted 1:20,000 in the same buffer. The signal was detected using the CDP-Star system (Tropix) and the following alkaline phosphatase-conjugated secondary antibodies: goat anti-mouse immunoglobulin G (Tropix WL10MS; dilution, 1:10,000) and rabbit anti-goat immunoglobulin G (Sigma A4187; dilution, 1:30,000).
Cell culture and transfections.
Zebrafish ZF4 cells (ATCC) were cultured according to ATCC's instructions and transfected using FuGENE HD (Roche). The β-tubulin-2c sequence (accession no. NM_198809.1; residues 2021 to 2923) was amplified from genomic DNA and inserted between the EcoRI and XhoI sites of vector pEGFP-C1 (Clontech). Codons 33, 62, 90, and 130 of the β-tubulin open reading frame (ORF) were replaced with TAA stop codons using the QuikChange site-directed mutagenesis kit (Stratagene). Intron 3 was excised from the corresponding constructs by site-directed mutagenesis. Plasmid pEGFP-C1, without an insert, was used as a transfection control. All constructs were fully sequenced to confirm the presence of the PTCs and the absence of additional mutations. Plasmids allowing the expression of human SMG6 wild type or mutant have been described before (24). Cells were transfected with 0.8 μg of each green fluorescent protein (GFP)-β-tubulin reporter plasmid (with or without PTC) and 0.2 μg of pEGFP-C1 as a transfection control. In the experiments shown in Fig. 6E, 0.5 μg of SMG6 expression plasmids was included in the transfection mixtures as indicated. Cells were harvested 72 h after transfection.
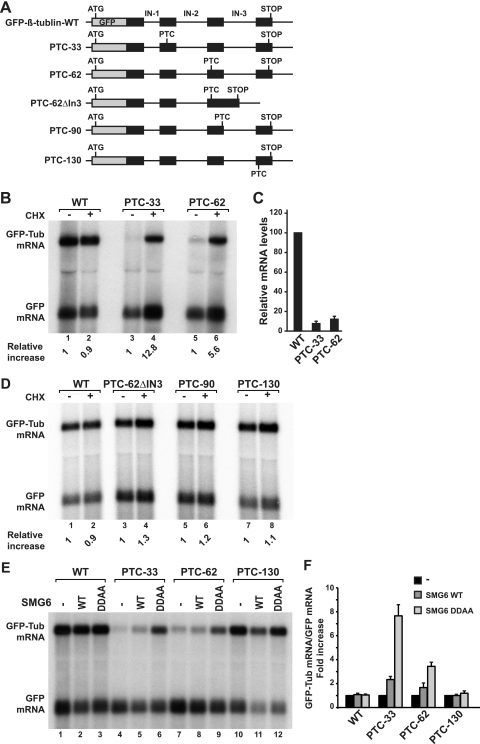
FIG. 6.
Splicing-dependent NMD in zebrafish. (A) Schematic representation of the GFP-β-tubulin reporters used in this study. The positions of PTCs and of the natural stop codon (STOP) are indicated. IN, intron. (B to D) Fish cells transiently expressing GFP-β-tubulin-WT or the indicated PTC reporters were mock treated (−) or incubated with cycloheximide (+ CHX, 100 μg/ml) for 45 min. Total RNA samples were isolated and analyzed by Northern blotting using a probe specific for GFP. The numbers below the lane numbers (relative increases) indicate the levels of GFP-β-tubulin transcripts normalized to those of the transfection control GFP mRNA. For each reporter, these values were set to 1 in mock-treated cells. (C) Normalized mRNA reporter levels relative to those of the wild-type reporter, which was set to 100. Mean values and standard deviations from three independent experiments are shown. (E and F) Fish cells were transiently transfected with a mixture of two plasmids, one expressing the GFP-β-tubulin reporters with or without PTC as indicated and another expressing GFP. Plasmids encoding SMG6 wild type (WT) or mutant (DDAA) were included in the transfection mixtures, as indicated. (F) Reporter mRNA levels were normalized to that of the GFP mRNA. For each reporter, these normalized values were set to 1 in the absence of SMG6. Mean values and standard deviations from three independent experiments are shown.
RNA isolation and Northern blots.
Total RNA was isolated using TriFast reagent (PeqLab), separated in denaturing formaldehyde agarose gels (5 to 20 μg/lane), and blotted onto positively charged nylon membranes (GeneScreen Plus; NEN Life Science).32P-labeled probes were generated by random priming or linear PCR using standard methods. Hybridizations were carried out overnight as described previously (17).
Nucleotide sequence accession numbers.
The following cDNA sequences encoding zebrafish NMD effectors were deposited in the EMBL/GenBank/DDBJ databases under the accession numbers in parentheses: upf1 (FM986817), upf2 (FM986818), upf3a (FM986819), upf3b (FM986820), smg1 (FM986821), smg5 (FM993108), smg6 (FM986822), and smg7 (FM986823).
RESULTS
NMD effectors are maternally provided and widely expressed in zebrafish embryos.
Using psi-BLAST searches (1), we identified single genes encoding Upf1, Upf2, Smg1, Smg5, Smg6, and Smg7 and two Upf3 paralogs in zebrafish genomic sequences. Alignment of the predicted sequences revealed that the zebrafish proteins harbor the same functional domains as do their human counterparts and share between 50 and 90% sequence identity (see Table S1A and B in the supplemental material). Throughout eukaryotes, the Upf1 protein is particularly well conserved: human and S. cerevisiae Upf1 share nearly 50% sequence identity, and between zebrafish, mouse, and human Upf1, the level of identity rises to over 90% (see Table S1A and B in the supplemental material).
To determine the spatiotemporal expression of NMD effectors during zebrafish early embryonic development, we carried out whole-mount in situ hybridization. We found that all NMD effectors are expressed ubiquitously during early cleavage (Fig. 1), as well as during gastrulation (see Fig. S1A in the supplemental material) and at 1 day postfertilization (1 dpf) (see Fig. S1B in the supplemental material). Detection of mRNAs encoding NMD effectors during early cleavage, before the onset of zygotic gene expression, indicates that they are provided maternally.
FIG. 1.
NMD factors are ubiquitously expressed and maternally provided during zebrafish embryogenesis. (A to P) mRNA expression patterns of NMD factors in embryos at two- to eight-cell stages revealed by antisense probes. Sense controls are shown on the right. Bars, 200 μm.
Upf1 is essential for zebrafish embryonic development.
Upf1 is a key effector of the NMD pathway (3, 12, 33) and is essential for embryonic development in A. thaliana (5, 54), D. melanogaster (38), and mice (37) but not in C. elegans (21). To address the physiological relevance of Upf1 during early zebrafish development, we depleted the protein using antisense MOs directed against either the translation initiation codon (Start-site MO) or the exon 1/intron 1 boundary of upf1 pre-mRNA (Splice-site MO). In both cases, a 5-bp mismatch MO was injected in parallel, to control for any nonspecific effects (control MOs).
At 1 dpf, embryos injected with the 5-bp mismatch control MOs showed no apparent morphological aberrations and were viable (Fig. 2A and E); in contrast, the Upf1 morphants injected with either the Splice-site or the Start-site MOs displayed overt phenotypes (Fig. 2B to D and F to H, respectively). Based on their severity, phenotypes were classified into three classes: weak (Fig. 2B and F), intermediate (Fig. 2C and G), and strong (Fig. 2D and H) (see Table S2 in the supplemental material for percentages of embryos in each class).
FIG. 2.
Upf1 is essential for zebrafish embryonic development and survival. (A to I) Lateral views of embryos injected with two different morpholinos (MOs) directed against either a splice site or the translational initiation codon (Start-site) of the upf1 transcript. (B to D and F to H) Representative phenotypes observed in Upf1 morphants at 1 dpf (mild to strong phenotypes). (A and E) Embryos injected with the corresponding 5-bp mismatch control MOs. Upf1 morphants exhibit extensive necrosis in the central nervous system (empty arrowheads), impaired eye development (filled arrowheads), abnormal somite morphogenesis (black arrows), and perturbation in the yolk sac extension (red arrows). (I) Uninjected wild-type embryo. Percentages of surviving embryos at 5 dpf are indicated below the panels. The total numbers of injected embryos are indicated in parentheses. Bar, 200 μm. (J to M) Lateral close-up views of heads and tails of Upf1 morphant and uninjected embryos shown in panels G and I, respectively. Arrows and arrowheads are as described above. (N to Q) Coinjection of human GFP-UPF1 mRNA rescued Upf1 morphants yielding embryos with weak and intermediate phenotypes and an increase in embryonic survival at 5 dpf. Note that only embryos showing a GFP signal were included in the analysis. (R and S) Western blot analysis of Upf1 in protein lysates from zebrafish embryos injected with Upf1 MOs or the respective controls. Protein lysates from human HeLa cells, zebrafish AB9 fin fibroblast cells, and uninjected wild-type embryos were analyzed in parallel. α-Tubulin served as a loading control. The migration of zebrafish Upf1 is consistent with its predicted size (122 kDa), which is slightly lower than that of human Upf1 (124 kDa).
Upf1 morphants were developmentally delayed, and their brain patterning was disturbed, particularly at the midbrain-hindbrain boundary, which was greatly diminished (Fig. 2B to D, F to H, and L versus I and J). In addition, the brain appeared opaque, which is indicative of extensive necrosis in the nervous system (Fig. 2B to D, F to H, and L versus I and J). Eye development was also aberrant (Fig. 2B to D, F to H, and L versus I and J). Moreover, somitogenesis was impaired, with the typical chevron-like arrangement of wild-type somites appearing stacked like rungs (Fig. 2B and D, F to H, and M versus I and K). The yolk sac extension as well as posterior axis elongation was perturbed in a fraction of Upf1-depleted embryos (Fig. 2). Furthermore, we observed high mortality rates of 80% to 85% 5 dpf.
To control for the specificity of the Upf1 MOs, we tested whether coinjecting an mRNA encoding GFP-tagged human UPF1 with the Upf1 Start-site MO could rescue the morphant phenotypes. Whereas 98% of Upf1 morphants injected in parallel showed all three phenotypic classes, with 42% of embryos showing a strong phenotype, coinjecting human UPF1 mRNA together with Upf1 MO yielded 32% morphants with weak phenotypes (Fig. 2N and O) and only 7% with strong phenotypes (see Table S3 in the supplemental material). Furthermore, the mortality rate was reduced to 18% at 5 dpf compared to 49% for embryos injected in parallel only with the Upf1 MO (see Table S3 in the supplemental material).
The effectiveness of the Upf1 morpholinos was tested by Western blotting and PCR analysis. In protein lysates from uninjected wild-type embryos or embryos injected with 5-bp mismatch control MOs, the antibodies recognized a protein of about 122 kDa (Fig. 2R and S, lanes 3 and 4). This band was strongly reduced in lysates from Upf1 morphants (Fig. 2R and S, lanes 5), indicating that the two Upf1 MOs interfere with Upf1 expression. In agreement with these results, by reverse transcription-PCR we observed that injecting the Upf1 Splice-site MO strongly reduced the levels of spliced upf1 mRNA at 1 dpf (see Fig. S2, lane 2, in the supplemental material), indicating that the morpholino did indeed interfere with intron 1 splicing.
As mentioned above, Upf1 morphants exhibited strong necrosis in the central nervous system. It was important to confirm that this phenotype was specific, because MOs were recently shown to nonspecifically induce neuronal cell death by activating p53 (46). These studies further showed that the nonspecific cell death could be abolished by depleting p53 (46). To this end, we coinjected the Upf1 Start-site MO together with the previously characterized p53 MO (46). In embryos coinjected with Upf1 and p53 MOs, we still observed extensive cell death in the central nervous system (see Fig. S3F to H and Table S2 in the supplemental material). In contrast, p53 morphants appeared phenotypically normal (data not shown), although their viability was slightly reduced to 87%. We conclude that the neuronal cell death observed in Upf1 morphants is a specific effect of the depletion.
In summary, our results indicate that the Splice-site and Start-site MOs downregulate Upf1 expression and result in developmental defects. The similarity of the phenotypes caused by these two different MOs provides further evidence that these phenotypes can be attributed to the depletion of Upf1. Furthermore, these results reveal an essential role for Upf1 during zebrafish embryogenesis.
Zebrafish embryonic development requires Upf2, Smg5, Smg6, and Smg7.
To further investigate the physiological role of NMD in zebrafish development, we depleted additional effectors of this pathway by using antisense MOs directed against either the translation initiation codon of upf2, upf3a, smg5, and smg6 mRNAs or exon-intron boundaries of smg1, smg7, and upf3b pre-mRNAs. We tested the effectiveness of the Splice-site MOs by PCR and confirmed that they indeed interfere with splicing of the targeted pre-mRNAs (data not shown).
Similar to the Upf1 knockdown, we observed an 80 to 90% reduction of viability at 5 dpf in embryos depleted of Upf2, Smg5, Smg6, and Smg7. The phenotypes caused by Upf2, Smg5, and Smg6 depletions resembled that of Upf1-depleted embryos (Fig. 3A, B, D, and E; see also Fig. S4 and S5 in the supplemental material for an overview of the three phenotypic classes and Table S2 in the supplemental material for percentages of embryos falling into each class). Indeed, as in Upf1 morphants, in Upf2, Smg5, and Smg6 morphants, the somites appeared stacked as opposed to the chevron-like shape of the wild-type somites (Fig. 3A, B, D, and E versus O), the brain was necrotic (Fig. 3), and eye development was impaired (Fig. 3). In Upf2-, Smg5-, and Smg6-depleted embryos, we observed a reduction in posterior axis elongation and an attenuated yolk sac extension as in Upf1 morphants (Fig. 3A, B, D, and E versus O). The control injections using the corresponding control MOs did not cause morphological aberrations and had no significant effect on embryonic viability (Fig. 3H to L).
FIG. 3.
Upf2, Smg5, Smg6, and Smg7 are essential during zebrafish embryogenesis. (A to O) Lateral views of embryos injected with antisense MOs directed against either translation initiation codons (Start-site) of upf1, upf2, upf3a, smg5, and smg6 mRNAs or splice sites of smg7 and upf3b pre-mRNAs. (A to G) Representative phenotypes observed in morphant embryos at 1 dpf. (H to N) Embryos injected with the corresponding 5-bp mismatch control MOs. (O) Uninjected wild-type embryo. Percentages of surviving embryos at 5 dpf are indicated below the panels. The total numbers of injected embryos are indicated in parentheses. Arrows and arrowheads are as described for Fig. 2. Bars, 200 μm.
In contrast, Smg7 morphants displayed phenotypes with specific features that were not observed in Upf1, Upf2, Smg5, and Smg6 morphants (Fig. 3F; see also Fig. S5 in the supplemental material). These embryos showed an elongated hindbrain; the appearance of the midbrain-hindbrain boundary was altered, but the boundary was still established (Fig. 3F; see also Fig. S5M to O in the supplemental material). Only the strong phenotype showed stacked somites comparable to the phenotypes observed in Upf1, Upf2, Smg5, and Smg6 morphants (Fig. 3F), whereas in the weak and intermediate phenotypes the chevron-like form of the wild-type somites was retained but the tail was bent or kinky (see Fig. S5N in the supplemental material).
Finally, depletion of Upf3a resulted in display of a very mild phenotype, only weakly affecting brain patterning (Fig. 3C). Beyond that, the Upf3a knockdown did not interfere with viability. This finding suggests that Upf3a is not essential for NMD, consistent with the modest activity that Upf3a displays in NMD in human cells (10, 30). Alternatively, this could be explained by possible functional redundancies between Upf3a and Upf3b. To distinguish between these possibilities, we depleted Upf3b using a Splice-site MO. Embryos depleted of Upf3b had no detectable phenotype (data not shown); in contrast, 97% of embryos lacking both Upf3a and Upf3b had phenotypes comparable to the weak phenotype observed in Upf1 morphants. The strong phenotype exhibited mild necrosis in the brain as well as brain patterning defects (Fig. 3G; see also Fig. S4H to J in the supplemental material). In the intermediate phenotype, the midbrain-hindbrain boundary was reduced (Fig. 3G; see also Fig. S4H to J in the supplemental material). Further, the hindbrain seemed to be elongated in the weak and intermediate phenotypes (see Fig. S4H and I in the supplemental material) as observed in Smg7 morphants. In contrast to all other morphants, we did not detect changes in somite structure. Furthermore, embryonic survival was not strongly compromised, as only 19% of Upf3a and Upf3b morphants died at 5 dpf. Finally, no phenotype was observed in embryos depleted of Smg1.
In summary, the phenotypic similarities between Upf1, Upf2, Smg5, and Smg6 morphants suggest that these proteins act in the same pathway and that the observed phenotypes are likely due to the inhibition of NMD. Smg7, in contrast, may have acquired additional functions. In all cases, though, the effects of the knockdowns were first detected in the brain.
Zebrafish Upf1 is essential for NMD.
To test directly whether zebrafish Upf1 is required for NMD in vivo, we analyzed the expression levels of an endogenous PTC-containing mRNA in Upf1-depleted embryos; specifically, we took advantage of the zebrafish golden mutant (31). The golden mutant allele (golb1) causes a hypopigmentation phenotype that arises from a nonsense mutation in the slc24a5 gene, which encodes a putative cation exchanger that functions in zebrafish pigmentation (31). The golb1 mutation is a nonsense codon at Tyr208; this nonsense codon lies upstream of three exon-exon boundaries. Since the transcript carrying this mutation is not detectable by in situ hybridization, it has been proposed that it undergoes NMD (31).
We tested whether the expression of the slc24a5 mRNA in golden mutant embryos could be restored by depleting Upf1. In wild-type embryos, slc24a5 expression begins at 1 dpf in the retinal pigment epithelium and in the melanophore precursors that are located dorsal of the floor plate (Fig. 4A). The melanophore precursors then divide and migrate throughout the body (Fig. 4A). In the golden mutant, slc24a5 mRNA is undetectable even at 36 h postfertilization (Fig. 4F) (31).
FIG. 4.
Depletion of Upf1 suppresses NMD in vivo. (A to J) Expression of slc24a5 mRNA in wild-type and golb1 embryos injected with upf1 Splice-site or Start-site MOs (B to E and G to J). Uninjected embryos are also shown (A and F). Empty arrowheads indicate detectable expression levels in the retinal pigment epithelium. Filled arrowheads show detectable expression in the melanophores. There is no detectable slc24a5 mRNA in uninjected golb1embryos (F). slc24a5 mRNA expression is partially restored in golden embryos injected with the Splice-site (G and H) or the Start-site (I and J) upf1 MO. Percentages of embryos displaying the phenotype shown in the panel are indicated in the top right corner. The total numbers of injected embryos are indicated in parentheses. Bars, 200 μm.
We depleted Upf1 both in wild-type and in golden mutant embryos. Because depleting Upf1 strongly affects embryonic development, its depletion reduces slc24a5 mRNA expression in wild-type embryos. Indeed, compared to uninjected embryos (or embryos injected with the control MOs), injecting the Upf1 Splice-site or Start-site MOs reduced slc24a5 expression in wild-type embryos (Fig. 4B to E versus A and data not shown). Nevertheless, slc24a5 expression was still detectable in the body, as well as in the retinal pigment epithelium (Fig. 4B to E). In about 50% of injected wild-type embryos, the expression was restricted to the retina (Fig. 4C and E). About 1% of injected embryos did not display any staining, whereas only 15% showed a wild-type pattern, indicating that Upf1 depletion does indeed affect slc24a5 expression directly or indirectly by interfering with cell differentiation in wild-type embryos.
Injecting Upf1 MOs into the golden mutant embryos rescued the expression of the nonsense slc24a5 transcript, rendering it detectable in the body and/or the retina in 65% to 70% of these embryos (Fig. 4G to J). In contrast, slc24a5 expression was undetectable in uninjected golden mutant siblings (Fig. 4F). Thus, depleting Upf1 rescues the expression of slc24a5 transcript in the golden background, allowing the transcript to be expressed at a level similar to that of the wild-type transcript in embryos similarly depleted of Upf1. Note that, although transcript levels are restored, the hypopigmentation phenotype cannot be rescued because the nonsense mutation prevents the synthesis of full-length SLC24A5 protein.
We performed quantitative PCR to quantify the effect of depleting Upf1 on expression levels of the PTC-containing slc24a5 transcript. We observed that, compared to the wild-type strain, the expression of slc24a5 mRNA is downregulated about fivefold in the golden mutant (Fig. 5A and B). In addition, whereas injecting control MOs did not change the levels of the aberrant transcript, depleting Upf1 with the Start-site MO restored the expression of the aberrant transcript fourfold; with the Splice-site MO, the transcript was expressed at wild-type levels (Fig. 5A and B); control injections did not alter the levels of the aberrant transcript (Fig. 5A and B). These results indicate that, in zebrafish, Upf1 is required for degradation of PTC-containing mRNAs.
FIG. 5.
Upf1 depletion restores the expression levels of the PTC-containing slc24a5 mRNA. (A and B) Expression of slc24a5 mRNA was analyzed by quantitative real-time PCR in uninjected wild-type and golb1 embryos as well as in embryos injected with Upf1 MOs (Start-site or Splice-site) or the corresponding 5-bp mismatch control MOs. slc24a5 mRNA levels were normalized to that of β-actin, which served as an internal control. These normalized values were then divided by those observed in wild-type embryos. In the lower panels, the products obtained after quantitative PCR amplification were analyzed on 0.8% agarose gels. The numbers on the right indicate the positions of DNA size markers in kilobases.
The NMD pathway is active in zebrafish cells.
To investigate the mechanisms of PTC definition in zebrafish and to gain further insights into the evolution of NMD mechanisms in eukaryotes, we designed a reporter gene in which the genomic coding region of the zebrafish β-tubulin-2c gene is cloned downstream of the GFP ORF and upstream of a polyadenylation site derived from simian virus 40 (Fig. 6A, GFP-β-tubulin-WT). This reporter produces a pre-mRNA with three introns (Fig. 6A, IN-1, IN-2, and IN-3). The introns lie between codons 19 and 20, 55 and 56, and 92 and 93 of the β-tubulin-2c ORF, respectively (Fig. 6A).
We introduced PTCs by site-directed mutagenesis at codons 33 and 62 in exons 2 and 3, respectively (Fig. 6A, PTC-33 and PTC-62). These PTCs are located more than 55 nt upstream of an exon-exon boundary. These reporter constructs were cotransfected into the zebrafish embryonic fibroblast cell line ZF4 (ZF4 cells) along with plasmid pEGFP-C1, which served as a transfection control. We analyzed the steady-state levels of the corresponding transcripts by Northern blotting, using a probe complementary to GFP mRNA (thus detecting both the reporters and the transfection control).
Wild-type GFP-β-tubulin mRNA accumulated at much higher levels than did the PTC-containing reporters, suggesting that the NMD pathway is active in these cells (Fig. 6B, lane 1 versus lanes 3 and 5). After normalization to the GFP transfection control, the levels of PTC-containing transcripts were reduced to about 10% of the wild-type levels (Fig. 6C).
To confirm that our reporters were in fact targeted by the NMD pathway, we examined them in the presence of cycloheximide, because it was previously shown that mRNAs degraded through the NMD pathway can be stabilized by inhibiting translation (9). Indeed, when cells expressing the GFP-β-tubulin reporters were treated with cycloheximide for 45 min, the steady-state levels of PTC-33 and PTC-62 mRNAs increased 12.8- and 5.6-fold, respectively (Fig. 6B, lanes 3 to 6), indicating that their decay requires ongoing translation as expected for bona fide targets of the NMD pathway.
NMD is strongly dependent on exon-exon boundaries in zebrafish.
To investigate whether exon-exon boundaries play a role in defining PTCs in zebrafish, we deleted intron 3 (IN-3) in the PTC-62 reporter, thereby removing the only intron located downstream of the PTC (PTC-62ΔIN-3, Fig. 6A). This PTC-containing reporter was now expressed at levels comparable to those of the wild-type reporter (Fig. 6D, lane 3 versus lane 1). Thus, the ability of PTC-62 to trigger NMD is strongly dependent on the presence of a downstream exon-exon boundary. Additionally, PTC-62ΔIN-3 mRNA was not stabilized by cycloheximide treatment (Fig. 6D, lane 3 versus lane 4).
To validate these results further and to test whether PTC definition in zebrafish follows the −50- to 55-nt rule (i.e., PTCs trigger NMD when located at least 50 to 55 nt upstream of an exon-exon boundary [39]), we inserted a PTC at codon 90 (PTC-90) in exon 3, 6 nt upstream of the final exon-exon boundary. We also inserted a PTC into the last exon (PTC-130), so that no introns lie downstream of this PTC. We observed that the transcripts carrying PTC-90 and PTC-130 were both expressed at levels comparable to those of the wild-type transcript (Fig. 6D, lanes 1, 5, and 7). Furthermore, the expression levels of PTC-90 and PTC-130 were not affected by cycloheximide treatment, indicating that these PTCs do not trigger efficient NMD in zebrafish cells (Fig. 6D, lanes 5 to 8). Thus, as in mammals, in zebrafish exon-exon boundaries strongly enhance NMD when they lie at least 50 to 55 nt downstream from a PTC.
A catalytically active SMG6-PIN domain is required for NMD in zebrafish cells.
Recent studies have shown that SMG6 functions as an endonuclease in the NMD pathway and contributes to the degradation of NMD targets in both D. melanogaster and human cells (14, 24). The catalytic activity of SMG6 resides on its C-terminal PIN domain, which is also present in other proteins with nuclease activity (19). PIN domains are characterized by a triad of acidic residues, which are crucial for catalysis (19). The conservation of these catalytic residues in zebrafish SMG6 prompted us to investigate the role of this protein in NMD.
Using the reporters described above, we observed that an SMG6 protein carrying alanine substitutions of two of the three catalytic aspartates inhibited NMD in a dominant-negative manner, leading to an upregulation of the PTC-containing reporters within the four- to eightfold range (Fig. 6E and F). Wild-type SMG6 slightly increased PTC-33 mRNA levels and had no effect on PTC-62 mRNA (Fig. 6E and F). Furthermore, SMG6 wild-type or mutant expression did not affect the levels of wild-type or PTC-130 mRNAs, indicating that the effects are specific for the reporters containing a nonsense codon (Fig. 6E and F). These results provide additional evidence that PTC-33 and PTC-62 reporters are regulated by NMD, and suggest that degradation of PTC-containing mRNAs in zebrafish also requires an active SMG6-PIN domain.
DISCUSSION
NMD is an mRNA quality control mechanism that degrades mRNAs that carry nonsense mutations and regulates the expression of about 10% of the transcriptome. In this study, we show that all known core NMD factors (Upf1 to Upf3, Smg1, and Smg5 to Smg7) are encoded in the zebrafish genome. During early embryogenesis, these factors are maternally provided and ubiquitous. Depleting Upf1, Upf2, Smg5, Smg6, and Smg7 causes embryonic lethality, indicating that these proteins are required for zebrafish embryonic development. Finally we show that, as in mammals, NMD is coupled to splicing in zebrafish.
Evolution of the NMD pathway.
By comparison of the NMD pathways in several organisms, it becomes clear that NMD is predominantly splicing independent in invertebrates and S. cerevisiae but is coupled to splicing in mammals. Although studies with mammals have provided a few examples in which PTCs are recognized without exon-exon boundaries lying downstream, in these cases the steady-state levels of the mRNAs were only moderately affected (15, 49). Notably, the splicing-dependent and splicing-independent NMD mechanisms coexist in plants, suggesting that both of these mechanisms were probably already present in the common ancestor of extant eukaryotes (22, 27, 28, 48, 52).
To gain further insight into the evolution of the NMD pathway, we have investigated NMD in a basal vertebrate. We show that, in zebrafish, NMD is strongly stimulated when exon-exon boundaries lie downstream of a PTC. These results do not rule out the possibility that the intron-independent NMD pathway may also be active in zebrafish but show that the NMD pathway in zebrafish is similar to that in mammals and plants and distinct from the NMD pathway in C. elegans, D. melanogaster, and S. cerevisiae.
In the splicing-dependent mode, efficient NMD requires additional proteins; these are the components of the exon junction complex (EJC), which marks the position of exon-exon boundaries in the cytoplasm (32). Indeed, depletion of the EJC proteins Y14, MAGOH, eIF4AIII, Barentsz, and RNPS1 inhibits NMD in human cells (12, 33). Similarly, Y14 and MAGO are required for splicing-dependent NMD in A. thaliana (27). In contrast, although these proteins are conserved in C. elegans and D. melanogaster, they are not required for NMD (17, 36).
Taken together, these observations suggest that, over the course of evolution in invertebrates, EJC components lost the ability to enhance NMD and may no longer interact productively with NMD effectors. Consequently, in organisms in which NMD and splicing have become uncoupled, EJC components may have acquired additional functions. Conversely, in organisms in which EJC components function in NMD, it appears that the NMD pathway has diversified. Indeed, Upf2- and Upf3-independent NMD pathways have been described in human cells (10, 18), suggesting that EJC components and/or associated proteins can, in some cases, functionally substitute for Upf2 or Upf3. Thus, it will be interesting to determine whether the mild phenotypes displayed by Upf3a- and Upf3b-deficient embryos reflect the existence of Upf3-independent NMD in zebrafish. In the future, comparing how EJC components interact with NMD effectors in animals and how they interact with those in plants should illuminate the molecular mechanisms and the evolution of NMD.
Physiological relevance of the NMD pathway.
Depletion of effectors of the NMD pathway leads to different phenotypes across species. In S. cerevisiae NMD effectors are not essential (3). Similarly in C. elegans, inhibiting the NMD pathway does not affect viability, but the worms do display defects in the male bursa and the hermaphrodite vulva (21). In contrast, D. melanogaster requires upf1 for larval development (38) and requires UPF1 and UPF2 proteins at the cellular level, since D. melanogaster Schneider cells depleted of these proteins are arrested at the G2/M phase of the cell cycle (43). In A. thaliana Upf1 and Smg7 are essential genes, and mutant alleles of Upf3 have pleiotropic effects (5, 45, 54). In mice, upf1 and upf2 null animals die early in embryonic development (37, 51). Consistently, in cultured human cells, depletion of UPF1 inhibits cell cycle progression (6).
Phenotypes associated with the depletion of Smg1 and Smg5 to Smg7 have not yet been described in vertebrates. Without information on the importance of these other NMD effectors in the context of a vertebrate organism, an important question arises: is the embryonic lethality in upf1 or upf2 knockout mice caused by the inhibition of the NMD pathway or the inhibition of an entirely separate function that these proteins acquired during evolution? In this study we show that Upf1, Upf2, Smg5, Smg6, and Smg7 are essential for embryonic development of a basal vertebrate. Furthermore, we show that depleting Smg5 and Smg6 has phenotypical consequences similar to those of Upf1 or Upf2 depletion, suggesting that these phenotypes result from inhibition of the NMD pathway. In contrast, the phenotypes caused by Smg7 depletion are different: the brain shows primarily patterning defects, the midbrain-hindbrain boundary is altered in shape, and the hindbrain is longer than that in wild-type siblings. The chevron-like structure of the wild-type somites is kept in the intermediate and weak phenotypes, but somites appear stacked in the strong phenotypes, as in Upf1 morphants. This suggests that Smg7 may have acquired additional functions. Accordingly, in addition to its role in NMD, A. thaliana Smg7 is essential for the progression from anaphase to telophase in the second meiotic division in developing floral buds (45).
The observation that NMD is essential in some organisms but not others may be related to the lack of conservation of the physiological targets. Indeed, NMD targets represent a heterogenous class of mRNAs. NMD targets include transcripts that acquired a PTC through inefficient, inaccurate, or alternative splicing, as well as transcripts derived from pseudogenes and transposable elements (25, 44). In addition, in vertebrates, mRNAs transcribed from unproductively rearranged immunoglobulin and T-cell receptor genes also represent an important physiological class of NMD substrates (34, 51), because about two-thirds of such recombination events produce frameshifted genes whose transcripts are degraded by NMD.
In addition to these PTC-containing mRNAs, NMD also regulates a large set of “PTC-free” transcripts (25, 44). These include, for instance, mRNAs undergoing leaky translation initiation, mRNAs harboring an upstream ORF, mRNAs encoding selenoproteins, and mRNAs subject to programmed frameshift. In organisms in which NMD is splicing dependent, transcripts harboring introns at least 50 nt downstream of the natural stop codon are also regulated by NMD as, in this case, the natural stop is redefined as a PTC. The structural features that trigger NMD (e.g., upstream ORFs, leaky scanning, and introns in 3′ untranslated regions) are often not retained in orthologous transcripts, which might explain why NMD targets are not conserved among different organisms.
It is also possible that, for some organisms, the essential role of NMD effectors reflects a functional diversification. This appears to have happened at least in mammals. Indeed, mammalian UPF1, SMG1, and SMG5 to SMG7 are implicated in cellular processes distinct from NMD (25, 44). These include roles in telomere maintenance, DNA repair, stress response, and degradation of specific classes of mRNAs through a mechanism unrelated to NMD (25, 44). It is currently unknown whether these additional roles of NMD effectors are conserved in zebrafish and account in part for the pleiotropic effects of their depletions. However, depleting Upf1, Upf2, Smg5, and Smg6 in zebrafish does induce similar phenotypes, arguing that the same pathway is affected in each and also that these proteins regulate a common set of endogenous targets. Identifying these targets will help to determine to what extent the phenotypes displayed by embryos depleted of Upf1, Upf2, Smg5, or Smg6 are due to the misregulation of specific transcripts, to pleiotropic effects caused by the inhibition of NMD, or to any potential additional but unknown function of these proteins.
Supplementary Material
Acknowledgments
We thank C. Nüsslein-Volhard and members of her laboratory for invaluable support.
This study was supported by the Max Planck Society and by a grant from the Deutsche Forschungsgemeinschaft (DFG, FOR855). N.W. is the recipient of a fellowship from the Christiane Nüsslein-Volhard-Foundation.
Footnotes
Published ahead of print on 4 May 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amrani, N., R. Ganesan, S. Kervestin, D. A. Mangus, S. Ghosh, and A. Jacobson. 2004. A faux 3′-UTR promotes aberrant termination and triggers nonsense-mediated mRNA decay. Nature 432112-118. [DOI] [PubMed] [Google Scholar]
- 3.Amrani, N., M. S. Sachs, and A. Jacobson. 2006. Early nonsense: mRNA decay solves a translational problem. Nat. Rev. Mol. Cell Biol. 7415-425. [DOI] [PubMed] [Google Scholar]
- 4.Anders, K. R., A. Grimson, and P. Anderson. 2003. SMG-5, required for C. elegans nonsense-mediated mRNA decay, associates with SMG-2 and protein phosphatase 2A. EMBO J. 22641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arciga-Reyes, L., L. Wootton, M. Kieffer, and B. Davies. 2006. UPF1 is required for nonsense-mediated mRNA decay (NMD) and RNAi in Arabidopsis. Plant J. 47480-489. [DOI] [PubMed] [Google Scholar]
- 6.Azzalin, C. M., and J. Lingner. 2006. The human RNA surveillance factor UPF1 is required for S phase progression and genome stability. Curr. Biol. 16433-439. [DOI] [PubMed] [Google Scholar]
- 7.Behm-Ansmant, I., D. Gatfield, J. Rehwinkel, V. Hilgers, and E. Izaurralde. 2007. A conserved role for cytoplasmic poly(A)-binding protein 1 (PABPC1) in nonsense-mediated mRNA decay. EMBO J. 261591-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behm-Ansmant, I., and E. Izaurralde. 2006. Quality control of gene expression, a stepwise assembly pathway for the surveillance complex that triggers nonsense-mediated mRNA decay. Genes Dev. 20391-398. [DOI] [PubMed] [Google Scholar]
- 9.Carter, M. S., J. Doskow, P. Morris, S. Li, R. P. Nhim, S. Sandstedt, and M. F. Wilkinson. 1995. A regulatory mechanism that detects premature nonsense codons in T-cell receptor transcripts in vivo is reversed by protein synthesis inhibitors in vitro. J. Biol. Chem. 27028995-29003. [DOI] [PubMed] [Google Scholar]
- 10.Chan, W. K., L. Huang, J. P. Gudikote, Y. F. Chang, J. S. Imam, J. A. MacLean, and M. F. Wilkinson. 2007. An alternative branch of the nonsense-mediated decay pathway. EMBO J. 261820-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu, S. Y., G. Serin, O. Ohara, and L. E. Maquat. 2003. Characterization of human Smg5/7a: a protein with similarities to Caenorhabditis elegans SMG5 and SMG7 that functions in the dephosphorylation of Upf1. RNA 177-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conti, E., and E. Izaurralde. 2005. Nonsense-mediated mRNA decay: molecular insights and mechanistic variations across species. Curr. Opin. Cell Biol. 17316-325. [DOI] [PubMed] [Google Scholar]
- 13.Denning, G., L. Jamieson, L. E. Maquat, E. A. Thompson, and A. P. Fields. 2001. Cloning of a novel phosphatidylinositol kinase-related kinase: characterization of the human SMG-1 RNA surveillance protein. J. Biol. Chem. 27622709-22714. [DOI] [PubMed] [Google Scholar]
- 14.Eberle, A. B., S. Lykke-Andersen, O. Mühlemann, and T. H. Jensen. 2009. SMG6 promotes endonucleolytic cleavage of nonsense mRNA in human cells. Nat. Struct. Mol. Biol. 1649-55. [DOI] [PubMed] [Google Scholar]
- 15.Eberle, A. B., L. Stalder, H. Mathys, R. Z. Orozco, and O. Mühlemann. 2008. Posttranscriptional gene regulation by spatial rearrangement of the 3′ untranslated region. PLoS Biol. 6e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuhara, N., J. Ebert, L. Unterholzner, D. Lindner, E. Izaurralde, and E. Conti. 2005. SMG7 is a 14-3-3-like adaptor in the nonsense-mediated mRNA decay pathway. Mol. Cell 18537-547. [DOI] [PubMed] [Google Scholar]
- 17.Gatfield, D., L. Unterholzner, F. D. Ciccarelli, P. Bork, and E. Izaurralde. 2003. Nonsense-mediated mRNA decay in Drosophila: at the intersection of the yeast and mammalian pathways. EMBO J. 223960-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gehring, N. H., J. B. Kunz, G. Neu-Yilik, S. Breit, M. H. Viegas, M. W. Hentze, and A. E. Kulozik. 2005. Exon-junction complex components specify distinct routes of nonsense-mediated mRNA decay with differential cofactor requirements. Mol. Cell 2065-75. [DOI] [PubMed] [Google Scholar]
- 19.Glavan, F., I. Behm-Ansmant, E. Izaurralde, and E. Conti. 2006. Structures of the PIN domains of SMG6 and SMG5 reveal a nuclease within the mRNA surveillance complex. EMBO J. 255117-5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimson, A., S. O'Connor, C. L. Newman, and P. Anderson. 2004. SMG-1 is a phosphatidylinositol kinase-related protein kinase required for nonsense-mediated mRNA decay in Caenorhabditis elegans. Mol. Cell. Biol. 177483-7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodgkin, J., A. Papp, R. Pulak, V. Ambros, and P. Anderson. 1989. A new kind of informational suppression in the nematode Caenorhabditis elegans. Genetics 123301-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hori, K., and Y. Watanabe. 2007. Context analysis of termination codons in mRNA that are recognized by plant NMD. Plant Cell Physiol. 481072-1078. [DOI] [PubMed] [Google Scholar]
- 23.Hori, K., and Y. Watanabe. 2005. UPF3 suppresses aberrant spliced mRNA in Arabidopsis. Plant J. 43530-540. [DOI] [PubMed] [Google Scholar]
- 24.Huntzinger, E., I. Kashima, M. Fauser, J. Saulière, and E. Izaurralde. 2008. SMG6 is the catalytic endonuclease that cleaves mRNAs containing nonsense codons in metazoa. RNA 142609-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isken, O., and L. E. Maquat. 5 August 2008. The multiple lives of NMD factors: balancing roles in gene and genome regulation. Nat. Rev. Genet. 9699-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashima, I., A. Yamashita, N. Izumi, N. Kataoka, R. Morishita, S. Hoshino, M. Ohno, G. Dreyfuss, and S. Ohno. 2006. Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes Dev. 20355-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerényi, Z., Z. Mérai, L. Hiripi, A. Benkovics, P Gyula, C. Lacomme, E. Barta, F. Nagy, and D. Silhavy. 2008. Inter-kingdom conservation of mechanism of nonsense-mediated mRNA decay. EMBO J. 271585-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kertesz, S., Z. Kerenyi, Z. Merai, I. Bartos, T. Palfy, E. Barta, and D. Silhavy. 2006. Both introns and long 3′-UTRs operate as cis-acting elements to trigger nonsense-mediated decay in plants. Nucleic Acids Res. 346147-6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimmel, C. B., W. W. Ballard, S. R. Kimmel, R. Ullmann, and T. F. Schilling. 1995. Stages of embryonic development of the zebrafish. Dev. Dyn. 203253-310. [DOI] [PubMed] [Google Scholar]
- 30.Kunz, J. B., G. Neu-Yilik, M. W. Hentze, A. E. Kulozik, and N. H. Gehring. 2006. Functions of hUpf3a and hUpf3b in nonsense-mediated mRNA decay and translation. RNA 121015-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamason, R. L., M. A. Mohideen, J. R. Mest, A. C. Wong, H. L. Norton, M. C. Aros, M. J. Jurynec, X. Mao, V. R. Humphreville, J. E. Humbert, et al. 2005. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 3101782-1786. [DOI] [PubMed] [Google Scholar]
- 32.Le Hir, H., E. Izaurralde, L. E. Maquat, and M. J. Moore. 2000. The spliceosome deposits multiple proteins 20-24 nucleotides upstream of mRNA exon-exon junctions. EMBO J. 196860-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lejeune, F., and L. E. Maquat. 2005. Mechanistic links between nonsense-mediated mRNA decay and pre-mRNA splicing in mammalian cells. Curr. Opin. Cell Biol. 17309-315. [DOI] [PubMed] [Google Scholar]
- 34.Li, S., and M. F. Wilkinson. 1998. Nonsense surveillance in lymphocytes? Immunity 8135-141. [DOI] [PubMed] [Google Scholar]
- 35.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 36.Longman, D., R. H. Plasterk, I. L. Johnstone, and J. F. Caceres. 2007. Mechanistic insights and identification of two novel factors in the C. elegans NMD pathway. Genes Dev. 211075-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medghalchi, S. M., P. A. Frischmeyer, J. T. Mendell, A. G. Kelly, A. M. Lawler, and H. C. Dietz. 2001. Rent1, a trans-effector of nonsense-mediated mRNA decay, is essential for mammalian embryonic viability. Hum. Mol. Genet. 1099-105. [DOI] [PubMed] [Google Scholar]
- 38.Metzstein, M. M., and M. A. Krasnow. 2006. Functions of the nonsense-mediated mRNA decay pathway in Drosophila development. PLoS Genet. 2e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagy, E., and L. E. Maquat. 1998. A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends Biochem. Sci. 23198-199. [DOI] [PubMed] [Google Scholar]
- 40.Ohnishi, T., A. Yamashita, I. Kashima, T. Schell, K. R. Anders, A. Grimson, T. Hachiya, M. W. Hentze, P. Anderson, and S. Ohno. 2003. Phosphorylation of hUPF1 induces formation of mRNA surveillance complexes containing hSMG-5 and hSMG-7. Mol. Cell 121187-1200. [DOI] [PubMed] [Google Scholar]
- 41.Page, M. F., B. Carr, K. R. Anders, A. Grimson, and P. Anderson. 1999. SMG-2 is a phosphorylated protein required for mRNA surveillance in Caenorhabditis elegans and related to Upf1p of yeast. Mol. Cell. Biol. 195943-5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pal, M., Y. Ishigaki, E. Nagy, and L. E. Maquat. 2001. Evidence that phosphorylation of human Upf1 protein varies with intracellular location and is mediated by a wortmannin-sensitive and rapamycin-sensitive PI 3-kinase-related kinase signaling pathway. RNA 75-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rehwinkel, J., I. Letunic, J. Raes, P. Bork, and E. Izaurralde. 2005. Nonsense-mediated mRNA decay factors act in concert to regulate common mRNA targets. RNA 111530-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rehwinkel, J., J. Raes, and E. Izaurralde. 2006. Nonsense-mediated mRNA decay: target genes and functional diversification of effectors. Trends Biochem. Sci. 31639-646. [DOI] [PubMed] [Google Scholar]
- 45.Riehs, N., S. Akimcheva, J. Puizina, P. Bulankova, R. A. Idol, J. Siroky, A. Schleiffer, D. Schweizer, D. E. Shippen, and K. Riha. 2008. Arabidopsis SMG7 protein is required for exit from meiosis. J. Cell Sci. 1212208-2216. [DOI] [PubMed] [Google Scholar]
- 46.Robu, M. E., J. D. Larson, A. Nasevicius, S. Beiraghi, C. Brenner, S. A. Farber, and S. C. Ekker. 2007. p53 activation by knockdown technologies. PLoS Genet. 3c78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulte-Merker, S., R. K. Ho, B. G. Herrmann, and C. Nüsslein-Volhard. 1992. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development 1161021-1032. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz, A. M., T. V. Komarova, M. V. Skulachev, A. S. Zvereva, I. L. Dorokhov, and J. G. Atabekov. 2006. Stability of plant mRNAs depends on the length of the 3′-untranslated region. Biochemistry (Moscow) 711377-1384. [DOI] [PubMed] [Google Scholar]
- 49.Singh, G., I. Rebbapragada, and J. Lykke-Andersen. 2008. A competition between stimulators and antagonists of Upf complex recruitment governs human nonsense-mediated mRNA decay. PLoS Biol. 6e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unterholzner, L., and E. Izaurralde. 2004. SMG7 acts as a molecular link between mRNA surveillance and mRNA decay. Mol. Cell 16587-596. [DOI] [PubMed] [Google Scholar]
- 51.Weischenfeldt, J., I. Damgaard, D. Bryder, K. Theilgaard-Mönch, L. A. Thoren, F. C. Nielsen, S. E. Jacobsen, C. Nerlov, and B. T. Porse. 2008. NMD is essential for hematopoietic stem and progenitor cells and for eliminating by-products of programmed DNA rearrangements. Genes Dev. 221381-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu, J., J. H. Kang, C. Hettenhausen, and I. T. Baldwin. 2007. Nonsense-mediated mRNA decay (NMD) silences the accumulation of aberrant trypsin proteinase inhibitor mRNA in Nicotiana attenuata. Plant J. 51693-706. [DOI] [PubMed] [Google Scholar]
- 53.Yamashita, A., T. Ohnishi, I. Kashima, Y. Taya, and S. Ohno. 2001. Human SMG-1, a novel phosphatidylinositol 3-kinase-related protein kinase, associates with components of the mRNA surveillance complex and is involved in the regulation of nonsense-mediated mRNA decay. Genes Dev. 152215-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoine, M., T. Nishii, and K. Nakamura. 2006. Arabidopsis UPF1 RNA helicase for nonsense-mediated mRNA decay is involved in seed size control and is essential for growth. Plant Cell Physiol. 47572-580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.