Abstract
Despite extensive study, the role of phosphatidylinositol 3-kinase (PI3-kinase) activation in CD28 function has been highly contentious. To definitively address this question, we generated knock-in mice expressing mutations in two critical domains of the cytoplasmic tail of CD28. Mutation of the proximal tyrosine motif interrupted PI3-kinase binding and prevented CD28-dependent phosphorylation of protein kinase B (PKB)/Akt; however, there was no detectable effect on interleukin-2 (IL-2) secretion, expression of Bcl-XL, or on T-cell function in vivo. Furthermore, we demonstrate that signaling initiated by the C-terminal proline motif is directly responsible for tyrosine phosphorylation of phosphoinosotide-dependent kinase 1, protein kinase Cθ, and glycogen synthase kinase 3β, as well as contributing to threonine phosphorylation of PKB. T cells mutated in this domain were profoundly impaired in IL-2 secretion, and the mice had marked impairment of humoral responses as well as less severe disease manifestations in experimental allergic encephalomyelitis. These data demonstrate that the distal proline motif initiates a critical nonredundant signaling pathway, whereas direct activation of PI3-kinase by the proximal tyrosine motif of CD28 is not required for normal T-cell function.
CD28 and T-cell receptor (TCR)-derived signals act synergistically, leading to optimal T-cell proliferation, cytokine secretion, and cell survival (for a review, see reference 32). The importance of CD28 in vivo is evidenced by impaired responses of CD28-deficient mice in a number of model systems, including allergic airway inflammation and experimental allergic encephalomyelitis (EAE) (13, 34). In addition, the recent development of inhibitors of CD28 as effective therapeutics for autoimmune disease and transplant immunosuppression further emphasizes the critical role of this receptor in human disease (21, 57).
Despite extensive study, the biochemical mechanism(s) that mediates CD28 function remains incompletely understood. Specific motifs within the cytoplasmic tail of CD28 have been identified that trigger distinct signaling pathways. Binding and activation of Src family kinases to the distal proline motif (sequence PYAP) initiates signaling, whereas the proximal tyrosine motif (sequence YMNM) binds and activates the p85 subunit of phosphatidylinositol 3-kinase (PI3-kinase) as well as other adaptor proteins, including Grb2 and GADS (12, 27, 28, 33, 42, 48, 51). Studies have suggested that both motifs contribute to CD28-dependent interleukin-2 (IL-2) secretion and proliferation but that the upregulation of Bcl-XL is uniquely dependent on PI3-kinase activation by the proximal tyrosine at position 170 (11, 25, 43). The potential for extensive overlap between pathways initiated by each motif exists, as well as overlap between CD28 and TCR-derived signals, making it unclear as to whether CD28 initiates any critical, nonredundant signaling pathway.
We generated gene-targeted knock-in mice expressing either wild-type CD28 or mutations in the proximal tyrosine-based motif or the distal proline-based motif to determine the importance of these both biochemically and functionally. Our data demonstrate that the distal proline motif initiates an essential signaling pathway required for normal regulation of IL-2 secretion and CD28-dependent responses in vivo. Mutation of this motif resulted in the failure to normally phosphorylate phosphoinosotide-dependent kinase 1 (PDK1), glycogen synthase kinase 3β (GSK3β), and protein kinase Cθ (PKCθ), implicating these kinases as critical downstream mediators of CD28 function. In contrast, mutation of the proximal tyrosine motif resulted in no detectable defect in T-cell function, despite impaired binding of the p85 subunit of PI3-kinase and a lack of CD28-dependent serine phosphorylation of protein kinase B (PKB/Akt). These data strongly suggest that loss of the proximal tyrosine motif can be overcome by compensatory pathways, whereas no such redundancy exists for signaling initiated by the distal proline motif.
MATERIALS AND METHODS
Mice.
CD28 knock-in mice were generated as previously described for CD28-AYAA mice (20). A knock-in mouse expressing nonmutated CD28 was generated using a construct encoding wild-type CD28. For the CD28-Y170F knock-in mice, oligonucleotide-directed site-specific mutagenesis was performed to generate a specific mutation (TAC to TTC) resulting in substitution of phenylalanine for the tyrosine at position 170. The sequence of the entire exon was verified by direct sequence analysis. The construct was transfected into 129/Sv embryonic stem cells (line RW4; provided by the Siteman Cancer Center, Washington University, St. Louis, MO), and neomycin-resistant clones were screened for homologous recombination by Southern blotting of BglII- and EcoRI-digested DNA using 5′ and 3′ external probes, respectively (see Fig. S1 in the supplemental material). The mutation was confirmed by direct sequencing of exon IV following PCR amplification of genomic DNA, as well as by restriction digestion with Bsph1, as the mutation results in the generation of a new BspH1 site (data not shown). Germ line transmission was verified by Southern blotting using both 5′ and 3′ external probes. The presence of the mutation was further confirmed by reverse transcription-PCR amplification of the mRNA using primers specific for exon III and the 3′ untranslated region and direct sequence analysis as well as restriction digestion of the PCR product. The mice were back-bred into C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME). Genetic background was monitored by DNA microsatellite analysis (Rheumatic Diseases Speed Congenics Core, Washington University, St. Louis, MO) and confirmed to be 100% C57BL/6J. The mice were also crossed to D011.10 OVA TCR transgenic mice (generously provided by K. Murphy, Washington University School of Medicine, St. Louis, MO) for five generations and then intercrossed to generate homozygous mutants that were used for experiments. CD28-deficient mice were originally obtained from C. Thompson (University of Pennsylvania, Philadelphia). C57BL/6J and BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed under specific-pathogen-free conditions at Washington University School of Medicine. All protocols were reviewed and approved by the Washington University School of Medicine Animal Studies Committee.
Antibodies.
Anti-CD3ɛ (clone 145-2c11; hamster immunoglobulin G [IgG]) and all other fluorescently conjugated antibodies used for staining were purchased from either eBioscience (San Diego, CA) or BD Biosciences (San Jose, CA). For T-cell stimulations, anti-CD28 (clone 37.51; hamster IgG) was purchased from BD Bioscience. For immunoprecipitation, anti-CD28 (clone PV-1; hamster IgG) was purchased from Southern Biotechnology (Birmingham, AL). For Western blotting, anti-CD28 (clone M2; goat IgG) was purchased from R&D Systems (Minneapolis, MN). Antibodies directed against phosphoserine PKB/Akt (S473; clone 193H12; rabbit IgG), phosphothreonine PKB/Akt (T308; clone 244F9; rabbit monoclonal), total PKB/Akt (polyclonal rabbit IgG), phospho-p44/42 mitogen-activated protein (MAP) kinase (Erk1/2 T202/Y204; rabbit polyclonal), and total p44/42 MAP kinase (Erk1/2; rabbit polyclonal), phospho-GSK3α/β (S21/9; rabbit polyclonal), phospho-serine PDK1 (S241; rabbit polyclonal), total PDK1 (rabbit polyclonal), PI3-kinase p85 subunit (rabbit polyclonal), phospho-PKCθ (T538; rabbit polyclonal), and total PKCθ (rabbit polyclonal) were all purchased from Cell Signaling (Danvers, MA). Phospho-tyrosine PDK1 (Y9; rabbit polyclonal) was purchased from ECM Biosciences (Versailles, KY). Anti-Bcl-XL (clone 2H12; mouse IgG) was purchased from Southern Biotechnology (Birmingham, AL). Total GSK3α/β (mouse IgG) was purchased from Biosource International (Camarillo, CA). Murine CTLA4 Ig was generously provided by Wyeth Pharmaceuticals.
Proliferation and cytokine assays.
Bulk splenocytes were isolated from 6- to 8-week-old mice of each genotype by density gradient centrifugation over Lympholyte-M (Cedarlane Labs, Ontario, Canada), plated at 1 × 105 cells per well in round-bottom 96-well tissue culture plates, and stimulated as indicated for 48 h. For proliferation, each well was pulsed with 1 μCi tritiated thymidine overnight and harvested the following morning. For IL-2 assays, supernatants were harvested and IL-2 was determined by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Splenocytes from mice on the DO11.10 OVA TCR transgenic background were incubated with OVA(323-339) peptide for 72 h prior to pulsing with tritiated thymidine. All conditions were plated in quadruplicate and the means ± standard deviations of the replicate wells are reported. All experiments were repeated a minimum of three times, and representative data are presented.
For determination of proliferation in vivo, splenocytes from mice of each genotype on the DO11.10 background were isolated and labeled with 2.5 μM carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, Invitrogen Corp., Carlsbad, CA). A total of 5 × 106 labeled cells were transferred by intravenous (i.v.) injection into naïve BALB/c mice. The following day, the mice were injected intraperitoneally (i.p.) with 50 μg OVA adsorbed to 2 mg alum. Three days after OVA immunization, the mesenteric lymph node cells were isolated and stained with allophycocyanin (APC)-conjugated CD4, phycoerythrin (PE)-conjugated KJ1-26 monoclonal antibody (recognizing the clonotypic TCR), and the CFSE profiles of the CD4+/KJ1-26-positive cells were determined by flow cytometry.
For measurement of IL-4, splenocytes were isolated from mice of each genotype on the DO11.10 background and stimulated with 0.3 μM OVA(323-339) in the presence of recombinant murine IL-4 and anti-IL-12 for 7 days. The cells were then washed and rested overnight in fresh medium without cytokine or antibody and restimulated 24 h later with peptide antigen for 48 h. IL-4 in the culture supernatant was determined by ELISA (R&D Systems, Minneapolis, MN).
Radiation-induced cell death.
Splenic T cells were purified from mice of each genotype by negative selection using the Stem Sep mouse T-cell enrichment kit (Stem Cell Technologies, Vancouver, BC, Canada) and an AutoMacs magnetic separator (Miltenyi Biotech, Auburn, CA). The cells were rested overnight in serum-free medium and then left either unstimulated or stimulated with anti-CD3 alone or with anti-CD3 and anti-CD28. The cells were then washed and resuspended at 2 × 105 cells/ml in serum-free medium and exposed to γ-irradiation (3 Gy). At 24 h following irradiation, viability of the T-cell population was determined by staining with anti-CD4-PE, annexin-APC, and 7-amino-actinomycin D and analyzed by flow cytometry.
Allergic airway inflammation.
Mice were immunized i.p. on days 0 and 7 with 8 μg OVA absorbed to 2 mg alum (Sigma-Aldrich, St. Louis, MO) as previously described (34, 35). On day 14, the mice were intranasally challenged with 50 μl of 2% OVA in phosphate-buffered saline and specimens were collected on the third day following challenge. Bronchoalveolar lavage was performed by intratracheal installation of 1% bovine serum albumin in phosphate-buffered saline. Cell differentials were performed on cytospin preparations stained with a modified Wright-Giemsa stain. For histology, the lungs were inflated with neutral buffered formalin to 25 cm water pressure and fixed overnight. Samples were progressively dehydrated in ethanol and processed for sectioning and hematoxylin and eosin (H&E) staining. Serum was collected at the time of sacrifice, and antigen-specific immunoglobulin titers were determined by ELISA as previously described (10). Spleens were also harvested and frozen in Tissue-Tek optimal cutting temperature compound (Sakura Finetek, Torrance, CA) on dry ice. Frozen sections were prepared, fixed in acetone, and stained with peanut agglutinin-biotin and rat anti-mouse IgD followed by detection with alkaline phosphatase-streptavidin and goat anti-rat IgG (H+L)-horseradish peroxidase to identify germinal centers. Sections were examined for the number of germinal centers, and the mean number of germinal centers per 10× field was determined.
Experimental allergic encephalomyelitis.
Mice were injected subcutaneously with 50 μg myelin oligodendrocyte peptide in incomplete Freund's adjuvant and 500 μg mycobacterium on day zero. Pertussis toxin (300 ng) was injected i.v. on days 1 and 3. Mice were monitored daily for symptoms and scored as follows: 0, normal without overt signs of disease; 1, limp tail; 2, hind limb weakness; 3, paralysis of one hind limb; 4, paralysis of both hind limbs; 5, moribund state, death due to EAE, or euthanized for humane reasons. Central nervous system (CNS) tissues were removed from the mice at the termination of the experiment and frozen, sectioned, and stained with hematoxylin and eosin for histological analysis.
Determination of Bcl-XL expression.
For determination of Bcl-XL expression, splenocytes were isolated and stimulated for 24 h. Bcl-XL expression was measured by intracellular staining and flow cytometry or by Western blotting as previously described (20). For flow cytometry, the cells were stained with APC-conjugated anti-CD4, peridinin chlorophyll protein-conjugated anti-CD8, and PE-conjugated Bcl-XL, and expression levels of CD4- and CD8-positive cells are presented.
Western blotting and immunoprecipitation studies.
Purified splenic T cells were isolated by negative selection using immunolabeling and magnetic bead separation as described above. Following purification the cells were rested at a density of 4 × 106 cells/ml overnight at 37°C, 5% CO2 in medium containing 2% fetal bovine serum. The following day, the cells were rested in serum-free medium on ice for 1 h at a density of 2 × 106 cells/100 μl and then incubated with primary antibody(s) at the indicated concentration for 30 min on ice. The cells were then pelleted and resuspended in prewarmed serum-free medium containing 4.5 μg/ml each of anti-Syrian hamster IgG and anti-Armenian hamster IgG (Jackson Immunoresearch, West Grove, PA) and incubated at 37°C for the indicated times. The cells were then pelleted, resuspended, and boiled in 25 μl 4× sodium dodecyl sulfate (SDS) loading buffer for 10 min. Proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride membrane, and analyzed by Western blotting. Densitometry was performed using ImageJ analysis software (National Institutes of Health, Bethesda, MD). The integrated pixel density was determined for each band and the density of the phosphorylated band was normalized to that of the total protein. The normalized result was then divided by that obtained for the unstimulated condition, and the ratio of the stimulated to unstimulated condition was determined.
For immunoprecipitation, splenocytes were harvested from mice of each genotype in the DO11.10 background and cultured with 3 μM OVA(323-339) peptide for 72 h. Equal numbers of cells from each genotype were collected and lysed in radioiummunoprecipitation assay (RIPA) buffer containing both protease and phosphatase inhibitors. After clarification of the insoluble material by high-speed centrifugation, the supernatants were collected and brought to 500 μl with lysis buffer. Five micrograms of anti-CD28 (clone PV-1) or isotype control antibody was added to each sample and incubated at 4°C with rocking overnight. Protein G-Sepharose beads were preincubated with 5 μg AffiniPure goat anti-hamster IgG for 1 h and washed, and 30 μl was added to each sample and further incubated for 45 min at 4°C with gentle rocking. The beads were then collected by centrifugation, washed four times with RIPA buffer, resuspended, and boiled in 30 μl of 4× SDS sample buffer for analysis. All autoradiographs were scanned on a Powerlook 1120 scanner (UMAX Corporation, Dallas, TX) using Silverfast Ai software (Lasersoft Imaging, Longboat Key, FL). Final images were processed with Adobe Photoshop software (Adobe Systems Inc., San Jose, CA).
RESULTS
IL-2 secretion and proliferation depend on the distal proline motif but not the proximal tyrosine motif of CD28.
To determine the effects of specific mutations in the cytoplasmic domain of CD28 on T-cell function, we generated knock-in mice expressing either wild-type CD28 (CD28+/+), CD28 with a phenylalanine-for-tyrosine substitution in the proximal motif at position 170 (CD28-Y170F), or CD28 with alanine-for-proline substitutions in the distal motif at positions 187 and 190 (CD28-AYAA) (see Fig. S1 in the supplemental material). All strains were bred to homozygosity of the knock-in allele and backcrossed into the C57BL/6 background. The CD28+/+ knock-in mice were extensively compared to commercially purchased C57BL/6J mice, and no differences were detected. Unless otherwise indicated, the wild-type knock-in mice were used as controls in all experiments. CD28-deficient mice were bred and maintained as previously described (24).
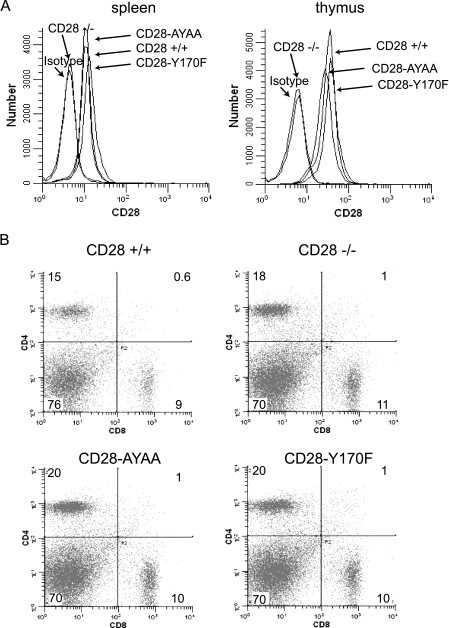
All strains expressed CD28 at similar levels and on both thymocytes and peripheral T cells (Fig. 1A). Activation-induced upregulation of CD28 expression was also similar between mutant and wild-type strains (data not shown). In addition, we detected no differences in the number and distribution of CD4 and CD8 cells in either the spleen or thymus of all genotypes (Fig. 1B and data not shown). Therefore, neither the specific mutations nor the genetic manipulation detectably altered T-cell development or CD28 expression.
FIG. 1.
(A) Expression of CD28 on CD4+ splenocytes and thymocytes from the wild-type and mutant knock-in mice. (B) CD4 and CD8 expression on splenocytes isolated from wild-type and CD28 mutant knock-in mice.
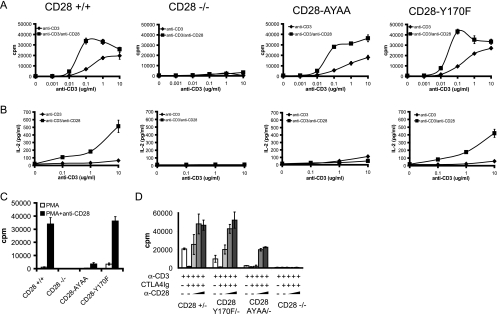
Given the hallmark importance of CD28 in T-cell proliferation and IL-2 secretion, we first examined the effect of the mutations on these responses. CD28+/+, CD28-Y170F, and CD28-AYAA cells all proliferated to similar levels following stimulation, whereas the CD28-deficient mice had markedly impaired proliferation (Fig. 2A). There was no defect observed in IL-2 secretion in T cells from the CD28-Y170F knock-in mice, in contrast to the CD28-AYAA mutation and CD28-deficient mice, which both had greatly reduced IL-2 secretion (Fig. 2B). When activated with phorbol myristate acetate (PMA) and anti-CD28, the wild-type and Y170F cells proliferated vigorously whereas the CD28-deficient and CD28-AYAA mouse cells failed to respond. All strains proliferated in response to PMA plus ionomycin stimulation (Fig. 2C and data not shown).
FIG. 2.
Proliferation and IL-2 secretion depend on the distal proline motif and not the proximal tyrosine motif. Splenocytes were isolated from mice of each genotype and stimulated with graded doses of anti-CD3 alone or in combination with anti-CD28 antibody (1 μg/ml). (A) Proliferation was determined by tritiated thymidine incorporation. (B) IL-2 was measured in culture supernatants by ELISA. (C) Cells were stimulated with PMA (5 ng/ml) alone or in combination with anti-CD28 (1 μg/ml), and proliferation was determined by tritiated thymidine incorporation. (D) Splenocytes were isolated from mice heterozygous for wild-type or mutant CD28 on the CD28−/− background, therefore expressing only one CD28 allele, and stimulated with anti-CD3 (0.1 μg/ml) alone, in combination with CTLA4 Ig (10 μg/ml), or with both CTLA4 Ig and increasing doses of anti-CD28 (0.03, 0.3, or 1.0 μg/ml). Proliferation was determined by tritiated thymidine incorporation. All experiments were repeated a minimum of three times and representative data are presented. Data are the means ± standard deviations of quadruplicate wells.
Our previous work had demonstrated that both the level of CD28 expression and the degree of receptor engagement were important determinants of whether cells expressing the mutant allele mounted a normal or reduced proliferative response (20). This was evident by examination of cells from mice expressing only one mutant allele on the CD28-deficient background and by using limiting amounts of anti-CD28 antibody. However, in contrast to the CD28-AYAA mice, there was no detectable difference between cells expressing one copy versus two copies of the Y170F allele at either low or high levels of CD28 cross-linking (Fig. 2D).
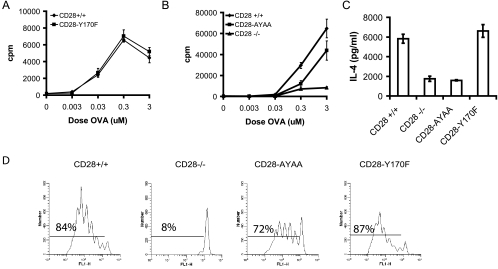
To examine additional another physiologic antigen receptor engagement, we bred the mice onto the DO11.10 TCR transgenic background and measured proliferation and cytokine secretion in response to peptide antigen. Stimulation with OVA(323-339) peptide led to similar proliferative responses in cells expressing wild-type or CD28-Y170F alleles (Fig. 3A). In contrast, T cells expressing CD28-AYAA had slightly, but reproducibly, reduced proliferation (Fig. 3B). IL-2 and IL-4 secretion was also reduced in cultures from CD28-AYAA-expressing cells but not from the CD28-Y170F cultures (Fig. 3C and data not shown). To examine proliferation in vivo we adoptively transferred CFSE-labeled splenocytes isolated from each genotype on the DO11.10 background into naïve BALB/c mice. Only the cells isolated from CD28-deficient mice manifested impaired proliferation in vivo; however, fewer cells from the CD28-AYAA mice divided beyond four generations than in the wild type or CD28-Y170F, although the significance of this observation is not clear (Fig. 3D).
FIG. 3.
Proliferation and IL-4 secretion in response to antigen is impaired in the CD28-AYAA knock-in mice but not CD28-Y170F mice. Splenocytes were isolated from mice of each genotype in the DO11.10 background. (A and B) Cells were stimulated with OVA(323-339) peptide and proliferation was measured after 72 h by tritiated thymidine incorporation. (C) Cells were cultured with OVA(323-339) peptide plus recombinant IL-4 and anti-IL-12 for 7 days. The cells were then washed, rested overnight, and restimulated with peptide for 48 h. The culture supernatant was collected and IL-4 content was assayed by ELISA. (D) Splenocytes were isolated from mice of each genotype and labeled with CFSE. A total of 5 × 106 cells were then injected i.v. into naïve BALB/c mice. The following day, the recipient mice were injected with OVA-alum (50 μg) i.p. Mesenteric lymph nodes were collected 72 h later, stained for CD4 and KJ1-26, and analyzed by flow cytometry. The CFSE profile of the KJ1-26-positive cells is presented. Representative data of three independent experiments are presented.
The Y170F mutation does not alter Bcl-XL expression or T-cell survival.
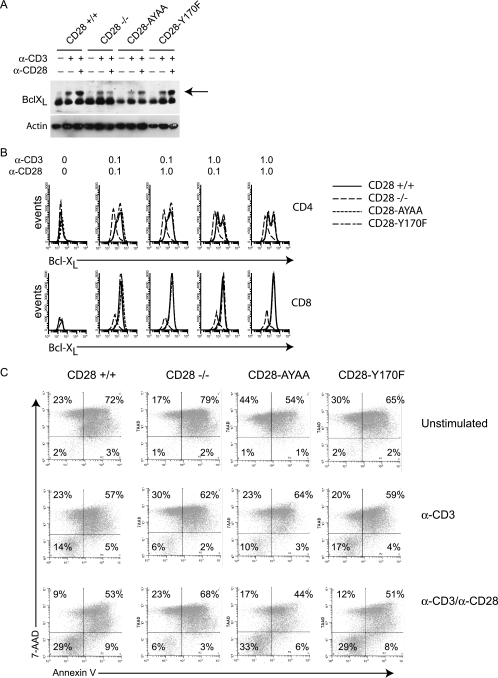
The proximal tyrosine-based motif has been shown by several groups to be essential for CD28-induced Bcl-XL upregulation, which has been shown to improve T-cell survival (9, 11, 43). Therefore, we determined both Bcl-XL expression and resistance to an apoptotic stimulus in each of the CD28 knock-in mouse groups. Surprisingly, Bcl-XL expression was not impaired in cells from the CD28-Y170F knock-in mice (Fig. 4A and B). To determine if this was related to the strength of the signal through either CD3 or CD28, we tested both low and high doses of each antibody and determined expression in both CD4- and CD8-positive cells. As expected, there was no CD28-dependent upregulation in the CD28-deficient mice. Cells from both knock-in strains upregulated Bcl-XL expression comparably to cells expressing wild-type CD28 at both 24 and 48 h following activation (Fig. 4A and B and data not shown). We directly tested the resistance of the cells to radiation-induced apoptosis (Fig. 4C). No difference was detected between the cells expressing CD28+/+, CD28-Y170F, or CD28-AYAA, whereas there were markedly fewer live CD28−/− cells. We also measured the survival of resting CD4+ T cells in culture over time. After 96 h, 83% of CD28+/+ cells were viable as determined by propidium iodide staining. In contrast 68% of CD28−/− cells were alive. Survival of the CD28-AYAA and CD28-Y170F cells was 85% and 83%, respectively. Thus, neither mutation altered cell survival, suggesting that no single motif was individually responsible for mediating the antiapoptotic effect of CD28.
FIG. 4.
Neither the proximal nor distal motif is absolutely required for T-cell survival or Bcl-XL expression. (A) Splenocytes were cultured in medium or stimulated with anti-CD3 alone (1.0 mg/ml) or with anti-CD28 (1.0 mg/ml). After 48 h, the cells were lysed and Bcl-XL expression levels were determined by Western blotting. The arrow points to the specific band. (B) Splenocytes from mice of each genotype were isolated and cultured either with medium alone or stimulated with anti-CD3 (0.1 or 1.0 μg/ml) and anti-CD28 (0.1 or 1.0 μg/ml) in the presence of CTLA4 Ig (10 μg/ml) for 48 h. The cells were surface stained with anti-CD4 or anti-CD8 followed by an intracellular stain for Bcl-XL. Shown is the Bcl-XL expression of CD4+ (top row) and CD8+ (bottom row) cells. (C) Purified T cells were stimulated as indicated for 18 h and then washed, and equal numbers of cells were exposed to γ-irradiation (3 Gy). After 24 h, cell viability was determined by staining with CD4-PE, annexin V-APC, and 7-amino-actinomycin D (7-AAD) and analyzed by flow cytometry. Representative data of three independent experiments are presented.
PI3-kinase binding and PKB/Akt phosphorylation are impaired in the CD28-Y170F mutant but are intact in the CD28-AYAA mutant.
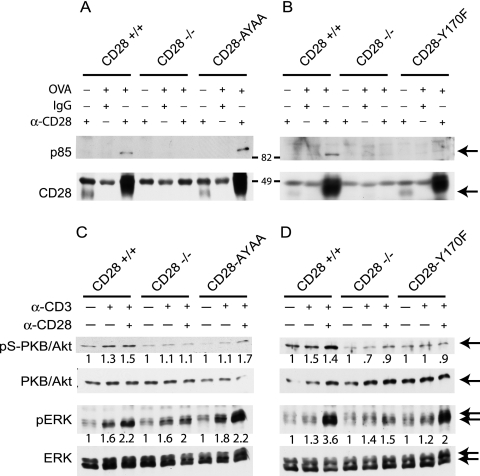
The p85 subunit of PI3-kinase binds to phosphorylated tyrosine at position 170, although it may also bind other sites in CD28 (44, 51). We directly assayed whether binding was preserved in the knock-in mice. The p85 subunit of PI3-kinase was coimmunoprecipitated with CD28 in lysates from CD28+/+ or CD28-AYAA cells, but not from CD28−/− cells or CD28-Y170F-expressing cells (Fig. 5A and B). Greater amounts of CD28 were immunoprecipitated from the activated cells, consistent with upregulation of the receptor upon cell activation, but this was not different between wild-type and mutant cells. All samples contained similar amounts of total p85 as determined by Western blotting of whole-cell lysates (data not shown).
FIG. 5.
p85 binding and serine phosphorylation of PKB/Akt are impaired in the CD28-Y170F mutant. (A and B) Splenocytes from mice of each genotype in the DO11.10 background were isolated and cultured with OVA(323-339) peptide for 72 h. Equal numbers of cells were isolated and lysed in RIPA buffer, and CD28 was immunoprecipitated. The immunoprecipitated protein was separated by SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride, and blotted for p85 or CD28. Arrows indicate the specific bands. (C and D) Purified T cells were isolated from mice of each genotype and cultured with either medium alone or stimulated for 5 min with anti-CD3 (0.1 μg/ml) alone or in combination with anti-CD28 (8 μg/ml) with cross-linking antibodies as described in Materials and Methods. Following stimulation, Western blot analysis was performed for each protein as indicated. Densitometry was performed as described in Materials and Methods, and the ratio with the unstimulated control is shown.
Activation of PKB/Akt has been shown to be a consequence of CD28-dependent activation of PI3-kinase and is thought to be essential for upregulation of Bcl-XL expression (29, 46). CD28-dependent serine phosphorylation of PKB/Akt was evident in cells from CD28+/+ mice at suboptimal levels of anti-CD3 stimulation but was absent in the cells expressing the CD28-Y170F mutant (Fig. 5C and D). Phosphorylation of PKB/Akt was preserved in the CD28-AYAA knock-in mutant cells, although at a slightly reduced intensity. We also examined more distal signaling by examining activation of the MAP kinase extracellular signal-regulated kinase (ERK). CD28 costimulation augmented ERK phosphorylation in all genotypes except for CD28−/− (Fig. 5C and D).
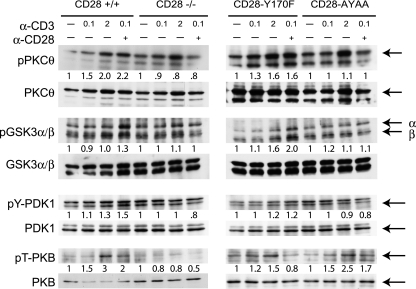
Phosphorylation of the downstream kinases, PDK1, PKCθ, and GSK3β, depends on the distal proline motif but not the proximal tyrosine motif of CD28.
Both PKCθ and GSK3β have been shown to be important elements of the signal transduction cascade initiated by CD28. PKC family members can phosphorylate and activate GSK3β, which results in nuclear trapping of NFAT, leading to prolonged transcription of NFAT responsive genes (5, 15, 18, 23, 41, 56). PKCθ signaling also contributes to the regulation of Bcl-XL expression (39). Cells expressing wild-type CD28 exhibited a CD28-dependent phosphorylation of PKCθ and GSK3β, as did the CD28-Y170F-expressing cells (Fig. 6). However, CD28-dependent phosphorylation of PCKθ and GSK3β was not evident in CD28-AYAA or CD28-deficient cells, although at higher doses of anti-CD3 both proteins were phosphorylated in the absence of CD28 ligation (Fig. 6).
FIG. 6.
Phosphorylation of PKCθ, GSK3β, and PDK1 depends on the distal proline motif but not the proximal tyrosine motif of CD28, whereas threonine phosphorylation of PKB requires both motifs. Purified T cells were isolated from mice of each genotype and cultured with either medium alone or stimulated for 5 min with the indicated doses of anti-CD3 alone or in combination with anti-CD28 (8 μg/ml) with cross-linking antibodies as described in Materials and Methods. Following stimulation, Western blot analysis was performed for each protein as indicated. Arrows indicate the specific bands. The lane order for the CD28-AYAA and CD28-Y170F pT-PKB and total PKB samples was modified from the original blot by using Adobe Photoshop software to be consistent with the other samples presented in this figure. In the original gel, the samples were loaded with CD28-AYAA in lanes 1 to 4 and CD28-Y170F in lanes 5 to 8. Densitometry was performed as described in Materials and Methods, and the ratio with the unstimulated control is shown.
We also examined GSK3β and ERK phosphorylation in each strain following stimulation with a low (0.1 μg/ml) or high (2.0 μg/ml) dose of anti-CD3 or anti-CD28 at 5, 20, and 60 min. CD28-dependent phosphorylation of GSK3β was detectable only at 5 min in cells from CD28+/+ and CD28-Y170F-expressing cells following low-dose stimulation but was not detectable in the CD28-deficient or CD28-AYAA-expressing T cells (data not shown). ERK phosphorylation was detected at 5 min but was not sustained. However, there may be important kinetic differences in signaling in vivo that are not detectable by this in vitro assay system. Previous work had suggested that PDK1 is important in signals initiated by the distal proline motif as well as in PI3-kinase-dependent pathways, thereby providing a potential linkage between signaling initiated by these motifs (3, 40). PDK1 has two phosphorylation sites, serine 241 (S241) and tyrosine 9 (Y9) (54). S241 is thought to be regulated primarily by autophosphorylation, whereas Y9 is dependent on upstream kinase activity. Consistent with this, we did not detect any effect of CD28 signaling on phosphorylation of the S241 residue (data not shown). In contrast, phosphorylation of Y9 was augmented by CD28 and was dependent upon the distal proline-based motif but not the proximal tyrosine motif (Fig. 6). PKB/Akt is an important substrate of PDK1, phosphorylating it on T308 (1). Although T308 phosphorylation was CD28 dependent, it was decreased in both mutant strains, suggesting both motifs are necessary for this site to be normally regulated (Fig. 6).
The distal proline-based motif, but not the proximal tyrosine-based motif, regulates humoral immunity in allergic airway inflammation and disease severity in EAE.
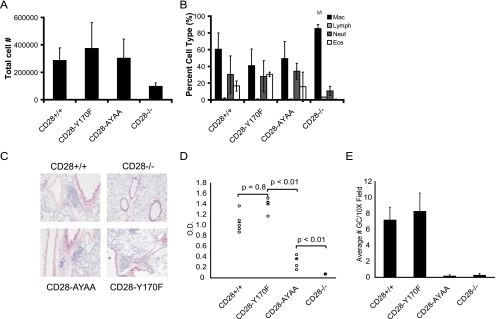
We studied how the knock-in mice responded to allergic airway inflammation and EAE, two distinct CD28-dependent disease models (13, 34). In the allergic airway inflammation model, only the CD28-deficient mice had a reduction in lung inflammation, as assayed by bronchoalveolar lavage cell counts and differentials and histology (Fig. 7A, B, and C). Both germinal center formation and isotype switching require CD28. As previously reported, both were markedly impaired in the CD28-AYAA mice and the CD28−/− mice; however, no defect was observed in the CD28-Y170F mice (Fig. 7D and E).
FIG. 7.
Allergic airway inflammation in the CD28 knock-in mice. Mice of each genotype were sensitized and given an inhaled challenged of OVA. (A and B) Total cell number (A) and differential analysis of cells (B) recovered in the bronchoalveolar lavage fluid. Mac, macrophages; Lymph, lymphocytes; Neut, neutrophils; Eos, eosinophils. (C) Histologic sections of lung tissue stained with H&E. (D) OVA-specific IgG1 titers in the serum of mice of each genotype following systemic sensitization. (E) Germinal center number as determined by staining sections of spleens with peanut agglutinin and anti-IgD.
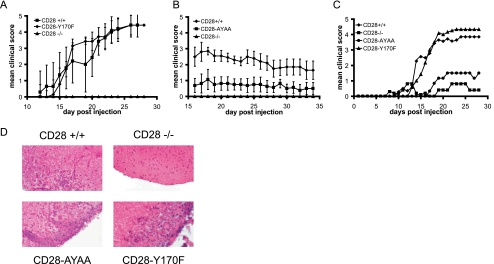
Experimental autoimmune encephalomyelitis results from self-reactive T cells that infiltrate the CNS and cause demyelination and a progressive ascending paralysis that models the human disease multiple sclerosis (16, 22). CD28-deficient mice manifest less severe disease than wild-type mice, highlighting the importance of this pathway in the response. When we tested the CD28-Y170F and CD28-AYAA mice in this model, we found that the CD28-AYAA mice had attenuated disease, whereas the CD28-Y170F mice manifested a clinical score similar to wild-type mice (Fig. 8). Despite differences in the clinical score, all genotypes, with the exception of the CD28-deficient mice, had inflammatory infiltrates in the CNS (Fig. 8D).
FIG. 8.
Disease severity in EAE is reduced in CD28-AYAA mice. Mice of each genotype were immunized with myelin oligodendrocyte protein as described in Materials and Methods. The clinical score was determined in a blinded fashion. For each experiment 8 to 10 mice of each genotype were included. Independent experiments were performed comparing CD28+/+ and CD28−/− mice with either CD28-Y170F (A) or CD28-AYAA (B) or all genotypes together (C). For clarity, error bars are not shown in this panel. There were no statistically significant differences in disease severity for the CD28+/+ and CD28-Y170F mice at any point. At later time points, there was a significant difference between the CD28-AYAA and CD28−/− mice. (D) Representative sections of brain stained with H&E.
DISCUSSION
CD28 is unique among costimulatory receptors in its potency to increase IL-2 secretion. Our data demonstrate that net IL-2 secretion is critically dependent on the distal proline motif of CD28 but not on the proximal tyrosine motif. CD28 regulates IL-2 by both transcriptional and posttranscriptional mechanisms (19, 30, 37, 53). Transcriptional control is exerted by the binding of multiple transcription factors to the IL-2 locus, including NFAT, AP-1, and NF-κB. Multiple pathways converge at these transcription factors, making them likely points of signal integration.
Among the many signaling intermediates downstream of CD28, recent data have emphasized the importance of PKB/Akt (29, 31, 40, 46, 58). PKB activation by CD28 is mediated by binding of PI3-kinase to the proximal tyrosine motif and is thought to be essential for upregulation of Bcl-XL as well as important in the regulation of IL-2 and gamma interferon secretion (31, 43, 46). Consistent with this, we found that the Y170F mutant failed to bind the p85 subunit of PI3-kinase and did not phosphorylate PKB on Ser473. However, despite these defects, expression of Bcl-XL was normal in this mutant. Thus, our data strongly suggest that there are at least two overlapping pathways that can lead to Bcl-XL expression. One is mediated by the proximal tyrosine-based motif binding PI3-kinase and subsequent activation of PKB/Akt. However, when this pathway is no longer operative, redundant pathways are sufficient to drive Bcl-XL expression.
Full activation of PKB/Akt requires not only serine phosphorylation but also phosphorylation by PDK1 at T308 (1, 7, 14). PDK1, in part, depends upon PI3-kinase for activation. Binding of PIP2 facilitates the membrane localization of PDK1 and subsequent autophosphorylation of S241. However, tyrosine phosphorylation of PDK1 by Src kinases is an additional regulatory mechanism (45). The importance of CD28 in tyrosine phosphorylation of PDK1 has not previously been reported. Given that PDK1 is implicated in activating PKB/Akt by phosphorylation of T308, we also examined this phosphorylation event in the knock-in strains. When examined in our knock-in mice, we found a CD28 dependence to tyrosine phosphorylation of PDK1 and that this required the distal proline motif. CD28 did regulate T308 phosphorylation of PKB, however; both mutants were defective in this, suggesting that the pathways cooperate to fully activate PKB. These data support a model in which CD28 can activate PKB by multiple mechanisms, including via Lck-mediated activation of PDK1.
PKCθ, a nonconventional member of the protein kinase C family, has been demonstrated to be a critical downstream effector of CD28 (50). We found that CD28-dependent phosphorylation of PKCθ was defective only in the CD28-AYAA and CD28-deficient mice. PKCθ serves a nonredundant function in T-cell activation, being important in NF-κB-, AP-1-, and possibly NFAT-mediated activation of IL-2 gene transcription (26, 36, 47, 52). In addition to its effect on IL-2, PKCθ also regulates Bcl-XL expression (39). PKCθ-deficient mice are unable to upregulate Bcl-XL in response to CD3/CD28 stimulation, and ectopic expression of PKCθ can drive expression of a Bcl-XL reporter via NF-κB and AP-1 transcription factors. Thus, the profound defect in IL-2 observed in the CD28-AYAA knock-in mice is consistent with a failure to activate PKCθ. Furthermore, preserved activation of PKCθ in the CD28-Y170F mutant suggests that this pathway might compensate for defective PKB/Akt activation and mediate expression of Bcl-XL.
Phosphorylation of PKCθ occurs at several sites and can be potentially mediated by several kinases, including PKB/Akt, PDK1, and Lck (6, 8, 17, 38). Our data demonstrate that phosphorylation of T538 depends on the distal proline motif, suggesting an important role for PDK1 in activating PKCθ. In addition, the proximal tyrosine motif and PI3-kinase activation have been implicated in the normal activation and localization of PKCθ. When this motif was disrupted, PKCθ was not recruited to the cSMAC and CD28-dependent transcriptional upregulation of IL-2 gene expression was lost (49). However, there was no change in net IL-2 secretion. As we did not directly examine IL-2 gene transcription or the localization of PKCθ, we cannot exclude these effects, and the alterations we observed in IL-2 secretion remain consistent with these results.
Recently, GSK3β has been implicated in CD28 signaling (4, 15, 58). Phosphorylation of the regulatory serine residue of GSK3β results in its inactivation (2). Active GSK3β promotes the nuclear export of the transcription factor NFAT; thus, its inactivation by phosphorylation promotes persistence of NFAT in the nucleus and increased transcription of NFAT-regulated genes (15). When examined in the knock-in mutant strains, GSK3β phosphorylation was preserved in the CD28-Y170F mutant but was absent in the CD28-AYAA mutant. Thus, one mechanism by which the proline mutant might impair IL-2 gene expression is through the failure to properly regulate NFAT.
Both PKB/Akt and PKC isoforms have been implicated in phosphorylating GSK3β; however, despite impaired activation of PKB/Akt, GSK3β phosphorylation remained intact in the CD28-Y170F mutant (5, 18, 23, 55, 56). Thus, alternative kinases capable of regulating GSK3β must remain operative. It has been demonstrated in several systems that members of the PKC family are able to phosphorylate GSK3β, although we are unaware of direct evidence demonstrating this for PKCθ (18, 23). Nonetheless, our data are consistent with a role for PKCθ in the regulation of GSK3β.
In vitro systems are necessarily reductionist and as such may not accurately reflect events that occur in vivo. Using two independent models of T-cell immunity, we found that only the CD28-AYAA and CD28-deficient mice manifested alterations in T-cell responses in vivo. There was a profound defect in humoral responses in the CD28-AYAA mice but not the CD28-Y170F mice in an allergic airway inflammation model. Similarly, there was a marked reduction in EAE disease severity in the CD28-AYAA mice but normal clinical responses in the CD28-Y170F mice. In both models, the defect in the CD28-AYAA mice was not as severe as that observed in the CD28-deficient mice, suggesting residual receptor function. The CD28-AYAA mice had evidence of tissue inflammation, but disease severity was lessened. This would be consistent with intact cell recruitment but impaired effector function. Nonetheless, the in vivo observations are consistent with our in vitro experiments demonstrating impairment in only the CD28-AYAA mutation.
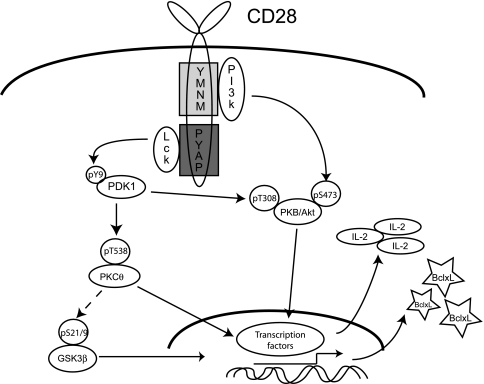
Taken together, our data strongly support a model for CD28 signaling and function in which two partially overlapping biochemical pathways exist (Fig. 9). In the wild-type setting, PI3-kinase is activated by binding to the proximal tyrosine motif, resulting in serine phosphorylation and activation of PKB/Akt. Concomitantly, activation of Lck by the distal proline domain results in the tyrosine phosphorylation of PDK1, which in turn also acts on PKB/Akt. Our data demonstrate that phosphorylation of PKCθ and GSK3β requires the proline motif and suggest that PDK1 is upstream of these effects, although we do not exclude a role for other upstream kinases. Importantly, we identify PDK1 as a point capable of linking signaling pathways initiated by the proximal tyrosine-based and distal proline-based motifs. Although published data suggest that there is significant cross talk in both directions of this pathway, our data suggest that in fact such compensation is predominantly unidirectional, as only the distal proline mutant manifests a detectable phenotype.
FIG. 9.
Model of CD28 signaling pathways initiated by the distal and proximal motifs. Pathways mediated by the proximal tyrosine motif are initiated by the binding of the p85 subunit of PI3-kinase, leading to S473 phosphorylation of PKB/AKT, which can activate gene transcription through NF-κB. Additionally, the distal proline motif activates Lck, which in turn results in tyrosine phosphorylation of PDK1. PDK1 can then phosphorylate PKB on T308 as well as PKCθ on T538. Phosphorylation of GSK3β is also dependent on the distal proline motif, perhaps through PKCθ. Both PKCθ phosphorylation and GSKβ phosphorylation lead to increased gene transcription through NF-κB, NFAT, and AP-1. The critical, nonredundant signaling pathway is primarily provided by the distal proline motif, in that mutation of the proximal tyrosine motif does not functionally impair T-cell responses, whereas mutation of the proline severely impairs CD28 function.
Supplementary Material
Acknowledgments
This work was supported by a grant to J.M.G. from the NIH (HL062683). L.F.D. and C.M.D. are supported by NIH training grant T32HL07317. We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO, for the use of the Embryonic Stem Cell Core, which provided embryonic stem cell transfection services. The Siteman Cancer Center is supported in part by an NCI Cancer Center Support grant (P30 CA91842).
We thank A. Shaw, W. Swat, and A. Gelman for helpful discussion and critical review of the manuscript. We thank Ron McCarthy for expert microinjections.
We have no conflicting financial interests.
L.D.F. contributed to the data presented in Fig. 1, 2, 3, 4, 7, and 8. J.S.B. contributed to the data presented in Fig. 5, 6, and 9. C.D. contributed to data presented in Fig. 4, 7, and 8. D.D.S. designed and generated the knock-in construct and performed all Southern analysis and verifications and contributed to the data presented in Fig. S1 in the supplemental material. T.B. maintained the breedings and contributed to the data presented in Fig. S1 of the supplemental material. J.S. and J.H.R. contributed to the data presented in Fig. 8. J.M.G. oversaw and directed all phases of this project. All authors have reviewed the final version of the manuscript and concur with the submission.
Footnotes
Published ahead of print on 27 April 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7261-269. [DOI] [PubMed] [Google Scholar]
- 2.Ali, A., K. P. Hoeflich, and J. R. Woodgett. 2001. Glycogen synthase kinase-3: properties, functions, and regulation. Chem. Rev. 1012527-2540. [DOI] [PubMed] [Google Scholar]
- 3.Andres, P. G., K. C. Howland, A. Nirula, L. P. Kane, L. Barron, D. Dresnek, A. Sadra, J. Imboden, A. Weiss, and A. K. Abbas. 2004. Distinct regions in the CD28 cytoplasmic domain are required for T helper type 2 differentiation. Nat. Immunol. 5435-442. [DOI] [PubMed] [Google Scholar]
- 4.Appleman, L. J., A. A. van Puijenbroek, K. M. Shu, L. M. Nadler, and V. A. Boussiotis. 2002. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J. Immunol. 1682729-2736. [DOI] [PubMed] [Google Scholar]
- 5.Ballou, L. M., P.-Y. Tian, H.-Y. Lin, Y.-P. Jiang, and R. Z. Lin. 2001. Dual regulation of glycogen synthase kinase-3β by the α1A-adrenergic receptor. J. Biol. Chem. 27640910-40916. [DOI] [PubMed] [Google Scholar]
- 6.Bauer, B., N. Krumbock, F. Fresser, F. Hochholdinger, M. Spitaler, A. Simm, F. Uberall, B. Schraven, and G. Baier. 2001. Complex formation and cooperation of protein kinase Cθ and Akt1/protein kinase Bα in the NF-kappa B transactivation cascade in Jurkat T cells. J. Biol. Chem. 27631627-31634. [DOI] [PubMed] [Google Scholar]
- 7.Bayascas, J. R., and D. R. Alessi. 2005. Regulation of Akt/PKB Ser473 phosphorylation. Mol. Cell 18143-145. [DOI] [PubMed] [Google Scholar]
- 8.Bi, K., Y. Tanaka, N. Coudronniere, K. Sugie, S. Hong, M. J. van Stipdonk, and A. Altman. 2001. Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat. Immunol. 2556-563. [DOI] [PubMed] [Google Scholar]
- 9.Boise, L. H., A. J. Minn, P. J. Noel, C. H. June, M. A. Accavitti, T. Lindsten, and C. B. Thompson. 1995. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-xL. Immunity 387-98. [DOI] [PubMed] [Google Scholar]
- 10.Burr, J. S., S. L. Kimzey, D. R. Randolph, and J. M. Green. 2001. CD28 and CTLA4 coordinately regulate airway inflammatory cell recruitment and T-helper cell differentiation after inhaled allergen. Am. J. Respir. Cell. Mol. Biol. 24563-568. [DOI] [PubMed] [Google Scholar]
- 11.Burr, J. S., N. D. L. Savage, G. E. Messah, S. L. Kimzey, A. S. Shaw, R. H. Arch., and J. M. Green. 2001. Cutting edge: distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J. Immunol. 1665331-5335. [DOI] [PubMed] [Google Scholar]
- 12.Cai, Y. C., D. Cefai, H. Schneider, M. Raab, N. Nabavi, and C. E. Rudd. 1995. Selective CD28pYMNM mutations implicate phosphatidylinositol 3-kinase in CD86-CD28-mediated costimulation. Immunity 3417-426. [DOI] [PubMed] [Google Scholar]
- 13.Chang, T. T., C. Jabs, R. A. Sobel, V. K. Kuchroo, and A. H. Sharpe. 1999. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J. Exp. Med. 190733-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, R., O. Kim, J. Yang, K. Sato, K. M. Eisenmann, J. McCarthy, H. Chen, and Y. Qiu. 2001. Regulation of Akt/PKB activation by tyrosine phosphorylation. J. Biol. Chem. 27631858-31862. [DOI] [PubMed] [Google Scholar]
- 15.Diehn, M., A. A. Alizadeh, O. J. Rando, C. L. Liu, K. Stankunas, D. Botstein, G. R. Crabtree, and P. O. Brown. 2002. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc. Natl. Acad. Sci. USA 9911796-11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dittel, B. N. 2008. CD4 T cells: balancing the coming and going of autoimmune-mediated inflammation in the CNS. Brain Behav. Immun. 22421-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutil, E. M., A. Toker, and A. C. Newton. 1998. Regulation of conventional protein kinase C isozymes by phosphoinositide-dependent kinase 1 (PDK-1). Curr. Biol. 81366-1375. [DOI] [PubMed] [Google Scholar]
- 18.Fang, X., S. Yu, J. L. Tanyi, Y. Lu, J. R. Woodgett, and G. B. Mills. 2002. Convergence of multiple signaling cascades at glycogen synthase kinase 3: Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol. Cell. Biol. 222099-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser, J. D., B. A. Irving, G. R. Crabtree, and A. Weiss. 1991. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science 251313-316. [DOI] [PubMed] [Google Scholar]
- 20.Friend, L. D., D. D. Shah, C. Deppong, J. Lin, T. L. Bricker, T. I. Juehne, C. M. Rose, and J. M. Green. 2006. A dose-dependent requirement for the proline motif of CD28 in cellular and humoral immunity revealed by a targeted knockin mutant. J. Exp. Med. 2032121-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genovese, M. C., J. C. Becker, M. Schiff, M. Luggen, Y. Sherrer, J. Kremer, C. Birbara, J. Box, K. Natarajan, I. Nuamah, T. Li, R. Aranda, D. T. Hagerty, and M. Dougados. 2005. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N. Engl. J. Med. 3531114-1123. [DOI] [PubMed] [Google Scholar]
- 22.Gold, R., C. Linington, and H. Lassmann. 2006. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 1291953-1971. [DOI] [PubMed] [Google Scholar]
- 23.Goode, N., K. Hughes, J. R. Woodgett, and P. J. Parker. 1992. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J. Biol. Chem. 26716878-16882. [PubMed] [Google Scholar]
- 24.Green, J. M., P. J. Noel, A. I. Sperling, T. L. Walunas, G. S. Gray, J. A. Bluestone, and C. B. Thompson. 1994. Absence of B7-dependent responses in CD28-deficient mice. Immunity 1501-508. [DOI] [PubMed] [Google Scholar]
- 25.Harada, Y., M. Tokushima, Y. Matsumoto, S. Ogawa, M. Otsuka, K. Hayashi, B. D. Weiss, C. H. June, and R. Abe. 2001. Critical requirement for the membrane-proximal cytosolic tyrosine residue for CD28-mediated costimulation in vivo. J. Immunol. 1663797-3803. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi, K., and A. Altman. 2007. Protein kinase C theta (PKCθ): a key player in T cell life and death. Pharmacol. Res. 55537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holdorf, A. D., J. M. Green, S. D. Levin, M. F. Denny, D. B. Straus, V. Link, P. S. Changelian, P. M. Allen, and A. S. Shaw. 1999. Proline residues in CD28 and the Src homology (SH)3 domain of Lck are required for T cell costimulation. J. Exp. Med. 190375-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchcroft, J. E., and B. E. Bierer. 1996. Signaling through CD28/CTLA-4 family receptors: puzzling participation of phosphatidylinositol-3 kinase. J. Immunol. 1564071-4074. [PubMed] [Google Scholar]
- 29.Jones, R. G., M. Parsons, M. Bonnard, V. S. Chan, W. C. Yeh, J. R. Woodgett, and P. S. Ohashi. 2000. Protein kinase B regulates T lymphocyte survival, nuclear factor κB activation, and Bcl-XL levels in vivo. J. Exp. Med. 1911721-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.June, C. H., J. A. Ledbetter, M. M. Gillespie, T. Lindsten, and C. B. Thompson. 1987. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol. Cell. Biol. 74472-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kane, L. P., P. G. Andres, K. C. Howland, A. K. Abbas, and A. Weiss. 2001. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat. Immunol. 237-44. [DOI] [PubMed] [Google Scholar]
- 32.Keir, M. E., and A. H. Sharpe. 2005. The B7/CD28 costimulatory family in autoimmunity. Immunol. Rev. 204128-143. [DOI] [PubMed] [Google Scholar]
- 33.Kim, H.-H., M. Tharayil, and C. E. Rudd. 1998. Growth factor receptor-bound protein 2 SH2/SH3 domain binding to CD28 and its role in co-signaling. J. Biol. Chem. 273296-301. [DOI] [PubMed] [Google Scholar]
- 34.Kimzey, S. L., P. Liu, and J. M. Green. 2004. Requirement for CD28 in the effector phase of allergic airway inflammation. J. Immunol. 173632-640. [DOI] [PubMed] [Google Scholar]
- 35.Kung, T. T., H. Jones, G. K. Adams III, S. P. Umland, W. Kreutner, R. W. Egan, R. W. Chapman, and A. S. Watnick. 1994. Characterization of a murine model of allergic pulmonary inflammation. Int. Arch. Allergy. Immunol. 10583-90. [DOI] [PubMed] [Google Scholar]
- 36.Li, Y., C. E. Sedwick, J. Hu, and A. Altman. 2005. Role for protein kinase Cθ (PKCθ) in TCR/CD28-mediated signaling through the canonical but not the non-canonical pathway for NF-κB activation. J. Biol. Chem. 2801217-1223. [DOI] [PubMed] [Google Scholar]
- 37.Lindsten, T., C. H. June, J. A. Ledbetter, G. Stella, and C. B. Thompson. 1989. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science 244339-343. [DOI] [PubMed] [Google Scholar]
- 38.Liu, Y., S. Witte, Y. C. Liu, M. Doyle, C. Elly, and A. Altman. 2000. Regulation of protein kinase Cθ function during T cell activation by Lck-mediated tyrosine phosphorylation. J. Biol. Chem. 2753603-3609. [DOI] [PubMed] [Google Scholar]
- 39.Manicassamy, S., S. Gupta, Z. Huang, and Z. Sun. 2006. Protein kinase Cθ-mediated signals enhance CD4+ T cell survival by up-regulating Bcl-xL. J. Immunol. 1766709-6716. [DOI] [PubMed] [Google Scholar]
- 40.Nirula, A., M. Ho, H. Phee, J. Roose, and A. Weiss. 2006. Phosphoinositide-dependent kinase 1 targets protein kinase A in a pathway that regulates interleukin 4. J. Exp. Med. 2031733-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohteki, T., M. Parsons, A. Zakarian, R. G. Jones, L. T. Nguyen, J. R. Woodgett, and P. S. Ohashi. 2000. Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3. J. Exp. Med. 19299-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okkenhaug, K., and R. Rottapel. 1998. Grb2 forms an inducible protein complex with CD28 through a Src homology 3 domain-proline interaction. J. Biol. Chem. 27321194-21202. [DOI] [PubMed] [Google Scholar]
- 43.Okkenhaug, K., L. Wu, K. M. Garza, J. La Rose, W. Khoo, B. Odermatt, T. W. Mak, P. S. Ohashi, and R. Rottapel. 2001. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat. Immunol. 2325-332. [DOI] [PubMed] [Google Scholar]
- 44.Pagès, F., M. Ragueneau, S. Klasen, M. Battifora, D. Couez, R. Sweet, A. Truneh, S. G. Ward, and D. Olive. 1996. Two distinct intracytoplasmic regions of the T-cell adhesion molecule CD28 participate in phosphatidylinositol 3-kinase association. J. Biol. Chem. 2719403-9409. [DOI] [PubMed] [Google Scholar]
- 45.Park, J., M. M. Hill, D. Hess, D. P. Brazil, J. Hofsteenge, and B. A. Hemmings. 2001. Identification of tyrosine phosphorylation sites on 3-phosphoinositide-dependent protein kinase-1 and their role in regulating kinase activity. J. Biol. Chem. 27637459-37471. [DOI] [PubMed] [Google Scholar]
- 46.Parry, R. V., K. Reif, G. Smith, D. M. Sansom, B. A. Hemmings, and S. G. Ward. 1997. Ligation of the T cell co-stimulatory receptor CD28 activates the serine-threonine protein kinase protein kinase B. Eur. J. Immunol. 272495-2501. [DOI] [PubMed] [Google Scholar]
- 47.Pfeifhofer, C., K. Kofler, T. Gruber, N. G. Tabrizi, C. Lutz, K. Maly, M. Leitges, and G. Baier. 2003. Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J. Exp. Med. 1971525-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raab, M., Y. C. Cai, S. C. Bunnell, S. D. Heyeck, L. J. Berg, and C. E. Rudd. 1995. p56Lck and p59Fyn regulate CD28 binding to phosphatidylinositol 3- kinase, growth factor receptor-bound protein GRB-2, and T cell-specific protein-tyrosine kinase ITK: implications for T-cell costimulation. Proc. Natl. Acad. Sci. USA 928891-8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez-Lockhart, M., E. Marin, B. Graf, R. Abe, Y. Harada, C. E. Sedwick, and J. Miller. 2004. Cutting edge: CD28-mediated transcriptional and posttranscriptional regulation of IL-2 expression are controlled through different signaling pathways. J. Immunol. 1737120-7124. [DOI] [PubMed] [Google Scholar]
- 50.Sedwick, C. E., and A. Altman. 2004. Perspectives on PKCθ in T cell activation. Mol. Immunol. 41675-686. [DOI] [PubMed] [Google Scholar]
- 51.Stein, P. H., J. D. Fraser, and A. Weiss. 1994. The cytoplasmic domain of CD28 is both necessary and sufficient for costimulation of interleukin-2 secretion and association with phosphatidylinositol 3′-kinase. Mol. Cell. Biol. 143392-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun, Z., C. W. Arendt, W. Ellmeier, E. M. Schaeffer, M. J. Sunshine, L. Gandhi, J. Annes, D. Petrzilka, A. Kupfer, P. L. Schwartzberg, and D. R. Littman. 2000. PKC-theta is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature 404402-407. [DOI] [PubMed] [Google Scholar]
- 53.Thompson, C. B., T. Lindsten, J. A. Ledbetter, S. L. Kunkel, H. A. Young, S. G. Emerson, J. M. Leiden, and C. H. June. 1989. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc. Natl. Acad. Sci. USA 861333-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toker, A., and A. C. Newton. 2000. Cellular signaling: pivoting around PDK-1. Cell 103185-188. [DOI] [PubMed] [Google Scholar]
- 55.van Weeren, P. C., K. M. de Bruyn, A. M. de Vries-Smits, J. van Lint, and B. M. Burgering. 1998. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J. Biol. Chem. 27313150-13156. [DOI] [PubMed] [Google Scholar]
- 56.Vilimek, D., and V. Duronio. 2006. Cytokine-stimulated phosphorylation of GSK-3 is primarily dependent upon PKCs, not PKB. Biochem. Cell. Biol. 8420-29. [DOI] [PubMed] [Google Scholar]
- 57.Vincenti, F., C. Larsen, A. Durrbach, T. Wekerle, B. Nashan, G. Blancho, P. Lang, J. Grinyo, P. F. Halloran, K. Solez, D. Hagerty, E. Levy, W. Zhou, K. Natarajan, and B. Charpentier. 2005. Costimulation blockade with belatacept in renal transplantation. N. Engl. J. Med. 353770-781. [DOI] [PubMed] [Google Scholar]
- 58.Wood, J. E., H. Schneider, and C. E. Rudd. 2006. TcR and TcR-CD28 engagement of protein kinase B (PKB/AKT) and glycogen synthase kinase-3 (GSK-3) operates independently of guanine nucleotide exchange factor VAV-1. J. Biol. Chem. 28132385-32394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.