Abstract
Several ETS transcription factors, including ELF4/MEF, can function as oncogenes in murine cancer models and are overexpressed in human cancer. We found that Elf4/Mef activates Mdm2 expression; thus, lack of or knockdown of Elf4/Mef reduces Mdm2 levels in mouse embryonic fibroblasts (mef's), leading to enhanced p53 protein accumulation and p53-dependent senescence. Even though p53 is absent in Elf4−/− p53−/− mef's, neither oncogenic H-RasV12 nor c-myc can induce transformation of these cells. This appears to relate to the INK4a/ARF locus; both p19ARF and p16 are increased in Elf4−/− p53−/− mef's, and expression of Bmi-1 or knockdown of p16 in this context restores H-RasV12-induced transformation. Thus, ELF4/MEF promotes tumorigenesis by inhibiting both the p53 and p16/Rb pathways.
MEF (also known as ELF4) belongs to the ETS family of transcription factors, which contains over 30 family members (39). ETS proteins function as transcriptional activators or repressors and regulate critical aspects of cellular differentiation, proliferation, and transformation. ELF4/MEF activates expression of a variety of cytokine genes (13, 27); it also regulates cell cycle progression, promoting the transition of cells from G1 to S phase, and the movement of hematopoietic stem cells from quiescence to G1 phase (18, 26). In addition to its effects on the cell cycle, several studies have implicated ELF4/MEF in tumorigenesis (37). Models of retrovirus-induced insertional mutagenesis have identified Elf4/Mef as a gene that cooperates in transformation (e.g., in INK4a/ARF-deficient mice and eμ-myc mice that lack pim1 and pim2) (22, 25). While ELF4/MEF transforms NIH 3T3 cells (43), it is not known how ELF4/MEF functions in tumorigenesis.
To address this issue, we examined the behavior of mouse embryo fibroblasts (mef's) isolated from Elf4/Mef null mice and detected enhanced DNA damage-initiated senescence with accumulation of p53 protein. This suggested that Elf4/Mef can suppress senescence, which prompted us to examine its effects on oncogene-induced senescence (6) as well. The tumor suppressor p53 is known as a “guardian of the genome” because of its critical ability to promote cell cycle arrest, apoptosis, and senescence (40). p53 plays a critical role in triggering oncogene-induced and DNA damage-induced senescence and it is regulated by a variety of posttranscriptional modifications and by the Mdm2 and p19ARF proteins. Mdm2 plays a central role in downregulating p53 function by its E3 ubiquitin ligase activity, which destabilizes p53 protein (14). p53 protein and activity are tightly regulated in cells; when p53 is induced by DNA damage or oncogenic stress, it promotes the transcriptional activation of Mdm2 expression, establishing a negative feedback loop (41). The INK4a/ARF locus contains two distinct genes encoding the p16INK4a and p19ARF (known as p14ARF in humans) proteins (30). While p16 inhibits Cdk4 and Cdk6 activity, leading to activation of RB, p19ARF stabilizes p53 protein levels by binding to Mdm2 and promoting its degradation (15). This tight regulation of p53 protein stability is lost when p53 is mutated.
To explain how p53 accumulates in the absence of Elf4/Mef, we found that Elf4/Mef binds to and activates the Mdm2 promoter and that Elf4/Mef null mef's have decreased Mdm2 expression and enhanced senescence. Increasing Elf4/Mef levels increases Mdm2 expression, and transformation by Elf4/Mef requires this increased Mdm2 expression. Elf4/Mef also has p53-independent effects on tumorigenesis, as the p53 null/Elf4 null mef's (that we generated) are resistant to oncogene-induced transformation, at least in part due to accumulation of p16. We identify several components of this resistance to transformation and by using both murine and human tumor models show the relevance of ELF4/MEF to tumorigenesis. Our studies demonstrate that a single transcription factor, ELF4/MEF, can promote transformation by inhibiting both the p53 and p16/Rb pathways.
MATERIALS AND METHODS
Mice.
Elf4/Mef knockout (KO) mice were generated as described previously (17), whereas the p53 KO mice and the p16/p19ARF KO mice were kindly provided by Harold Varmus's lab and Eric C. Holland's lab, respectively. We generated p53/Elf4 double-KO (DKO) mice by crossing p53 KO mice with Elf4/Mef KO mice. DKO mice and control single KO mice were identified by PCR-based genotyping. All mice were maintained in the MSKCC Animal Core Facility according to IACUC-approved protocols. Tumor sections were assessed by hematoxylin-eosin (H&E) staining and immunohistochemistry (IHC). The primary antibodies were p16 (NA29; Calbiochem, Darmstadt, Germany), Mdm2 (ab16895; Abcam, Cambridge, MA), and Ki67 (TEC-3; Dako, Carpinteria, CA). Two pathologists (G. Sashida and S. Shimizu) evaluated stained tissues in a blinded fashion and determined scores based on the percentage of positively staining tumor cells. Prism software (Graphpad) was used to perform all statistical analyses.
Lymphoma patient samples.
The study comprised 19 follicular lymphoma, 16 diffuse large B-cell lymphoma, and 3 mantle cell lymphoma biopsies at the Tokyo Medical University Hospital. Patient anonymity was ensured, and the study was approved by the Institutional Ethics Review Committee. The histological diagnosis was based on H&E and IHC staining. The primary antibody was p53 (DO-7; Dako). RNA was extracted from frozen tissue at diagnosis.
Plasmids and transfections.
Several retroviral vectors (pBabe-puromycin-H-RasV12, pBabe-hygromycin-H-RasV12, and pWZL-hygromycin-c-myc) were kindly provided by Pier Paolo Pandolfi. The human ELF4/MEF cDNA was subcloned into the pBabe vector. shRNA directed against murine p16, Elf4/Mef, or Mdm2 mRNA was expressed using the lentivirus vector pLKO.1, which has a puromycin-selectable marker (Openbiosystems, Huntsville, AL) and transfected into 293T cells together with the pMD.GVSVG and pCMVΔ8.9 helper vectors. Either 293T cells or Phoenix ecotropic cells were transfected with the relevant viruses, using a standard calcium phosphate method. Cultured virus supernatants were added to passage 2 or 3 mef's with 8 μg/ml Polybrene and incubated for 4 h. The infection procedure was repeated a total of three times over a 2-day interval. Drug selection was started 24 h after the last infection.
Luciferase reporter assay.
The Mdm2 and p16 promoter luciferase plasmids are described in the Methods section of the supplemental material. A 0.5-μg aliquot of either pCMV5-empty, pCMV5-p53, pCMV-Ets1, pCMV-Ets2, or pCMV5-ELF4/MEF was transfected into NIH 3T3 cells plated in 12-well dishes, using FuGENE 6 (Roche) together with 100 ng of pGL3 reporter plasmid and 1 ng of pTKRL-CMV plasmid, which was used to control for the transfection efficiency. Cell lysates were prepared 48 h after transfection and were assessed for dual luciferase activity (Promega).
Real-time PCR.
Total RNA was extracted using the RNeasy minikit (Qiagen, Valencia, CA), and cDNA synthesis was preformed using an oligo(dT) primer. PCR was performed using either TaqMan or SYBR green primers (shown in the Methods section of the supplemental material) and the 7500 Real-Time PCR system (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was measured to provide an internal standard. All data are expressed as the n-fold enrichment divided by the level of GAPDH expression.
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed according to the manufacturer's instructions (ChIP assay kit; Upstate Biotechnology). Approximately 1 × 107 mef's were used for each immunoprecipitation. The following antibodies were used for the immunoprecipitation reactions: anti-RNA polymerase II antibody (sc-9001; Santa Cruz Biotechnology, Santa Cruz, CA), anti-Elf4/Mef rabbit polyclonal antibody (described previously [26]), and preimmune rabbit immunoglobulin G. DNA was amplified by PCR, using the primers shown in the Methods section of the supplemental material. The reaction was resolved using agarose gels with ethidium bromide staining.
RESULTS
Elf4/Mef prevents cellular senescence by suppressing the p53 pathway.
Expression of oncogenic Ras in mef's can trigger both p53- and p16-dependent responses, thereby inducing cellular senescence, which is a critical initial barrier against tumorigenesis (2, 7, 35). Given the transforming activity of ELF4/MEF in NIH 3T3 cells (43), we determined if ELF4/MEF could prevent oncogene-induced senescence, monitoring expression of the senescence marker SA β-galactosidase (SA β-Gal) (8). First, we transduced oncogenic H-RasV12 (alone or with ELF4/MEF) into wild-type mef's; mef's transduced with both ELF4/MEF and H-RasV12 showed significantly less SA β-Gal-positive cells than H-RasV12 alone-transduced mef's (19% ± 9.1% versus 49% ± 9.8% [means ± standard deviations]; P = 0.0003) (Fig. 1A). We next compared the senescent phenotype of Elf4/Mef KO and wild-type (WT) mef's. Although both Elf4/Mef KO and WT mef's cease to grow at passage 8 (when passaged according to a 3T9 protocol), Elf4/Mef KO mef's rapidly lost their proliferative ability, with slower doubling rates than WT mef's. Elf4/Mef KO cells begin to show SA β-Gal-positive cells at passage 5, with a clear increase in the number of SA β-Gal-positive cells at passage 6 (54% ± 5.8% versus 35% ± 2.6% for WT mef's; P = 0.007) (Fig. 1B). Commensurate with this enhanced senescence, we found a senescent morphology in Elf4/Mef KO mef's at passage 6 (see Fig. S1 in the supplemental material) and detected a 1.8-fold decrease in bromodeoxyuridine (BrdU) incorporation into passage 6 Elf4/Mef KO mef's (3.7% ± 0.5% versus 6.8% ± 1.8% for WT; P = 0.020) (Fig. 1C), with accumulation of cells in the G1 phase of the cell cycle (data not shown).
FIG. 1.
ELF4/MEF can prevent cellular senescence by suppressing the p53 pathway. (A) ELF4/MEF overexpression blocks oncogenic Ras-induced senescence. SA β-galactosidase staining of control vector, ELF4/MEF alone, H-RasV12 alone, and ELF4/MEF plus H-RasV12-transduced WT mef's was assessed. ELF4/MEF plus H-RasV12-transduced mef's showed less SA β-Gal-positive staining than the H-RasV12-alone-transduced mef's (P = 0.003, n = 5). (B) The enhanced senescence seen in cells lacking Elf4/Mef is dependent on p53. SA β-galactosidase staining of WT, Elf4/Mef KO, p53 KO, and p53/Elf4 DKO mef's was assessed at passages 5 and 6, and Elf4/Mef KO mef's showed significantly more positive staining cells at passage 6 (54% versus 35% for WT mef's; P = 0.007; n = 3). DKO mef's showed very few positive cells (0.5% ± 1.0%; n = 3). (C) The rate of DNA synthesis was examined using BrdU incorporation into WT and Elf4/Mef KO mef's at passage 6. BrdU incorporation was 1.8-fold less in Elf4/Mef KO mef's than in WT mef's (P = 0.020; n = 3). (D) Elf4/Mef loss promotes p53 accumulation. The levels of cell cycle regulators were examined at passages 4 and 6 in WT and Elf4/Mef KO mef's. The level of p16 accumulation over time was the same in the WT and Elf4/Mef KO mef's. Elf4/Mef KO mef's showed greater accumulation of both p53 and p21 at passage 6 than WT mef's. The level of Mdm2 protein showed a slight increase in passage 6 Elf4/Mef KO mef's compared to WT mef's. (E) Knockdown of Elf4/Mef by shRNA enhances senescence at passages 5 and 6. Growth curve assays showed that the Elf4/Mef-directed shRNA transduced WT mef's cease to grow at passage 5. (F) SA β-galactosidase staining of Elf4/Mef-directed shRNA-transduced and control empty vector-transduced WT mef's were assessed at passages 5 and 6, and Elf4/Mef-directed shRNA-transduced mef's showed significantly more positive staining cells at passage 5 (33.9% versus 3.3% for the control; P = 0.0098, n = 3). (G) Acute knockdown of Elf4/Mef activates the p53 pathway. The levels of p19ARF, Mdm2, p53, p21, and p16 were examined at passage 5 in Elf4/Mef-directed shRNA-transduced and control (empty vector)-transduced WT mef's. The Elf4/Mef-directed shRNA-transduced cells showed increases in p53, p21, and p16 and a slight increase in p19ARF and Mdm2 protein levels. (H) Elf4/Mef expression is not induced by serum in WT mef's that undergo senescence (at passage 5). Passage 3 WT mef's, but not passage 5 WT mef's, upregulate Elf4/Mef mRNA expression 4 hours after serum restimulation, leading to increased Mdm2 protein expression.
To define why Elf4/Mef KO mef's display more senescence, we assessed the expression of the cell cycle regulators p53, p21, and p16 at passages 4 and 6. We saw a marked accumulation of p53 protein (but not p53 mRNA) and p21 protein in Elf4/Mef KO mef's at passage 6 (Fig. 1D) but no significant change in the level of p16 protein. To determine whether Elf4/Mef KO mef's have activated p53, we assessed the expression of several p53 target genes, including p21 and the growth arrest- and DNA damage-inducible gene Gadd45. We found a 2.1-fold increase in p21 mRNA and a 7.8-fold increase in Gadd45 mRNA in passage 5 Elf4/Mef KO mef's (compared to WT mef's) (see Fig. S2 in the supplemental material).
To exclude the possibility that developmental adaptation to the lack of Elf4/Mef resulted in enhanced senescence, we acutely knocked down Elf4/Mef using shRNA in wild-type mef's at passage 2. Whereas control (empty vector)-transduced wild-type mef's kept growing with few SA β-Gal-positive cells, the Elf4/Mef-directed shRNA-transduced mef's ceased to grow (Fig. 1E) and showed markedly increased numbers of SA β-Gal-positive cells at passage 5 (34% ± 11% versus 3.3% ± 0.5% for the control; P = 0.0098) and passage 6 (Fig. 1F). The shRNA-transduced mef's had 97% knockdown of Elf4/Mef mRNA (see Fig. S3 in the supplemental material). This resulted in a significant accumulation of p53, p21, and p16 protein at passage 5 (Fig. 1G), as well as a significant accumulation of p21 and Gadd45 mRNA at passage 5 (see Fig. S3 in the supplemental material). Thus, both the acute loss and chronic absence of Elf4/Mef substantially enhances senescence with activation of p53 function, leading to p21 and Gadd45 accumulation.
To determine whether restoring Elf4/Mef expression can prevent the enhanced senescence, we reintroduced MEF into Elf4/Mef KO mef's. Indeed, we found that MEF-transduced Elf4/Mef KO mef's had a significant decrease in SA β-Gal-positive cells at passage 6 (56% ± 3.0% versus 32% ± 5.8% for the control; P < 0.001) (see Fig. S4 in the supplemental material).
To assess whether changes in Elf4/Mef expression normally accompany senescence, we examined Elf4/Mef mRNA levels in early- and late-passage WT mef's and found a 6.1-fold reduction in Elf4/Mef mRNA at passage 6 versus passage 3 (1.3 ± 1.0 [relative amount] versus 7.9 ± 1.9; P = 0.006) (see Fig. S2 in the supplemental material). As senescence is accompanied by an insensitivity to extracellular growth signals (36), we also compared the serum-inducible expression of Elf4/Mef in WT mef's at passage 3 versus passage 5. WT mef's were serum starved for 4 days and then restimulated with 10% serum. Passage 3 mef's upregulate Elf4/Mef mRNA expression 4 hours after serum restimulation (followed by increased Mdm2 expression), whereas passage 5 mef's do not upregulate either (Fig. 1H). While passage 3 mef's eventually entered the S phase of the cell cycle, passage 5 mef's had fewer S-phase cells 24 h after serum stimulation (21% ± 5% versus 30% ± 5% for the passage 3 mef's) (data not shown). Thus, induction of Elf4/Mef expression by growth factors is lost as cells undergo senescence.
To determine whether the enhanced senescence in Elf4/Mef KO mef's is dependent on p53, we generated p53/Mef DKO mice (crossing Elf4/Mef KO mice with p53 KO mice) and isolated p53/Elf4 DKO mef's. DKO mef's grow continuously, as do p53 KO mef's (which are known to be immortalized), and indeed the DKO mef's do not display senescence, as demonstrated by minimal SA β-Gal staining of the DKO cells at passage 6 (0.5% ± 1.0%) (Fig. 1B). Thus, p53 is required for the enhanced senescence observed in Elf4/Mef KO mef's.
Elf4/Mef binds the Mdm2 promoter and activates its expression.
To explain why p53 protein accumulates in Elf4/Mef KO mef's, we investigated whether the expression of Mdm2, which inhibits p53 by inducing its degradation, is altered in the Elf4/Mef KO mef's. Indeed, we saw decreased Mdm2 protein in the earlier-passage Elf4/Mef KO mef's (passage 4), but not in the senescent Elf4/Mef KO mef's, which have activated p53 that can secondarily upregulate Mdm2 expression (Fig. 1D). We also found a twofold reduction in Mdm2 mRNA in early-passage Elf4/Mef KO mef's (1.3 ± 1.2 for Elf4 KO mef's versus 2.6 ± 1.1 for WT mef's; P = 0.030) (see Fig. S2 in the supplemental material). Furthermore, although Ras can activate both Mdm2 and p19ARF to regulate p53 expression, the induction of Mdm2 mRNA (and protein levels) by H-RasV12 was much lower in the Elf4/Mef KO mef's than in WT mef's (11.2 ± 0.5 versus 21.6 ± 1.2 for H-RasV12-transduced WT mef's; P = 0.008) (Fig. 2A). While p19ARF protein level showed no significant change in H-RasV12-transduced mef's, induction of Mdm2 protein by H-RasV12 was also much lower in Elf4/Mef KO mef's than in WT mef's (Fig. 2A). Thus, the lack of Elf4/Mef enhances the accumulation of p53 via reduced transcriptional activation of Mdm2, rather than via effects on p19ARF expression.
FIG. 2.
Elf4/Mef directly and positively regulates Mdm2 expression. (A) Elf4/Mef loss impairs H-RasV12-induced Mdm2 expression. H-RasV12-transduced KO mef's express 2.0-fold less Mdm2 mRNA and markedly less Mdm2 protein than H-RasV12-transduced WT mef's (P = 0.008; n = 3). The level of p19ARF protein was the same in the Elf4/Mef KO mef's as the WT mef's, in the presence or absence of H-RasV12. (B) ELF4/MEF expression induces Mdm2, but not p19ARF, thereby reducing p53 expression, while H-RasV12 induces p19, Mdm2, and p53 expression (as determined by densitometry studies). (C) Elf4/Mef is bound to the Mdm2 promoter in vivo. ChIP assays were performed using WT mef's. Protein-DNA complexes were precipitated with an anti-RNA polymerase II antibody, anti-Mef antibody, or preimmune rabbit immunoglobulin G (for a negative control). PCR was performed using primers specific for regions of the Mdm2 promoter: either 200 bp (upper row) or 1.5 kb (lower row) upstream of the start site. Binding of Elf4/Mef (and RNA polymerase II) to the MDM2 promoter was visualized by gel electrophoresis. (D) ELF4/MEF and p53 transactivate the Mdm2 promoter. The 200-bp MDM2 promoter region includes two ETS binding sites (GGAAG) and two p53 binding sites, the 100-bp promoter fragment contains only p53 binding sites, and the bp −200 to −100 promoter fragment contains two ETS binding sites (and TATA box). Transient transfection of p53 activated the 100-bp promoter activity 41-fold, the 200-bp promoter 10.1-fold, and the bp −200 to −100 promoter 0.4-fold (n = 6). Transient transfection of MEF activated the 100-bp promoter 3.0-fold, the 200-bp promoter 7.2-fold, and the bp −200 to −100 promoter 2.3-fold compared to the control vector (n = 6). (E) ELF4/MEF transactivates the Mdm2 promoter more potently than Ets1 or Ets2. MEF transfection activated the 200-bp Mdm2 promoter 7.2-fold, whereas Ets1 and Ets2 activated the 200-bp Mdm2 promoter 3.4-fold and 2.5-fold, respectively (n = 6). (F) Transient expression of ELF4/MEF increases the endogenous level of Mdm2 protein in a dose-dependent manner. The levels of Mdm2 protein were examined by Western blot analysis 24 h after the transfection of pCMV5-MEF or an empty vector control.
To understand how Elf4/Mef regulates the p53 pathway, we examined the expression of the p53 regulators p19ARF and Mdm2 following overexpression of either ELF4/MEF or H-RasV12 in WT mef's. MEF induced Mdm2 protein expression, but not p19ARF, resulting in reduced p53 protein expression (Fig. 2B). H-RasV12 expression increased the levels of Mdm2, p21, and Gadd45 mRNA (data not shown) and of endogenous Elf4/Mef expression as well (Fig. 2B), while overexpression of MEF suppressed expression of the p53 target genes p21 and Gadd45 (see Fig. S5 in the supplemental material). Thus, Elf4/Mef appears to suppress the p53 pathway, through activating Mdm2 expression.
To determine if Elf4/Mef directly regulates Mdm2 expression, we first examined whether Elf4/Mef is bound to the Mdm2 promoter in vivo. The Mdm2 promoter contains several consensus ETS binding sites (GGAAG), so we performed ChIP assays, using PCR primers that amplify genomic DNA located either 200 bp or 1.5 kb upstream of the Mdm2 transcriptional initiation site (31). We found Elf4/Mef bound to the Mdm2 promoter 200 bp, but not 1.5 kb, upstream of the coding region in WT mef's that contain Elf4/Mef (Fig. 2C), but not in the Elf4/Mef KO mef's (data not shown). We next tested whether MEF can upregulate Mdm2 promoter activity by transiently transfecting an Mdm2 promoter-driven luciferase reporter plasmid into NIH 3T3 cells. We generated three different Mdm2 promoter plasmids which contained either a 100-bp promoter region (that has only p53 binding sites and no ETS binding sites), a 200-bp promoter region (that includes two ETS binding sites and two p53 binding sites), or a bp −200 to −100 promoter region (that has two ETS binding sites but has the p53 binding sites deleted). p53 overexpression activated the 100-bp Mdm2 promoter fragment 41-fold and the 200-bp promoter fragment 10.1-fold (Fig. 2D), whereas MEF activated the 100-bp Mdm2 promoter fragment 3.0-fold and the 200-bp Mdm2 promoter fragment 7.2-fold (compared to control vector) (Fig. 2D). To assess the importance of the p53 binding sites for the activation of Mdm2 promoter activity by MEF or p53, we used a p53 binding site-deleted Mdm2 promoter. MEF activated this construct 2.3-fold, but p53 did not activate the p53 binding site-deleted promoter (Fig. 2D). Thus, the effect of ELF4/MEF does not require the p53 binding sites; however, activation is greater when the 200-bp region is intact. Ets1 and Ets2 are both known to be downstream targets of Ras signaling, which can lead to increases in Mdm2 promoter activity (31). Therefore, we compared the ability of ELF4/MEF to activate the Mdm2 promoter with that of Ets1 and Ets2. MEF activated the 200-bp Mdm2 promoter 7.2-fold, while Ets1 and Ets2 activated it 3.4-fold and 2.5-fold, respectively (Fig. 2E).
Lastly, when we transiently expressed MEF in NIH 3T3 cells, we observed a dose-dependent upregulation of expression of the endogenous Mdm2 protein (Fig. 2F). Thus, Elf4/Mef can bind the Mdm2 promoter and promote Mdm2 expression; in the absence of Elf4/Mef, Mdm2 levels are reduced.
Mdm2 is required for the transforming capability of Elf4/Mef.
Having shown that ELF4/MEF can transform NIH 3T3 cells, which have wild-type p53 (43), and promote Mdm2 expression, we examined whether this transformation ability depends on Mdm2 upregulation in cells lacking p53. We screened several shRNA to find an shRNA that could efficiently decrease the level of Mdm2 mRNA (see Fig. S6 in the supplemental material) and protein (Fig. 3A) and then knocked down Mdm2 expression in ELF4/MEF-transduced p53 KO mef's. Cells expressing ELF4/MEF and the shRNA directed against Mdm2 grew at a significant slower rate than ELF4/MEF alone-transduced mef's (Fig. 3B). Furthermore, shRNA directed against Mdm2 significantly decreased the transformation of p53 KO mef's by ELF4/MEF, as measured by soft agar colony-forming ability (10 ± 0.8 versus 20 ± 2.5 for the ELF4/MEF alone-transduced p53 KO mef's; P < 0.005) (Fig. 3C). The shRNA directed against Mdm2 has less of an impact on colony formation by H-RasV12 transduced p53 KO mef's (472 ± 51 for the control versus 338 ± 40 for the shRNA-transduced p53 KO mef's) (data not shown). Thus, ELF4/MEF can enhance Mdm2 expression in the absence of p53 (Fig. 3A), and p53-independent effects of Mdm2 play a role in transformation by ELF4/MEF.
FIG. 3.
Mdm2 is required for the transformation of p53 KO mefs by ELF4/MEF. (A) Elf4/Mef, Mdm2, and p21 protein levels were assessed in p53 KO mef's after transduction with control vectors, Mdm2-directed shRNA alone, ELF4/MEF alone, or ELF4/MEF plus Mdm2-directed shRNA. The ELF4/MEF plus Mdm2-directed shRNA-transduced mef's showed increased p21 protein expression compared to the ELF4/MEF-alone or the shRNA-alone-transduced mef's. (B) Growth curves of the p53 KO mef's transduced with ELF4/MEF- and/or Mdm2-directed shRNA. The ELF4/MEF-alone-transduced p53 KO mef's grew continuously, as did the control mef's. The Mdm2-directed shRNA severely impaired the cell growth in the presence or absence of ELF4/MEF expression. The vertical axis represents relative cell numbers as measured by light absorption. A representative experiment is shown. (C) Mdm2 knockdown partially blocks transformation of p53 KO mef's by ELF4/MEF. The ELF4/MEF alone-transduced p53 KO mef's formed many more colonies in soft agar than the ELF4/MEF plus Mdm2-directed shRNA-transduced mef's (P < 0.005; n = 4). (D) Analysis of ELF4/MEF and MDM2 mRNA levels in 38 human lymphoma patient tissue samples, stratified by p53 IHC staining and level of MDM2 expression. The highest level of ELF4/MEF expression was seen in the samples with high MDM2 expression and wild-type p53.
To define the possible clinical relevance of the ability of ELF4/MEF to activate MDM2, we examined the levels of ELF4/MEF and MDM2 mRNA in 38 lymphoma patient lymph node tissue samples. Mutant p53 proteins are not usually degraded by MDM2, and they lack the ability to fully upregulate MDM2 expression (5, 24). Mutant p53 can be detected by IHC, whereas wild-type p53 usually cannot (21). We found that 26 of the 38 human lymphoma samples were negative by IHC. Of these samples, where p53 was presumably wild type, 6 had high MDM2 expression levels (compared to control reactive lymph node tissue) (see Fig. S7 in the supplemental material) and 20 had normal MDM2 expression. The samples with high MDM2 expression had much higher MEF expression than the samples with normal MDM2 expression (3.10 ± 0.90 versus 1.09 ± 0.72; P < 0.0001) (Fig. 3D). This suggests that MEF overexpression leads to MDM2 activation in cells with wild-type p53, promoting their transformation by reducing p53 function. (Of the 12 samples with “mutant” p53, 11 had neither elevated MDM2 nor MEF mRNA levels.)
Elf4/Mef loss prevents transformation of p53 null mef's due to enhanced senescence.
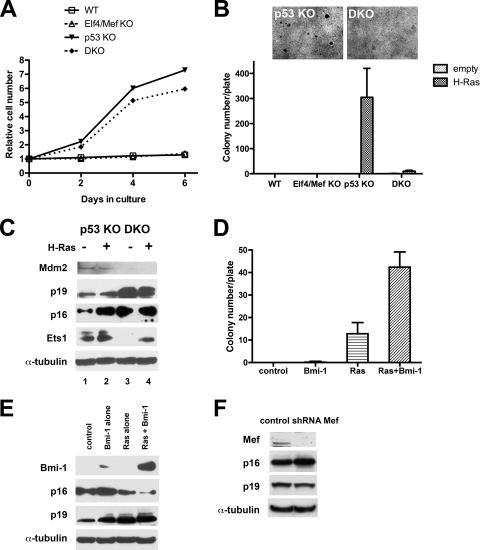
The absence of Elf4/Mef clearly promotes p53 function via reduced Mdm2 expression, but to determine whether Elf4/Mef plays an Mdm2-independent, additional role in oncogene-induced transformation, we further studied p53 KO mef's and p53/Elf4 DKO mef's. We transduced H-RasV12 into p53 KO and p53/Elf4 DKO mef's (and WT and Elf4/Mef KO mef's, as well). As expected, both the WT and Elf4/Mef KO mef's ceased to grow after the introduction of Ras (Fig. 4A), with increased senescence (demonstrated by SA β-Gal staining) (data not shown). While both the H-RasV12-transduced p53 KO and DKO mef's grew continuously (Fig. 4A), we saw significantly more SA β-Gal-positive cells among the Ras transduced DKO mef's (11.0%±2.3%) than the Ras-transduced p53 KO mef's (1.0%±1.1%) or the empty control-transduced DKO mef's (3.5%±0.5%) (data not shown). When we compared the transformation of mef's lacking p53 alone to those lacking p53 and Elf4/Mef, we found 2.9-fold-less focus formation for the H-RasV12-transformed DKO mef's than the p53 KO mef's, with a >96% reduction in anchorage-independent growth in the soft agar colony assay (10 ± 3.6 versus 305 ± 115 for the H-RasV12-transduced p53 KO mef's; P < 0.005) (Fig. 4B). Thus, mef's lacking both p53 and Elf4/Mef resist oncogenic transformation by H-RasV12, likely due to enhanced senescence.
FIG. 4.
Elf4/Mef is required for transformation in the absence of p53. (A) Growth curves of WT, Elf4/Mef KO, p53 KO, and p53/Elf4 DKO mef's transduced with H-RasV12. WT and Elf4/Mef KO mef's cease to grow after the introduction of H-RasV12, whereas the Ras-transduced DKO and p53 KO mef's grow continuously. The vertical axis represents relative cell numbers measured by light absorption. A representative experiment is shown. (B) Oncogenic H-RasV12 can transform p53 KO mef's but not p53/Elf4 DKO mef's. A soft agar assay examined WT, Elf4/Mef KO, p53 KO, and DKO mef's transduced either with H-RasV12 or control vector. H-RasV12 transforms p53 KO mef's but not DKO mef's (P < 0.005; n = 4). Representative figures show photomicrographs of H-RasV12-transduced p53 KO and DKO mef's. (C) Elf4/Mef loss promotes H-RasV12-dependent p16 induction but impairs Mdm2 induction in cells lacking p53. The levels of Mdm2, p19ARF, MDM2, p16, and Ets1 protein were examined in p53 KO and p53/Elf4 DKO mef's transduced with either the control vector or H-RasV12. The p53/Elf4 DKO mef's showed a marked accumulation of p16 protein and also reduced Mdm2 and Ets1 levels compared to p53 KO mef's in the presence H-RasV12. The DKO mef's expressed more p19ARF protein than the p53 KO mef's. (D) Bmi-1 expression can restore transformation of p53/Elf4 DKO mef's by H-RasV12. DKO mef's were transduced with control vector, Bmi-1 alone, H-RasV12 alone, or H-RasV12 plus Bmi-1. While the Bmi-1 alone-transduced mef's showed very few soft agar colonies, the H-RasV12 plus Bmi-1-transduced p53/Elf4 DKO mef's showed more soft agar colonies than the H-RasV12-alone-transduced mef's (P < 0.005; n = 4). (E) Bmi-1 suppresses p16 expression but not p19ARF expression in p53/Elf4 DKO mef's. The levels of p16, p19ARF, and Bmi-1 were examined in the DKO mef's. (F) shRNA directed against Elf4/Mef led to the absence of detectable Elf4/Mef protein in the p53 KO mef's. The acute knockdown of Elf4/Mef led to a moderate increase in the level of p16, but not p19ARF, protein.
To explore whether oncogenes other than H-RasV12 can transform the DKO mef's, we also introduced the c-myc proto-oncogene into p53 KO and p53/Elf4 DKO mef's. Consistent with their resistance to H-RasV12-induced transformation, the DKO mef's transduced with c-myc showed very few soft agar colonies, compared to the c-myc-transduced p53 KO mef's (0.5 ± 0.7 versus 14 ± 1.0; P < 0.005) (data not shown). Thus, the absence of Elf4/Mef confers important resistance against transformation by multiple oncogenes.
To understand the mechanism of the resistance of p53/Elf4 DKO mef's to transformation, we examined the expression of cell cycle regulators Mdm2, p19ARF, and p16. While the DKO mef's did not express Mdm2 protein in the presence or absence of H-RasV12, p53 KO mef's transduced with H-RasV12 showed a slight increase in Mdm2 expression compared to the control-transduced p53 KO mef's (Fig. 4C). Given that both the p53 and p16/RB pathways potently suppress oncogenesis by triggering the senescence response, we hypothesized that p16 accumulation could contribute to the resistance to transformation seen in the p53 null/Elf4 null mef's. p16 associates with CDK4 and inhibits its activity, leading to decreased phosphorylation of RB and thereby inhibition of cell cycle progression (34). p16 is required to trigger Ras-induced senescence, and when p16 is expressed senescence becomes irreversible (1, 3).
Elevated levels of p16 are found in the absence of p53 (compared to WT mef's) (data not shown), but we found a further accumulation of p16 protein in the DKO mef's compared to the p53 KO mef's (Fig. 4C, compare lanes 3 and 1). Several ETS family members (including Ets1) have been shown to activate p16 expression and thereby promote Ras-induced senescence (29). As expected, H-RasV12-transduced mef's express more Ets1 protein than control vector-transduced cells (Fig. 4C, compare even lanes with odd lanes). However, in the absence of Elf4/Mef we detected less Ets1 protein in the H-RasV12-transduced cells (Fig. 4C, compare lanes 4 and 2), even though p16 expression was higher. We also saw more p19ARF protein in the DKO mef's than the p53 KO mef's (Fig. 4C, compare lanes 3 and 1).
To determine whether the accumulation of p16 and p19ARF suppresses Ras-induced transformation in the DKO mef's that lack p53 (and Elf4/Mef), we attempted to reduce both p16 and p19ARF expression in the p53/Elf4 DKO mef's by expressing Bmi-1. Bmi-1 itself did not transform the DKO mef's (we observed no soft agar colonies), and expressing Bmi-1 in H-RasV12-transduced p53 KO mef's had no significant effect on soft agar colony formation (112 ± 20 versus 119 ± 35 for the H-RasV12 alone-transduced p53 KO mef's; P = 0.328) (data not shown). However, Bmi-1 plus H-RasV12-transduced DKO mef's showed a 3.1-fold increase in soft agar colony formation compared to H-RasV12 alone-transduced DKO mef's (45 ± 7.9 versus 15 ± 5.5; P = 0.0028) (Fig. 4D). While the Bmi-1 plus H-RasV12-transduced DKO mef's had decreased p16 expression (compared to singly transduced DKO cells), we saw little change in p19ARF expression (Fig. 4E). Thus, despite not really downregulating p19ARF expression, Bmi-1 still promoted the transformation of the Ras-transduced DKO mef's. Furthermore, by examining the effect of acute knockdown of Elf4/Mef in p53 KO mef's (which reduced Mdm2 mRNA expression 5.0-fold [see Fig. S8 in the supplemental material), we found an increase in p16 expression and no change in p19ARF protein expression compared to the control (Fig. 4F). This suggests that the marked accumulation of p16 (rather than p19ARF) may mediate the transformation resistance of p53/Elf4 DKO mef's.
Elf4/Mef suppresses oncogenic Ras-induced p16 activation, leading to transformation.
We next examined directly whether reductions of p16 levels would restore the transformation potential to the p53/Elf4 DKO mef's, and after screening several shRNAs, we found an shRNA directed against p16 that potently reduced p16, but not p19ARF, protein expression (Fig. 5A). When we transduced DKO mef's with H-RasV12 and p16-directed shRNA, we observed a 7.5-fold increase in soft agar colonies compared to the H-RasV12 plus control shRNA-transduced DKO mef's (36 ± 3.6 versus 4.8 ± 0.8; P < 0.005) (Fig. 5A), despite a decrease in the rate of cell growth (compare H-RasV12 with H-RasV12 plus shRNA p16 [see Fig. S9 in the supplemental material]). As shRNA directed against p16 promotes the transformation of p53/Elf4 DKO mef's by oncogenic H-RasV12, it appears that the absence of Elf4/Mef leads to p16 accumulation, which suppresses oncogene-induced transformation.
FIG. 5.
The resistance of p53/Elf4 DKO mef's to transformation depends on p16. (A) p16 knockdown allows transformation of p53/Elf4 DKO mef's by H-RasV12. The protein levels of p16 and p19ARF were examined in p53/Elf4 DKO mef's after transduction with the control vector, shRNAp16 alone, H-RasV12 alone, or H-RasV12 plus shRNAp16. Soft agar transformation assays were performed using p53/Elf4 DKO mef's transduced with control vector, shRNAp16 alone, H-RasV12 alone, or H-RasV12 plus shRNAp16. Introduction of shRNAp16 plus H-RasV12 resulted in an 8.3-fold increase in soft agar colonies (compared to H-RasV12-alone-transduced mef's; P < 0.005; n = 4). (B) Knockdown of p16 promotes transformation of p53 KO mef's by ELF4/MEF. The protein levels of p16 and Elf4/Mef were examined in p53 KO mef's after transduction with the control vector, shRNAp16 alone, ELF4/MEF alone, or ELF4/MEF plus shRNAp16. Introduction of shRNAp16 plus ELF4/MEF resulted in a 1.5-fold increase in soft agar colony formation compared to ELF4/MEF alone-transduced p53 KO mef's (P = 0.008; n = 4). (C) Dose-dependent reduction of Ets1-induced p16 promoter activation by ELF4/MEF. Ets1, but not ELF4/MEF, activated the p16 promoter activity, and increasing amounts of ELF4/MEF inhibited Ets1-induced p16 promoter activity by 80% (n = 3). (D) ELF4/MEF expression can rescue the transformation of p53/Elf4 DKO mef's by H-RasV12. When p53/Elf4 DKO mef's were transduced with control vector, ELF4/MEF alone, H-RasV12 alone, or ELF4/MEF plus H-RasV12, the ELF4/MEF plus H-RasV12-transduced DKO mef's showed more soft agar colonies than the H-RasV12-alone-transduced DKO mef's (P < 0.005; n = 4). (E) ELF4/MEF expression suppresses p16 expression but enhances Mdm2 expression in p53/Elf4 DKO mef's. The protein levels of Ets1, p16, Rb, phospho-Rb (Ser 807/811), p19ARF, and Mdm2 were examined in the DKO mef's. ELF4/MEF plus H-RasV12-transduced DKO mef's showed a 1.4-fold decrease in p16 protein, compared to H-RasV12-alone-transduced DKO mef's (as determined by densitometries of two independent studies), despite increased Ets1 expression. The ELF4/MEF plus H-RasV12-transduced DKO mef's showed a significant increase in phospho-Rb protein, compared to the H-RasV12 alone. The ELF4/MEF plus H-RasV12-transduced DKO mef's also showed a significant increase in Mdm2 protein expression compared to the H-RasV12 alone. (F) ELF4/MEF expression does not promote transformation of p16/p19ARF DKO mef's by H-RasV12. p16/p19ARF DKO mef's were transduced with control vector, ELF4/MEF alone, H-RasV12 alone, or ELF4/MEF plus H-RasV12. The ELF4/MEF plus H-RasV12-transduced cells had similar soft agar colony-forming abilities as the H-RasV12-alone-transduced p16/p19ARF DKO mef's (18.7 ± 6.3 versus 15.5 ± 5.8; P = 0.19; n = 4). (G) ELF4/MEF induction suppresses p53 expression in p16/p19ARF DKO mef's. The protein levels of Mef, Mdm2, p53, and p21 were examined in p16/p19ARF DKO mef's. ELF4/MEF plus H-RasV12-transduced p16/p19ARF DKO mef's showed reduced levels of p53 and p21 protein compared to H-RasV12-alone-transduced mef's.
As overexpression of ELF4/MEF can transform p53 KO mef's, we examined whether p16 also plays a role in suppressing MEF-induced transformation in this setting. We transduced p53 KO mef's with ELF4/MEF and p16-directed shRNA and observed increased soft agar colony formation compared to the ELF4/MEF plus control shRNA-transduced p53 KO mef's (18 ± 3.5 versus 12 ± 1.8; P = 0.008) (Fig. 5B). Furthermore, MEF overexpression suppressed the level of p16 protein to a modest degree (Fig. 5B, compare lanes 1 to 3 and 2 to 4).
Despite the lower level of Ets1 seen in the absence of Elf4/Mef (Fig. 4C), we found an accumulation of p16 and similarly found that ELF4/MEF overexpression suppresses p16 protein expression. Therefore, we directly examined whether MEF can reduce basal or Ets1-induced p16 promoter activity. MEF did not repress basal p16 promoter activity in NIH 3T3 cells; however, we did observe a dose-dependent downregulation of Ets1-induced p16 promoter activity (Fig. 5C). Given the abundance of Ets1 in the p53 KO mef's, this may explain how Elf4/Mef can suppress p16 promoter activity and p16 expression.
We next examined whether restoring MEF expression promotes transformation of the p53/Elf4 DKO mef's by H-RasV12. ELF4/MEF and H-RasV12 doubly transduced DKO mef's had fewer SA β-Gal-positive cells and more soft agar colonies than H-RasV12 alone-transduced DKO mef's (11.0 ± 2.3 versus 1.0 ± 1.0; P < 0.005) (Fig. 5D). Thus, ELF4/MEF can restore the ability of H-RasV12 to transform DKO mef's, at least in part by blocking cellular senescence. In this setting, an increase in Mdm2 protein levels is seen only in the ELF4/MEF plus H-RasV12-transduced DKO mef's (Fig. 5E), implying that expression of H-RasV12 may potentiate the ability of Elf4/Mef to upregulate Mdm2. Modest decreases in p16 expression were seen when ELF4/MEF was expressed in the DKO mef's, alone or with H-RasV12, accompanied by a significant increase in phosphorylated Rb protein, even though cells containing ELF4/MEF showed H-RasV12-induced Ets1 activation (Fig. 5E). This suggests that Elf4/Mef functions to reduce p16 expression (and promote Rb phosphorylation) when it is activated by H-RasV12-induced Ets1 expression.
To determine the importance of p16 expression in transformation by H-RasV12 and/or ELF4/MEF, we transduced ELF4/MEF and H-RasV12 into p16 null/p19ARF null mef's. We saw few soft agar colonies when ELF4/MEF alone was introduced into the p16/p19ARF DKO mef's (Fig. 5F), and while H-RasV12 transformed the p16/p19ARF DKO mef's, ELF4/MEF plus H-RasV12-transduced p16/p19ARF DKO mef's had no more soft agar colonies than H-RasV12 alone (18.7 ± 6.3 versus 15.5 ± 5.8; P = 0.19) (Fig. 5F). Expression of ELF4/MEF reduced the level of p53 protein, leading to reduced p21 expression in the H-RasV12-transduced p16/p19ARF DKO mef's (Fig. 5G). Nonetheless, no significant change in the rate of cell growth was observed (compare H-RasV12 versus H-RasV12 plus ELF4/MEF) (see Fig. S10 in the supplemental material). Thus, ELF4/MEF expression can block Ras-induced senescence and enhance its transformation. Taken together, ELF4/MEF plays a crucial role not only in promoting transformation by preventing senescence via suppression of Ets1-induced p16 activation but also by activating Mdm2 expression when p53 null cells are challenged by oncogenic Ras.
p53/Elf4 DKO mice have better survival than p53 KO mice due to delayed tumor onset.
Given the critical role of Elf4 in the oncogenic transformation of mef's, we next examined how Elf4/Mef contributes to tumorigenesis in vivo by examining the incidence of “spontaneous” tumor formation in the p53/Elf4 DKO mice. Tumor formation was impaired in the DKO mice; thus, the DKO mice had significantly longer median survival than the p53 KO mice (142 days versus 102 days; P = 0.001) (Fig. 6A). We defined the types of tumors that occurred in the p53 KO versus the DKO mice and found that although all of the p53 KO mice had significant tumors at autopsy, 6 of the 48 DKO mice died with no detectable tumor. (Elf4/Mef KO mice do not develop tumors, and all p53 KO mice developed tumors within the expected time frame [9].) We found that 31 of 48 DKO mice had lymphoma, versus 15 of 25 p53 KO mice, indicating no significant difference in the incidence of lymphoma when MEF was present or absent (65% versus 60%). However, the median survival of the p53 KO mice with lymphoma was 105 days, versus 149 days for the DKO mice with lymphoma (P = 0.036) (Fig. 6B). Furthermore, while 19 of 48 DKO mice developed sarcomas, only 5 of 25 p53 KO mice did (40% versus 20%; P < 0.005) (12 DKO mice had both sarcoma and lymphoma).
FIG. 6.
p53/Elf4 DKO mice survive longer than p53 KO mice. (A) Kaplan-Meier survival curves show longer median survival for the p53/Elf4 DKO mice than the p53 KO mice (142 days versus 102 days; P = 0.001). (B) Time to death from lymphoma is shown by Kaplan-Meier curves for the p53 KO and the p53/Elf4 DKO mice. Results show the median survival of the p53 KO mice, who develop lymphoma in 105 days, versus 149 days for the p53/Elf4 DKO mice who develop lymphoma (P = 0.036). (C) Similar Ki67 indexes in lymphomas isolated from p53/Elf4 DKO and p53 KO mice (44% ± 19% versus 39% ± 18%; P = 0.25). (D) H&E staining of splenic lymphoma tissue in a p53 KO mouse and a p53/Elf4 DKO mouse, together with representative staining of Mdm2 and p16 protein by IHC. (E) Correlation between Mdm2 and p16 expression in lymphoma tissue obtained from p53 KO and p53/Elf4 DKO mice. Each score shows the number of lymphoma samples with low, moderate (mod), and high Mdm2 staining or with negative and positive p16 staining by IHC.
To assess whether the better survival of the DKO mice was caused by the delayed initiation or the slower growth of the tumors, we examined Ki67 expression in the lymphomas of the DKO and the p53 KO mice by IHC. We did not find a significant difference in Ki67 staining of the lymphomas of DKO mice (44% ± 19%) versus the p53 KO mice (39% ± 18%; P = 0.25) (Fig. 6C). We also analyzed tumor- or event-free survival in the p53 KO mice versus the DKO mice and found that the DKO mice had significantly longer median tumor-free survival than did the p53 KO mice (137 days versus 96 days; P = 0.002) (see Fig. S11 in the supplemental material). This implies that Elf4/Mef loss in the absence of p53 results primarily in delayed tumor onset, rather than slower tumor growth.
Given that Elf4/Mef loss leads to impaired Mdm2 expression and elevated p16 expression in cultured mef's, we examined by IHC whether the lymphomas of the DKO mice and the p53 KO mice expressed Mdm2 and/or p16 protein. The lymphoma cells of the p53 KO mice more often showed strong staining for Mdm2 and no staining for p16 than the lymphoma cells of the DKO mice (Fig. 6D). Overall 3 of 10 lymphomas from the p53 KO mice versus 2 of 18 from the DKO mice had high Mdm2 expression, indicating that Mdm2 expression is reduced when Elf4/Mef is absent (30% versus 11%; P = 0.018) (Fig. 6E). The lymphoma tissue from 5 of 18 DKO mice showed p16 expression, whereas none of the lymphoma tissue from the p53 KO mice expressed p16 (27% versus 0%; P = 0.017) (Fig. 6E). The inhibitory effects of Elf4/Mef on p16 are yet another means for it to promote the G1-to-S transition (18, 20). Elf4/Mef loss can impair tumor cell initiation in vitro and in vivo, and it controls (or at least correlates with) Mdm2 expression in murine and human lymphoma tissue specimens.
DISCUSSION
We have identified the dual capability of a single transcription factor, ELF4/MEF, to promote transformation via effects on cellular senescence and tumor cell growth by regulating the p53/Mdm2/p19ARF and the p16/Rb pathways. Senescence is a critical initial barrier against tumorigenesis (2, 7), and we have found that both the acute loss of Elf4/Mef and its chronic absence enhance senescence (confirmed by increased SA β-Gal-positive cells and decreased BrdU incorporation) via marked accumulation of p53 protein. The importance of Elf4/Mef expression in regulating cell senescence is further demonstrated by the decrease in Elf4/Mef mRNA expression that occurs with serial passage of wild-type mef's, as well as the impaired Elf4/Mef expression found in response to extracellular growth signals as presenescent mef's age. This decreased Elf4/Mef expression could serve to trigger the senescence machinery.
In response to DNA damage or oncogenic stress, cells activate p53 function to exit the cell cycle and promote cellular senescence (1). p53 activation also triggers cells to undergo apoptosis or to arrest in the G1 phase, depending on the cell type and the nature of stress signals (10, 40). Thus, loss of p53 or mutations that prevent its activation allow cells to escape apoptosis or senescence and become transformed (42). Using p53/Elf4 DKO mef's, we found that the enhanced senescence seen in Elf4/Mef KO mef's is dependent on p53, which is perhaps not surprising given the critical role of p53 in promoting senescence in mef's.
Our observations in mef's establish a critical link between ELF4/MEF and p53 expression, as overexpression of ELF4/MEF reduces p53 protein levels (but not p19ARF, which also regulates p53 levels [15]), leading to decreased p21 expression. In other studies, we recently showed that p53 is required for the enhanced hematopoietic stem cell quiescence seen in Elf4/Mef KO mice (19). To define how Elf4/Mef inversely regulates p53 levels, we first examined Mdm2, which is the major negative regulator of p53 (4, 28). Early-passage Elf4/Mef KO mef's have low Mdm2 mRNA and protein levels, leading to their enhanced senescence (due to activation of p53 function). We found that Elf4/Mef not only directly binds the Mdm2 promoter (using ChIP assays), but also it activates Mdm2 promoter function to the same degree as p53. The moderate increase in Mdm2 protein seen in the senescent Elf4/Mef KO mef's may be due to p53 activation, which is known to upregulate Mdm2, as part of a well-described negative feedback loop between p53 and Mdm2. But, in the absence of detectable (mutant) p53 protein, both human lymphoma tissue and murine lymphomas showed a significant positive correlation between the levels of ELF4/MEF and MDM2 expression. Thus, our data show that Elf4/Mef prevents senescence and suppresses p53 activity by directly activating Mdm2, rather than by inhibiting p19ARF (this model is shown in Fig. 7).
FIG. 7.
Model for how ELF4/MEF functions to suppress senescence. The model shows how Elf4/Mef can regulate Mdm2 expression and also Ets1-induced p16 expression. Arrows represent the expression levels of the genes indicated. (Left) In Elf4/Mef null cells, Elf4/Mef loss reduces Mdm2 expression, which leads to an accumulation of p53. Together with p53, p16 promotes Ras-induced senescence when it is activated by Ras-induced Ets1 expression. (Right) In the Elf4/Mef-overexpressing cells, Elf4/Mef not only enhances Mdm2 expression (overriding the opposite effect of p19ARF on p53) but also inhibits Ets1-induced p16 induction, which is provoked by oncogenic Ras. Lastly, Elf4/Mef overexpression inhibits oncogene-induced senescence and promotes tumorigenesis.
Several studies have identified a role for ELF4/MEF in tumorigenesis (22, 25), and we did find that the transforming ability of Elf4/Mef is dependent on Mdm2 (knocking down Mdm2 expression suppresses MEF-induced transformation in p53 null mef's). This indicates that ELF4/MEF also promotes cellular transformation via p53-independent effects of Mdm2.
Oncogenic Ras transforms cells that have inactivated their senescence program (which is controlled by the p53 and p16/Rb pathways [32, 35]). As loss of p53 function delays or abrogates senescence, p53 null mef's can be transformed by a single oncogene, and neither oncogenic Ras nor c-myc induces senescence in cells lacking p53 (12, 38). However, neither H-RasV12 nor c-myc was able to transform p53/Elf4 DKO mef's. This defect is due to a lack of Elf4/Mef, as introduction of H-RasV12 plus ELF4/MEF can transform p53/Elf4 DKO mef's (an effect accompanied by increased Mdm2 expression). Expression of the endogenous Elf4/Mef gene is potently upregulated by H-RasV12, and we have also found that Elf4/Mef can be phosphorylated by the ERK family of mitogen-activated protein kinases (unpublished data) which function downstream of Ras. Thus, Ras-induced activation of Elf4/Mef or at least the presence of Elf4/Mef appears to be needed to transform p53 null mef's.
We found marked increased expression of both p16 and p19ARF proteins following introduction of H-RasV12 into p53/Elf4 DKO mef's and that either Bmi-1 expression or knock down of p16 expression restored the transforming ability of p53/Elf4 DKO mef's. Furthermore, knocking down p16 also promoted ELF4/MEF-induced transformation of p53 KO mef's. This suggests that enhanced p16 activation plays a major role in the resistance to transformation of cells lacking p53 and Elf4/Mef. p16 is required for Ras-induced senescence, and oncogenic Ras induces p16 expression by activating Ets1 or Ets2 (16, 29). We have found that in the absence of p53 ELF4/MEF can reduce p16 expression that occurs in response to Ras-induced Ets1 expression and that ELF4/MEF represses Ets1-induced p16 promoter activity in a dose-dependent manner. While we can detect Elf4/Mef on the p16 promoter in ChIP assays (unpublished data), we have not fully defined how Elf4/Mef inhibits p16 expression. But, by transducing ELF4/MEF and H-RasV12 into mef's that lack p16/p19ARF, we found no effect of ELF4/MEF on Ras-induced transformation, suggesting that ELF4/MEF enhancement of Ras-induced transformation requires the presence of p16. Thus, ELF4/MEF overexpression can overcome Ras-induced p16 upregulation, which normally limits oncogenesis, in certain cell types that lack p53 (as shown in Fig. 7).
The importance of Elf4/Mef in transformation is clearly seen in vivo, as p53/Mef DKO mice have better survival and are less tumor-prone than p53 KO mice. We did not see a significant difference in the cell growth of the lymphomas of the p53/Mef DKO mice compared to the p53 KO mice (by Ki67 staining or surveillance of palpable tumors). However, Elf4/Mef loss appeared to delay the onset of lymphomas in vivo, perhaps due to activated p16 expression, as in vivo studies have indicated that p16 can suppress the development of spontaneous tumors (38). Certainly, we found increased p16 expression in lymphoma tissues from some of the DKO mice, but not in the lymphomas that arose in the p53 KO mice.
We have described several mechanisms by which ELF4/MEF can function as an oncogene. The several studies that suggest that ELF4/MEF may play a potential tumor suppressor role in lung cancer and acute myeloid leukemia (11, 23, 33) may have identified quite context-dependent activities. Given that lack of Elf4/Mef enhances HSC quiescence and diminishes chemotherapy-induced myelosuppression (18, 19), further study is required to determine whether low ELF4/MEF expression may be selected for by cancer cells over time, thereby contributing to their resistance to cytotoxic therapies. Nonetheless, ELF4/MEF can participate in tumorigenesis as a nodal point in the cross talk between the two major tumor suppressor pathways, p53 and p16/Rb.
Supplementary Material
Acknowledgments
We thank Robert Benezra for his critical reading of the manuscript, Shigeki Shimizu and Hiroko Shinoda for their help with the mouse lymphoma IHC studies, and the members of the Nimer lab for their helpful ideas and discussion. We are also grateful to the staff of the Mouse Genotyping Core Facility and Comparative Pathology Facility at MSKCC.
This work was supported by NIH grant R01 DK52208 (S.D.N.) and the Maynard Parker Leukemia Research Fund. Equipment used for these studies was funded by the William H. and Alice Goodwin and the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center of MSKCC.
Footnotes
Published ahead of print on 20 April 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Beausejour, C. M., A. Krtolica, F. Galimi, M. Narita, S. W. Lowe, P. Yaswen, and J. Campisi. 2003. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 224212-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braig, M., S. Lee, C. Loddenkemper, C. Rudolph, A. H. Peters, B. Schlegelberger, H. Stein, B. Dorken, T. Jenuwein, and C. A. Schmitt. 2005. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436660-665. [DOI] [PubMed] [Google Scholar]
- 3.Brookes, S., J. Rowe, M. Ruas, S. Llanos, P. A. Clark, M. Lomax, M. C. James, R. Vatcheva, S. Bates, K. H. Vousden, D. Parry, N. Gruis, N. Smit, W. Bergman, and G. Peters. 2002. INK4a-deficient human diploid fibroblasts are resistant to RAS-induced senescence. EMBO J. 212936-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks, C. L., and W. Gu. 2006. p53 ubiquitination: Mdm2 and beyond. Mol. Cell 21307-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buschmann, T., T. Minamoto, N. Wagle, S. Y. Fuchs, V. Adler, M. Mai, and Z. Ronai. 2000. Analysis of JNK, Mdm2 and p14ARF contribution to the regulation of mutant p53 stability. J. Mol. Biol. 2951009-1021. [DOI] [PubMed] [Google Scholar]
- 6.Campisi, J. 2005. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120513-522. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Z., L. C. Trotman, D. Shaffer, H. K. Lin, Z. A. Dotan, M. Niki, J. A. Koutcher, H. I. Scher, T. Ludwig, W. Gerald, C. Cordon-Cardo, and P. P. Pandolfi. 2005. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436725-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimri, G. P., X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E. E. Medrano, M. Linskens, I. Rubelj, O. Pereira-Smith, et al. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 929363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donehower, L. A., M. Harvey, B. L. Slagle, M. J. McArthur, C. A. Montgomery, Jr., J. S. Butel, and A. Bradley. 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356215-221. [DOI] [PubMed] [Google Scholar]
- 10.Fridman, J. S., and S. W. Lowe. 2003. Control of apoptosis by p53. Oncogene 229030-9040. [DOI] [PubMed] [Google Scholar]
- 11.Fukushima, T., Y. Miyazaki, H. Tsushima, C. Tsutsumi, J. Taguchi, S. Yoshida, K. Kuriyama, D. Scadden, S. Nimer, and M. Tomonaga. 2003. The level of MEF but not ELF-1 correlates with FAB subtype of acute myeloid leukemia and is low in good prognosis cases. Leuk. Res. 27387-392. [DOI] [PubMed] [Google Scholar]
- 12.Harvey, M., A. T. Sands, R. S. Weiss, M. E. Hegi, R. W. Wiseman, P. Pantazis, B. C. Giovanella, M. A. Tainsky, A. Bradley, and L. A. Donehower. 1993. In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene 82457-2467. [PubMed] [Google Scholar]
- 13.Hedvat, C. V., J. Yao, R. A. Sokolic, and S. D. Nimer. 2004. Myeloid ELF1-like factor is a potent activator of interleukin-8 expression in hematopoietic cells. J. Biol. Chem. 2796395-6400. [DOI] [PubMed] [Google Scholar]
- 14.Honda, R., H. Tanaka, and H. Yasuda. 1997. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 42025-27. [DOI] [PubMed] [Google Scholar]
- 15.Kamijo, T., F. Zindy, M. F. Roussel, D. E. Quelle, J. R. Downing, R. A. Ashmun, G. Grosveld, and C. J. Sherr. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91649-659. [DOI] [PubMed] [Google Scholar]
- 16.Kim, W. Y., and N. E. Sharpless. 2006. The regulation of INK4/ARF in cancer and aging. Cell 127265-275. [DOI] [PubMed] [Google Scholar]
- 17.Lacorazza, H. D., Y. Miyazaki, A. Di Cristofano, A. Deblasio, C. Hedvat, J. Zhang, C. Cordon-Cardo, S. Mao, P. P. Pandolfi, and S. D. Nimer. 2002. The ETS protein MEF plays a critical role in perforin gene expression and the development of natural killer and NK-T cells. Immunity 17437-449. [DOI] [PubMed] [Google Scholar]
- 18.Lacorazza, H. D., T. Yamada, Y. Liu, Y. Miyata, M. Sivina, J. Nunes, and S. D. Nimer. 2006. The transcription factor MEF/ELF4 regulates the quiescence of primitive hematopoietic cells. Cancer Cell 9175-187. [DOI] [PubMed] [Google Scholar]
- 19.Liu, Y., S. E. Elf, Y. Miyata, G. Sashida, Y. Liu, G. Huang, S. Di Giandomenico, J. M. Lee, A. Deblasio, S. Menendez, J. Antipin, B. Reva, A. Koff, and S. D. Nimer. 2009. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell 437-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, Y., C. V. Hedvat, S. Mao, X. H. Zhu, J. Yao, H. Nguyen, A. Koff, and S. D. Nimer. 2006. The ETS protein MEF is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCFSkp2. Mol. Cell. Biol. 263114-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lossos, I. S., and D. Morgensztern. 2006. Prognostic biomarkers in diffuse large B-cell lymphoma. J. Clin. Oncol. 24995-1007. [DOI] [PubMed] [Google Scholar]
- 22.Lund, A. H., G. Turner, A. Trubetskoy, E. Verhoeven, E. Wientjens, D. Hulsman, R. Russell, R. A. DePinho, J. Lenz, and M. van Lohuizen. 2002. Genome-wide retroviral insertional tagging of genes involved in cancer in Cdkn2a-deficient mice. Nat. Genet. 32160-165. [DOI] [PubMed] [Google Scholar]
- 23.Mao, S., R. C. Frank, J. Zhang, Y. Miyazaki, and S. D. Nimer. 1999. Functional and physical interactions between AML1 proteins and an ETS protein, MEF: implications for the pathogenesis of t(8;21)-positive leukemias. Mol. Cell. Biol. 193635-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Midgley, C. A., and D. P. Lane. 1997. p53 protein stability in tumour cells is not determined by mutation but is dependent on Mdm2 binding. Oncogene 151179-1189. [DOI] [PubMed] [Google Scholar]
- 25.Mikkers, H., J. Allen, P. Knipscheer, L. Romeijn, A. Hart, E. Vink, and A. Berns. 2002. High-throughput retroviral tagging to identify components of specific signaling pathways in cancer. Nat. Genet. 32153-159. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki, Y., P. Boccuni, S. Mao, J. Zhang, H. Erdjument-Bromage, P. Tempst, H. Kiyokawa, and S. D. Nimer. 2001. Cyclin A-dependent phosphorylation of the ETS-related protein, MEF, restricts its activity to the G1 phase of the cell cycle. J. Biol. Chem. 27640528-40536. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki, Y., X. Sun, H. Uchida, J. Zhang, and S. Nimer. 1996. MEF, a novel transcription factor with an Elf-1 like DNA binding domain but distinct transcriptional activating properties. Oncogene 131721-1729. [PubMed] [Google Scholar]
- 28.Momand, J., G. P. Zambetti, D. C. Olson, D. George, and A. J. Levine. 1992. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 691237-1245. [DOI] [PubMed] [Google Scholar]
- 29.Ohtani, N., Z. Zebedee, T. J. Huot, J. A. Stinson, M. Sugimoto, Y. Ohashi, A. D. Sharrocks, G. Peters, and E. Hara. 2001. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature 4091067-1070. [DOI] [PubMed] [Google Scholar]
- 30.Quelle, D. E., F. Zindy, R. A. Ashmun, and C. J. Sherr. 1995. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 83993-1000. [DOI] [PubMed] [Google Scholar]
- 31.Ries, S., C. Biederer, D. Woods, O. Shifman, S. Shirasawa, T. Sasazuki, M. McMahon, M. Oren, and F. McCormick. 2000. Opposing effects of Ras on p53: transcriptional activation of mdm2 and induction of p19ARF. Cell 103321-330. [DOI] [PubMed] [Google Scholar]
- 32.Sarkisian, C. J., B. A. Keister, D. B. Stairs, R. B. Boxer, S. E. Moody, and L. A. Chodosh. 2007. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat. Cell Biol. 9493-505. [DOI] [PubMed] [Google Scholar]
- 33.Seki, Y., M. A. Suico, A. Uto, A. Hisatsune, T. Shuto, Y. Isohama, and H. Kai. 2002. The ETS transcription factor MEF is a candidate tumor suppressor gene on the X chromosome. Cancer Res. 626579-6586. [PubMed] [Google Scholar]
- 34.Serrano, M., G. J. Hannon, and D. Beach. 1993. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366704-707. [DOI] [PubMed] [Google Scholar]
- 35.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88593-602. [DOI] [PubMed] [Google Scholar]
- 36.Seshadri, T., and J. Campisi. 1990. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science 247205-209. [DOI] [PubMed] [Google Scholar]
- 37.Seth, A., and D. K. Watson. 2005. ETS transcription factors and their emerging roles in human cancer. Eur. J. Cancer 412462-2478. [DOI] [PubMed] [Google Scholar]
- 38.Sharpless, N. E., N. Bardeesy, K. H. Lee, D. Carrasco, D. H. Castrillon, A. J. Aguirre, E. A. Wu, J. W. Horner, and R. A. DePinho. 2001. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 41386-91. [DOI] [PubMed] [Google Scholar]
- 39.Sharrocks, A. D. 2001. The ETS-domain transcription factor family. Nat. Rev. Mol. Cell Biol. 2827-837. [DOI] [PubMed] [Google Scholar]
- 40.Vogelstein, B., D. Lane, and A. J. Levine. 2000. Surfing the p53 network. Nature 408307-310. [DOI] [PubMed] [Google Scholar]
- 41.Wu, X., J. H. Bayle, D. Olson, and A. J. Levine. 1993. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 71126-1132. [DOI] [PubMed] [Google Scholar]
- 42.Xue, W., L. Zender, C. Miething, R. A. Dickins, E. Hernando, V. Krizhanovsky, C. Cordon-Cardo, and S. W. Lowe. 2007. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445656-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao, J. J., Y. Liu, H. D. Lacorazza, R. A. Soslow, J. M. Scandura, S. D. Nimer, and C. V. Hedvat. 2007. Tumor promoting properties of the ETS protein MEF in ovarian cancer. Oncogene 264032-4037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.