Abstract
Signaling via the Pyk2-Src-Cbl complex downstream of integrins contributes to the assembly, organization, and dynamics of podosomes, which are the transient adhesion complexes of highly motile cells such as osteoclasts and dendritic cells. We previously demonstrated that the GTPase dynamin is associated with podosomes, regulates actin flux in podosomes, and promotes bone resorption by osteoclasts. We report here that dynamin associates with Pyk2, independent of dynamin's GTPase activity, and reduces Pyk2 Y402 phosphorylation in a GTPase-dependent manner, leading to decreased Src binding to Pyk2. Overexpressing dynamin decreased the macrophage colony-stimulating factor- and adhesion-induced phosphorylation of Pyk2 in osteoclastlike cells, suggesting that dynamin is likely to regulate Src-Pyk2 binding downstream of integrins and growth factor receptors with important cellular consequences. Furthermore, catalytically active Src promotes dynamin-Pyk2 association, and mutating specific Src-phosphorylated tyrosine residues in dynamin blunts the dynamin-induced decrease in Pyk2 phosphorylation. Thus, since Src binds to Pyk2 through its interaction with phospho-Y402, our results suggest that Src activates a negative-feedback loop downstream of integrin engagement and other stimuli by promoting both the binding of dynamin to Pyk2-containing complexes and the dynamin-dependent decrease in Pyk2 Y402 phosphorylation, ultimately leading to the dissociation of Src from Pyk2.
Podosomes are specialized transient actin-containing adhesion structures (11, 14, 37, 60) that are found in highly motile cells, such as osteoclasts, macrophages, dendritic cells, transformed metastatic cells, and v-src-transformed cells (37, 43), where they are thought to play important roles in cellular migration and invasion (34). In resorbing osteoclasts on bone, podosomes are concentrated within the sealing zone, a beltlike actin-rich structure that is important for adhesion and which delineates the resorptive region of the cell known as the ruffled border. Unlike focal adhesions, which are relatively stable structures (11, 60), the assembly and disassembly of podosomes occurs within minutes (t1/2 = 2 to 4 min) and involves the recruitment and activation of integrins, signaling proteins and scaffolding proteins (11, 14, 35, 47, 60). However, the mechanisms of action of key signaling proteins involved in podosome assembly and disassembly are only partially understood.
The focal adhesion kinase Pyk2 has been linked to the proliferation, migration, and activity of a variety of mesenchymal, epithelial, and hematopoietic cell types. Several groups, including our own, have reported the importance of Pyk2 in podosome belt organization, cell spreading, and bone-resorbing activity in osteoclasts (18, 26, 31, 40, 65, 66). Pyk2 is recruited to activated β2 and β3 integrins (9, 20) at adhesion sites and is autophosphorylated at Y402 (17, 47, 50) via an intermolecular trans-acting mechanism (46). Although Pyk2 is partially activated by integrin-induced Ca2+ signaling (20, 50), the induction of Pyk2's full catalytic activity requires the binding of Src via its SH2 domain to autophosphorylated Pyk2 Y402 and the subsequent phosphorylation of Pyk2 at functionally distinct sites, including Y579, Y580, and Y881 (17, 31, 46). The binding of Src to phosphorylated Pyk2, which leads to the formation of a multiprotein signaling complex at adhesion sites (17, 40, 50), is critical for Pyk2 activity, as demonstrated by the fact that Pyk2 phosphorylation and activity are significantly reduced in osteoclasts derived from Src−/− mice (17, 40). Src−/− osteoclasts also exhibit decreased motility (50) and decreased bone-resorbing activity (40, 54, 59), and we recently demonstrated that Src promotes both podosome formation and disassembly, as well as actin flux into existing podosomes and the organization of podosomes into a peripheral belt in osteoclasts (15).
We have also demonstrated that the GTP-hydrolyzing protein dynamin-2, which is ubiquitously expressed and well known for its role in endocytosis (53), regulates actin remodeling in the podosomes of osteoclasts and Rous sarcoma virus-transformed baby hamster kidney cells (43). In addition, a dynamin-2 mutant that binds GTP with reduced affinity (dynK44A) (12) decreased the flux of actin into podosomes (43) and disrupted podosome belt formation in osteoclasts, thereby affecting osteoclast migration and bone-resorbing activity (8). The dynamin proteins, of which there are three homologous isoforms (3), contain several protein domains: a GTP-hydrolyzing domain (GTPase), a plextrin homology domain that mediates binding to phosphoinositides, a GTPase effector domain (GED), and a C-terminal proline-rich domain (PRD) (38, 45, 55) through which dynamin binds a number of functionally diverse SH3-containing molecules, such as Src, cortactin, Grb2, and N-Wasp (1, 7, 27, 39, 58). We previously reported that dynamin-2 partially colocalizes and associates with the E3-ubiquitin ligase Cbl within the podosome belt/sealing zone of osteoclasts, as well as in SYF cells, which lack the Src family kinases Src, Yes, and Fyn, and in HEK 293 cells that stably express the vitronectin receptor (293VnR) (8). Protein complexes containing dynamin-2 and Cbl, which are both substrates of Src (1, 2, 23, 50, 56), were disrupted in the presence of activated Src and stabilized in the absence of Src (8), demonstrating a key role of Src in regulating the formation of signaling complexes in osteoclasts downstream of integrins.
In the present study, we sought to determine whether dynamin, which regulates podosome actin dynamics and bone resorption in osteoclasts, also associates with Pyk2 and/or regulates Pyk2's activities in osteoclasts. We report here that dynamin associates with Pyk2 and promotes the dephosphorylation of Pyk2 Y402 and that catalytically active Src promotes both dynamin's association with Pyk2 and the dynamin-induced dephosphorylation of Pyk2 Y402, resulting, in turn, in the decreased binding of Src to Pyk2. Thus, we propose that dynamin regulates podosome dynamics and osteoclast bone-resorbing activity by promoting the disassembly of the Pyk2-Src-Cbl complex that is formed in osteoclasts downstream of β3 integrin activation.
MATERIALS AND METHODS
Materials.
Anti-dynamin-2 (dynamin II; epitope 274-555) and anti-Pyk2 antibodies used for Western blot analyses were obtained from Transduction Laboratories (Lexington, KY). Anti-human dynamin (hudy2), anti-avian Src and anti-phosphotyrosine (clone 4G10) antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). Anti-Pyk2-phospho-Y402 and anti-Src-phospho-Y416 antibodies were obtained from Biosource International. Polyclonal and monoclonal anti-green fluorescent protein (anti-GFP) antibodies (Living Colors) were from Clontech (Palo Alto, CA). Antihemagglutinin (anti-HA) was from Bethyl Laboratories (Montgomery, TX). Horseradish peroxidase-conjugated anti-rabbit and anti-mouse secondary antibodies were obtained from Fisher Scientific (Fairlawn, NJ). PP2, SU6656, and collagenase A were purchased from Calbiochem (San Diego, CA). Orthovanadate was purchased from Sigma-Aldrich (St. Louis, MO). Fluorescein-conjugated secondary antibodies were from Molecular Probes (Eugene, OR). Collagen was obtained from Nitta Gelatin Co. (Osaka, Japan).
Plasmids.
A construct of rat dynamin-2 (splice variant aa) (10) and the dynamin-2 mutant dynK44A inserted into the pEGFP-N1 vector were generated as previously described (3, 12). Dynamin-2 point mutations were generated by site-directed mutagenesis (Stratagene, La Jolla, CA) with the following primer combinations: dynY231F (5′-CTT GAG AAG AGG CTT CAT CGG CGT GGT TAAC and 5′-GTT AAC CAC GCC GAT GAA GCC TCT TCT CAAG) and dynY597F (5′-CAG AGG AAC GTC TTC AAG GAC CTG CGAC and 5′-GTC GCA GGT CCT TGA AGA CGT TCC TCTG). The c-Src construct was provided by P. Schwartzberg (National Institutes of Health, Bethesda, MD). pBK-SrcW118K and pBKSrcR175L constructs were made by site-directed mutagenesis as described elsewhere (15). Wild-type human Pyk2 cDNA was provided by J. Schlessinger (Yale University, New Haven, CT). Full-length Pyk2 and Pyk2 point mutants Y402F and K457A were generated as previously described (16, 33, 57). Grb2-myc was provided by I. Dikic (Goethe University Medical School, Frankfurt, Germany). DynΔPRD was kindly provided by P. De Camilli (Yale University, New Haven, CT). Src-SH2-glutathione S-transferase (GST) and Src-SH3-GST constructs were generated and prepared as previously described (51). All cDNA mutations were confirmed by sequencing.
Transient transfections.
Transient transfections were performed using FuGENE 6 (Roche, Indianapolis, IN) according to the manufacturer's specifications using a total of 6 μg of DNA and 12 μl of FuGENE per 100-mm2 tissue culture plate. In all experiments, the total amount of cDNA transfected was kept constant by the addition of empty pcDNA3.1 vector. Transient transfections were performed in 293VnR cells (HEK293 human embryonic kidney cells that stably overexpress the αvβ3 integrin/VnR, the major integrin found on osteoclasts), a cell line that we have validated as a suitable model system and used extensively to elucidate Pyk2-Src-Cbl intermolecular interactions (40, 50) and the interaction of dynamin with Cbl (8). Transiently transfected cells were maintained in α-minimal essential medium containing 10% fetal bovine serum (FBS) for 72 h after transfection.
Coimmunoprecipitation assays.
Coimmunoprecipitations were performed as previously described (8, 50). Briefly, cells were lysed in modified radioimmunoprecipitation assay buffer containing 50 mM Tris-Cl, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, and protease and phosphatase inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM NaF, 1 mM sodium orthovanadate, and 10 μg each of leupeptin, aprotinin, and pepstatin/ml). Lysates were sonicated and the supernatant clarified by centrifugation. Because dynamin-2 is endogenously expressed in 293VnR cells and in osteoclasts, transient transfections were performed with GFP-tagged dynamin-2. Immunoprecipitations were performed with a polyclonal antibody against GFP to distinguish between endogenous and recombinant dynamin. Pyk2 is not expressed in 293VnR cells, and recombinant Pyk2 was therefore immunoprecipitated with antibodies to Pyk2. Typically, 500 μg of cell lysate protein was incubated with 5 μg of primary antibody for 2 to 16 h at 4°C. Immunoprecipitations were performed with protein G-agarose beads for 1 h. After binding and washing, ca. 80% of the target antigen protein remained bound to the beads. The samples were heated to 100°C in sample buffer containing sodium dodecyl sulfate (SDS) and β-mercaptoethanol and resolved by SDS-polyacrylamide gel electrophoresis (PAGE). For most samples, 100% of the immune complex was loaded on the gels. However, in 293VnR experiments in which Pyk2 was overexpressed in the absence of dynamin, only 30% of the immunoprecipitated Pyk2 (Pyk2 control) was loaded onto gels due to the high phosphorylation state of Pyk2 and the potential for Western blot saturation. Total cell lysates (TCLs) (20 μg) were also resolved alongside immunoprecipitates and blotted as indicated. Western blot analysis was performed by using an enhanced chemiluminescence detection kit (Amersham) with multiple exposure times. The phospho-Y402 antibody was specific for Pyk2 as determined by Western blot analysis of immunoprecipitated Pyk2 or the Pyk2-Y402F mutant (see Fig. S1 in the supplemental material). After Western blotting, membranes were stripped with a solution containing 6.25 mM Tris, 2% SDS (pH 6.7), and 0.07% β-mercaptoethanol at 50°C for 40 min prior to reblotting. Films of Western blots were scanned by using an Epson Stylus Photo RX100 printer/scanner, and images were processed with Adobe Photoshop. All experiments were performed a minimum of three times, and representative blots or images are shown. A comparison of band intensity was performed by densitometric analysis using Scion Image analysis software or ImageJ (National Institutes of Health freeware).
Preparation of authentic osteoclasts and OCLs.
Authentic osteoclasts were isolated from the long bones of 2- to 4-day-old neonatal mice. Bones were dissected free of adherent tissues, placed in α-minimal essential medium containing 5% FBS, and minced into small pieces. After vigorous pipetting to release osteoclasts, the bone particles were allowed to sediment, and the remaining cell suspension containing osteoclasts was seeded onto serum-coated coverslips. For all biochemical analyses (both coimmunoprecipitations and Western blots), mouse osteoclastlike cells (OCLs) were used. OCLs were generated using the murine coculture system (62) by culturing neonatal primary calvarial osteoblasts with spleen and marrow cells in the presence of 1,25-dihyrodroxyvitamin D3 and prostaglandin E2. After 4 to 5 days in culture, the osteoblast layer was removed by gentle pipetting of medium over the surface of the cell layer, leaving purified OCLs behind. Suspensions of serum-starved (1% FBS, 1 h) OCLs were obtained by treating the purified OCLs with 10 mM EDTA for 5 min at 37°C. Cells were then flushed off the culture dishes, washed once in serum-free medium, and then resuspended in serum-free medium (63). For experiments involving macrophage colony-stimulating factor (M-CSF) stimulation, bone marrow cells were plated on collagen gel (41) and differentiated with M-CSF (20 ng/ml) and RANKL (100 ng/ml) for 7 days (61). For experiments examining the effects of activating integrin signaling, OCLs generated by the coculture system were replated on culture plates precoated with mouse vitronectin (25 μg/ml for 2 h at 37°C). For confocal studies after adenovirus infections, OCLs were generated by using the coculture method on collagen gel-coated dishes. Collagen was digested with 0.1% collagenase, and the cells were replated onto coverslips or dentin slices after collagenase treatment. Animal protocols were approved by the Yale University Institutional Animal Care and Use Committee.
Confocal microscopy.
Authentic osteoclasts and adenovirus-infected OCLs were seeded onto FBS-coated coverslips and incubated for 2 to 24 h at 37°C followed by fixing with 3.7% formaldehyde in phosphate-buffered saline for 10 min. Fixed cells were visualized by using a Zeiss 510 Meta laser scanning confocal microscope. Coverslips for actin labeling were extracted in ice-cold acetone for 3 to 5 min, incubated in a 1:40 dilution of rhodamine phalloidin stock solution (Molecular Probes) for 1 h, washed with phosphate-buffered saline, and mounted in FluorSave (Calbiochem). All other coverslips were permeabilized in 0.05% saponin for 30 min, blocked in 5% normal goat serum (Roche) for 30 min, and then incubated in appropriate primary antibodies. Coverslips were then washed again, incubated with fluorescent secondary antibody (Alexa Fluor 488, green; Alexa Fluor 568, red; and Alexa Fluor 647, blue), washed again, and mounted in FluorSave. Images were obtained at room temperature by using a Zeiss LSM 510 Meta laser scanning microscope with a ×63 water immersion lens and Zeiss LSM 510 software. Images were recorded, composites were compiled, and total image enhancements were performed using Adobe Photoshop 6.0 To quantify the effect of dynamin protein levels of Pyk2 phosphorylation, multiple preparations of cells were labeled and imaged by confocal microscopy using constant imaging parameters for time of exposure, light intensity, and magnification, and the relative intensities were then determined.
Generation of recombinant adenovirus.
Recombinant adenovirus expressing dynamin-2 (ba splice variant) or the GTP-binding mutant dynamin-2 (dynK44A) containing HA tags were made using the tetracycline-inhibited Cre-lox recombination system as described elsewhere (13) and were kindly provided by S. Schmid (Scripps Research Institute, La Jolla, CA). To express dynamin in OCLs, adenoviruses expressing the tetracycline transcription activator (tTA; 20 μl) and dynamin-HA (0 to 400 μl) were added in combination (3). Infection of mouse OCLs with adenoviruses was performed on immature OCLs after 3 days in culture. Cells were infected using a multiplicity of infection score of 100, which results in a two- to threefold overexpression of dynamin relative to endogenous dynamin. After infection, OCLs were either replated onto coverslips for 24 h and processed for confocal immunofluorescence microscopy or harvested directly for biochemical analyses.
Adenovirus vectors expressing dynamin-2 shRNA or scrambled shRNA (scb-shRNA) were generated by using a Block-iT U6 RNAi entry vector kit and the Block-iT adenoviral RNAi expression system (Invitrogen), according to the manufacturer's instructions. Dynamin-2 primers for shRNA generation were previously published (28). OCLs were infected with shRNA adenovirus (multiplicity of infection of 100 to 300) for 3 days prior to harvesting. Protein depletion was confirmed by Western blotting and confocal immunofluorescence.
RESULTS
Dynamin associates with Pyk2 in osteoclasts.
We previously demonstrated that dynamin partially colocalized with Cbl, Src, and several actin-binding proteins in the peripheral podosome belt of osteoclasts. We found that dynamin formed a protein complex with Src and Cbl in osteoclasts as well as in 293VnR cells, and that the interaction of dynamin with Cbl was negatively regulated by Src's kinase activity (8). Given these observations and our previous finding that the Pyk2-Src-Cbl complex plays an important role in osteoclast function (50), we sought to determine whether dynamin associates with Pyk2 and, if so, what are the functional consequences of their interaction.
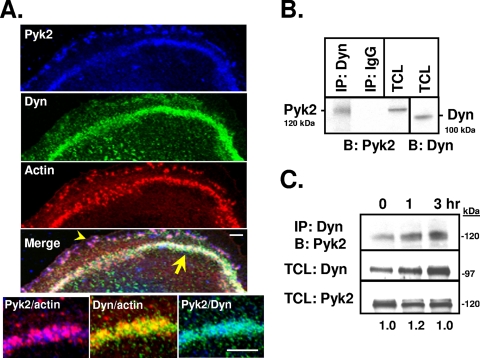
Consistent with previous reports (17, 26, 66), confocal immunofluorescence analysis of authentic osteoclasts showed the presence of Pyk2 together with actin and dynamin in the podosome belt (Fig. 1A, arrow), similar to what we reported for Cbl (8), and at the cell periphery (arrowhead). Coimmunoprecipitation and Western blot analysis of OCL lysates revealed that dynamin and Pyk2 formed a molecular complex in OCLs (Fig. 1B), a finding consistent with the overlapping expression of these proteins in the podosome belt. Since Pyk2 is activated in osteoclasts by the engagement of integrins (20, 50), we examined whether the association of Pyk2 and dynamin was altered by activating the αvβ3 integrin (VnR). There was little difference in the association of dynamin with Pyk2 in OCLs that were kept in suspension and then replated on vitronectin for up to 3 h (Fig. 1C), suggesting that the association of dynamin and Pyk2 was not dependent on the activation of the VnR.
FIG. 1.
Dynamin and Pyk2 colocalize within the actin podosome belt and form a molecular complex in osteoclasts. (A) Confocal immunofluorescence microscopy of authentic mouse osteoclasts plated on glass and labeled for Pyk2 (blue), dynamin (green), and actin (rhodamine-phalloidin; red). Images from the three fluorescence channels were merged using Adobe Photoshop 6.0. The yellow arrow indicates overlapping expression of dynamin, actin, and Pyk2 within the podosome belt, while the arrowhead shows peripherally localized Pyk2. Also shown are high-magnification images showing the colocalization of Pyk2 with actin, dynamin with actin, and Pyk2 with dynamin. Scale bars, 5 μm. (B) Dynamin was immunoprecipitated from mouse OCLs and resolved by SDS-PAGE. Western blotting was performed with an anti-Pyk2 antibody. As a control for nonspecific binding, immunoprecipitations were also performed using an isotype-matched immunoglobulin G (IgG). TCLs were blotted for dynamin or Pyk2. Reciprocal experiments in which the anti-Pyk2 antibody was used for immunoprecipitation and the dynamin antibody was used for blotting were also performed, with similar results (data not shown). (C) OCLs were removed from the plates as described in Materials and Methods and kept in suspension in medium supplemented with 1% FBS for 1 h. An equal number of cells was replated onto culture plates previously coated with vitronectin and allowed to reattach for 0, 1, or 3 h. A total of 500 μg of cell lysates were subjected to immunoprecipitation as indicated. Immune complexes were resolved by SDS-PAGE and blotted as indicated. Portions (10 μg) of TCLs were blotted for dynamin or Pyk2. Molecular mass markers are indicated. Protein bands were quantified by densitometry. Numbers are in arbitrary units and reflect the normalized ratios of band intensity in blot 1 (top, IP:Dyn/B:Pyk2) to blot 2 (middle, TCLs blotted for dynamin). Total Pyk2 levels were not significantly changed by replating (blot 3, bottom). Similar results were obtained when immunoprecipitations were performed with an anti-Pyk2 antibody, followed by blotting for endogenous dynamin (data not shown). Labels: IP, immunoprecipitation; B, Western blot; Dyn, dynamin.
Dynamin decreases Pyk2 Y402 phosphorylation.
The interaction of dynamin with some of its binding partners is regulated by GTP binding and activity (53), while the interaction of Pyk2 with its downstream effectors is modulated by Pyk2's catalytic activity and the phosphorylation of its tyrosine residues (4). We therefore examined whether dynamin's GTPase activity affected dynamin's association with Pyk2, using the dynK44A mutant (12), and also compared the binding of wild-type Pyk2, the autophosphorylation site mutant (Pyk2-Y402F) and kinase-inactive Pyk2 (Pyk2-K457A) to the two dynamin proteins (see Fig. S2 in the supplemental material). Similar amounts of wild-type dynamin and dynK44A coimmunoprecipitated with all three Pyk2 variants, suggesting that neither dynamin's GTPase activity nor Pyk2's catalytic activity and phosphorylation at Y402 are critical for the dynamin-Pyk2 association. Together, these findings are consistent with the observation that dynamin's association with Pyk2 in OCLs appeared to be independent of the engagement of the VnR (Fig. 1C), which leads to Y402 phosphorylation and Pyk2 activation (20, 50).
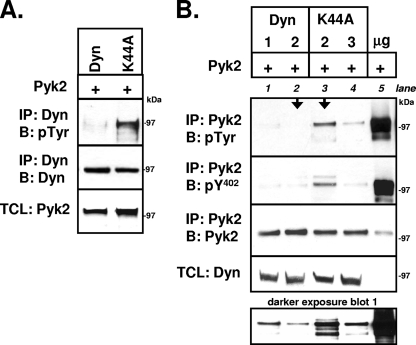
We next examined whether dynamin was phosphorylated by Pyk2. 293VnR cells were cotransfected with Pyk2 and either dynamin-GFP or dynK44A-GFP. The GFP-tagged dynamin proteins were immunoprecipitated, and the protein complexes were subjected to Western blot analysis to detect phosphotyrosine. The phosphorylation of both wild-type dynamin and dynK44A was detected in the presence but not in the absence of cotransfected Pyk2. Interestingly, the overall phosphorylation of wild-type dynamin was significantly less than that of dynK44A (Fig. 2A). We therefore examined whether the phosphorylation of Pyk2, which correlates with Pyk2 activity (46), was lower when dynamin was overexpressed (Fig. 2B). Both wild-type dynamin and dynK44A dose dependently reduced the total tyrosine phosphorylation of Pyk2 (blot 1) and the phosphorylation of the autophosphorylated Y402 (blot 2), with wild-type dynamin having a greater effect with equivalent amounts of transfected cDNA (arrows). The reduced effect of the GTPase-deficient dynK44A suggested that the dynamin-induced decrease in Pyk2 phosphorylation was at least partly dependent on dynamin's GTPase activity.
FIG. 2.
Dynamin reduces phosphorylation of Pyk2-Y402. (A) 293VnR cells were transiently cotransfected with Pyk2 and either dyn-GFP or dynK44A-GFP. Dynamin and dynK44A were immunoprecipitated with anti-GFP and resolved by SDS-PAGE, and Western blot analysis was performed with an antiphosphotyrosine (anti-pTyr) antibody. Immunoprecipitates were reblotted for dyn-GFP with anti-GFP, while TCLs were blotted for Pyk2. Representative blots of 10 independent experiments are shown. (B) Increasing amounts of dyn-GFP (1 and 2 μg) or dynK44A-GFP cDNA (2 and 3 μg) were transiently cotransfected with constant amounts of Pyk2 cDNA (3 μg) into 293VnR cells. Empty vector was used to keep total transfected cDNA constant. Immunoprecipitation of Pyk2 was performed, followed by SDS-PAGE. Western blot analyses of immunoprecipitates, followed by stripping and reblotting was performed consecutively with an anti-pTyr), an anti-Pyk2-phospho-Y402 (pY402), and an anti-Pyk2 antibody. Transiently expressed Pyk2 undergoes robust phosphorylation in the absence of dynamin proteins (lane 5) (30% of the immunoprecipitate was loaded). However, coexpression of wild-type dynamin (lanes 1 and 2) or mutant dynamin (lanes 3 and 4) caused a dose-dependent decrease in total phosphorylated Pyk2 (blot 1, top) and phosphorylated Pyk2-Y402 (blot 2, bottom). Dynamin reduced Pyk2 phosphorylation to a greater degree than dynK44A when similar amounts of cDNA were transfected (arrows). Representative blots from five independent experiments are shown. Molecular mass markers are indicated. Labels: IP, immunoprecipitation; B, Western blot; Dyn, dynamin.
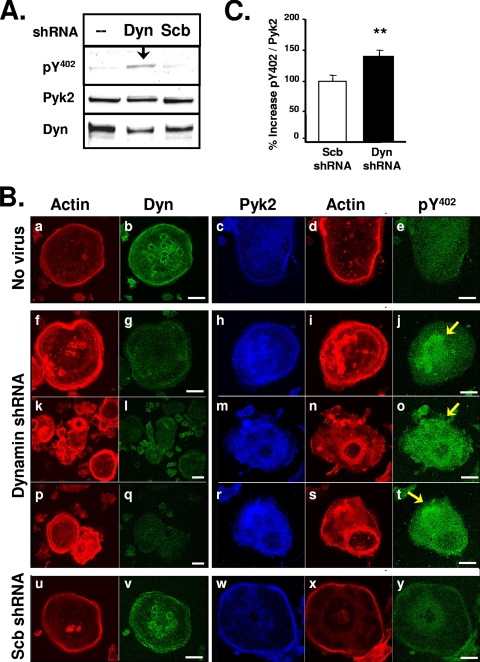
We next sought to determine whether endogenous dynamin affected the function of Pyk2 in osteoclasts. We first examined the effect of reducing endogenous dynamin levels in OCLs using adenovirus-expressed dynamin-2 shRNA (dyn-shRNA). Western blots showed that the dynamin-2 shRNA, but not the control scrambled shRNA (scb-shRNA), significantly reduced dynamin levels in OCLs (Fig. 3A, blot 3). Furthermore, Western blot analysis showed that the level of phospho-Y402 was increased in the dyn-shRNA-infected OCLs (Fig. 3A, blot 1). The decrease in dynamin protein expression by dyn-shRNA was also observed by confocal immunofluorescence microscopy (Fig. 3Bg, l, and q). In OCLs infected with the control scb-shRNA (Fig. 3Bu to y), the peripheral podosome belt appeared similar to uninfected OCLs (Fig. 3Ba to e), whereas the podosome belt in the dyn-shRNA-infected OCLs (Fig. 3Bf to t) was considerably wider than control cells, an effect similar to what was seen in OCLs that overexpress dynK44A (8). The actin belt matures by the disassembly of podosomes at the center of the cell and the assembly of podosomes at the cell periphery (14). Therefore, we hypothesize that podosome disassembly and the maturation of the actin belt is disrupted in the dyn-shRNA-infected cells.
FIG. 3.
Dyn-shRNA increases Pyk2 Y402 phosphorylation in OCLs. (A) OCLs were prepared as described in Materials and Methods and were left uninfected or were infected with dyn-shRNA or scrambled (scb)-shRNA adenoviruses. Cell lysates were resolved by SDS-PAGE and blotted with antibodies to phospho-Pyk2-Y402, Pyk2 or dynamin. (B) OCLs were uninfected (a to e) or infected with adenoviruses expressing shRNA to dynamin-2 (dyn-shRNA) (f to t) or a scrambled sequence (scb-shRNA) (u to y) for 3 days. Infected cells were replated on glass coverslips for 24 h, fixed, and immunolabeled for dynamin, Pyk2, phosphorylated Pyk2-Y402, or actin (rhodamine phalloidin, red) as indicated. Cells were visualized by scanning confocal immunofluorescence microscopy. Scale bars, 20 μm. Experiments were repeated at least three times and representative cell images are shown. The cells shown in panels f to j, k to o, and p to t are separate representative images of dyn-shRNA-infected cells. Arrows indicate increased levels of phospho-Y402 in dyn-shRNA-infected cells. (C) The change in the phosphorylation of Pyk2 Y402 in dyn-shRNA-infected OCLs was determined by normalizing the fluorescence intensity of anti-phospho-Y402 staining to the fluorescence intensity of anti-Pyk2 staining in double-labeled dyn-shRNA-infected OCLs. The fluorescence intensity of a minimum of seven cells from three different experiments was measured (error bars represent the standard error of the mean [SEM]; *, P < 0.05 [Student t test]). The specificity of the phospho-Y402 antibody for Pyk2 that was used for labeling the OCLs was confirmed by Western blot analysis of Pyk2 and Pyk2-Y402F expressed in 293VnR cells (see Fig. S1 in the supplemental material). Dyn, dynamin.
In the untreated OCLs and scb-shRNA-infected OCLs, Pyk2 was enriched in the podosome belt and present at lower levels in the central part of the cells (Fig. 3Bc and w). In contrast, Pyk2 in the dyn-shRNA-infected OCLs (Fig. 3Bh, m, and r) partially relocalized away from the cell periphery, and the enrichment of Pyk2 in the belt was often not observed. The level of phosphorylated Pyk2 Y402 appeared to be higher in the dyn-shRNA-infected OCLs (Fig. 3Bj, o, and t) than in control (Fig. 3Be) or scb-shRNA-infected cells (Fig. 3By), particularly in the centrally located actin patches (arrows). Quantification of the phosphorylated Pyk2-Y402 and Pyk2 fluorescence intensities revealed that phospho-Y402, normalized to Pyk2, increased by 40% in the dyn-shRNA-infected OCLs (Fig. 3C), indicating that dynamin indeed downregulates Pyk2 phosphorylation in osteoclasts.
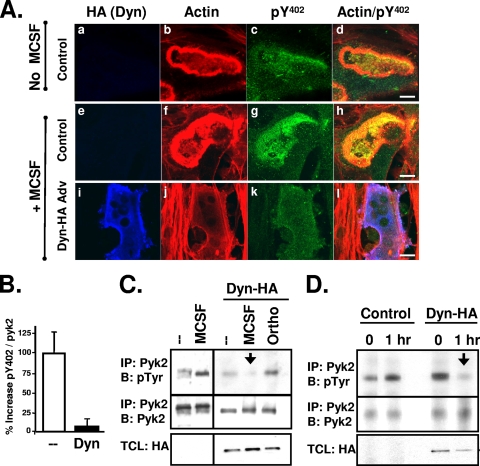
Pyk2 phosphorylation is increased in osteoclasts by the cytokine M-CSF or by the engagement of β2 and β3 integrins (19, 20, 31, 40, 50). We therefore examined the effect of overexpressing dynamin on the stimulus-induced increases in Pyk2 phosphorylation in OCLs. OCLs were infected with either a control adenovirus or an adenovirus encoding wild-type dynamin (dyn-HA) and then treated with M-CSF or vehicle. As shown in Fig. 4A, M-CSF treatment of the control-infected OCLs increased the width of the actin-rich podosome belt/sealing zone (compare Fig. 4Ab and f) and significantly increased the phosphorylation of Pyk2 Y402, particularly in the podosome belt/sealing zone (compare Fig. 4Ac and g). In contrast, Pyk2 Y402 phosphorylation remained low in M-CSF-treated dynamin-HA adenovirus-infected OCLs (Fig. 4Ak). We also observed a thinning of the podosome belt in the dynamin-overexpressing OCLs (Fig. 4Aj) as previously reported (8). Quantification of the fluorescence intensities of phospho-Y402 and Pyk2 in double-labeled OCLs confirmed that the amount of phosphorylated Y402, normalized to Pyk2, was significantly lower in the M-CSF-treated dynamin-HA-infected cells relative to M-CSF-treated control-infected cells (Fig. 4B). Immunoprecipitation studies confirmed that M-CSF treatment increased Pyk2 phosphorylation in control OCLs and provided additional evidence that overexpression of dynamin prevented the M-CSF-induced increase in Pyk2 phosphorylation (Fig. 4C). Furthermore, treating the dynamin-overexpressing OCLs with the broad-spectrum tyrosine phosphatase inhibitor orthovanadate prevented the dynamin-induced decrease in Pyk2 phosphorylation, indicating that an unidentified protein tyrosine phosphatase, possibly PTP-PEST (36), contributes to the decrease in Pyk2 phosphorylation that is induced by dynamin.
FIG. 4.
Dynamin decreases stimulus-induced Pyk2 phosphorylation. (A) OCLs were generated on collagen with M-CSF and RANKL (see Materials and Methods) and infected with control adenovirus (tTA) (a to h) or adenovirus expressing dyn-HA (i to l) for 3 days. Mature OCLs were lifted by digestion with collagenase and replated onto dentin slices in the presence or absence of 50 μg of M-CSF/ml. Cells were incubated for 1 h prior to fixing and labeling. Exogenous dynamin expression was detected with anti-HA (blue). Actin was labeled with rhodamine-phalloidin (red), and phosphorylated Pyk2-Y402 was labeled with an anti-phospho-Y402 antibody (green). Scale bars, 10 μm. (B) The decrease in Pyk2 Y402 phosphorylation in the presence of overexpressed dyn-HA was quantified as in Fig. 3. Experiments were repeated three times, and error bars represent the SEM. (C) OCLs infected with dyn-HA expressing adenovirus or a control virus (tTA) were serum starved for 30 min and then stimulated with M-CSF for 1 h prior to harvesting and lysis. Alternatively, OCLs were treated with 4 mM orthovanadate (Ortho) for 1 h prior to harvesting. Pyk2 was immunoprecipitated and analyzed for phosphotyrosine by Western blotting. The membranes were then stripped and reblotted for total Pyk2. TCLs were blotted with an anti-HA antibody to detect adenovirus-expressed dyn-HA. Similar results were obtained in duplicate experiments. (D) OCLs were infected with an adenovirus expressing dyn-HA or a control virus (tTA) for 3 days. After removal of osteoblasts, mature OCLs were detached with trypsin-EDTA, washed and kept in suspension in serum-free media for 60 min and were either replated on plates coated with mouse vitronectin and harvested immediately (0 h) or allowed to attach for 1 h. Immunoprecipitations were performed with an anti-Pyk2 antibody, followed by Western blot analysis with an anti-pTyr antibody. Blots were stripped and blotted for Pyk2. TCLs were blotted with an anti-HA antibody to detected adenovirus-expressed dyn-HA. Similar results were obtained in duplicate experiments. Labels: IP, immunoprecipitation; B, Western blot; Dyn, dynamin.
Finally, we examined the effect of dynamin on the attachment-induced increase in Pyk2 phosphorylation in OCLs. OCLs infected with either the dyn-HA adenovirus or the control adenovirus were kept in suspension and then replated for 1 h on vitronectin-coated plates to activate the VnR. The tyrosine phosphorylation of Pyk2 was then examined by Western blotting (Fig. 4D). As previously shown (50), Pyk2 phosphorylation was increased by replating control OCLs on vitronectin. However, in the OCLs that overexpressed dynamin, replating for 1 h failed to increase Pyk2 phosphorylation (Fig. 4D, arrow), suggesting that dynamin decreases integrin-induced Pyk2 phosphorylation.
Taken together, these results indicate that dynamin downregulates Pyk2 Y402 phosphorylation in osteoclasts, both under basal conditions and following physiologically important stimuli that activate Pyk2. Furthermore, these findings support the functional importance of the dynamin-Pyk2 interaction in the context of the regulation of osteoclast function.
The dynamin-induced decrease in Pyk2 Y402 phosphorylation reduces Pyk2-Src binding.
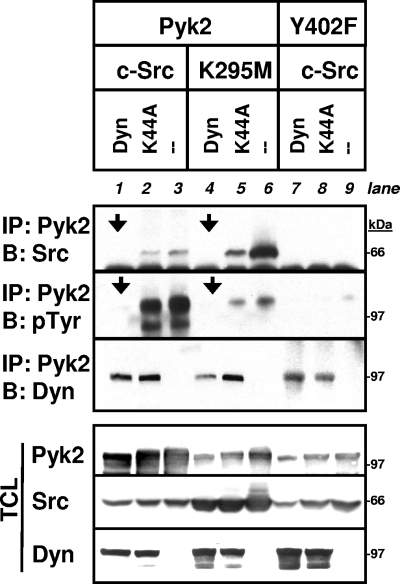
Src binds to phosphorylated Pyk2 Y402 and phosphorylates other functionally important Pyk2 tyrosine residues (16, 40, 46, 50, 52). Therefore, we predicted that the dynamin-induced decrease in Pyk2 Y402 phosphorylation would also reduce the binding of Src to Pyk2 and the Src-catalyzed phosphorylation of Pyk2. To test this, dynamin or dynK44A were coexpressed with Pyk2 and either wild-type Src or kinase-inactive SrcK295M in 293VnR cells, and Pyk2-Src association was analyzed (Fig. 5). In the absence of transfected dynamin, both wild-type Src and SrcK295M bound to Pyk2 (Fig. 5, blot 1 [i.e., the top immunoprecipitation blot], lanes 3 and 6). The Y402F mutation abolished the interaction of Pyk2 and Src (Fig. 5, blot 1, lane 9) and, as a consequence, nearly eliminated the tyrosine phosphorylation of Pyk2 (Fig. 5, blot 2 [i.e., the middle immunoprecipitation blot], lane 9), confirming this important function of Y402 (46). Total Pyk2 phosphorylation was markedly decreased when catalytically inactive SrcK295M was coexpressed, notwithstanding the fact that more SrcK295M bound to Pyk2, confirming the reported Src-catalyzed phosphorylation of Pyk2 (46). As predicted, the Pyk2-Src complex (blot 1, arrows) and Pyk2's phosphorylation (Fig. 5, blot 2, arrows) were significantly reduced when dynamin was also coexpressed. dynK44A had less of an effect on both the association of Src with Pyk2 (Fig. 5, top immunoprecipitation blot, lane 2) and the total phosphorylation of Pyk2 (Fig. 5, middle immunoprecipitation blot, lane 2), a finding consistent with its more limited ability to reduce Pyk2 Y402 phosphorylation. Together, these results indicate that dynamin reduces Pyk2-Src complex formation, possibly as a consequence of decreasing Pyk2 Y402 phosphorylation.
FIG. 5.
Dynamin reduces the binding of Pyk2 to Src. 293VnR cells were cotransfected with empty vector, dyn-GFP, or dynK44A-GFP in combination with Pyk2 or Pyk2-Y402F, as well as wild-type c-Src or kinase-inactive SrcK295M as indicated. Immunoprecipitation and Western blots were performed as indicated. Transfected dynamin was detected with an anti-GFP antibody. The dynamin-mediated decrease in Pyk2 phosphorylation (blot 2 [middle immunoprecipitation], arrows) was accompanied by a corresponding loss in Src-Pyk2 binding (blot 1 [top], arrows). Pyk2-Y402 failed to bind Src, as expected, and phosphorylation was barely detectable at this exposure and less dynamin bound to Pyk2-Y402 than to wild-type Pyk2. Labels: IP, immunoprecipitation; B, Western blot; Dyn, dynamin.
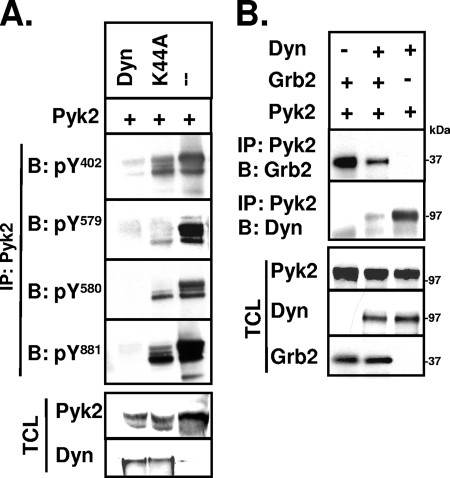
Since Src catalyzes the phosphorylation of Y579 and Y580 within Pyk2's catalytic domain and Y881 within its C-terminal domain once it binds to phosphorylated Y402 (52), the dynamin-induced decrease in Src binding should reduce the phosphorylation of these functionally important Pyk2 tyrosine residues. Analysis of immunoprecipitated Pyk2 with site-specific antibodies revealed that the phosphorylation of Pyk2 Y579, Y580, and Y881 were indeed decreased when dynamin and Pyk2 were overexpressed in 293VnR cells (Fig. 6A). dynK44A also decreased Pyk2 phosphorylation at all three sites, but to a lesser extent than wild-type dynamin. The decreased phosphorylation of the catalytically important Y579 and Y580 would reduce the Pyk2 kinase activity (36, 46, 52), possibly explaining the decreased tyrosine phosphorylation of dynamin relative to the dynK44A that we initially observed. On the other hand, phosphorylated Pyk2 Y881 is a known binding site for the signaling proteins Grb2 (22) and c-Abl (67), and we found that overexpression of dynamin in 293VnR cells also decreased Grb2 binding to Pyk2 (Fig. 6B), a finding consistent with the decreased phosphorylation of Y881. Thus, the dynamin-induced reduction of Pyk2 Y402 phosphorylation potentially affects both Pyk2's kinase activity and its subsequent binding to downstream effector proteins, further supporting a functional importance of the dynamin-Pyk2 association.
FIG. 6.
Dynamin decreases Pyk2 Y881 phosphorylation and Pyk2's binding to Grb2. (A) 293VnR cells were transiently cotransfected with equal amounts of cDNAs encoding Pyk2 and dyn-GFP, dynK44A-GFP, or empty vector as indicated. Pyk2 was immunoprecipitated, resolved by SDS-PAGE, and blotted with phospho-specific antibodies to Pyk2 tyrosine residues Y402, Y579, Y580, and Y881. TCLs were blotted for Pyk2 or dynamin (GFP) as indicated. (B) 293VnR cells were cotransfected with Pyk2, dyn-GFP or Grb2-myc as indicated. Pyk2 was immunoprecipitated and blotted for Grb2 (myc) or dynamin (GFP). TCLs were blotted to confirm protein expression in these cells. Representative blots from three independent experiments are shown. The positions of molecular mass markers are indicated. Labels: IP, immunoprecipitation; B, Western blot; Dyn, dynamin.
Src promotes the association of dynamin and Pyk2 and dynamin-induced Pyk2 dephosphorylation.
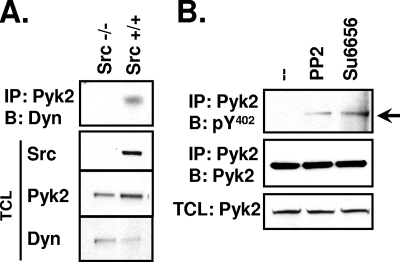
Eliminating the binding of Src to Pyk2 by mutating Pyk2 Y402 and inhibiting Src catalytic activity decreased the amount of Pyk2-associated dynamin in 293VnR cells (Fig. 5 and Fig. S3 in the supplemental material, respectively), suggesting that Src might promote Pyk2-dynamin association. We also observed that Pyk2-associated dynamin was reduced in OCLs lacking Src (Src−/−) (Fig. 7A), further supporting such a role for Src.
FIG. 7.
Src promotes dynamin-Pyk2 association and reduced Pyk2 Y402 phosphorylation. (A) OCLs were generated from Src+/+ or Src−/− mice and lysed in modified radioimmunoprecipitation assay buffer. Immunoprecipitation of endogenous Pyk2 was performed, followed by Western blotting for endogenous dynamin with the hudy2 antibody. TCLs were blotted as indicated. Experiments were performed three times. (B) OCLs were treated with either dimethyl sulfoxide, PP2 (15 μM, 1 h) or SU6656 (10 μM, 2 h). Pyk2 was immunoprecipitated and blotted for phosphorylated Y402. Membranes were stripped and reblotted for Pyk2. Labels: IP, immunoprecipitation; B, Western blot; Dyn, dynamin.
The association of Pyk2 and dynamin could involve either the catalytic activity or the adaptor function of Src or both. We first examined how Src's kinase activity affected dynamin-Pyk2 association and the phosphorylation of Pyk2 Y402. We found that inhibiting Src catalytic activity, using two Src family kinase inhibitors (PP2 and SU6656) with different patterns of inhibitory effects on non-Src family kinases (5), increased the phosphorylation of Pyk2 Y402 in OCLs (Fig. 7B), indicating that Src-catalyzed phosphorylation indeed promotes the dynamin-induced dephosphorylation of Py2 Y402.
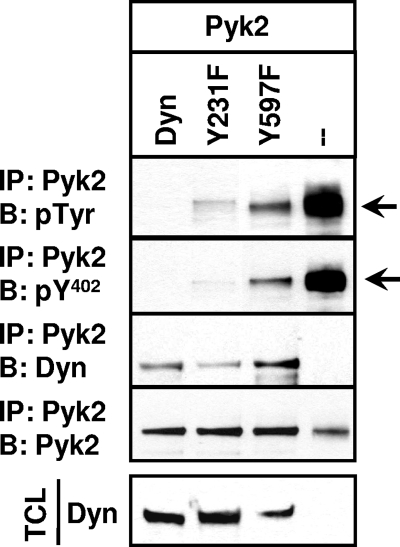
Src-catalyzed phosphorylation of dynamin-1 on tyrosine residues Y231 and Y597, which are conserved in dynamin-2, increases dynamin's GTPase activity (1, 2, 56). Our observations that reducing dynamin's GTPase activity partially disabled dynamin's ability to induce Pyk2 Y402 dephosphorylation (Fig. 2B) and that Src's catalytic activity promoted the association of dynamin and Pyk2 raised the possibility that the Src-catalyzed phosphorylation of dynamin might promote dynamin's ability to decrease Pyk2 phosphorylation. To test this, we mutated the Src-phosphorylated Y231 and Y597 residues in dynamin-2, coexpressed the dynamin mutants with Pyk2 in 293VnR cells, and analyzed their effect on Pyk2 phosphorylation and binding. Although both mutants bound Pyk2, the mutations of the Src-phosphorylated tyrosines, in particular the Y597F mutation, partially blunted the ability of dynamin to decrease total Pyk2 phosphorylation and the phosphorylation of Pyk2 Y402 (Fig. 8), although only a fraction of the phosphorylation was restored. Thus, the phosphorylation of dynamin by Src appears to enhance the dynamin-induced decrease in Pyk2 Y402 phosphorylation and the resulting decrease in Src's binding to Pyk2, possibly by increasing dynamin GTPase activity.
FIG. 8.
Mutation of dynamin's Src-phosphorylated tyrosines reduces dynamin's ability to downregulate Pyk2 Y402 phosphorylation. 293VnR cells were cotransfected with Pyk2 and either wild-type dyn-GFP, the Src phosphorylation site mutants dynY231F-GFP and dynY597F-GFP, or empty vector as indicated. Pyk2 was immunoprecipitated, and the immune complexes were analyzed for Pyk2 by Western blotting. Membranes were stripped and reblotted for phospho-Y402. Pyk2 was also immunoprecipitated and blotted with dynamin and Pyk2. TCLs were blotted for transfected dyn-GFP. Experiments were reproduced four times, and the results of a representative experiment are shown. Labels: IP, immunoprecipitation; B, Western blot; Dyn, dynamin.
Src acts as an adaptor to mediate the association of dynamin and Pyk2.
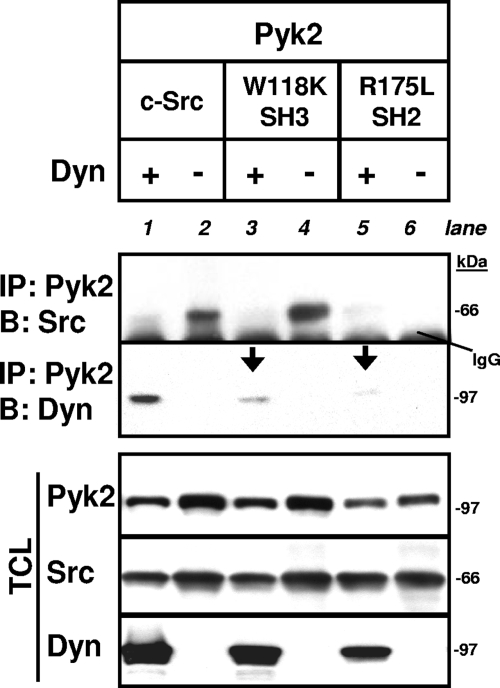
Published studies show that Src's SH2 domain binds Pyk2 phospho-Y402 (16) and suggest that the SH3 domain of Src interacts with dynamin's C-terminal PRD (25, 27). We therefore sought to determine whether Src's ability to act as an adaptor contributed to the dynamin-Pyk2 association by determining the effects of disabling Src's SH3 (SrcW118K) or SH2 (SrcR175L) domains on the association of dynamin and Pyk2 (Fig. 9). Disabling either of Src's binding domains reduced the amount of dynamin that coimmunoprecipitated with Pyk2 (Fig. 9, blot 2 [i.e., the lower immunoprecipitation blot], lanes 3 and 5), indicating that Src may indeed act as an adaptor in the association of dynamin and Pyk2. In the absence of cotransfected dynamin, both wild-type Src and SrcW118K, which retains a functional SH2 domain, bound to Pyk2 (Fig. 9, blot 1 [i.e., the upper blot], lanes 2 and 4), whereas disabling Src's SH2 domain eliminated the Pyk2-Src interaction (Fig. 9, blot 1, lane 6), confirming the known role of Src's SH2 domain in binding Pyk2. Furthermore, coexpression of dynamin with Pyk2 and Src markedly reduced the association of Pyk2 with both wild-type Src and SrcW118K (Fig. 9, blot 1, lanes 1 and 3), a finding consistent with the dynamin-induced reduction of the phosphorylation of Y402 and, thereby, the binding of Src's SH2 domain to Pyk2.
FIG. 9.
Src SH2 and SH3 domains mediate the dynamin-Pyk2 association. 293VnR cells were cotransfected with Pyk2, dyn-GFP, or empty vector in combination with either wild-type c-Src or Src mutants with a disabled SH3 or SH2 domain (SrcW118K and SrcR175L, respectively), as indicated. Immunoprecipitation and Western blots were performed as indicated. Exogenously expressed Src proteins were detected with an anti-avian Src antibody that does not detect endogenous Src. Disabling either the SH3 domain or the SH2 domain significantly reduced the Pyk2-dynamin association (arrows). Molecular mass markers and the position of IgG band are indicated. Labels: IP, immunoprecipitation; B, Western blot; Dyn, dynamin.
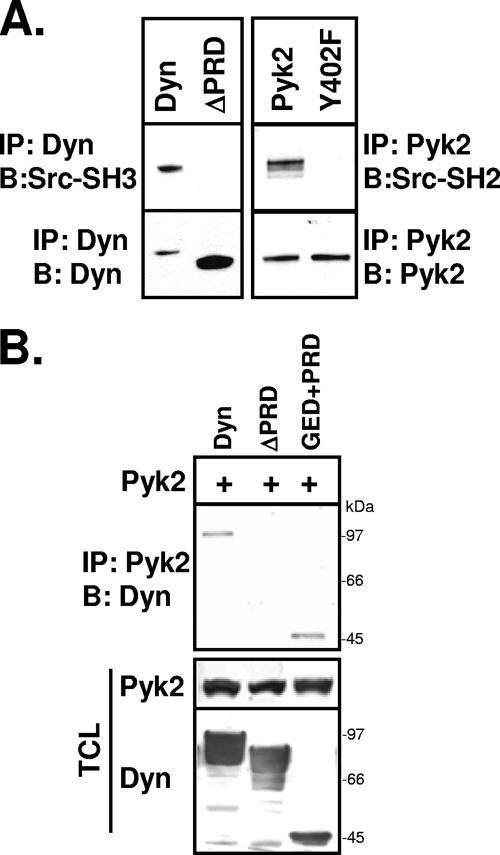
Having demonstrated that Src's adaptor function contributes to the association of dynamin and Pyk2, we next sought to identify the dynamin domain involved in the complex formation. We first tested the reported binding of the Src SH3 domain to the dynamin PRD. We performed far-Western analyses of the binding of a GST-conjugated Src-SH3 domain with full-length dynamin and a C-terminally truncated dynamin lacking the PRD (ΔPRD). As shown in Fig. 10A, the Src-SH3 domain bound to dynamin but not to dynΔPRD, confirming that Src can bind dynamin's PRD but not other dynamin domains. Similarly, we confirmed that the Src-SH2 domain requires Pyk2 phospho-Y402 for binding.
FIG. 10.
Dynamin's PRD is required for the dynamin-Pyk2 association. (A) 293VnR cells were cotransfected with dynamin, dynΔPRD, Pyk2, or Pyk2-Y402F. Dynamin and Pyk2 were immunoprecipitated and blotted with bacterially expressed GST-Src-SH3 or GST-Src-SH2 fusion proteins, respectively. Blots were stripped and reblotted for dynamin or Pyk2 as indicated. (B) 293VnR cells were cotransfected with Pyk2 and either full-length dynamin, dynΔPRD or dyn(GED+PRD). Pyk2 was immunoprecipitated, and the immune complexes were analyzed for the presence of the dynamin construct by Western blotting. The expression of all three dynamin proteins was also confirmed by Western blotting. Labels: IP, immunoprecipitation; B, Western blot; Dyn, dynamin.
We also demonstrated that the PRD was required for the dynamin-Pyk2 association. Pyk2 was coexpressed with full-length dynamin, dynΔPRD, or a dynamin fragment consisting of only the GED and the PRD (GED+PRD). Pyk2 formed a complex with full-length dynamin and the GED+PRD fragment but not with dynΔPRD (Fig. 10B), indicating that the binding of Src (or possibly another SH3 domain-containing adaptor protein) to dynamin's PRD mediates the dynamin-Pyk2 association.
DISCUSSION
The adhesion, migration, and function of osteoclasts and other highly motile cells are critically dependent on the proper assembly, organization and disassembly of podosomes, the dynamic integrin- and actin-containing adhesion structures that characterize such cells (29, 34, 37). We and others have shown that podosome organization and function in osteoclasts is regulated in part by Src and Pyk2 (15, 18, 26, 31, 35, 40, 50, 65) and that Src−/− osteoclasts fail to form a podosome belt and do not resorb bone (17, 32, 40, 42, 50, 54). Integrin engagement and activation induces the formation of a Pyk2-, Src-, and Cbl-containing signaling complex by the sequential increase in cytosolic free Ca2+ levels, activation and autophosphorylation of Pyk2 at Y402, and recruitment of Src and Src-associated Cbl to the phosphorylated Pyk2 Y402 (40, 50). Once the complex is assembled, Src phosphorylates Pyk2 and Cbl on tyrosine residues, which promotes the binding of other important signaling elements, such as phosphatidylinositol 3-kinase, Vav, and Grb2 (4, 64). The absence or inhibition of Src decreases the number of podosomes and the rate of actin flux into existing podosomes, while increasing the podosome life span (15). The absence of Pyk2 similarly decreases the flux of actin into existing podosomes, but without affecting podosome life span, and prevents the transition of podosome organization from small rings at the center of the cell to a mature podosome belt at the cell periphery (26).
Our findings from this and earlier studies indicate that the GTPase dynamin is also involved in the regulation of podosome organization and turnover in osteoclasts, in part by controlling Pyk2's phosphorylation/dephosphorylation cycle. We found that dynamin associates with Pyk2, Src and Cbl and decreases Pyk2 tyrosine phosphorylation in osteoclasts and in 293VnR cells (8; the present study). Whether dynamin prevents Pyk2's phosphorylation or, alternatively, promotes its rapid dephosphorylation by activating as-yet-unidentified protein tyrosine phosphatases (e.g., PTP-PEST [36]) remains to be determined. However, the prevention of the dynamin-induced loss of M-CSF-induced Pyk2 phosphorylation by orthovanadate (Fig. 4C) suggests the latter mechanism. Overexpressing dynamin-2 also led to the dephosphorylation of FAK at conserved tyrosine residues (data not shown), which has recently been reported to also modulate podosome function in osteoclasts (48). Thus, the dynamin-mediated regulation of the two focal adhesion kinases may have broad relevance to mechanisms that control signaling complex assembly/disassembly in adhesion structures in a variety of cellular types.
The dynamin-induced decrease in Pyk2 Y402 phosphorylation, in contrast to dynamin's association with Cbl, appears to involve the hydrolysis of dynamin-bound GTP. dynK44A, which binds and hydrolyzes GTP inefficiently (12, 13), binds Pyk2 at least as much as wild-type dynamin (see Fig. S2 in the supplemental material) but has less of an effect on Pyk2-Y402 dephosphorylation (Fig. 2B). Interestingly, overexpressed dynK44A reduced the rate of actin flux into formed podosomes in v-src-transformed BHK cells (43), similar to what we observed in osteoclasts that lack Pyk2 (26). We also found that dynamin's ability to decrease Pyk2 phosphorylation was partially disabled by mutating either of two tyrosine residues (Y231 or Y597) in dynamin that are required for the Src-induced increase in dynamin's GTPase activity (1, 2), providing further evidence for a possible role of the Src-regulated dynamin GTPase activity in the decrease in Pyk2 phosphorylation. Although further research will be required to characterize the mechanism by which GTP hydrolysis promotes Pyk2 dephosphorylation, these results suggest that the cycling of dynamin between its GTP-bound and GDP-bound states, regulated in part by changes in its phosphorylation state, drives Pyk2 dephosphorylation, just as it drives endocytosis (1, 2, 6, 12, 13, 56).
The reduction of Pyk2 Y402 phosphorylation by dynamin exerts a major effect on Pyk2- and Src-dependent signaling. We confirmed that the dynamin-induced loss of Pyk2 phospho-Y402 prevents the binding of Src to Pyk2 and, as a result, the Src-catalyzed phosphorylation of Pyk2 tyrosine residues 579, 580, and 881. The Src-catalyzed phosphorylation of Pyk2 Y579 and Y580 is necessary for maximal Pyk2 catalytic activity (36, 46, 52), and the phosphorylation of Pyk2 Y881 and the resulting binding of Grb2 to Pyk2 were decreased when dynamin was overexpressed. Thus, by inducing Pyk2 Y402 dephosphorylation and eliminating the Src binding site, dynamin is likely to both decrease Pyk2's catalytic activity and antagonize the recruitment of important signaling effectors such as Src, Abl, and Grb2 (16, 22, 67).
We found that Src regulates dynamin's association with Pyk2 in OCLs (Fig. 7). In contrast to Src's destabilizing effect on dynamin's association with Cbl (8), both the catalytic activity and the adaptor function of Src appear to contribute positively to the dynamin-Pyk2 association and to the dephosphorylation of Pyk2-Y402. In support of this conclusion, treatment with either PP2 or SU6656 increased Pyk2-Y402 phosphorylation in OCLs and decreased dynamin-Pyk2 association in 293VnR cells. Our findings also suggest that dynamin is a functionally important target of Src-catalyzed phosphorylation in the signaling complex, since Pyk2 Y402 phosphorylation increased when dynamin's Src-phosphorylated tyrosine residues were mutated (Fig. 8), although we cannot exclude the possibility that Src-catalyzed phosphorylation of other proteins also contributes to the regulation of dynamin-induced dephosphorylation of Pyk2 Y402.
In addition to the evidence that Src modulates dynamin-Pyk2 association and the dephosphorylation of Pyk2 by phosphorylating dynamin (and possibly other proteins in the complex), the decrease in dynamin-Pyk2 association observed when the Src-SH2 or SrcSH3 domain was disabled (Fig. 9), strongly supports a mechanism in which Src also acts as an adaptor between dynamin and Pyk2. Far Western analysis (Fig. 10A) confirmed that Src's SH2 and SH3 domains bind directly to Pyk2 and dynamin, respectively. However, our data show that the SH2-mediated binding of Src to Pyk2 is not required for the dynamin-Pyk2 association, since Pyk2-Y402F fails to bind Src but still associates with dynamin (Fig. 5). Although a mechanism in which Src directly bridges dynamin and Pyk2 is thus ruled out, a mechanism in which Src binds indirectly to Pyk2 via one or more other proteins (24, 30, 44, 49) is supported by our findings. Characterizing the proteins and intermolecular interactions that mediate the association of dynamin and Pyk2 will be the focus of future investigation.
Our results indicate that the Src-modulated downregulation of Pyk2 phosphorylation by dynamin is an important component of the mechanisms that regulate osteoclast activity. The dynamin-induced decrease in Pyk2 Y402 phosphorylation and the resulting prevention of Pyk2-Src binding provide an “off-switch” mechanism for disassembling the Pyk2-Src-Cbl complex and thereby terminating Src-dependent signaling downstream of activated Pyk2. Importantly, we showed that overexpressing dynamin reduced the phosphorylation of Pyk2 that is induced by integrin-mediated adhesion and by M-CSF treatment (Fig. 4), two stimuli that are key regulators of osteoclast function (21), while depleting dynamin increased steady-state phosphorylation of Pyk2 Y402 and altered the intracellular distribution of Pyk2 and the organization of the podosome belt (Fig. 3).
The Src-induced promotion of dynamin-Pyk2 association and the dynamin-induced dephosphorylation of Pyk2 Y402 in osteoclasts (Fig. 7) suggests the presence of a feedback mechanism by which Src that is activated downstream of Pyk2 also induces the downregulation of Pyk2- and Src-dependent signaling cascades. Thus, Src may be involved in two separate Pyk2-related functions in the complex: first, coupling activated Pyk2 to downstream signaling events by binding to Pyk2's phosphorylated Y402 and, second, initiating the disassembly of the Pyk2-Src complex by promoting (and possibly in part mediating) the association of dynamin-Pyk2 and the consequent decrease in Pyk2 phosphorylation. Additional investigation will be required to confirm this hypothesis and to further elucidate the molecular mechanism by which Src promotes dynamin's modulation of Pyk2 phosphorylation.
In conclusion, we have shown that dynamin associates with Pyk2 and reduces the phosphorylation of Pyk2 that occurs in response to functionally important stimuli such as M-CSF and integrin attachment in osteoclasts, leading to the disruption of Pyk2-Src binding. In addition, Src promotes the association of dynamin and Pyk2 and the subsequent loss of phosphorylated Pyk2 Y402, the site by which Src binds to Pyk2. Thus, dynamin may provide a Src-dependent “off-switch” that disassembles the Pyk2-Src-Cbl complex and downregulates its downstream signaling.
Supplementary Material
Acknowledgments
This study was supported in part by a Pilot and Feasibility Project grant from the Yale Core Center for Musculoskeletal Diseases (2P30 AR 46032-08) and funds from the Indiana University School of Dentistry to A.B. and grants from the National Institutes of Health (AR-42927 and AR-54450) to R.B.
We thank Archana Sanjay for providing Src cDNA mutant constructs; Pietro De Camilli, Shawn Ferguson, and Enkee Purev for constructs and helpful discussions; Karen Ford for maintaining mouse colonies; Gloria White for technical assistance; and Leslie Gourlay for administrative assistance.
Footnotes
Published ahead of print on 20 April 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ahn, S., J. Kim, C. L. Lucaveche, M. C. Reedy, L. M. Luttrell, R. J. Lefkowitz, and Y. Daaka. 2002. Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the epidermal growth factor receptor. J. Biol. Chem. 27726642-26651. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, S., S. Maudsley, L. M. Luttrell, R. J. Lefkowitz, and Y. Daaka. 1999. Src-mediated tyrosine phosphorylation of dynamin is required for β2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J. Biol. Chem. 2741185-1188. [DOI] [PubMed] [Google Scholar]
- 3.Altschuler, Y., S. M. Barbas, L. J. Terlecky, K. Tang, S. Hardy, K. E. Mostov, and S. L. Schmid. 1998. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J. Cell Biol. 1431871-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avraham, H., S.-Y. Park, K. Schinkmann, and S. Avraham. 2000. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 12123-133. [DOI] [PubMed] [Google Scholar]
- 5.Bain, J., H. McLauchlan, M. Elliott, and P. Cohen. 2003. The specificities of protein kinase inhibitors: an update. Biochem. J. 371199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bashkirov, P. V., S. A. Akimov, A. I. Evseev, S. L. Schmid, J. Zimmerberg, and V. A. Frolov. 2008. GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell 1351276-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop, A. L., and A. Hall. 2000. Rho GTPases and their effector proteins. Biochem. J. 348241-255. [PMC free article] [PubMed] [Google Scholar]
- 8.Bruzzaniti, A., L. Neff, A. Sanjay, W. C. Horne, P. De Camilli, and R. Baron. 2005. Dynamin forms a Src kinase-sensitive complex with Cbl and regulates podosomes and osteoclast activity. Mol. Biol. Cell 163301-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler, B., C. Gao, A. T. Mersich, and S. D. Blystone. 2006. Purified integrin adhesion complexes exhibit actin-polymerization activity. Curr. Biol. 16242-251. [DOI] [PubMed] [Google Scholar]
- 10.Cao, H., F. Garcia, and M. A. McNiven. 1998. Differential distribution of dynamin isoforms in mammalian cells. Mol. Biol. Cell 92595-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, W.-T. 1989. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J. Exp. Zool. 251167-185. [DOI] [PubMed] [Google Scholar]
- 12.Damke, H., T. Baba, D. E. Warnock, and S. L. Schmid. 1994. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127915-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damke, H., D. D. Binns, H. Ueda, S. L. Schmid, and T. Baba. 2001. Dynamin GTPase domain mutants block endocytic vesicle formation at morphologically distinct stages. Mol. Biol. Cell 122578-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Destaing, O., F. Saltel, J.-C. Geminard, P. Jurdic, and F. Bard. 2003. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell 14407-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Destaing, O., A. Sanjay, C. Itzstein, W. C. Horne, D. Toomre, P. De Camilli, and R. Baron. 2008. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol. Biol. Cell 19394-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dikic, I., G. Tokiwa, S. Lev, S. A. Courtneidge, and J. Schlessinger. 1996. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature 383547-550. [DOI] [PubMed] [Google Scholar]
- 17.Duong, L. T., P. T. Lakkakorpi, I. Nakamura, M. Machwate, R. M. Nagy, and G. A. Rodan. 1998. PYK2 in osteoclasts is an adhesion kinase, localized in the sealing zone, activated by ligation of αvβ3 integrin, and phosphorylated by Src kinase. J. Clin. Investig. 102881-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duong, L. T., I. Nakamura, P. T. Lakkakorpi, L. Lipfert, A. J. Bett, and G. A. Rodan. 2001. Inhibition of osteoclast function by adenovirus expressing antisense protein-tyrosine kinase 2. J. Biol. Chem. 2767484-7492. [DOI] [PubMed] [Google Scholar]
- 19.Elsegood, C. L., Y. Zhuo, G. A. Wesolowski, J. A. Hamilton, G. A. Rodan, and L. T. Duong. 2006. M-CSF induces the stable interaction of c-Fms with αvβ3 integrin in osteoclasts. Int. J. Biochem. Cell Biol. 381518-1529. [DOI] [PubMed] [Google Scholar]
- 20.Faccio, R., D. V. Novack, A. Zallone, F. P. Ross, and S. L. Teitelbaum. 2003. Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by β3 integrin. J. Cell Biol. 162499-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faccio, R., S. Takeshita, A. Zallone, F. P. Ross, and S. L. Teitelbaum. 2003. c-Fms and the αvβ3 integrin collaborate during osteoclast differentiation. J. Clin. Investig. 111749-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felsch, J. S., T. G. Cachero, and E. G. Peralta. 1998. Activation of protein tyrosine kinase PYK2 by the m1 muscarinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA 955051-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feshchenko, E. A., W. Y. Langdon, and A. Y. Tsygankov. 1998. Fyn, Yes, and Syk phosphorylation sites in c-Cbl map to the same tyrosine residues that become phosphorylated in activated T cells. J. Biol. Chem. 2738323-8331. [DOI] [PubMed] [Google Scholar]
- 24.Flynn, D. C., T.-H. Leu, A. B. Reynolds, and J. T. Parsons. 1993. Identification and sequence analysis of cDNAs encoding a 110-kilodalton actin filament-associated pp60src substrate. Mol. Cell. Biol. 137892-7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster-Barber, A., and J. M. Bishop. 1998. Src interacts with dynamin and synapsin in neuronal cells. Proc. Natl. Acad. Sci. USA 954673-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gil-Henn, H., O. Destaing, N. A. Sims, K. Aoki, N. Alles, L. Neff, A. Sanjay, A. Bruzzaniti, P. De Camilli, R. Baron, and J. Schlessinger. 2007. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2−/− mice. J. Cell Biol. 1781053-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herskovits, J. S., H. S. Shpetner, C. C. Burgess, and R. B. Vallee. 1993. Microtubules and Src homology 3 domains stimulate the dynamin GTPase via its C-terminal domain. Proc. Natl. Acad. Sci. USA 9011468-11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, F., A. Khvorova, W. Marshall, and A. Sorkin. 2004. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J. Biol. Chem. 27916657-16661. [DOI] [PubMed] [Google Scholar]
- 29.Jurdic, P., F. Saltel, A. Chabadel, and O. Destaing. 2006. Podosome and sealing zone: specificity of the osteoclast model. Eur. J. Cell Biol. 85195-202. [DOI] [PubMed] [Google Scholar]
- 30.Kanner, S. B., A. B. Reynolds, H.-C. R. Wang, R. R. Vines, and J. T. Parsons. 1991. The SH2 and SH3 domains of pp60src direct stable association with tyrosine phosphorylated proteins p130 and p110. EMBO J. 101689-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakkakorpi, P. T., A. J. Bett, L. Lipfert, G. A. Rodan, and L. T. Duong. 2003. PYK2 autophosphorylation, but not kinase activity, is necessary for adhesion-induced association with c-Src, osteoclast spreading, and bone resorption. J. Biol. Chem. 27811502-11512. [DOI] [PubMed] [Google Scholar]
- 32.Lakkakorpi, P. T., I. Nakamura, M. Young, L. Lipfert, G. A. Rodan, and L. T. Duong. 2001. Abnormal localization and hyperclustering of αVβ3 integrins and associated proteins in Src-deficient or tyrphostin A9-treated osteoclasts. J. Cell Sci. 114149-160. [DOI] [PubMed] [Google Scholar]
- 33.Lev, S., H. Moreno, R. Martinez, P. Canoll, E. Peles, J. M. Musacchio, G. D. Plowman, B. Rudy, and J. Schlessinger. 1995. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature 376737-745. [DOI] [PubMed] [Google Scholar]
- 34.Linder, S., and M. Aepfelbacher. 2003. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 13376-385. [DOI] [PubMed] [Google Scholar]
- 35.Luxenburg, C., J. T. Parsons, L. Addadi, and B. Geiger. 2006. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J. Cell Sci. 1194878-4888. [DOI] [PubMed] [Google Scholar]
- 36.Lyons, P. D., J. M. Dunty, E. M. Schaefer, and M. D. Schaller. 2001. Inhibition of the catalytic activity of cell adhesion kinase β by protein-tyrosine phosphatase-PEST-mediated dephosphorylation. J. Biol. Chem. 27624422-24431. [DOI] [PubMed] [Google Scholar]
- 37.Marchisio, P. C., D. Cirillo, A. Teti, A. Zambonin-Zallone, and G. Tarone. 1987. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp. Cell Res. 169202-214. [DOI] [PubMed] [Google Scholar]
- 38.McNiven, M. A., M. Baldassarre, and R. Buccione. 2004. The role of dynamin in the assembly and function of podosomes and invadopodia. Front. Biosci. 91944-1953. [DOI] [PubMed] [Google Scholar]
- 39.Miller, W. E., S. Maudsley, S. Ahn, K. D. Khan, L. M. Luttrell, and R. J. Lefkowitz. 2000. β-arrestin1 interacts with the catalytic domain of the tyrosine kinase c-SRC: role of β-arrestin1-dependent targeting of c-SRC in receptor endocytosis. J. Biol. Chem. 27511312-11319. [DOI] [PubMed] [Google Scholar]
- 40.Miyazaki, T., A. Sanjay, L. Neff, S. Tanaka, W. C. Horne, and R. Baron. 2004. Src kinase activity is essential for osteoclast function. J. Biol. Chem. 27917660-17666. [DOI] [PubMed] [Google Scholar]
- 41.Miyazaki, T., H. Takayanagi, M. Isshiki, T. Takahashi, M. Okada, Y. Fukui, H. Oda, K. Nakamura, H. Hirai, T. Kurokawa, and S. Tanaka. 2000. In vitro and in vivo suppression of osteoclast function by adenovirus vector-induced csk gene. J. Bone Miner Res. 1541-51. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura, I., E. Jimi, L. T. Duong, T. Sasaki, N. Takahashi, G. A. Rodan, and T. Suda. 1998. Tyrosine phosphorylation of p130Cas is involved in actin organization in osteoclasts. J. Biol. Chem. 27311144-11149. [DOI] [PubMed] [Google Scholar]
- 43.Ochoa, G.-C., V. I. Slepnev, L. Neff, N. Ringstad, K. Takei, L. Daniell, W. Kim, H. Cao, M. McNiven, R. Baron, and P. De Camilli. 2000. A functional link between dynamin and the actin cytoskeleton at podosomes. J. Cell Biol. 150377-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamura, H., and M. D. Resh. 1995. p80/85 cortactin associates with the Src SH2 domain and colocalizes with v-Src in transformed cells. J. Biol. Chem. 27026613-26618. [DOI] [PubMed] [Google Scholar]
- 45.Orth, J. D., and M. A. McNiven. 2003. Dynamin at the actin-membrane interface. Curr. Opin. Cell Biol. 1531-39. [DOI] [PubMed] [Google Scholar]
- 46.Park, S.-Y., H. K. Avraham, and S. Avraham. 2004. RAFTK/Pyk2 activation is mediated by trans-acting autophosphorylation in a Src-independent manner. J. Biol. Chem. 27933315-33322. [DOI] [PubMed] [Google Scholar]
- 47.Pfaff, M., and P. Jurdic. 2001. Podosomes in osteoclast-like cells: structural analysis and cooperative roles of paxillin, proline-rich tyrosine kinase 2 (Pyk2) and integrin αvβ3. J. Cell Sci. 1142775-2786. [DOI] [PubMed] [Google Scholar]
- 48.Ray, B. J., and A. H. Boughton. 2008. Investigations into the role of focal adhesion kinase (FAK) in bone marrow-derived osteoclasts. J. Bone Miner. Res. 23(Suppl. 1)S269. [Google Scholar]
- 49.Sakai, R., A. Iwamatsu, N. Hirano, S. Ogawa, T. Tanaka, H. Mano, Y. Yazaki, and H. Hirai. 1994. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 133748-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanjay, A., A. Houghton, L. Neff, E. Didomenico, C. Bardelay, E. Antoine, J. Levy, J. Gailit, D. Bowtell, W. C. Horne, and R. Baron. 2001. Cbl associates with Pyk2 and Src to regulate Src kinase activity, αvβ3 integrin-mediated signaling, cell adhesion, and osteoclast motility. J. Cell Biol. 152181-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanjay, A., T. Miyazaki, C. Itzstein, E. Purev, W. C. Horne, and R. Baron. 2006. Identification and functional characterization of an Src homology domain 3 domain-binding site on Cbl. FEBS J. 2735442-5456. [DOI] [PubMed] [Google Scholar]
- 52.Schlaepfer, D. D., C. R. Hauck, and D. J. Sieg. 1999. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71435-478. [DOI] [PubMed] [Google Scholar]
- 53.Schmid, S. L., M. A. McNiven, and P. De Camilli. 1998. Dynamin and its partners: a progress report. Curr. Opin. Cell Biol. 10504-512. [DOI] [PubMed] [Google Scholar]
- 54.Schwartzberg, P. L., L. Xing, O. Hoffmann, C. A. Lowell, L. Garrett, B. F. Boyce, and H. E. Varmus. 1997. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src−/− mutant mice. Genes Dev. 112835-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sever, S., H. Damke, and S. L. Schmid. 2000. Garrotes, springs, ratchets, and whips: putting dynamin models to the test. Traffic 1385-392. [DOI] [PubMed] [Google Scholar]
- 56.Shajahan, A. N., B. K. Timblin, R. Sandoval, C. Tiruppathi, A. B. Malik, and R. D. Minshall. 2004. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J. Biol. Chem. 27920392-20400. [DOI] [PubMed] [Google Scholar]
- 57.Sieg, D. J., D. Ilic, K. C. Jones, C. H. Damsky, T. Hunter, and D. D. Schlaepfer. 1998. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK− cell migration. EMBO J. 175933-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slepnev, V. I., and P. De Camilli. 2000. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat. Rev. Neurosci. 1161-172. [DOI] [PubMed] [Google Scholar]
- 59.Soriano, P., C. Montgomery, R. Geske, and A. Bradley. 1991. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64693-702. [DOI] [PubMed] [Google Scholar]
- 60.Stickel, S. K., and Y. Wang. 1987. Alpha-actinin-containing aggregates in transformed cells are highly dynamic structures. J. Cell Biol. 1041521-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takayanagi, H., S. Kim, and T. Taniguchi. 2002. Signaling crosstalk between RANKL and interferons in osteoclast differentiation. Arthritis Res. 4(Suppl. 3)S227-S232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka, S., M. Amling, L. Neff, A. Peyman, E. Uhlmann, J. B. Levy, and R. Baron. 1996. c-Cbl is downstream of c-Src in a signalling pathway necessary for bone resorption. Nature 383528-531. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka, S., T. Takahashi, H. Takayanagi, T. Miyazaki, H. Oda, K. Nakamura, H. Hirai, and T. Kurokawa. 1998. Modulation of osteoclast function by adenovirus vector-induced epidermal growth factor receptor. J. Bone Miner Res. 131714-1720. [DOI] [PubMed] [Google Scholar]
- 64.Thien, C. B. F., and W. Y. Langdon. 2005. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem. J. 391153-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, Q., Y. Xie, Q.-S. Du, X.-J. Wu, X. Feng, L. Mei, J. M. McDonald, and W.-C. Xiong. 2003. Regulation of the formation of osteoclastic actin rings by proline-rich tyrosine kinase 2 interacting with gelsolin. J. Cell Biol. 160565-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang, Z., L. Neff, A. L. M. Bothwell, R. Baron, and W. C. Horne. 2002. Calcitonin induces dephosphorylation of Pyk2 and phosphorylation of focal adhesion kinase in osteoclasts. Bone 31359-365. [DOI] [PubMed] [Google Scholar]
- 67.Zrihan-Licht, S., S. Avraham, S. Jiang, Y. Fu, and H. K. Avraham. 2004. Coupling of RAFTK/Pyk2 kinase with c-Abl and their role in the migration of breast cancer cells. Int. J. Oncol. 24153-159. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.