Abstract
Hypoxia promotes Na,K-ATPase endocytosis via protein kinase Cζ (PKCζ)-mediated phosphorylation of the Na,K-ATPase α subunit. Here, we report that hypoxia leads to the phosphorylation of 5′-AMP-activated protein kinase (AMPK) at Thr172 in rat alveolar epithelial cells. The overexpression of a dominant-negative AMPK α subunit (AMPK-DN) construct prevented the hypoxia-induced endocytosis of Na,K-ATPase. The overexpression of the reactive oxygen species (ROS) scavenger catalase prevented hypoxia-induced AMPK activation. Moreover, hypoxia failed to activate AMPK in mitochondrion-deficient ρ0-A549 cells, suggesting that mitochondrial ROS play an essential role in hypoxia-induced AMPK activation. Hypoxia-induced PKCζ translocation to the plasma membrane and phosphorylation at Thr410 were prevented by the pharmacological inhibition of AMPK or by the overexpression of the AMPK-DN construct. We found that AMPK α phosphorylates PKCζ on residue Thr410 within the PKCζ activation loop. Importantly, the activation of AMPK α was necessary for hypoxia-induced AMPK-PKCζ binding in alveolar epithelial cells. The overexpression of T410A mutant PKCζ prevented hypoxia-induced Na,K-ATPase endocytosis, confirming that PKCζ Thr410 phosphorylation is essential for this process. PKCζ activation by AMPK is isoform specific, as small interfering RNA targeting the α1 but not the α2 catalytic subunit prevented PKCζ activation. Accordingly, we provide the first evidence that hypoxia-generated mitochondrial ROS lead to the activation of the AMPK α1 isoform, which binds and directly phosphorylates PKCζ at Thr410, thereby promoting Na,K-ATPase endocytosis.
When exposed to low oxygen levels (hypoxia), cells develop adaptative strategies to maintain adequate levels of ATP (21). These strategies include increasing the efficiency of energy-producing pathways, mostly through anaerobic glycolysis, while decreasing energy-consuming processes such as Na,K-ATPase activity (30). Alveolar hypoxia occurs in many respiratory disorders, and it has been shown to decrease epithelial active Na+ transport, leading to impaired fluid reabsorption (37, 41, 42). Active Na+ transport and, thus, alveolar fluid reabsortion are effected mostly via apical sodium channels and the basolateral Na,K-ATPase (32, 38, 42). We have reported previously that hypoxia inhibits Na,K-ATPase activity by promoting its endocytosis from the plasma membrane by a mechanism that requires the generation of mitochondrial reactive oxygen species (ROS) and the phosphorylation of the Na,K-ATPase α subunit at Ser18 by protein kinase Cζ (PKCζ) (8, 9).
The 5′-AMP-activated protein kinase (AMPK) is a heterotrimeric Ser/Thr kinase composed of a catalytic α subunit and regulatory β and γ subunits. Both isoforms of the AMPK catalytic subunit (α1 and α2) form complexes with noncatalytic subunits. The α1 subunit is ubiquitously expressed, whereas the α2 subunit isoform is expressed predominantly in tissues like the liver, heart, and skeletal muscle (36). The α1 and α2 subunit isoforms have ∼90% homology in their N-terminal catalytic domains and ∼60% homology in their C-terminal domains (36), suggesting that they may have distinct downstream targets (31). AMPK activation requires phosphorylation at Thr172 in the activation loop of the α subunit by upstream kinases (12, 19). Findings from recent studies suggest that AMPK is an important signaling intermediary in coupling ion transport and metabolism (15). Indeed, it has been reported that the pharmacological activation of AMPK inhibits amiloride- and ouabain-sensitive epithelial Na+ transport (15). Moreover, the activities of the epithelial Na+ channel (ENaC) (2, 17), the Na,K-ATPase (40), and the cystic fibrosis transmembrane conductance regulator (17) have been shown to be inhibited by AMPK. Here, we provide evidence that hypoxia, via mitochondrial ROS, leads to AMPK activation and that AMPK binds to and directly phosphorylates PKCζ in an isoform-specific manner, thus promoting Na,K-ATPase endocytosis in alveolar epithelial cells (AEC).
MATERIALS AND METHODS
Reagents.
All cell culture reagents were from Mediatech Inc. Human AMPK α1 and AMPK α2 small interfering RNA (siRNA) duplexes and protein A/G PLUS-agarose beads were purchased from Santa Cruz Biotechnology Inc. A nonsilencing siRNA used as a negative control was purchased from Ambion. Lipofectamine RNAiMAX and Lipofectamine 2000 were from Invitrogen. Recombinant full-length human PKCζ-glutathione S-transferase (GST) fusion protein and a fusion protein comprising recombinant human AMPK α1 (amino acids M1 to C312) and GST were from Cell Signaling Technology. [γ-32P]ATP was purchased from PerkinElmer. Compound C and tert-butyl hydroperoxide (t-H2O2) were from Sigma-Aldrich. EZ-Link N-hydroxysuccinimide-SS-biotin and streptavidin-Sepharose beads were purchased from Pierce Biotechnology. 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) was purchased from Toronto Research Chemicals Inc. Catalase-expressing adenovirus was obtained from the Gene Transfer Vector Core, University of Iowa (26). All other chemicals were purchased from Calbiochem and were of the highest grade available.
ATII cell isolation and cell culture.
Alveolar epithelial type II (ATII) cells were isolated from the lungs of Sprague-Dawley rats at Cell Culture and Physiology Core B in the Division of Pulmonary and Critical Care Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL, as described previously (34) and were used 2 or 3 days after isolation.
Human A549 cells (ATCC CCL 185; a human adenocarcinoma cell line) and COS-7 cells (ATCC CRL 1651; a monkey kidney fibroblast cell line) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. A549 cells with reconstituted LKB1 (A549+LKB1 cells) (25) were grown in complete DMEM supplemented with 400 μg/ml of Geneticin. To generate mitochondrion-deficient ρ0-A549 cells, wild-type (WT) A549 cells were incubated in DMEM containing ethidium bromide (50 ng/ml) as described previously (9, 23). Cells were serum starved for 4 h prior to hypoxia exposure. Hypoxic conditions (1.5% O2, 93.5% N2, and 5% CO2) were achieved in a humidified workstation (Invivo O2; Ruskinn Technology) that continuously monitors the chamber's oxygen tension as described previously (9).
Adenoviral infection of ATII cells.
On day 2, ATII cells were infected with null adenovirus (Ad-null; 20 PFU/cell), with a hemagglutinin (HA)-tagged adenovirus carrying a dominant-negative, kinase-dead (K45R) variant of the AMPK α1 subunit (Ad-HA-DN AMPK-α; 20 PFU/cell) (16, 40), with an adenovirus expressing a constitutively active AMPK α variant (Ad-CA AMPK; 20 PFU/cell) in which Thr172 was replaced with aspartate in the truncated AMPK α subunit, comprising residues 1 to 312 (a generous gift from Kenneth Walsh, Boston University) (40), or with an adenovirus coding for catalase (Ad-catalase; 20 PFU/cell), an H2O2 scavenger. After 2 h of incubation, DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin was added to the cell culture plates. Experiments were performed 24 h later. In the experiments in which Ad-catalase and Ad-CA AMPK were used, the cells were infected with Ad-catalase or Ad-null on day 2 and then infected with Ad-CA AMPK or null virus 24 h later, and the experiments were performed after another 24 h. The levels of expression of catalase after 48 h were comparable to those obtained after 24 h, and there was no increase in cell death (data not shown).
Transfection of A549 cells with siRNA.
A549 cells were transfected with human AMPK α1 and AMPK α2 siRNA duplexes (60 pmol) by using Lipofectamine RNAiMAX according to the manufacturer's recommended protocol, and experiments were performed 48 h later. A nonsilencing siRNA, which did not influence the expression of the AMPK, was used as a negative control.
Biotinylation of cell surface proteins.
Cells were exposed to 21% O2 (normoxia) or 1.5% O2 (hypoxia) for 60 min; cell surface proteins were labeled for 20 min using 1 mg/ml EZ-Link N-hydroxysuccinimide-SS-biotin. Cells were lysed in modified RIPA buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and protease inhibitors). Samples of 50 μg of protein were incubated overnight at 4°C with end-over-end shaking in the presence of streptavidin beads and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting as described previously (9).
Isolation of 1% Triton X-100-soluble membrane fraction.
Cells were exposed to normoxia or hypoxia for 10 min, and the total membrane fraction was prepared as described previously (33). Briefly, cells were homogenized in homogenization buffer containing 1 mM EDTA, 1 mM EGTA, 10 mM Tris-HCl (pH 7.5), 1 μg/ml leupeptin, 100 μg/ml N-tosyl-l-phenylalanine chloromethyl ketone (TPCK), and 1 mM phenylmethylsulfonyl fluoride (PMSF) and centrifuged at 500 × g to discard nuclei and debris. The supernatant was then centrifuged at 100,000 × g for 1 h at 4°C. The pellet containing the crude membrane fraction was resuspended in homogenization buffer supplemented with 1% Triton X-100 and centrifuged at 100,000 × g for 30 min at 4°C. The supernatant was designated the 1% Triton X-100-soluble membrane fraction.
Cell lysis and immunoprecipitation.
For the detection of AMPK and acetyl coenzyme A carboxylase (ACC) phosphorylation after hypoxia exposure, ATII cells were washed in ice-cold phosphate-buffered saline and solubilized in lysis buffer. The lysates were cleared by centrifugation for 10 min at 14,000 × g. Samples containing equal amounts (50 to 75 μg) of proteins were resuspended in Laemmli sample buffer, boiled for 5 min, and subjected to Western blot analysis with specific antibodies as described below.
PKCζ was immunoprecipitated from ATII cell lysates in a buffer containing 150 mM NaCl, 50 mM Tris, 1% Triton X-100, 1 mM EGTA, 1 mM EDTA, 1 mM NaF, 1 mM Na-orthovanadate, 1 mM PMSF, 100 μg/ml TPCK, and 10 μg/ml leupeptin (pH 7.4). Equal amounts (300 μg) of protein were then incubated with anti-PKCζ antibody (1 μg) for 2 h at 4°C. Protein A/G PLUS-agarose beads were added to the samples, which were incubated overnight at 4°C. The beads were washed five times with lysis buffer, resuspended in 2× Laemmli sample buffer, and analyzed by SDS-PAGE and Western blotting.
A549 cells were exposed to hypoxia for 10 min and then harvested after being washed with phosphate-buffered saline. Cells were lysed by suspension in 2 volumes of lysis buffer containing 0.4 M NaCl, 20 mM HEPES, 0.1% NP-40, 5% (vol/vol) glycerol, 2 mM EGTA, 2 mM EDTA, 1 mM NaF, 1 mM Na-orthovanadate, 1 mM PMSF, 100 μg/ml TPCK, and 10 μg/ml leupeptin (pH 7.4). The salt concentration was brought down to 0.15 M NaCl by using the lysis buffer without NaCl. Equal amounts (500 μg) of protein were then incubated with either anti-AMPK (5-μg) or anti-PKCζ (5-μg) antibody for 2 h at 4°C. Protein A/G PLUS-agarose beads were added to the samples, which were incubated overnight at 4°C. The beads were washed five times with a solution of 0.15 M NaCl, 20 mM HEPES, 0.1% NP-40, 5% (vol/vol) glycerol, 2 mM EGTA, 2 mM EDTA, 1 mM NaF, 1 mM Na-orthovanadate, 1 mM PMSF, 100 μg/ml TPCK, and 10 μg/ml leupeptin (pH 7.4), resuspended in 40 μl of 2× Laemmli sample buffer, and subjected to SDS-PAGE and Western blotting.
In vitro kinase assay.
COS-7 cells were transiently transfected either with FLAG-tagged full-length WT PKCζ or with FLAG-tagged T410A mutant PKCζ by using Lipofectamine 2000 as described by the manufacturer, and the cells were cultured for 48 h. Cell lysates were obtained, and PKCζ was immunoprecipitated by incubating approximately 300 μg of protein with 1 μl of FLAG antibody in lysis buffer overnight. Immunocomplexes were pulled down with 50 μl of protein A/G-agarose beads and then washed in lysis buffer. Immunoprecipitated PKCζ or 150 ng of the recombinant full-length human PKCζ-GST fusion protein was incubated with a mixture containing 200 ng of recombinant human AMPK α1-GST fusion protein, 5 μCi [γ-32P]ATP, and 100 μM ATP for 30 min at 30°C in a solution of 50 mM Tris, 5 mM MgCl2, 2 mM EGTA, 1 mM dithiothreitol, and 10 mM β-glycerol phosphate (pH 7.5). The reactions were stopped by cooling the samples on ice and adding Laemmli sample buffer. The proteins were separated by SDS-PAGE, and radiolabeled PKCζ was detected by autoradiography.
Western blot analysis.
Protein concentrations were determined as described by Bradford (9) using a commercial dye reagent (Bio-Rad), and protein extracts were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Optitran; Schleider & Schuell) by using a semidry transfer apparatus (Bio-Rad). The following commercially available antibodies and dilutions were used for Western blotting: rabbit anti-phosphorylated PKCζ, anti-phosphorylated AMPK (anti-pAMPK), anti-AMPK, anti-phospho-ACC, and anti-ACC were from Cell Signaling Technology and were used at 1:1,000; mouse anti-Na,K-ATPase subunit α1 (clone 464.6; 1:1,000) and rabbit anti-AMPK α1 (1:1,000) were from Upstate Biotechnology; rabbit anti-AMPK α2 (1:2,000; Novus Biologicals), mouse anti-PKCζ (clone H-1; 1:500), and rabbit anti-E-cadherin (1:1,000) were from Santa Cruz Biotechnology Inc.; and mouse anti-GST tag (clone G018; 1:1,000 [Applied Biological Materials Inc.]), mouse anti-HA tag (clone 16B12; 1:1,000 [Covance]), mouse anti-FLAG (clone M2; 1:2,000), and mouse anti-β-actin (clone AC-15; 1:5,000) were from Sigma-Aldrich. Primary antibodies were detected by horseradish peroxidase-conjugated secondary goat anti-mouse antibodies (1:10,000; Bio-Rad) or goat anti-rabbit antibodies (1:2,000; Cell Signaling Technology) by using a chemiluminescence detection kit (PerkinElmer Life Sciences). Quantification of protein levels was performed by densitometric scanning with ImageJ 1.29X (NIH).
Statistics.
Data are presented as means ± standard errors of the means (SEM) and were statistically analyzed using one-way analysis of variance, followed by a multiple comparison with the Dunnet test. P values of less than 0.05 were considered statistically significant.
RESULTS
Hypoxia activates AMPK in rat ATII cells.
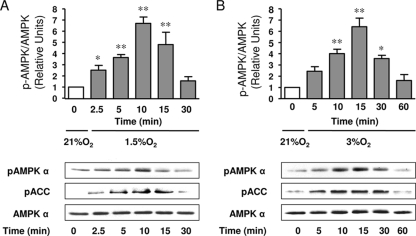
Hypoxia (1.5% O2) led to time-dependent AMPK activation in rat ATII cells as determined by assessing the phosphorylation of Thr172 with a phosphospecific antibody (Fig. 1A). This activation was transient, and AMPK levels returned to the baseline after 30 min of hypoxia exposure; a slight retardation in the migration of the AMPK forms after hypoxia could be observed, confirming the phosphorylation of AMPK. We also determined the phosphorylation state of Ser79 in ACC, a known substrate of AMPK (5, 6), which correlated with AMPK activation in response to hypoxia (Fig. 1A). Moreover, a milder hypoxic stimulus (3% O2) activated AMPK and ACC in a similar but slower manner than 1.5% O2 (Fig. 1B). Since the maximal activation of AMPK was achieved after 10 min of 1.5% O2 exposure, the subsequent cellular experiments were performed under these conditions.
FIG. 1.
Hypoxia activates AMPK in ATII cells. (A) ATII cells were exposed to 21% O2 (white bar) or 1.5% O2 (gray bars) for 2.5 to 30 min, and the levels of AMPK phosphorylated at Thr172 (pAMPK α) and ACC phosphorylated at Ser79 (pACC), as well as the total amount of AMPK α, were measured by Western blot analysis. The graph represents the pAMPK/AMPK ratios. Representative Western blots analyzing pAMPK α, pACC, and total AMPK α are shown. (B) ATII cells were exposed to 21% O2 (white bar) or 3% O2 (gray bars) for 5 to 60 min, and the levels of pAMPK α and pACC and the total amount of AMPK α were determined as described above. The graph represents the pAMPK/AMPK ratios. Representative Western blots analyzing pAMPK α, pACC, and total AMPK α are shown. Values are expressed as means ± SEM (n = 4). *, P < 0.05; **, P < 0.01.
AMPK regulates hypoxia-induced Na,K-ATPase endocytosis.
We assessed the abundance of the Na,K-ATPase α1 subunit protein at the plasma membrane in ATII cells infected with Ad-null or Ad-HA-DN AMPK-α prior to exposure to 1.5% O2. Consistent with our previously published data (9, 10), hypoxia decreased Na,K-ATPase α1 protein abundance by ∼40% in the cells infected with Ad-null, as determined from cell surface biotinylation. However, in cells infected with Ad-HA-DN AMPK-α, hypoxia-induced endocytosis was prevented (Fig. 2A). AMPK activation has been reported to decrease the number of ENaC molecules at the cell membrane (17), which may indirectly affect Na,K-ATPase activity. We conducted experiments to investigate whether Na,K-ATPase downregulation via AMPK was a secondary effect of ENaC-mediated changes in intracellular Na+ levels. After infection with Ad-null or Ad-HA-DN AMPK-α, cells were pretreated with the Na+ ionophore monensin (13) and exposed to 1.5% O2. As shown in Fig. 2B, the inhibition of AMPK prevented hypoxia-mediated Na,K-ATPase endocytosis even when the intracellular Na+ concentration was increased, suggesting that the effect of AMPK activation on Na,K-ATPase α1 abundance at the plasma membrane is not secondary to changes in intracellular Na+ levels.
FIG. 2.
AMPK activation is required for hypoxia-induced Na,K-ATPase endocytosis, independent of changes in intracellular Na+ concentrations. (A) ATII cells were infected with Ad-null (20 PFU/cell) or Ad-HA-DN AMPK-α (20 PFU/cell). After 24 h, the cells were exposed to 21 or 1.5% O2 for 60 min. Na,K-ATPase α1 subunit abundance at the plasma membrane was determined from cell surface biotinylation, followed by streptavidin pulldown and Western blot analysis with an α1 subunit-specific antibody. (B) ATII cells were infected as described above and, after 24 h, pretreated with monensin (2 μM) for 5 min and then exposed to 21 or 1.5% O2 for 60 min. Na,K-ATPase α1 subunit abundance at the plasma membrane was determined as described above. Representative Western blots analyzing the level of Na,K-ATPase subunit α1 at the plasma membrane and the level of HA-tagged AMPK expression are shown. E-cadherin was used as a loading control. Values shown are means ± SEM (n = 4). **, P < 0.01.
Mitochondrial ROS mediate hypoxia-induced AMPK activation.
AEC generate mitochondrial ROS when exposed to low oxygen concentrations (9). To investigate whether ROS are required for hypoxia-induced AMPK activation, ATII cells were infected with an adenovirus coding for catalase, a ROS scavenger, which prevented AMPK activation (Fig. 3A). The incubation of ATII cells with t-H2O2 caused time-dependent AMPK and ACC phosphorylation, similar to hypoxia (Fig. 3B). To assess the role of mitochondrion-generated ROS, we conducted experiments with ρ0-A549 cells, which are not capable of mitochondrial respiration because they lack key components of the electron transfer chain (7, 9). In a previous study, we have shown that these cells are unable to generate ROS during hypoxia (9). As shown in Fig. 3C, the hypoxia-induced phosphorylation of ACC observed in WT A549 cells was prevented in ρ0-A549 cells. To confirm that the lack of activation of AMPK in ρ0-A549 cells was caused by the absence of mitochondrial ROS and that these cells respond to ROS, the cells were treated with exogenous t-H2O2. As indicated in Fig. 3D, ρ0-A549 cells responded to t-H2O2 by increasing the phosphorylation of ACC.
FIG. 3.
ROS activate AMPK during hypoxia. (A) ATII cells were infected with Ad-null or Ad-catalase (20 PFU/cell) and then exposed to 21 or 1.5% O2 for 10 min; the levels of pAMPK α and total AMPK α were determined by Western blotting as described above. Representative Western blots analyzing pAMPK α, total AMPK, and catalase are shown (n = 3). (B) ATII cells were treated with 100 μM t-H2O2 for up to 30 min; the levels of pAMPK α, pACC, and total AMPK α were determined by Western blotting. Representative Western blots are shown (n = 3). (C) WT and ρ0-A549 cells were exposed to 21 or 1.5% O2 for 10 min, and the levels of pACC and total ACC were determined by Western blotting as described above (n = 3). (D) ρ0-A549 cells were treated with 100 μM t-H2O2 or vehicle (V) for 10 min, and the levels of pACC and total ACC were determined by Western blotting. Representative Western blots are shown (n = 3).
We have demonstrated previously that AMPK activation is sufficient to trigger Na,K-ATPase endocytosis (40). Figure 4A shows that while the overexpression of catalase prevents hypoxia-induced Na,K-ATPase endocytosis, it fails to prevent the endocytosis triggered by a constitutively active form of AMPK. Moreover, the introduction of constitutively active AMPK into ρ0-A549 cells restored Na,K-ATPase α1 subunit endocytosis, as illustrated in Fig. 4B; a Western blot demonstrating the overexpression of AMPK is also shown. These results suggest that AMPK acts downstream of the mitochondria and that, during hypoxia, mitochondrion-generated ROS mediate the activation of AMPK, leading to Na,K-ATPase endocytosis.
FIG. 4.
AMPK activation promotes Na,K-ATPase endocytosis in the absence of ROS. (A) ATII cells were infected with Ad-catalase (Ad-CAT; 20 PFU/cell) and Ad-CA AMPK as described in Materials and Methods. After infection, the cells were exposed to 21 or 1.5% O2 for 60 min. Na,K-ATPase α1 abundance at the plasma membrane (PM) was determined from cell surface biotinylation, followed by streptavidin pulldown and Western blot analysis with an α1 subunit-specific antibody. Representative Western blots analyzing the expression of Na,K-ATPase α1 at the plasma membrane and in the cell lysates (TCL) as a loading control and the expression of catalase and constitutively active AMPK (CA-AMPK) in the cell lysates are shown. (B) ρ0-A549 cells were infected with Ad-null (20 PFU/cell) or Ad-CA AMPK (20 PFU/cell), and the amount of Na,K-ATPase was determined. Representative Western blots analyzing Na,K-ATPase α1 at the plasma membrane, E-cadherin as a loading control, and the expression of CA-AMPK are shown. Values are expressed as means ± SEM (n = 4). **, P < 0.01.
AMPK regulates PKCζ during hypoxia.
Since PKCζ directly phosphorylates Na,K-ATPase during hypoxia, we examined whether AMPK activation regulates PKCζ activity. Hypoxia led to PKCζ translocation to the plasma membrane, which was prevented by compound C (an AMPK inhibitor) and also by Ad-HA-DN AMPK-α (Fig. 5A), suggesting that AMPK activation occurs upstream of the hypoxia-induced PKCζ activation. To determine whether PKCζ is a direct target of AMPK phosphorylation, we first examined whether hypoxia led to the binding of AMPK and PKCζ in vivo. ATII cells were exposed to hypoxia, endogenous AMPK was immunoprecipitated from cell lysates, and the presence of PKCζ in the immunocomplex was analyzed. As depicted in Fig. 5B, there was no association between AMPK and PKCζ under normoxic conditions and the two kinases associated only during hypoxia exposure (Fig. 5B, top). Importantly, AMPK phosphorylation was required for this interaction to occur (Fig. 5B, middle). The reverse experiments (the immunoprecipitation of PKCζ and Western blotting with pAMPK) yielded the same results (Fig. 5C). Moreover, in an in vitro assay, catalytically active AMPK phosphorylated purified full-length PKCζ-GST fusion protein (Fig. 5D, left lane) to a level much higher than the basal level of PKCζ autophosphorylation (Fig. 5D, right lane). These results suggest that during hypoxia AMPK is upstream of PKCζ and that PKCζ is a direct substrate of AMPK.
FIG. 5.
AMPK binds to and activates PKCζ in ATII cells during hypoxia. (A) ATII cells were infected with Ad-null (20 PFU/cell) or Ad-HA-DN AMPK-α (DN AMPK-α; 20 PFU/cell) or were pretreated with compound C (20 μM for 30 min) and exposed to 21 or 1.5% O2 for 10 min. A 1% Triton X-100-soluble membrane fraction was obtained, and PKCζ translocation was evaluated by Western blotting. Representative Western blots for PKCζ and E-cadherin (as a loading control) at the plasma membrane and for the expression levels of AMPK in the cell lysates are shown. The graph represents the PKCζ/E-cadherin ratios at the plasma membrane. Values are expressed as means ± SEM (n = 4). **, P < 0.01. (B and C) ATII cells were exposed to 21 or 1.5% O2 for 10 min. At the end of the incubation, cells were lysed and equal amounts (500 μg) of proteins were immunoprecipitated (IP) with anti-AMPK antibody or rabbit immunoglobulin G (IgG) as a control (B) or with anti-PKCζ antibody or mouse IgG (C). Immunocomplexes were analyzed by Western blotting with antibodies specific for pAMPK, total AMPK, and total PKCζ. Representative Western blots are shown. (D) PKCζ-GST fusion protein (150 ng) was incubated in the presence (+) or absence (−) of the constitutively active AMPK α1-GST fusion protein (200 ng) and [γ-32P]ATP. A representative autoradiograph of phosphorylated PKCζ and Western blots (WB) analyzing PKCζ and AMPK are shown (n = 3).
AMPK phosphorylation of PKCζ at the Thr410 residue is required for hypoxia-induced Na,K-ATPase endocytosis.
The phosphorylation of Thr410 in the PKCζ activation loop is critical for PKCζ activity (27). To further characterize the interaction between AMPK and PKCζ, we evaluated whether AMPK phosphorylates PKCζ at Thr410. We inhibited AMPK pharmacologically (with compound C) or genetically (with a dominant-negative AMPK construct) and then exposed the cells to 1.5% O2 for 10 min, immunoprecipitated PKCζ, and analyzed the phosphorylation state of PKCζ residue T410 using a phospho-T410 antibody. As shown in Fig. 6A, the inhibition of AMPK with either compound C or dominant-negative AMPK prevented hypoxia-induced PKCζ phosphorylation at T410, suggesting that AMPK is required for PKCζ T410 phosphorylation. Moreover, the treatment of ATII cells with AICAR (Fig. 6B, left) or the overexpression of constitutively active AMPK (Fig. 6B, right) also led to the phosphorylation of PKCζ at T410. These results were confirmed in a kinase assay in which COS-7 cells were transiently transfected with either FLAG-WT PKCζ or FLAG-T410A mutant PKCζ and PKCζ was immunoprecipitated 48 h later. In vitro, AMPK phosphorylated WT PKCζ but failed to phosphorylate T410A mutant PKCζ (Fig. 6C).
FIG. 6.
AMPK phosphorylation of PKCζ at the Thr410 residue is required for hypoxia-induced downregulation of Na,K-ATPase. (A) ATII cells were infected with Ad-null (20 PFU/cell) or Ad-HA-DN AMPK-α (DN-AMPK α; 20 PFU/cell) or pretreated with compound C (2 μM for 30 min) and exposed to 21 or 1.5% O2 for 10 min. PKCζ was immunoprecipitated from the cell lysates as described in Materials and Methods. PKCζ phosphorylated at Thr410 [pPKCζ (T410)] and the total amount of PKCζ were measured by Western blot analysis. Representative Western blots for pPKCζ (T410), PKCζ, and HA-AMPK in the cell lysates are shown (n = 4). (B) ATII cells were treated with AICAR (2 mM) for 15 min or Ad-CA AMPK (CA-AMPK; 20 PFU/cell). PKCζ was immunoprecipitated from the cell lysate as described in Materials and Methods. pPKCζ (T410), pACC as a control for AMPK activation, and the total amount of PKCζ were measured by Western blot analysis (n = 4). Representative Western blots are shown. V, vehicle. (C) COS-7 cells were transiently transfected with full-length FLAG-WT PKCζ, FLAG-T410A mutant PKCζ, or empty vector (EV); 48 h later, PKCζ was immunoprecipitated with 1 μl of FLAG antibody as described in Materials and Methods and incubated with AMPK α1-GST fusion protein (200 ng) and [γ-32P]ATP. A representative autoradiograph of the phosphorylated PKCζ and a Western blot (WB) analyzing FLAG-PKCζ are shown (n = 3). (D) COS-7 cells were transiently transfected with a vector expressing full-length FLAG-WT PKCζ or FLAG-T410A mutant PKCζ or an empty vector; 48 h later, cells were exposed to 21 or 1.5% O2 for 60 min. Na,K-ATPase α1 subunit abundance at the plasma membrane was determined from cell surface biotinylation, followed by streptavidin pulldown and Western blot analysis using specific antibodies. Representative Western blots analyzing the Na,K-ATPase α1 subunit at the plasma membrane (PM) and the Na,K-ATPase α1 subunit (as a loading control) and PKCζ in the cell lysates (TCL) are shown. Values are expressed as means ± SEM (n = 4). **, P < 0.01.
We next studied the role of the phosphorylation of PKCζ T410 in hypoxia-induced Na,K-ATPase endocytosis. Hypoxia led to Na,K-ATPase endocytosis in COS-7 cells transfected with an empty vector or with a vector expressing WT PKCζ; however, in cells transfected with the mutant PKCζ construct, endocytosis was prevented (Fig. 6D). We confirmed the overexpression of these constructs by Western blotting with a PKCζ antibody (Fig. 6D, bottom). In the lanes corresponding to extracts from cells infected with an empty vector, there is a faint band representing endogenous rat PKCζ. Collectively, these results suggest that AMPK can directly phosphorylate PKCζ at T410 and that this phosphorylation is physiologically relevant, leading to Na,K-ATPase endocytosis.
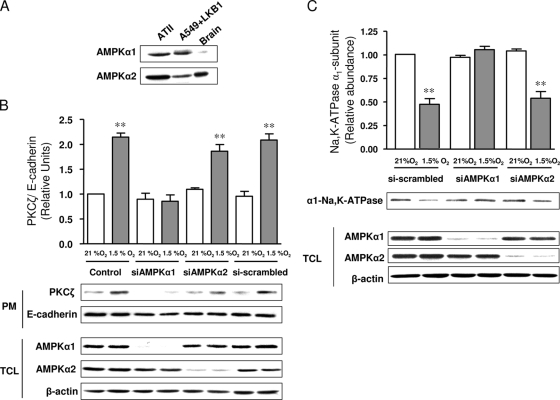
AMPK α1 is required for hypoxia-induced PKCζ activation.
Rat ATII and human A549 cells express both the α1 and α2 isoforms of AMPK (Fig. 7A). Because WT A549 cells do not express LKB1, we performed experiments with A459 cells carrying reconstituted LKB1. To determine whether both AMPK α subunits contribute to hypoxia-induced PKCζ activation, we transfected A549+LKB1 cells with siRNA to selectively knock down each isoform. As depicted in Fig. 7B, the transient transfection of cells with siRNA targeting AMPK α1 or AMPK α2 reduced AMPK α1 (by ∼92%) or AMPK α2 (by 85%), respectively, while transfection with a scrambled siRNA duplex did not affect the level of expression of either AMPK α1 or AMPK α2. The knockdown of AMPK α1 prevented hypoxia-induced PKCζ translocation to the plasma membrane, whereas the depletion of AMPK α2 had no effect (Fig. 7B). As expected, hypoxia-mediated PKCζ translocation in cells transfected with the scrambled siRNA duplex was comparable to that in nontransfected control cells (Fig. 7B). Moreover, the knockdown of AMPK α1 prevented hypoxia-induced Na,K-ATPase endocytosis, while the knockdown of AMPK α2 had no effect (Fig. 7C).
FIG. 7.
AMPK α1 is required for hypoxia-induced PKCζ activation. (A) Western blot showing the expression of AMPK α1 and α2 in rat ATII and A549+LKB1 cells and a brain cell lysate as a positive control. (B) A549+LKB1 cells were transfected with siRNA against AMPK α1 (siAMPKα1) or AMPK α2 (siAMPKα2) or with scrambled siRNA (si-scrambled); 48 h later, cells were exposed to 21 or 1.5% O2 for 10 min. PKCζ plasma membrane translocation was assessed as described above. The graph represents the PKCζ/E-cadherin ratios at the plasma membrane (PM). Representative Western blots analyzing PKCζ, E-cadherin (loading-control), AMPK α1, AMPK α2, and β-actin expression levels in the cell lysates (TCL) are shown. Values are expressed as means ± SEM (n = 4). **, P < 0.01. (C) A549+LKB1 cells were transfected with siRNA against AMPK α1 or AMPK α2 or with scrambled siRNA; 48 h later, cells were exposed to 21 or 1.5% O2 for 10 min. Na,K-ATPase was evaluated as described above. The graph represents the abundance of Na,K-ATPase at the plasma membrane. Representative Western blots analyzing Na,K-ATPase, AMPK α1 and AMPK α2, and β-actin expression levels in whole-cell lysates (TCL) are shown. Values are expressed as means ± SEM (n = 4). **, P < 0.01.
DISCUSSION
Here, we provide evidence that hypoxia induces Na,K-ATPase endocytosis in AEC via mitochondrial ROS and AMPK α1 activation. Moreover, we identify AMPK as a novel upstream kinase responsible for PKCζ phosphorylation at Thr410.
Hypoxia leads to rapid and transient AMPK activation in AEC and to the phosphorylation of ACC, a hallmark of AMPK activation (18, 36). The inhibition of AMPK activity via the overexpression of a catalytically inactive mutant of AMPK α prevents hypoxia-induced Na,K-ATPase endocytosis, suggesting that AMPK is necessary for this process. Because AMPK was reported previously to inhibit the ENaC, we investigated whether the observed effects of AMPK on Na,K-ATPase were secondary to changes in intracellular Na+ levels (2). Monensin leads to changes in intracellular Na+ concentrations and Na,K-ATPase catalytic activity without altering the incorporation of new molecules into the plasma membrane (1). Pretreatment with monensin did not prevent hypoxia-induced Na,K-ATPase endocytosis in cells infected with Ad-HA-DN AMPK-α, suggesting that AMPK has a direct effect on Na,K-ATPase trafficking rather than a secondary effect due to changes in Na+ concentrations. In addition to hypoxia, high CO2 levels lead to the endocytosis of Na,K-ATPase via AMPK (40). Interestingly, while these two stimuli seem to activate different signaling pathways (CO2 does not increase the generation of ROS), both converge in the phosphorylation of AMPK.
An important finding of the present study is that ROS generated at the mitochondrial level are required for the activation of AMPK. ROS are typically described as toxic by-products of metabolism, but at low levels ROS can act as signaling molecules, influencing key physiological processes (11). We have shown previously that ROS generated by mitochondrial complex III are required for hypoxia-induced Na,K-ATPase endocytosis and that H2O2 can restore the endocytosis of the Na,K-ATPase in [rho0] cells (9, 10). In the present study, we provide evidence that hypoxia-induced AMPK activation is prevented in cells overexpressing catalase and also in cells depleted of mitochondrial DNA and that H2O2 leads to ACC phosphorylation in these cells, suggesting that mitochondrial ROS act upstream of AMPK. The fact that in ρ0-A549 cells or in cells overexpressing catalase, constitutively active AMPK restores the ability of the cells to bring about Na,K-ATPase endocytosis provides a physiological link between the production of ROS and the activation of AMPK during hypoxia. Mitochondria have previously been proposed to act as oxygen sensors (4, 29). Low oxygen concentrations lead to superoxide generation at the Qo site of complex III (24); superoxide is then converted to H2O2 and diffuses into the cytosol, activating cellular signaling pathways (44). AMPK activity is regulated by upstream kinases; whether H2O2 acts directly on these enzymes or regulates the activity of a specific phosphatase that in turn regulates the AMPK kinase remains unclear and is outside the scope of this investigation. These results are in agreement with the findings of a recent study of mouse embryonic fibroblasts in which mitochondrion-generated ROS led to AMPK activation (14).
We have reported previously that PKCζ phosphorylates the Na,K-ATPase α1 subunit at Ser18, triggering Na,K-ATPase endocytosis (3, 9). Here, we observe that after activation by hypoxia, AMPK interacts with and phosphorylates PKCζ, as evidenced by the following: (i) pharmacologic and genetic inhibition of AMPK prevented PKCζ phosphorylation and translocation to the plasma membrane, (ii) AMPK and PKCζ were found in a complex only during hypoxia, and (iii) in vitro-purified catalytically active AMPK phosphorylated PKCζ. These data are consistent with the results of a previous study in which AICAR and the overexpression of constitutively active AMPK led to PKCζ activation and Na,K-ATPase endocytosis (40).
PKCζ consists of an N-terminal regulatory domain and a C-terminal kinase domain (20). Phosphorylation in the activation loop of the kinase domain at Thr410 exposes the kinase domain for further phosphorylation and is essential for PKCζ activity (20). In cells transfected with a T410A mutant PKCζ construct, the phosphorylation of PKCζ by AMPK was lost (Fig. 6A). To confirm that AMPK phosphorylates PKCζ at T410, we inhibited AMPK activity, which prevented the hypoxia-mediated phosphorylation at T410 (Fig. 6A), suggesting that phosphorylation at T410 is AMPK dependent. Consistent with these results, AMPK activation led to robust phosphorylation of PKCζ at T410 and hypoxia-induced Na,K-ATPase endocytosis was prevented in cells transfected to express T410A mutant PKCζ. The level of endocytosis achieved with the T410 mutant was comparable to the levels obtained using specific pharmacological inhibitors or the Ad-HA-DN AMPK-α construct, suggesting that phosphorylation at T410 is essential for PKCζ activation and Na,K-ATPase endocytosis. Phosphorylation at T410 by 3-phosphoinositide-dependent protein kinase 1 is required for insulin-induced GLUT4 exocytosis, and until now, 3-phosphoinositide-dependent protein kinase 1 has been the only kinase reported to phosphorylate PKCζ (35).
Our data show that AEC express both α1 and α2 AMPK catalytic subunits and that PKCζ activation and Na,K-ATPase endocytosis during hypoxia may be preferentially regulated by AMPK α1. Several kinases have been shown previously to phosphorylate AMPK; among them, LKB1 is involved in transducing signals generated by changes in the cellular energy status, and Ca2+/calmodulin-dependent kinase kinase responds to changes in intracellular calcium levels (22, 43). AMPK α2 activation has been shown previously to play a role in the regulation of metabolic homeostatic mechanisms and is associated with an increase in the AMP/ATP ratio. AMPK α1 is known to be less sensitive to AMP than AMPK α2 and is activated under conditions such as oxidative stress, and specifically, peroxynitrate and H2O2 have been reported to activate AMPK α1 (39, 45). We have reported previously that short-term hypoxia decreases Na,K-ATPase activity but does not change ATP levels (9). However, prolonged hypoxia exposure leads to AMPK activation and changes in the AMP/ATP ratio (28). The fact that AMPK α1 is involved in PKCζ activation and Na,K-ATPase endocytosis is consistent with ROS generation upstream of AMPK activation and suggests that the identity of the AMPK catalytic subunit activated during hypoxia may be time dependent, with short-term exposures activating the α1 subunit.
In conclusion, as depicted schematically in Fig. 8, we have shown that AMPK α1 activation (via mitochondrial ROS) is necessary for Na,K-ATPase endocytosis during hypoxia and that PKCζ is a target substrate for AMPK, which is of biological relevance.
FIG. 8.
Schematic illustration of the hypoxia-induced signaling pathway leading to Na,K-ATPase downregulation. Hypoxia increases mitochondrial ROS production and leads to AMPK α1 phosphorylation. Activated AMPK directly phosphorylates PKCζ at T410, and PKCζ in turn phosphorylates the Na,K-ATPase α1 subunit at Ser18, triggering its endocytosis.
Acknowledgments
We acknowledge Lynn Welch for the valuable insights to the manuscript.
This work was supported in part by NIH grants HL093014 and PO1-HL071643.
Footnotes
Published ahead of print on 20 April 2009.
REFERENCES
- 1.Bertorello, A. M., Y. Komarova, K. Smith, I. B. Leibiger, R. Efendiev, C. H. Pedemonte, G. Borisy, and J. I. Sznajder. 2003. Analysis of Na+,K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol. Biol. Cell 141149-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhalla, V., N. M. Oyster, A. C. Fitch, M. A. Wijngaarden, D. Neumann, U. Schlattner, D. Pearce, and K. R. Hallows. 2006. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J. Biol. Chem. 28126159-26169. [DOI] [PubMed] [Google Scholar]
- 3.Briva, A., I. Vadasz, E. Lecuona, L. C. Welch, J. Chen, L. A. Dada, H. E. Trejo, V. Dumasius, Z. S. Azzam, P. M. Myrianthefs, D. Batlle, Y. Gruenbaum, and J. I. Sznajder. 2007. High CO2 levels impair alveolar epithelial function independently of pH. PLoS ONE 2e1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunelle, J. K., E. L. Bell, N. M. Quesada, K. Vercauteren, V. Tiranti, M. Zeviani, R. C. Scarpulla, and N. S. Chandel. 2005. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 1409-414. [DOI] [PubMed] [Google Scholar]
- 5.Carling, D., V. A. Zammit, and D. G. Hardie. 1987. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 223217-222. [DOI] [PubMed] [Google Scholar]
- 6.Carlson, C. A., and K. H. Kim. 1973. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J. Biol. Chem. 248378-380. [PubMed] [Google Scholar]
- 7.Chandel, N. S., E. Maltepe, E. Goldwasser, C. E. Mathieu, M. C. Simon, and P. T. Schumacker. 1998. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 9511715-11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comellas, A. P., L. A. Dada, E. Lecuona, L. M. Pesce, N. S. Chandel, N. Quesada, G. R. Budinger, G. J. Strous, A. Ciechanover, and J. I. Sznajder. 2006. Hypoxia-mediated degradation of Na,K-ATPase via mitochondrial reactive oxygen species and the ubiquitin-conjugating system. Circ. Res. 981314-1322. [DOI] [PubMed] [Google Scholar]
- 9.Dada, L. A., N. S. Chandel, K. M. Ridge, C. Pedemonte, A. M. Bertorello, and J. I. Sznajder. 2003. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J. Clin. Investig. 1111057-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dada, L. A., E. Novoa, E. Lecuona, H. Sun, and J. I. Sznajder. 2007. Role of the small GTPase RhoA in the hypoxia-induced decrease of plasma membrane Na,K-ATPase in A549 cells. J. Cell Sci. 1202214-2222. [DOI] [PubMed] [Google Scholar]
- 11.D'Autreaux, B., and M. B. Toledano. 2007. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8813-824. [DOI] [PubMed] [Google Scholar]
- 12.Dyck, J. R., G. Gao, J. Widmer, D. Stapleton, C. S. Fernandez, B. E. Kemp, and L. A. Witters. 1996. Regulation of 5′-AMP-activated protein kinase activity by the noncatalytic beta and gamma subunits. J. Biol. Chem. 27117798-17803. [DOI] [PubMed] [Google Scholar]
- 13.Efendiev, R., A. M. Bertorello, R. Zandomeni, A. R. Cinelli, and C. H. Pedemonte. 2002. Agonist-dependent regulation of renal Na+,K+-ATPase activity is modulated by intracellular sodium concentration. J. Biol. Chem. 27711489-11496. [DOI] [PubMed] [Google Scholar]
- 14.Emerling, B. M., F. Weinberg, C. Snyder, Z. Burgess, G. M. Mutlu, B. Viollet, G. R. Budinger, and N. S. Chandel. 2009. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic. Biol. Med. 461386-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallows, K. R. 2005. Emerging role of AMP-activated protein kinase in coupling membrane transport to cellular metabolism. Curr. Opin. Nephrol Hypertens. 14464-471. [DOI] [PubMed] [Google Scholar]
- 16.Hallows, K. R., G. P. Kobinger, J. M. Wilson, L. A. Witters, and J. K. Foskett. 2003. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am. J. Physiol. Cell Physiol. 284C1297-C1308. [DOI] [PubMed] [Google Scholar]
- 17.Hallows, K. R., V. Raghuram, B. E. Kemp, L. A. Witters, and J. K. Foskett. 2000. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J. Clin. Investig. 1051711-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardie, D. G., D. Carling, and M. Carlson. 1998. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67821-855. [DOI] [PubMed] [Google Scholar]
- 19.Hawley, S. A., M. Davison, A. Woods, S. P. Davies, R. K. Beri, D. Carling, and D. G. Hardie. 1996. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 27127879-27887. [DOI] [PubMed] [Google Scholar]
- 20.Hirai, T., and K. Chida. 2003. Protein kinase Cζ (PKCζ): activation mechanisms and cellular functions. J. Biochem. 1331-7. [DOI] [PubMed] [Google Scholar]
- 21.Hochachka, P. W., L. T. Buck, C. J. Doll, and S. C. Land. 1996. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc. Natl. Acad. Sci. USA 939493-9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorgensen, S. B., and A. J. Rose. 2008. How is AMPK activity regulated in skeletal muscles during exercise? Front. Biosci. 135589-5604. [DOI] [PubMed] [Google Scholar]
- 23.King, M. P., and G. Attardi. 1996. Isolation of human cell lines lacking mitochondrial DNA. Methods Enzymol. 264304-313. [DOI] [PubMed] [Google Scholar]
- 24.Klimova, T., and N. S. Chandel. 2008. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 15660-666. [DOI] [PubMed] [Google Scholar]
- 25.Klimova, T. A., E. L. Bell, E. H. Shroff, F. D. Weinberg, C. M. Snyder, G. P. Dimri, P. T. Schumacker, G. R. S. Budinger, and N. S. Chandel. 2009. Hyperoxia-induced premature senescence requires p53 and pRb, but not mitochondrial matrix ROS. FASEB J. 23783-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam, E. W. N., R. Zwacka, E. A. Seftor, D. R. C. Nieva, B. L. Davidson, J. F. Engelhardt, M. J. C. Hendrix, and L. W. Oberley. 1999. Effects of antioxidant enzyme overexpression on the invasive phenotype of hamster cheek pouch carcinoma cells. Free Radic. Biol. Med. 27572-579. [DOI] [PubMed] [Google Scholar]
- 27.Le Good, J. A., and D. N. Brindley. 2004. Molecular mechanisms regulating protein kinase Cζ turnover and cellular transformation. Biochem. J. 37883-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, L., T. P. Cash, R. G. Jones, B. Keith, C. B. Thompson, and M. C. Simon. 2006. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol. Cell 21521-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansfield, K. D., R. D. Guzy, Y. Pan, R. M. Young, T. P. Cash, P. T. Schumacker, and M. C. Simon. 2005. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 1393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michiels, C. 2004. Physiological and pathological responses to hypoxia. Am. J. Pathol. 1641875-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minokoshi, Y., Y. B. Kim, O. D. Peroni, L. G. Fryer, C. Muller, D. Carling, and B. B. Kahn. 2002. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415339-343. [DOI] [PubMed] [Google Scholar]
- 32.Mutlu, G. M., and J. I. Sznajder. 2005. Mechanisms of pulmonary edema clearance. Am. J. Physiol. Lung Cell. Mol. Physiol. 289L685-695. [DOI] [PubMed] [Google Scholar]
- 33.Ridge, K. M., L. Dada, E. Lecuona, A. M. Bertorello, A. I. Katz, D. Mochly-Rosen, and J. I. Sznajder. 2002. Dopamine-induced exocytosis of Na,K-ATPase is dependent on activation of protein kinase C-ɛand -δ. Mol. Biol. Cell 131381-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridge, K. M., W. G. Olivera, F. Saldias, Z. Azzam, S. Horowitz, D. H. Rutschman, V. Dumasius, P. Factor, and J. I. Sznajder. 2003. Alveolar type 1 cells express the α2 Na,K-ATPase, which contributes to lung liquid clearance. Circ. Res. 92453-460. [DOI] [PubMed] [Google Scholar]
- 35.Standaert, M. L., G. Bandyopadhyay, Y. Kanoh, M. P. Sajan, and R. V. Farese. 2001. Insulin and PIP3 activate PKC-zeta by mechanisms that are both dependent and independent of phosphorylation of activation loop (T410) and autophosphorylation (T560) sites. Biochemistry 40249-255. [DOI] [PubMed] [Google Scholar]
- 36.Stapleton, D., K. I. Mitchelhill, G. Gao, J. Widmer, B. J. Michell, T. Teh, C. M. House, C. S. Fernandez, T. Cox, L. A. Witters, and B. E. Kemp. 1996. Mammalian AMP-activated protein kinase subfamily. J. Biol. Chem. 271611-614. [DOI] [PubMed] [Google Scholar]
- 37.Sznajder, J. I. 2001. Alveolar edema must be cleared for the acute respiratory distress syndrome patient to survive. Am. J. Respir. Crit. Care Med. 1631293-1294. [DOI] [PubMed] [Google Scholar]
- 38.Sznajder, J. I., W. G. Olivera, K. M. Ridge, and D. H. Rutschman. 1995. Mechanisms of lung liquid clearance during hyperoxia in isolated rat lungs. Am. J. Respir. Crit. Care Med. 1511519-1525. [DOI] [PubMed] [Google Scholar]
- 39.Tzatsos, A., and P. N. Tsichlis. 2007. Energy depletion inhibits phosphatidylinositol 3-kinase/Akt signaling and induces apoptosis via AMP-activated protein kinase-dependent phosphorylation of IRS-1 at Ser-794. J. Biol. Chem. 28218069-18082. [DOI] [PubMed] [Google Scholar]
- 40.Vadasz, I., L. A. Dada, A. Briva, H. E. Trejo, L. C. Welch, J. Chen, P. T. Toth, E. Lecuona, L. A. Witters, P. T. Schumacker, N. S. Chandel, W. Seeger, and J. I. Sznajder. 2008. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J. Clin. Investig. 118752-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vivona, M. L., M. Matthay, M. B. Chabaud, G. Friedlander, and C. Clerici. 2001. Hypoxia reduces alveolar epithelial sodium and fluid transport in rats: reversal by beta-adrenergic agonist treatment. Am. J. Respir. Cell Mol. Biol. 25554-561. [DOI] [PubMed] [Google Scholar]
- 42.Ware, L. B., and M. A. Matthay. 2005. Clinical practice. Acute pulmonary edema. N. Engl. J. Med. 3532788-2796. [DOI] [PubMed] [Google Scholar]
- 43.Witters, L. A., B. E. Kemp, and A. R. Means. 2006. Chutes and ladders: the search for protein kinases that act on AMPK. Trends Biochem. Sci. 3113-16. [DOI] [PubMed] [Google Scholar]
- 44.Xu, D., I. I. Rovira, and T. Finkel. 2002. Oxidants painting the cysteine chapel: redox regulation of PTPs. Dev. Cell 2251-252. [DOI] [PubMed] [Google Scholar]
- 45.Zou, M. H., and Y. Wu. 2008. AMP-activated protein kinase activation as a strategy for protecting vascular endothelial function. Clin. Exp. Pharmacol. Physiol. 35535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]