Abstract
LKB1, a master kinase that controls at least 13 downstream protein kinases including the AMP-activated protein kinase (AMPK), resides mainly in the nucleus. A key step in LKB1 activation is its export from the nucleus to the cytoplasm. Here, we identified S307 of LKB1 as a putative novel phosphorylation site which is essential for its nucleocytoplasmic transport. In a cell-free system, recombinant PKC-ζ phosphorylates LKB1 at S307. AMPK-activating agents stimulate PKC-ζ activity and LKB1 phosphorylation at S307 in endothelial cells, hepatocytes, skeletal muscle cells, and vascular smooth muscle cells. Like the kinase-dead LKB1 D194A mutant (mutation of Asp194 to Ala), the constitutively nucleus-localized LKB1 SL26 mutant and the LKB1 S307A mutant (Ser307 to Ala) exhibit a decreased association with STRADα. Interestingly, the PKC-ζ consensus sequence surrounding LKB1 S307 is disrupted in the LKB1 SL26 mutant, thus providing a likely molecular explanation for this mutation causing LKB1 dysfunction. In addition, LKB1 nucleocytoplasmic transport and AMPK activation in response to peroxynitrite are markedly reduced by pharmacological inhibition of CRM1, which normally facilitates nuclear export of LKB1-STRAD complexes. In comparison to the LKB1 wild type, the S307A mutant complexes show reduced association with CRM1. Finally, adenoviral overexpression of wild-type LKB1 suppresses, while the LKB1 S307A mutant increases, tube formation and hydrogen peroxide-enhanced apoptosis in cultured endothelial cells. Taken together, our results suggest that, in multiple cell types the signaling pathways engaged by several physiological stimuli converge upon PKC-ζ-dependent LKB1 phosphorylation at S307, which directs the nucleocytoplasmic transport of LKB1 and consequent AMPK activation.
LKB1 is a tumor suppressor (3, 25, 33, 42, 59) that is mutated in Peutz-Jeghers cancer syndrome (20, 24). This serine/threonine protein kinase phosphorylates and activates at least 13 downstream kinases, which in turn regulate multiple cellular processes, including the cell cycle, cellular proliferation, apoptosis, and energy metabolism (1, 30). One of the key downstream kinases of LKB1 is the 5′-AMP-activated protein kinase (AMPK), a serine/threonine kinase that serves as a master regulator of energy metabolism (18, 19, 28). LKB1 is ubiquitously expressed in adult and fetal tissue, particularly pancreatic, liver, testicular, cardiac, and skeletal muscle tissue (21, 25, 43, 60). In humans, LKB1 comprises 433 amino acids (436 residues in mouse LKB1) and is located predominantly in the nucleus due to its nuclear localization signal in the N-terminal noncatalytic region (residues 38 to 43) (36, 53). Paradoxically, LKB1 activation takes place predominantly in the cytoplasm, after it complexes with STRAD (STE-related adapter) and MO25 (mouse protein 25). As a result, the nucleocytoplasmic transport and subsequent association of LKB1 with STRAD and MO25 in the cytoplasm are required for full activation of LKB1 (2, 5) and its downstream kinases, including AMPK. Consistent with this theory, 12 mutants of LKB1 (including the SL26 mutants) found in patients with Peutz-Jeghers cancer syndrome are constitutively nuclear (5, 6). Further, a recent study from Macara's group (13) shows that STRAD regulates LKB1 localization by blocking access to importin and by association with CRM1 and exportin-7, two nuclear protein exportins.
LKB1 is phosphorylated at S325, T366, and S431 by upstream kinases. In addition, LKB1 autophosphorylates at S31, T185, T189, T336, and S404 (1). Mutation of any of these phosphorylation sites to Ala (to abolish phosphorylation) or Glu (to mimic phosphorylation) does not significantly affect the in vitro catalytic activity of LKB1 or its intracellular localization (5, 44, 45). Recently, we demonstrated that phosphorylation of LKB1 S428 is required for metformin-enhanced AMPK activation (56). Nevertheless, several questions such as the precise mechanism(s) underlying LKB1 activation, the relevant phosphorylation sites, and the upstream activating kinase(s) remain unclear. While it has been shown that LKB1 S428 phosphorylation is required for nucleocytoplasmic transport of LKB1, the translocation of LKB1 to the cytosol could be further regulated by unknown mechanisms. Here, we have identified S307 as a novel phosphorylation site in LKB1 and provide evidence that, in multiple cell types, phosphorylation of this site by protein kinase C ζ (PKC-ζ) induces nucleocytoplasmic transport of LKB1 and subsequent activation of AMPK and suppression of angiogenesis and apoptosis. Importantly, we provide a molecular explanation for the constitutive nuclear localization of the LKB1 SL26 mutant. Taken together, our results suggest that the phosphorylation of LKB1 S307 by PKC-ζ is essential for LKB1 regulation of cell cycle progression, proliferation, angiogenesis, and apoptosis.
MATERIALS AND METHODS
Materials.
Human umbilical vein endothelial cells (HUVECs) were obtained from Cascade Biologics (Portland, OR). Bovine aortic endothelial cells (BAECs) and cell culture media were obtained from Cambrex Bio Science Walkersville, Inc. (Walkersville, MD). A549 cells were from ATCC (Manassas, VA). The SAMS peptide was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). Antibodies against phospho-AMPK (T172), AMPK-α, phospho-LKB1 (S428), phospho-acetyl-coenzyme A carboxylase (ACC) (Ser79), and phospho-PKC-ζ were obtained from Cell Signaling, Inc. (Beverly, MA). PKC-ζ and PKC-λ/ι, LKB1, STRADα, and MO25α small interfering RNA (siRNA) and antibodies against PKC-ζ, PKC-λ/ι, LKB1 (D-19), CRM-1 (C-1), and STRAD (N-13) were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). MO25 rabbit monoclonal antibody was purchased from Epitomics, Inc. (Burlingame, CA), protein A Sepharose CL-48 was from Amersham Biosciences (Uppsia, Sweden), and cell-permeable myristoylated PKC-ζ pseudosubstrate (PKC-ζ-PS) was from Biosource International (Camarillo, CA). All other chemicals and organic solvents were of the highest grade and were obtained from Sigma-Aldrich (St. Louis, MO).
Recombinant AMPK and LKB1 protein complexes.
Recombinant AMPK His-α1β1γ1, was prepared as described previously (35, 51) LKB1-MO25α-STRADα complexes were bacterially expressed and purified up to the heparin step. Construction of the LKB1 S428A mutant was described previously (34).
Cell culture and adenoviral infection.
Adenoviral vectors expressing constitutively active PKC-ζ (PKC-ζ-CA); dominant-negative PKC-ζ (PKC-ζ-DN); wild-type (WT) LKB1 or LKB1 S307A, LKB1 S428A, and LKB1 D194A mutants; or green fluorescent protein (GFP) were used, as described previously (10, 57, 62). A replication-defective adenoviral vector expressing GFP (Ad-GFP) served as a negative control. In these experiments, infection efficiency typically exceeded 80%, as determined by GFP expression.
siRNA silencing of PKC-ζ in HUVECs.
HUVECs were transfected with human-specific PKC-ζ siRNA or a corresponding scrambled siRNA for 48 h using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. Infected cells were starved in serum-free medium for 6 h and then exposed to the indicated concentrations of metformin.
PKC-ζ activity.
PKC-ζ was first immunoprecipitated with PKC-ζ-specific antibody, and PKC-ζ activity was assayed with PKC-ζ-specific peptides, as described previously (23).
Immunoprecipitation and Western blotting.
Cells were homogenized in lysis buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride. The resulting lysates were sonicated twice for 10 s in an Ultrasonic Dismemberator with 10% output (Model 500; Fisher Scientific) and then centrifuged at 14,000 × g for 20 min at 4°C. The supernatants were subjected to immunoprecipitation with the indicated antibodies. Immunoprecipitates or whole-cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, and probed with specific antibodies, as described previously (57, 63).
Quantification of Western blots.
The intensities (density × area) of individual bands were measured by densitometry (Model GS-700, Imaging Densitometer, Bio-Rad). The background was subtracted from the calculated area.
In vitro kinase assays.
Recombinant hexahistidine-tagged AMPK His-α1β1γ1 was incubated with various concentrations of recombinant PKC-ζ or PKC-βII for 15 min at 37°C in the presence of [32P]ATP (1 μCi) and the SAMS peptide (200 μM), with or without AMP (200 μM). The reaction mixture was supplemented with 20 μl 3× sample buffer to terminate the reaction, boiled for 5 min at 95°C, and separated by 12% SDS-PAGE. The dried gels were subjected to autoradiography to analyze changes in protein phosphorylation. Aliquots of the reaction mixture were also subjected to scintillation counting to determine AMPK activity, or more specifically, 32P incorporation into the SAMS peptide. In other experiments, the activity of recombinant LKB1 was indirectly measured by assaying AMPK-dependent [32P]ATP incorporation into the SAMS peptide.
Construction of plasmid DNA vectors of LKB1 mutants and adenoviral LKB1 mutation vectors.
S307 or S428 of human LKB1 was mutated to Ala; catalytic site Asp 194 was replaced by Ala using the QuikChange kit (Stratagene), according to the manufacturer's instructions. Oligonucleotides used for point mutation are listed in Table S3 in the supplemental material. The S307A S428A double mutant was prepared by the sequential mutation strategy. All mutations were confirmed by DNA sequencing. Plasmid DNA was extracted on a large scale using Qiagen's EndoFree plasmid maxikit (cat. no. 12362) and transfected into HeLa-S3 cells or A549 using the Lipofectamine 2000 kit (Invitrogen, catalog no. 11668-019), according to the instructions provided by the supplier. After transfection, cells underwent a 24-h incubation before receiving any additional treatments. Cells transfected with the LacZ expression vector as well as untreated cells served as controls.
Adenovirus encoding WT LKB1 or the LKB1 S307A, S428A, and D194A mutants was prepared as instructed by the provider (Q-Biogene, Inc.). In brief, the WT and KLB1 S307A, S428A, and D194A mutants were released from their DNA vector with EcoRI/NotI and subcloned into the EcoRI/NotI site of pCR259. The resulting plasmids were transformed into the HighQ-1 Transpose-Ad 294 Escherichia coli strain to recombine with the adenovirus genome followed by characterization, amplification, and titration in mammalian 293A cells.
Immunocytochemical staining of LKB1 and TUNEL staining.
HUVECs and A549 cells transfected with WT LKB1 and mutated plasmids were cultured on coverslips and then fixed with 4% paraformaldehyde. After blocking, the HUVECs were incubated with goat anti-LKB1 antibody (Santa Cruz, Biotechnology, Inc., Santa Cruz, CA) overnight. Since all tested commercially available antibodies against LKB1 did not work for A549 cells and LKB1 plasmids encoded an N-terminal His Tag, the LKB1 proteins in A549 were detected with a mouse anti-His antibody (Upstate Cell Signaling Solutions, Temecula, CA). After three washes, the slides were incubated with a fluorescein isothiocyanate (FITC)-conjugated donkey anti-goat antibody and an FITC-conjugated donkey anti-mouse antibody (Jackson ImmunoResearch Lab, Inc., West Grove, PA), respectively, at a dilution of 1:150 for 1 h. The slides were then rinsed, counterstained with 4′,6-diamidino-2-phenylindole (DAPI), mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and viewed on an SLM 510 laser-scanning confocal microscope (Carl Zeiss Meditec, Inc., Jena, Germany). Apoptosis was assessed by terminal deoxynucleotidyltransferas-mediated dUTP-biotin nick end labeling (TUNEL) staining (TMR red) using a kit from Roche (Mannheim, Germany) following the instruction manual provided. The percentage of apoptotic cells was calculated from the number of TUNEL-positive cells divided by the total number of cells counted.
Preparation of subcellular fractions.
Cellular cytosolic and nuclear fractions were prepared as described previously (56, 58).
Tube formation assay.
HUVECs were transfected with adenoviral vectors expressing WT LKB1, the LKB1 D194A, LKB1 S307A, and LKB1 S428A mutants, or GFP 2 days prior to plating on Matrigel-coated culture dishes. The formation of vascular-like structures by HUVECs was performed on Matrigel (BD Biosciences) as follows. Six-well culture plates were coated with Matrigel, according to the manufacturer's instructions. The indicated HUVECs were seeded on coated plates at 5 × 105 cells/well in endothelial cell growth medium and incubated at 37°C for 8 h. The degree of tube formation was quantified by measuring the length of tubes in three randomly chosen fields from each well using the NIH Image Program.
Statistical analysis.
Results were analyzed using a one-way analysis of variance. All values are expressed as means ± standard errors. P values of <0.05 were considered statistically significant.
RESULTS
AMPK-activating stimuli also activate PKC-ζ in intact cells.
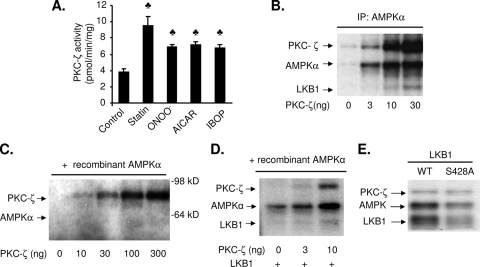
AMPK is activated by diverse stimuli, such as 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), oxidants, hormones, and drugs. As shown in Fig. 1A, exposure of BAECs to peroxynitrite (ONOO−), metformin, AICAR, statin, or the thromboxane receptor agonist IBOP [[1S-(1α,2β(5Z),3 α(1E,3R),4 α)]-7-[3-(3-hydroxy-4-(4′-iodophenoxy)-1-butenyl)-7-oxabicyclo-[2.2.1] heptan-2-yl]-5-heptenoic acid] increases the activity of PKC-ζ, as measured by in vitro kinase assays with a PKC-ζ-specific substrate. In all cases, increased PKC-ζ activity was associated with the translocation of PKC-ζ from the cytosol to the plasma membrane (data not shown), confirming the activation of PKC-ζ by these stimuli.
FIG. 1.
LKB1 is required for PKC-ζ stimulation of AMPK activation and phosphorylation in vitro. (A) PKC-ζ activity following exposure of BAECs to the indicated AMPK-activating agents. ♣, P < 0.05 vs. control. (B) AMPKα immunoprecipitated (IP) from BAECs was incubated with recombinant PKC-ζ for 15 min. 32P incorporation of PKC-ζ, LKB1, and AMPK was monitored by autoradiography. (C) AMPK 32P incorporation following coincubation of recombinant PKC-ζ and recombinant AMPK in the absence of exogenous LKB1. (D) AMPK 32P incorporation following incubation of recombinant LKB1 with PKC-ζ and AMPK. All blots are representative of four to six blots from four to six independent experiments (n = 4 or 6). (E) Mutation of LKB1 S428 into alanine partially blocks PKC-ζ-enhanced 32P incorporation into LKB1 and AMPK in vitro. Recombinant AMPK and PKC-ζ were incubated with recombinant WT LKB1 or LKB1 S428A for 15 min, and 32P incorporation into AMPK was monitored by autoradiography. The blot is a representative of three blots from three independent experiments (n = 3).
PKC-ζ-mediated activation of AMPK requires the phosphorylation of LKB1.
Our previous studies (10, 57) have shown that selective inhibition of PKC-ζ attenuates AMPK activation elicited by metformin or ONOO−, a potent oxidant formed by nitric oxide and superoxide anions. To determine if PKC-ζ phosphorylates AMPK, we assayed 32P incorporation of AMPK immunoprecipitated from BAECs in the presence or absence of recombinant PKC-ζ in vitro (Fig. 1B). In the absence of exogenous PKC-ζ, 32P was not incorporated into AMPK, showing that AMPK does not undergo autophosphorylation (Fig. 1B). The addition of increasing amounts of recombinant PKC-ζ (3 to 30 ng) to the reaction mixture progressively increased 32P incorporation into PKC-ζ (Fig. 1B), consistent with the fact that PKC-ζ undergoes autophosphorylation in the presence of ATP. Significantly increasing concentrations of PKC-ζ also resulted in a dose-dependent increase of 32P incorporation into AMPKα (Fig. 1B). In contrast to PKC-ζ, PKC-βII (300 ng) had only marginal effects on AMPK 32P incorporation (data not shown). Interestingly, repetition of this experiment with recombinant AMPKα1β1γ1 revealed that recombinant PKC-ζ did not cause 32P incorporation into recombinant AMPKα1β1γ1s in the absence of LKB1 (Fig. 1C). Similar results were also obtained with recombinant AMPKα2β1γ1 (Z. Xie, unpublished data). Taken together, these results suggest that PKC-ζ does not phosphorylate AMPK directly and that PKC-ζ-dependent AMPK activation is likely mediated by some unknown factor(s).
As shown in Fig. 1B, an unknown protein with a molecular mass of approximately 50 kDa was detected in the presence of high PKC-ζ concentrations (>10 ng). This molecular mass is close to that of LKB1, prompting us to investigate whether LKB1 may mediate AMPK activation by PKC-ζ. In vitro kinase assays were performed with AMPKα immunoprecipitates from the HeLa-S3 and A549 cell lines, both of which are deficient for endogenous LKB1 and express normal levels of AMPK. AMPKα immunoprecipitates from these cell lines did not undergo phosphorylation or become activated in the presence of PKC-ζ (data not shown), implying that LKB1 mediates AMPK activation by PKC-ζ.
These results were confirmed by repeating the in vitro kinase assays with recombinant AMPKα1β1γ1 in the presence of both PKC-ζ and LKB1. Inclusion of LKB1 in the reaction mixture restored the dose-dependent induction of AMPK phosphorylation and AMPK activity by PKC-ζ (Fig. 1D). Both effects were accompanied by a dose-dependent increase of 32P incorporation into both LKB1 and AMPK (Fig. 1D). Taken together, these results suggest that LKB1 is required for PKC-ζ-dependent AMPK activation, in vitro.
Identification of LKB1 S307 as a putative PKC-ζ phosphorylation site.
Previously, we had demonstrated that phosphorylation of LKB1 at S428 is required for LKB1 to activate AMPK (56). To determine if S428 is necessary for AMPK activation, in vitro kinase assays were performed with mouse WT LKB1 or the mutant LKB1 S431A (equal to Ser428 in human sequence). Each LKB1 variant was bacterially expressed as LKB1-MO25α-STRADα complexes and purified on a heparin column, as described previously (34). As shown in Fig. 1E, inclusion of PKC-ζ dramatically stimulated 32P incorporation into AMPKα and the LKB1 WT. When WT LKB1 was replaced with the LKB1 S428A mutant, incorporation of phosphate into AMPKα and LKB1 was markedly reduced (Fig. 1E). Since the LKB1 S428A mutant was phosphorylated by PKC-ζ, these results strongly suggest the existence of additional PKC-ζ phosphorylation sites in LKB1.
High-stringency searches for putative phosphorylation sites in LKB1 were performed using the web-based bioinformatics tools, NetPhos2.0 server (University of Denmark; http://www.cbs.dtu.dk/services/NetPhos/), KinasePhos (Taiwan, China; http://kinasephos.mbc.nctu.edu.tw/), and MotifScan (MIT, Cambridge, MA; https://scansite.mit.edu/). These searches identified S31, S142, S216, S307, S378, S428, T209, and Y60 as possible phosphorylation sites (see Tables S1 and S2 in the supplemental material). Moreover, the search results from MotifScan suggest that S307 of LKB1 could be directly phosphorylated by PKC-ζ (see Fig. S1 in the supplemental material), since the surrounding sequence (QIRQHS307WFRKKHP) is conserved across various species and has similarity to the PKC-ζ consensus phosphorylation site (see Tables S1 and S2 in the supplemental material). Importantly, the constitutively nuclear LKB1 SL26 mutant carries a 9-bp deletion of codons 303 to 306 that consequently alters the amino acid sequence immediately preceding S307 (IRQHS307→NS307) (21). Based on these findings, S307 was selected for further analysis.
LKB1 S307 phosphorylation is stimulated by PKC-ζ in vitro and by AMPK activators in multiple cell types.
To investigate S307 phosphorylation, we developed a specific antibody against phospho-S307 of LKB1 using the peptide Ac-C-(Ahx)-QIRQH(pS)WFRKKH-amide, in which S307 contains a phosphate group. The specificity of the antibody was tested by Western blot analysis of HUVEC lysates. The phospho-S307 antibody recognizes an ∼52-kDa species in ONOO−-treated HUVEC lysates, which was colabeled by LKB1 antibody (see Fig. S2A in the supplemental material) but did not produce any signal in the lysates of untreated HUVECs. Phospho-S307 labeling is completely blocked by the addition of phospho-peptides, but not a non-phosphopeptide or unrelated peptides (see Fig. S2B in the supplemental material). Furthermore, the phospho-S307 antibody does not react with the LKB1 S307A mutant (see Fig. S2C in the supplemental material). Taken together, these results confirm the specificity of the antibody for LKB1 phosphorylated at S307.
To determine whether PKC-ζ phosphorylates LKB1 at S307 in vitro, purified recombinant PKC-ζ was incubated with recombinant LKB1. Western blot analysis of the reaction products revealed that PKC-ζ increases LKB1 phosphorylation at S307 in a dose-dependent manner in vitro (see Fig. S2D in the supplemental material). This result is consistent with the finding that PKC-ζ increases 32P incorporation into LKB1 (Fig. 1B and D) and provides support for the involvement of LKB1 S307 phosphorylation in the PKC-ζ-LKB1-AMPK signaling pathway.
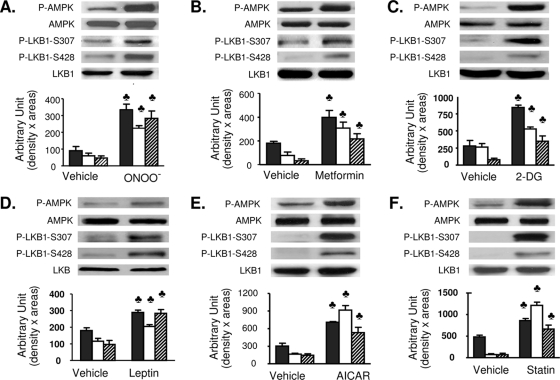
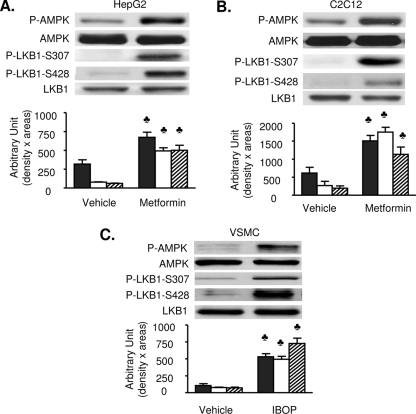
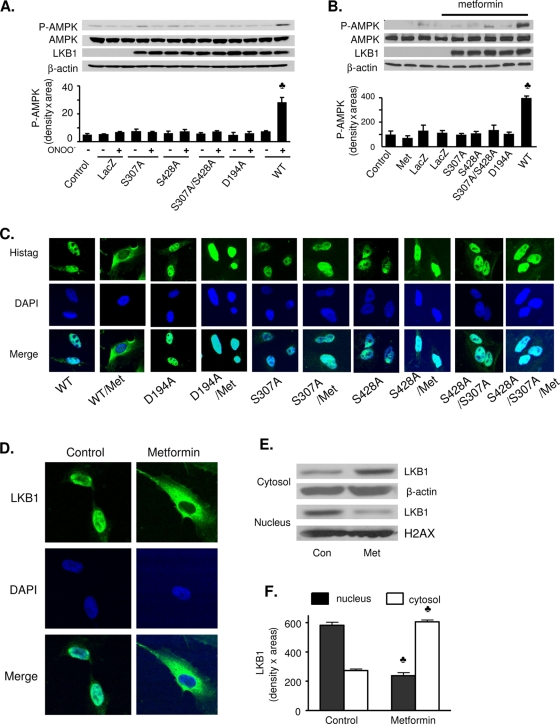
Next, we determined if LKB1 S307 phosphorylation is elevated in response to the AMPK activators ONOO−, metformin, 2-deoxyglucose, leptin, AICAR, or statin. Exposure of BAECs to each of these agents significantly increases AMPK T172 phosphorylation without altering AMPK expression (Fig. 2). Importantly, in all cases, in addition to increasing LKB1 S428 phosphorylation, LKB1 S307 phosphorylation is significantly increased. LKB1 S307 phosphorylation by AMPK activators is not limited to BAECs. Metformin (1 mM, 60 min) significantly elevated LKB1 S307 and AMPK T172 phosphorylation in both HepG2 (Fig. 3A) and C2C12 cells (Fig. 3B). Moreover, metformin (data not shown) and IBOP have similar effects in confluent vascular smooth muscle cells (Fig. 3C). Taken together, these findings suggest that, in multiple cell types, the signaling pathways engaged by several physiological stimuli converge upon LKB1 phosphorylation at both S307 and S428 for the activation of AMPK.
FIG. 2.
AMPK-activating agents stimulate phosphorylation of LKB1 at S307 and S428. Confluent BAECs were exposed to ONOO− (50 μM, 15 min) (A), metformin (1 mM, 60 min) (B), 2-deoxyglucose (2-DG; 40 mM, 15 min) (C), leptin (500 ng/ml, 30 min) (D), AICAR (2 mM, 120 min) (E), or statin (50 μM, 1 h) (F). Levels of total and phosphorylated (T172) AMPK, total and phosphorylated (S307) LKB1, and phosphorylated (S428) LKB1 were determined by Western blotting. Black bars, P-AMPK; white bars, P-LKB1 S307; and striped bars, P-LKB1 S428. All blots are representative of at least four blots from four independent experiments (n = 4 or 6). ♣, P < 0.05 versus control.
FIG. 3.
Both LKB1 S307 and LKB1 S428 are phosphorylated in metformin- or IBOP-treated cell lines. Confluent cultures of HepG2 (A) or C2C12 (B) cells were exposed to metformin, and vascular smooth muscle cells (VSMC) (C) were exposed to IBOP (1 μM, 60 min). Levels of total and phosphorylated (T172) AMPK, total and phosphorylated (S307) LKB1, as well as phosphorylated (S428) LKB1 were determined by Western blotting. Black bars, P-AMPK; white bars, P-LKB1 S307; and striped bars, P-LKB1 S428. All blots are representative of at least four to six blots from four to six independent experiments. ♣, P < 0.05 versus vehicle.
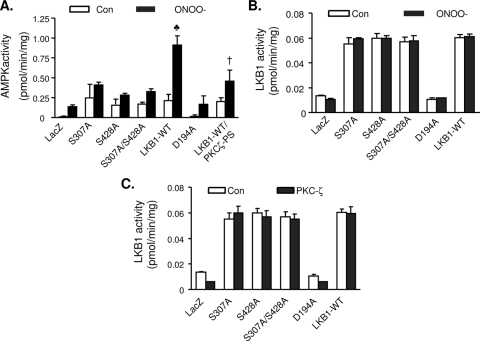
Ser307A point mutation reduces ONOO−-enhanced AMPK activation without affecting LKB1 activity.
To determine if S307 is necessary for AMPK activation, LKB1-deficient cell line A549 was transfected with either wild-type LKB1 or LKB1 mutants (S307A, S428A, S307A S428A, and D194A). After transfection, AMPK activity was assayed in the cells with or without ONOO− treatment. Compared to cells overexpressing either LacZ or with the D194A mutant, AMPK activity was elevated in the cells transfected with either WT LKB1 or LKB1 mutants (Ser307A, Ser428A, or S307A S428A (Fig. 4A). ONOO− did not alter AMPK activity in the cells transfected with either LacZ or D194A (Fig. 4A). However, ONOO− increased AMPK activity in the cells overexpressing WT LKB1 by threefold (Fig. 4A). Conversely, ONOO− did not alter AMPK activity in cells transfected with either the S307A or S428A mutant or the S307A S428A double mutant (Fig. 4A), implying that the phosphorylation of LKB1 at either S307 or S428 is required for AMPK activation by ONOO−. Inhibition of PKC-ζ with PKC-ζ-PS, a PKCζ-specific inhibitor, attenuated ONOO−-enhanced AMPK activity (Fig. 4A).
FIG. 4.
Effects of mutation of LKB1 Ser307 to alanine on AMPK and LKB1 activity.(A) LKB1-deficient A549 cells were transfected with WT LKB1 and LKB1 mutants. After the transfection, the cells were pretreated with PKC-ζ-PS (10 μM, 30 min) followed by treatment with ONOO− (50 μM, 15 min). AMPK was immunoprecipitated with AMPKα1/2 antibody, and AMPK activity was detected as described in Materials and Methods. n = 6. ♣, P < 0.01 for WT LKB1 treated with ONOO− versus untreated WT LKB1 and LKB1 mutants treated with ONOO−; †, P < 0.01 for ONOO−-treated WT LKB1 in the presence of PKC-ζ-PS compared with ONOO−-treated WT LKB1 in the absence of PKC-ζ-PS. con, control. (B) A549 cells were transfected with WT LKB1 and LKB1 mutants and treated with ONOO− (50 μM, 15 min). LKB1 was immunoprecipitated with specific antibody, and its activity was measured as described in Materials and Methods. n = 4. (C) LKB1 was immunoprecipitated from A549 cells transfected with WT LKB1 and LKB1 mutants, the immunoprecipitates were incubated with 100 ng of recombinant PKC-ζ, and LKB1 activity was assayed using LKB1-specific peptide. n = 3.
We next investigated whether the mutation of LKB1 serine 307, serine 428, or both sites into alanine altered its activity in response to ONOO−. Compared to WT LKB1, replacement of aspartic acid of 194 with alanine significantly reduced LKB1 activity. However, LKB1 activities were comparable between WT LKB1 and LKB1 mutants (S307A, S428A and S307A S428A) (Fig. 4B). Consistently, neither ONOO− nor the addition of PKC-ζ-PS altered LKB1 activity in the cells transfected with either WT LKB1 or the LKB1 mutants (Fig. 4B). Taken together, these data imply that the phosphorylation of LKB1 at either serine 307 or serine 428 did not alter its kinase activity.
To determine whether or not PKC-ζ affected LKB1 activity, LKB1 immunoprecipitated from the cells transfected with WT LKB1 or LKB1 mutants were incubated with recombinant PKC-ζ and the LKB1 activity was assayed by 32P incorporation into LKB1tide. As depicted in Fig. 4C, PKC-ζ had no effect on LKB1 activity when incubated with either WT LKB1 or LKB1 mutants (S307A, S428A, and S307A S428A) (Fig. 4C). Thus, PKC-ζ-mediated AMPK activation is not a result of the alteration of LKB1 activity.
S307 mutation blocks the sequestration of LKB1 in the cytoplasm.
The role of S307 in LKB1-dependent AMPK activation was further investigated by testing the effect of the S307A mutation on ONOO−- or metformin-induced AMPK phosphorylation. A549 cells were transfected with human WT LKB1 or an LKB1 S307A, LKB1 S428A, or LKB1 S307A S428A mutant. Asp194, which is essential for LKB1 activity (46), was mutated into Ala to serve as a positive control. As shown in Fig. 5A, ONOO− caused a fourfold increase in AMPK T172 phosphorylation in A549 cells transfected with WT LKB1, but not in nontransfected cells, LacZ-transfected cells, or mutant LKB1-transfected cells. Similar results were obtained in A549 cells treated with metformin (Fig. 5B).
FIG. 5.
LKB1 S307 mutation blocks metformin- and ONOO−-induced LKB1 nucleocytoplasmic transport and AMPK activation. LKB1-deficient A549 cells were transfected with WT LKB1 and LKB1 mutants, and cells were then treated with ONOO− (50 μM, 15 min) or metformin (Met; 1 mM for 1 h). (A and B) Western blot analysis of total and phosphorylated (T172) AMPK in ONOO− (A)- and metformin (B)-treated cells. Both blots are a representative of five blots from five independent experiments (n = 5 in panel A, and n = 4 in panel B). ♣, P < 0.05 for WT LKB1 treated with ONOO− or metformin versus LacZ, LKB1 mutants, or LKB1 mutants treated with ONOO− or metformin. (C) Immunocytochemical staining for LKB1, which was detected using a mouse anti-His tag antibody. Images of LacZ-transfected cells were omitted, as the antibody did not detect LacZ. (D) Immunocytochemical staining of translocation of LKB1 from nucleus to the cytosols caused by metformin in HUVECs. (E and F) Cytosolic and nuclear fractions were prepared as described in Materials and Methods, and the amount of LKB1 in the fractions was detected by Western blotting. β-Actin was used as a loading control (Con) for the cytoplasmic fraction, and histone H2AX was used as a loading control for the nuclear fraction. Metformin increases the amount of LKB1 in the cytosol but decreases LKB1 in the nuclei in HUVECs. The blot is a representative of five blots obtained from four independent experiments. ♣, P < 0.05 versus control.
The region spanning amino acids 88 to 433 may contribute to the cytoplasmic sequestration of LKB1 (36), which is required for the full activation of this kinase. Mutation of LKB1 at either Ser307 or Ser428 did not alter LKB1 activity but attenuated ONOO−-enhanced AMPK activation in intact cells. These results might emphasize the importance of LKB1 relocalization from the nucleus to the cytoplasm in order to achieve full activation of its downstream enzymes such as AMPK.
To determine whether S307 phosphorylation is involved in the regulation of nucleocytoplasmic shuttling of LKB1, we used immunocytochemistry and subcellular fractionation to investigate the effect of LKB1 S307A on the intracellular localization of LKB1 in A549 cells. As expected, WT LKB1, which was predominantly in the nuclear fraction of resting cells, translocated to the cytoplasm in response to metformin treatment (Fig. 5C; see Fig. S4A in the supplemental material) or ONOO− (see Fig. S3 in the supplemental material). However, treatment with either metformin (Fig. 5C; see Fig. S4A in the supplemental material) or ONOO− (see Fig. S3 in the supplemental material) failed to induce the cytoplasmic translocation of LKB1 S307A, LKB1 S428A, or the LKB1 S307A S428A double mutant, which remained in the nucleus.
We also assayed whether metformin treatment induces endogenous LKB1 translocation in HUVECs. As expected, LKB1 was primarily found in the nucleus of untreated HUVECs (Fig. 5D). In metformin-treated HUVECs, LKB1 translocated from the nucleus to cytoplasm. Metformin-enhanced LKB1 nucleocytoplasmic transport was further confirmed by Western blot analysis of LKB1 in subcellular fractionations. As shown in Fig. 5E and F, metformin significantly increases the total amount of LKB1 in the cytoplasm and markedly reduces the amount of LKB1 in the nucleus.
To further confirm whether LKB1 cytosolic translocation is essential for AMPK activation, we tested the cellular translocation of LKB1 SL26, a constitutively nuclear localized, kinase-active LKB1 mutant originally identified in Peutz-Jeghers syndrome patients (21). As expected, LKB1 SL26 was mainly localized in the nucleus when overexpressed in A549 cells (see Fig. S4B in the supplemental material). Furthermore, ONOO− treatment had no stimulatory effect on the translocation of LKB1 SL26 to the cytosol in A549 (see Fig. S4B in the supplemental material). In addition, overexpression of LKB1 SL26, LKB1 S428A, or LKB1 S307 reduced ONOO−-stimulated AMPK phosphorylation in A549 cells (see Fig. 4C in the supplemental material). Taken together, these results confirm that cytoplasmic translocation of LKB1 is critical for AMPK activation.
Inhibition of PKC-ζ blocks LKB1 S307 phosphorylation.
PKC-ζ loss-of-function studies were performed to further investigate whether PKC-ζ mediates LKB1 S307 phosphorylation in response to ONOO− or metformin. Treatment of BAECs with PKC-ζ-PS, which only inhibits the activity of the PKC-ζ isoform, prevented ONOO−- or metformin-induced phosphorylation of AMPK at T172 and phosphorylation of LKB1 at both S307 and S428, without affecting AMPK and LKB1 protein levels (Fig. 6A and B). Metformin-induced AMPK, LKB1 S307, and LKB1 S428 phosphorylation was also blocked by PKC-ζ-specific siRNA, which suppressed PKC-ζ expression by 60% (Fig. 6C). On the other hand, scrambled siRNA had no effect.
FIG. 6.
Inhibition of PKC-ζ blocks LKB1 S307 phosphorylation and LKB1 translocation from the nucleus to the cytoplasm. (A) Pharmacological inhibition of PKC-ζ with PKC-ζ-PS diminishes ONOO−-enhanced LKB1 phosphorylation in BAECs. Shown is Western blot analysis of AMPK and LKB1 phosphorylation in confluent BAECs that were preincubated with PKC-ζ-PS (PKC-PS) for 30 min followed by exposure to ONOO− (50 μM) for 15 min. The blot is a representative of six blots obtained from six individual experiments (n = 6). ♣, P < 0.05 versus control (Con). (B) Effect of PKC-ζ-PS on metformin-induced (Met, 1 mM, 1 h) LKB1 and AMPK phosphorylation in BAECs. The blot is representative of six blots obtained from six individual experiments (n = 6). ♣, P < 0.05 versus control; †, P < 0.05 versus Met. (C) Effect of PKC-ζ-specific siRNA on metformin-induced AMPK T172 phosphorylation in HUVECs (n = 5). ♣, P < 0.05 versus control; †, P < 0.05 versus metformin plus scrambled siRNA (S-siRNA). (D) Effect of PKC-λ/ι-specific siRNA on metformin-induced AMPK-T172 phosphorylation in HUVECs. The blot is representative of three blots obtained from three individual experiments (n = 3). ♣, P < 0.05 versus control. (E) Metformin-induced LKB1-S307 phosphorylation in BAECs overexpressing PKC-ζ-DN, PKC-ζ-CA, or GFP. The blot is representative of five blots from five individual experiments (n = 5). ♣, P < 0.05 versus control; †, P < 0.05 versus metformin. Black bars, P-AMPK; white bars, P-LKB1 S307; and striped bars, P-LKB1 S428. (F) LKB1 immunocytochemical staining in confluent HUVECs preincubated with or without PKC-ζ-PS (PKC-PS) and exposed to metformin (1 mM, 1 h). Nuclei were counterstained with DAPI. (G) Time course of phosphorylation of PKC-ζ, LKB1, and AMPK in HUVECs in response to metformin. HUVECs were treated with metformin (1 mM) at the times indicated. The phosphorylation of PKC-ζ, LKB1 S307, LKB1 S428, and AMPK was detected at the indicated times by Western blotting with respective antibodies. The blot is representative of three blots from three individual experiments.
PKC-ζ belongs to an atypical PKC family which shares ∼80% homology with other types of atypical PKCs, such as PKC-λ/ι. To determine if PKC-λ/ι contributes to the activation of LKB1 and AMPK signaling, we used PKC-λ/ι siRNA to inhibit PKC-λ/ι expression. As shown in Fig. 6D, transfection of PKC-λ/ι-specific siRNA, but not control siRNA, reduced the levels of PKC-λ/ι by ∼80%. Importantly, transfection of PKC-λ/ι siRNA did not alter metformin-enhanced phosphorylation of AMPK at T172 or the phosphorylation of LKB1 at S307 and S428. These results confirm that PKC-ζ, but not the closely related PKC-λ/ι, is involved in regulation of the LKB1-AMPK pathway.
Overexpression of adenoviral PKC-ζ-DN, but not Ad-GFP, prevented metformin-induced phosphorylation of AMPK and LKB1 S307 and S428 (Fig. 6E). Consistent with these results, selective PKC-ζ inhibition blocked ONOO−- and statin-induced phosphorylation of AMPK T172 and LKB1 S307 (8).
Inhibition of PKC-ζ blocks LKB1 nucleocytoplasmic transport.
LKB1 is a constitutively active protein kinase, and its translocation from the nucleus to the cytoplasm is essential for LKB1 physiologic functions (5, 53). Transfection of LKB1-specific siRNA but not scrambled siRNA in HUVECs markedly reduced the signals of LKB1, confirming this antibody was specific for LKB1 in both immunocytochemistry (Fig. 6F) and Western blots (see Fig. S6C in the supplemental material). Furthermore, immunohistochemical staining of LKB1 in HUVECs revealed that, as in LKB1-transfected A549 cells, LKB1 is mainly present in the nucleus in untreated cells, but translocates to the cytoplasm following stimulation with metformin (Fig. 6F). Analysis of the distribution of LKB1 in subcellular fractions confirmed this result, showing an accumulation of LKB1 in the cytosolic fraction, with a reciprocal decline in the nuclear fraction (data not shown), upon metformin treatment. Importantly, PKC-ζ-PS abolished metformin-induced LKB1 translocation from the nucleus to the cytoplasm (Fig. 6F). Similarly, selective PKC-ζ inhibition blocked the LKB1 nucleocytoplasmic transport induced by ONOO− (42) or statins (8).
We next determined the time course of metformin-induced phosphorylation of PKC-ζ, LKB1, and AMPK. Metformin significantly increased PKC-ζ phosphorylation within 1 min of exposure, and increased LKB1 phosphorylation at S428 and S307 after around 10 min. A significant increase in AMPK T172 phosphorylation was observed at 30 min and peaked at 60 min after metformin treatment (Fig. 6G).
Phosphorylation of LKB1 at S307 is required for its increased association with STRADα.
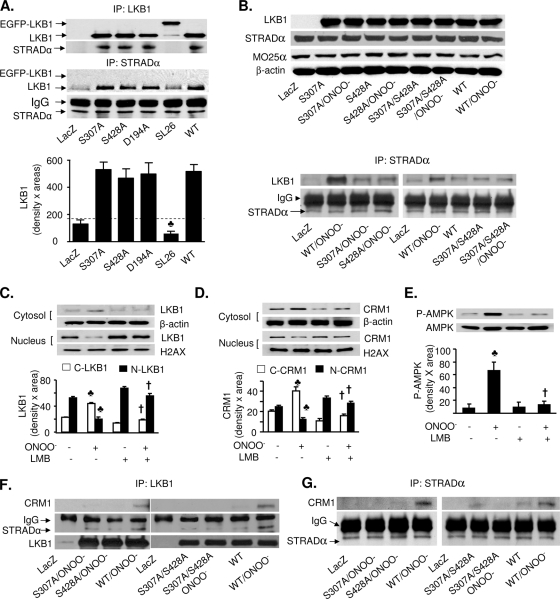
Recent evidence (13) suggests that LKB1 becomes fully activated only after it complexes with STRAD and CRM1, a nuclear protein exportin which participates in the nucleocytoplasmic shuttling of LKB1. Since the LKB1 S307A mutation blocks LKB1 export from the nucleus (Fig. 5C and D), we next investigated if phosphorylation of LKB1 at S307 alters the association of LKB1 with STRADα. To this end, STRADα was first immunoprecipitated from A549 cells transfected with either WT LKB1 or LKB1 mutants (S307A, S428A, S307A S428, D194A, or SL26), and the immunoprecipitates were analyzed by Western blotting with LKB1- or CRM1-specific antibodies. As expected, the transfection of LKB1 SL26, a mutant deficient in the STRADα binding motif, exhibited no association between LKB1 and STRADα (Fig. 7A). Interestingly, in unstimulated cells, STRADα was weakly coimmunoprecipitated with WT LKB1 or the LKB1 S307A, LKB1 S428A, and LKB1 D194A mutants (Fig. 7A), suggesting that unlike the LKB1 SL26 mutant, the mutations of Ser307, Ser428, or D194 do not alter the affinity of LKB1 with STRADα. Conversely, CRM1 was not coimmunoprecipitated with either STRADα or LKB1 in nonstimulated cells overexpressing WT LKB1 or LKB1 mutants (Z. Xie, unpublished data).
FIG. 7.
Mutation of either LKB1 S307 or S428 to alanine inhibits LKB1 binding to STRAD and CRM1. (A) A549 cells were transfected with WT LKB1 or mutant LKB1 S307A, LKB1 S428A, LKB1 D194A, or LKB1 SL26. STRADα or LKB1 was immunoprecipitated (IP) using a specific antibody, and LKB1 or STRADα was detected by Western analysis. The blot is representative of four blots from four individual experiments (n = 4). ♣, P < 0.05 versus WT LKB1 or mutant LKB1 S307A, LKB1 S428A, or D194A. IgG, immunoglobulin G. (B) Mutation of S307 and S428 reduced the ONOO−-enhanced association of LKB1 with STRADα. A549 cells were transfected with WT LKB1 or mutant LKB1 S307A, LKB1 S428A, or LKB1 S307A S428A and treated with ONOO− (50 μM) for 15 min. (Upper panel) Protein levels of LKB1, MO25α, and STRADα in total cell lysates were detected by Western blot analysis. (Bottom panel) STRADα was immunoprecipitated using a specific antibody, and LKB1 was detected by Western analysis. The blot is representative of four blots from four individual experiments (n = 4). (C and D) HUVECs were pretreated with LMB (200 nM) for 1 h followed by treatment with ONOO− (50 μM) for 15 min; subcellular fractions were prepared as described in Materials and Methods. The protein levels of LKB1 (C) and CRM1 (D) in each fraction were analyzed by Western blotting. β-Actin was used as the loading control for the cytoplasmic fraction, and histone H2AX was used as the loading control for the nuclear fraction. The blot is a representative of three blots from three individual experiments (n = 3). ♣, P < 0.05 versus control; †, P < 0.05 versus ONOO−. (E) Confluent HUVECs were pretreated with LMB followed by ONOO− treatment; cell lysates were prepared as described in Materials and Methods. Phosphorylation of AMPK at T172 was detected by Western blotting. The blot is a representative of three blots from three individual experiments (n = 3). ♣, P < 0.05 versus control; †, P < 0.05 versus ONOO−. (F and G) A549 cells transfected with WT LKB1 and LKB1 mutants were treated with ONOO− (50 μM) for 15 min. (F) LKB1 was immunoprecipitated, and CRM1 and STRADα were detected by Western blotting. (G) STRADα was immunoprecipitated with a specific antibody, and CRM1 was detected by Western analysis. The blot is a representative of four blots from four individual experiments (n = 4).
We next determined whether the phosphorylation of LKB1 at either S307 or S428 is required for the increased association of LKB1 with STRADα induced by ONOO−. As expected, there was no LKB1 expression in A549 cells transfected with LacZ, whereas both MO25 and STRADα were detected in the cells transfected with LacZ (Fig. 7B, top panel). Transfection of WT LKB1 and LKB1 mutants increased the levels of LKB1 at similar levels in A549 (Fig. 7B, top panel). ONOO− treatment did not alter the levels of LKB1 (Fig. 7B, top panel). In contrast, neither transfection of LKB1 constructs nor ONOO− treatment altered the protein levels of MO25α and STRADα in A549 cells. The specificity of the antibodies against STRADα, MO25α, and CRM1 was confirmed by Western blotting as the transfection of STRADα-, CRM1-, and MO25α-specific siRNA but not control siRNA markedly reduced the antibody staining (see Fig. S5A, B, and D and Fig. S6A and B in the supplemental material).
We next assayed the effects of ONOO− on the coimmunoprecipitation of LKB1 with STRADα and if LKB1 mutation at either Ser307 or Ser428 altered the association between LKB1 and STRADα. Although STRADα is only weakly coimmunoprecipitated with WT LKB1 or the LKB1 S307A, LKB1 S428A, and LKB1 D194A mutants in nonstimulated cells (Fig. 7A), ONOO− significantly increased the coimmunoprecipitation of WT LKB1 with STRADα (Fig. 7B, low panel). Overexpression of LKB1 S307A, LKB1 S428A, or the LKB1 S307A S428A double mutant markedly attenuated ONOO−-enhanced association between LKB1 and STRADα (Fig. 7B, bottom panels), implying that LKB1 phosphorylation at S307 and S428 is required for the association of LKB1 and STRADα.
Inhibition of CRM1 abolishes nucleocytoplasmic shuttling of CRM1 and LKB1 and AMPK activation caused by ONOO−.
CRM1, a nuclear protein exportin, is reported to be essential for STRAD-dependent LKB1 nucleoplasmic shuttling (13) and leptomycin B (LMB) is reported to be a specific inhibitor for CRM1 (17, 39). We next assayed if LMB altered ONOO−-induced nuclear export of LKB1 in HUVECs. As depicted in Fig. 7C and D, ONOO− significantly increased the cytoplasmic portion of both CRM1 and LKB1, whereas it lowered their nuclear contents. Further, LMB abolished ONOO−-induced nucleocytoplasmic shuttling of both LKB1 and CRM1 in HUVECs (Fig. 7C and D), implying that CRM1 is required for ONOO−-induced LKB1 nucleocytoplasmic transport. Furthermore, LMB abolished ONOO−-induced phosphorylation of AMPK Thr172 in HUVECs (Fig. 7E), implying that LKB1 nucleocytoplasmic transport is also required for AMPK activation.
Phosphorylation of LKB1 at Ser307 and Ser428 is required for the association of LKB1 with CRM1.
Although CRM1 was not coimmunoprecipitated with STRADα or LKB1 in unstimulated cells, it was interesting to determine if stimuli like ONOO− increased LKB1 nucleoplasmic shuttling by increasing the association of CRM1 with LKB1 or STRADα. As depicted in Fig. 7F, ONOO− markedly increased the association of both LKB1 and CRM1 in A549 cells transfected with WT LKB1, but not in the cells transfected with LacZ. We also found that ONOO− treatment increased the association between STRADα and CRM1 in the cells expressing WT LKB1, but not in cells expressing LacZ (Fig. 7G). Importantly, mutation of LKB1 at Ser307, Ser428, or both sites abolished the effects of ONOO− on the association between LKB1, CRM1, and STRAD (Fig. 7F and G).
Overexpression of LKB1 S307A decreases ONOO−-induced association with AMPK.
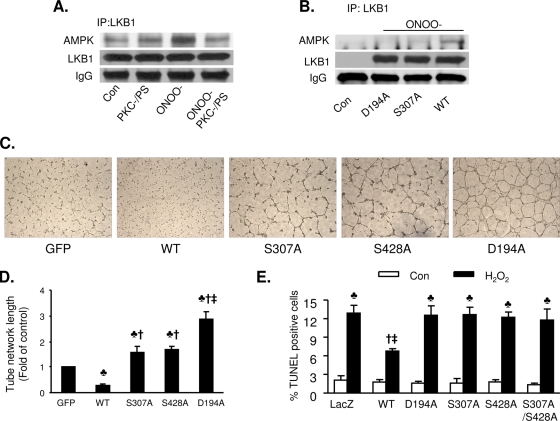
We had previously shown that PKC-ζ coimmunoprecipitates with LKB1 and that metformin exposure increases the association of LKB1 with both PKC-ζ and AMPK (56). In this study, we further investigated whether PKC-ζ inhibition alters the ONOO−-enhanced association of LKB1 and AMPK. As shown in Fig. 8A, inhibition of PKC-ζ by PKC-ζ-PS abolishes the ONOO−-enhanced coimmunoprecipitation of LKB1 and AMPK. These results further confirm that PKC-ζ is required for LKB1 translocation.
FIG. 8.
Mutation of LKB1 S307 to alanine potentiates angiognesis and increases H2O2-induced apoptosis. (A) Inhibition of PKC-ζ abolishes ONOO−-enhanced coimmunoprecipitation (IP) of AMPK and LKB1. Confluent BAECs were exposed to ONOO− (50 μM) for 15 min. After the treatment, LKB1 was immunoprecipitated using a specific antibody. AMPK and LKB1 were detected by Western blotting. The blot is a representative of three blots from three independent experiments. IgG, immunoglubulin G; Con, control. (B) Mutation of S307 to alanine abolished ONOO−-enhanced coimmunoprecipitation of LKB1 and AMPK. After transfection with WT LKB1 or an LKB1 D194A or LKB1 S307A mutant, A549 cells were exposed to ONOO− (50 μM) for 15 min and LKB1 was immunoprecipitated using a specific antibody. AMPK and LKB1 were detected by Western blotting. The blot is representative of three blots from three independent experiments. (C) Representative images displaying tube formation in HUVECs transfected with adenoviral vectors expressing WT LKB1 or LKB1 mutants at a multiplicity of infection of 25 PFU/cell. (D) Quantitative analysis of tube lengths. n = 5. ♣, P < 0.01 versus GFP; †, P < 0.01 versus WT; ‡, P < 0.05 for LKB1 D194A versus LKB1 S307A or LKB1 S428A. (E) Mutation of LKB1 S307 to alanine abolishes the antiapoptotic effects of LKB1 in response to H2O2. After transfection with WT LKB1 or an LKB1 D194A, LKB1 Ser428A, LKB1 S307A, or LKB1 S307A S428A mutant, A549 cells were exposed to H2O2 (100 μM) for 12 h. Apoptosis was detected by TUNEL staining. n = 6. ♣, P < 0.05 for H2O2 treatment versus the respective control; †, P < 0.05 for WT versus WT plus H2O2; ‡, P < 0.05 for WT plus H2O2 versus H2O2-treated LKB1 S307A or H2O2-treated LKB1 D194A.
LKB1 S428 phosphorylation is reported to be required for the metformin-enhanced association of LKB1 and AMPK (56, 57). We next examined whether phosphorylation of LKB1 at S307 by PKC-ζ contributes to the ONOO−-enhanced association of AMPK with LKB1. WT LKB1, kinase-dead LKB1, and LKB1 S307A were overexpressed in A549 cells. Overexpressed LKB1, kinase-dead LKB1, or LKB1 S307A alone did not coimmunoprecipitate with AMPK. ONOO− treatment increased the association of AMPK with LKB1 in A549 cells transfected with WT LKB1, but not LacZ or LKB1 S307A (Fig. 8B), indicating that LKB1 S307 phosphorylation might be required for ONOO−-enhanced AMPK activation.
S307A mutant LKB1 shows a reduced ability to suppress angiogenesis.
Ylikorkala et al. (60) reported that LKB1-deficient mice have vascular abnormalities and deregulated vascular endothelial growth factor (VEGF), which suggests that loss of LKB1 increases angiogenic potential in certain cell types. We next determined the roles of LKB1 S307 phosphorylation in angiogenesis using in vitro vascular-like structure assays. HUVECs were transfected with adenoviral vectors encoding WT LKB1, a mutant form of LKB1 (D194A, S307A, or S428A), or GFP 2 days prior to plating on Matrigel-coated culture dishes. Figure 8C and D show vascular-like structures formed in the cultured HUVECs after 8 h. Compared to the cells transfected with GFP, cells overexpressing WT LKB1 had reduced tube formation, whereas overexpression of the D194A mutant, a kinase-dead form of LKB1, significantly increased tube formation (Fig. 8C and D). Conversely, overexpression of either LKB1 S307A or LKB1 S428A modestly, though significantly, increased tube formation compared to the GFP control. In comparison to the cells overexpressing LKB1 D194A, tube formation in HUVECs overexpressing either LKB1 S307A or LKB1 S428A was significantly reduced. Taken together, our results suggest that LKB1 phosphorylation at residues S307 and S428 is required for LKB1-dependent suppression of endothelial cell angiogenesis.
Phosphorylation of LKB1 at S307 is required for the suppression of hydrogen peroxide-induced apoptosis in A549 cells.
Since LKB1 is known to protect cells from apoptosis induced by agents that elevate intracellular AMP, such as H2O2 and AICAR (47), we next determined if S307 phosphorylation is required for the antiapoptotic effects of LKB1. H2O2 (100 μM, 12 h) markedly increased apoptosis, as evidenced by an increased amount of TUNEL-positive cells in A549 cells transfected with LacZ or LKB1 mutants (D194A, S307A, S428A, and S307A S428A). In contrast, overexpression of WT LKB1 dramatically reduced the levels of H2O2-induced apoptosis (Fig. 8E). Taken together, these results suggest that phosphorylation of LKB1 at S307 is required for the antiapoptotic effects of LKB1.
DISCUSSION
The results presented here suggest that atypical PKC-ζ, a member of the AGC protein kinase family, functions as an LKB1 kinase and is a major regulator of the LKB1-AMPK pathway. We also demonstrate for the first time that PKC-ζ directly phosphorylates LKB1 at S307, resulting in increased association of LKB1 with STRADα and CRM1 and export of LKB1 from the nucleus to the cytoplasm, consequently activating AMPK, attenuating angiogenesis, and suppressing H2O2-induced apoptosis. In addition to the observation that LKB1 contains a putative PKC-ζ phosphorylation consensus sequence surrounding S307, the kinase directly interacts with PKC-ζ (10, 56), and LKB1 is directly phosphorylated by PKC-ζ in vitro, mutation of S307 to alanine markedly reduces the physiological functions of LKB1, including the suppression of angiogenesis and apoptosis. Overall, our findings indicate that S307 of LKB1 is a novel phosphorylation site which is essential for LKB1 nucleocytoplasmic shuttling and consequent AMPK activation.
The cytoplasmic localization of LKB1 is critical for its function (5, 53). Although neither the S307A nor the S428A mutation affects the normal nuclear localization of LKB1, both mutations prevent LKB1 translocation to the cytoplasm following metformin or ONOO− treatment, suggesting that S307 and S428 phosphorylation regulates LKB1 nucleocytoplasmic transport. The activity and subcellular distribution of LKB1 are controlled by its interaction with STRADα and the nuclear export protein CRM1. There is evidence (13) to suggest that STRADα induces nucleocytoplasmic shuttling of LKB1 by serving as an adaptor between LKB1, exportin CRM1, and exportin 7. MO25α stabilizes the STRADα-LKB1 interaction but does not facilitate its nucleocytoplasmic shuttling (13). Replacement of either S307 or S428 with alanine reduces the association of LKB1 and STRADα, which may uncouple this process by preventing the association of LKB1 with its substrates. Similar to overexpression of the kinase-inactive mutant LKB1 D194A, expression of LKB1 S307A diminishes metformin- and ONOO−-enhanced phosphorylation of AMPK in several mammalian cell lines. Intriguingly, the Peutz-Jeghers syndrome-causing LKB1 SL26 mutation is located immediately upstream of the S307 site that is predicted to disrupt the PKC-ζ consensus site. The observed nuclear localization of the SL26 mutant, therefore, could be due to the inability of PKC-ζ to target the S307 site in the SL26 mutant and the consequent blockade of nuclear export, which we have shown requires S307 phosphorylation. These findings support the hypothesis that phosphorylation of S307 regulates LKB1 function by altering its cellular localization or its ability to interact with regulatory or substrate proteins.
PKC-ζ was originally discovered as a unique PKC isoform and is classified as an atypical PKC (aPKC) based on its structural similarity to PKC-λ/ι (38). PKC-ζ has been implicated in the regulation of widespread cellular functions. Increasing evidence from studies using in vitro and in vivo systems indicates that PKC-ζ is a key regulator of critical intracellular signaling pathways induced by various extracellular stimuli (22, 31, 41). Intriguingly, PKC-ζ has similar functions to LKB1, including regulation of cell growth and survival (4), cell polarity (26, 37, 52), and metabolism (7, 29). Thus, we speculate that PKC-ζ and LKB1 interact under physiological conditions. Consistent with this notion, we found that PKC-ζ directly phosphorylates LKB1 at S307 in vitro, PKC-ζ inhibition attenuates stimulus-induced LKB1 S307 phosphorylation and downstream AMPK signaling, metformin increases the coimmunoprecipitation of LKB1 with AMPK, and inhibition of PKC-ζ decreases the metformin-enhanced association of LKB1 with AMPK (56). These results suggest that by promoting LKB1 cytosolic accumulation, thereby positioning LKB1 in proximity to AMPK, PKC-ζ activity toward LKB1 represents a limiting step for AMPK activation. Furthermore, our data suggest that PKC-ζ-mediated phosphorylation of S307 of LKB1, similar to LKB1 phosphorylation at S428, is important for AMPK activation mainly by increasing both nucleocytoplasmic shuttling of LKB1 and its affinity with AMPK.
Our previous work demonstrated that activation of PKC-ζ results in cytosolic localization of LKB1 and consequent AMPK activation concomitant with phosphorylation of LKB1 at S428 (49, 57). S428 is also targeted by protein kinase A (PKA) and p90 ribosomal S6 kinase (RSK) (9, 45), but neither nuclear export of LKB1 nor AMPK activation has been reported in response to stimuli that increase PKA or RSK activity. Here, we find that PKC-ζ also phosphorylates LKB1 at S307. In in vitro kinase assays, PKC-ζ phosphorylates S428 when S307 is mutated, and vice versa, suggesting that these two sites are independently phosphorylated by PKC-ζ. Indeed, ONOO− and metformin simultaneously increase LKB1 phosphorylation at S428 and S307 in HUVECs (Fig. 2A and B). Since mutation of either S307 or S428 to alanine blocks LKB1 nuclear export and AMPK activation in response to ONOO− or metformin (56) (see Fig. S3 and S5C in the supplemental material), our results suggest that phosphorylation of both S307 and S428 by PKC-ζ is required for LKB1 nuclear export and consequent AMPK activation. These results are further supported by the fact that ONOO− and metformin do not alter LKB1-dependent AMPK activity in A549 cells overexpressing LKB1 SL26, a natural mutant of LKB1, in the nucleus. Thus, we consider that PKC-ζ might be essential for LKB1-dependent AMPK activation through the nucleocytoplasmic shuttling of LKB1 at the cellular level. Whether or not other unknown phosphorylation sites in LKB1 result in increased LKB1-dependent AMPK activity warrants future investigation. Overall, we conclude that PKC-ζ is an LKB1 kinase.
The roles of LKB1 S428 phosphorylation in the regulation of the subcellular localization of LKB1 and AMPK activation remain controversial in the literature. Consistent with our findings, Dolinsky et al. found that in cardiomyocytes, electrophilic lipid peroxidation by-product 4-hydroxy-2-nonenal reduced phosphorylation of LKB1 at Ser428 could reduce AMPK activity (12). In contrast, several reports (11, 14, 15, 54, 61) published during the revision of this article have found that LKB1 Ser428 phosphorylation is not required or has an inhibitory effect on AMPK activation. Based on these results obtained with a short form of LKB1 (LKB1S) which lacks both a Ser428 phosphorylation site and a farnesylation site in its C terminal, two groups have concluded that the phosphorylation of S428/431 is not required for AMPK activation and cell cycle arrest (11, 15, 54). However, the results obtained with LKB1S should be interpreted with caution. Although the 63 amino acids in the C terminal of the long form of human LKB1, LKB1L, are replaced by 34 amino acids in human LKB1S, there are several potential phosphorylation sites in the C terminal of LKB1S which, like Ser428, can be phosphorylated by upstream kinases. Using bioinformatics analysis tools (NetPhos 2.0 Server and KinasePhos), we have found several new putative phosphorylation sites among the 34 new amino acids at the C terminal of human LKB1S: for example, Ser388 (GLQKSEGSD) and Ser394 (GSDLSGEEA). It remains to be determined if these putative phosphorylation sites in LKB1S might, like the S428 phosphorylation sites in human LKB1L, control the subcellular localization of LKB1 and consequent AMPK activation.
More recently, Zheng et al. (61) have reported that in melanoma cells LKB1 phosphorylation by extracellular signal-regulated kinase (ERK) and RSK, two kinases downstream of B-RAF, compromises its ability to bind and activate AMPK. Although this study is consistent with previous finding that C-terminal region of LKB1 is involved in AMPK activation (16), the overexpression of LKB1 Ser428A mutants in melanoma cells was found to activate AMPK under basal conditions or in response to AICAR. This observation is in contrast to an inhibitory effect of LKB1 Ser428A mutants on AMPK activation caused by metformin or ONOO− in endothelial cells or LKB1-deficient HeLa-S3 or A549 cells. AICAR was intentionally omitted in our experiments because we found AICAR activated AMPK in both HeLa-S3 and A549 cells (Xie et al., unpublished data). Consistent with our observations, several other groups report that AICAR treatment increases AMPK phosphorylation in LKB1-deficient HeLa cells (50) and LKB1 knockout embryo fibroblasts (40). Thus, the different effects of LKB1 mutants on AMPK activation between two publications might be explained by an LKB1-independent effect of AICAR. In addition, this discrepancy might also be related to cell types used, as the same group (61) showed that elevated activity of ERK, an upstream kinase responsible for LKB1 Ser325 and Ser428 phosphorylation, does not inhibit AMPK activation in some melanoma cell lines (MeWo and SK-MEL-31) that lack B-RAF mutations. Other explanations might include different phosphorylation sites of LKB1 by different upstream kinases in response to different stimuli in different cell types. Considering the controversial effects of LKB1 Ser428 phosphorylation on AMPK (no effect [11, 15, 54], activation [12], and inhibition [14, 61]) in different cell types, we speculate that the phosphorylation of Ser428 may recruit different signaling molecule partners depending on the cell types involved, resulting in different effects on AMPK. How LKB1 Ser428 phosphorylation regulates AMPK activation warrants further investigation.
Another important finding in this study is that the phosphorylation of LKB1 at S307 is involved in LKB1-mediated inhibition of angiogenesis and apoptosis induced by H2O2. LKB1 phosphorylates and activates a number of kinases, implicating it in the regulation of multiple signaling pathways (27). Recently, Ylikorkala et al. (60) reported that targeted disruption of LKB1 results in neural tube defects, mesenchymal cell death, and vascular abnormalities. These phenotypes are associated with tissue-specific deregulation of VEGF expression, including a marked increase in the amount of VEGF mRNA. These findings suggest that the vascular defects accompanying LKB1 deficiency are related to the alteration of VEGF expression. Of note is that LKB1-suppressed tube formation may be independent of AMPK, since AMPK signaling has little or no effect on endothelial cell migration, tube formation, or nitric oxide production when cultures are exposed to normoxia, although it may be essential for the angiogenic response to ischemic stress (32). The mechanism by which LKB1 regulates angiogenesis warrants further studies.
LKB1 functions as a tumor suppressor by inhibiting cellular proliferation and modulating cell polarity (6), as well as by acting upstream of AMPK to control cellular energy levels (30, 55). In addition to its tumor suppressor function, LKB1 regulates glucose homeostasis in the liver and mediates the therapeutic effects of metformin (48). Therefore, identification of PKC-ζ as a major activator of LKB1 may introduce a new set of potential strategies for targeting LKB1 activity in the treatment of cancer and diabetes. Furthermore, identification of phosphorylation sites associated with LKB1 activation and its corresponding upstream kinase is likely to provide important insights into the molecular mechanisms by which LKB1 functions as a tumor suppressor and metabolic regulator.
Supplementary Material
Acknowledgments
We thank Lily Q. Dong for providing the LKB1 SL26 construct. We also thank the reviewers for excellent suggestions.
This study was supported by funding from the following: NIH (HL079584, HL080499, HL074399, HL089920, and HL096032 to M.Z. and 1P20RR024215-01 to Z.X. and M.Z.), the American Heart Association (scientist development grant to Z.X.), the Juvenile Diabetes Research Foundation (M.Z.), the Oklahoma Center for the Advancement of Science and Technology (M.Z.), the American Diabetes Association (M.Z.), the Travis Endowed Chair of the University of Oklahoma Health Science Center (M.Z.), the “Innerschweizer Krebsliga” (D.N.), an ETH graduate student grant for R.S. (D.N. and Theo Wallimann), and the European Union FP6 program (EXGENESIS, LSHM-CT-2004-005272). M. H. Zou is a recipient of the National Established Investigator Award of the American Heart Association.
Footnotes
Published ahead of print on 4 May 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alessi, D. R., K. Sakamoto, and J. R. Bayascas. 2006. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 75137-163. [DOI] [PubMed] [Google Scholar]
- 2.Baas, A. F., J. Boudeau, G. P. Sapkota, L. Smit, R. Medema, N. A. Morrice, D. R. Alessi, and H. C. Clevers. 2003. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 223062-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardeesy, N., M. Sinha, A. F. Hezel, S. Signoretti, N. A. Hathaway, N. E. Sharpless, M. Loda, D. R. Carrasco, and R. A. DePinho. 2002. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature 419162-167. [DOI] [PubMed] [Google Scholar]
- 4.Berra, E., M. T. az-Meco, I. Dominguez, M. M. Municio, L. Sanz, J. Lozano, R. S. Chapkin, and J. Moscat. 1993. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell 74555-563. [DOI] [PubMed] [Google Scholar]
- 5.Boudeau, J., A. F. Baas, M. Deak, N. A. Morrice, A. Kieloch, M. Schutkowski, A. R. Prescott, H. C. Clevers, and D. R. Alessi. 2003. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 225102-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boudeau, J., G. Sapkota, and D. R. Alessi. 2003. LKB1, a protein kinase regulating cell proliferation and polarity. FEBS Lett. 546159-165. [DOI] [PubMed] [Google Scholar]
- 7.Canto, C., E. Suarez, J. M. Lizcano, E. Grino, P. R. Shepherd, L. G. Fryer, D. Carling, J. Bertran, M. Palacin, A. Zorzano, and A. Guma. 2004. Neuregulin signaling on glucose transport in muscle cells. J. Biol. Chem. 27912260-12268. [DOI] [PubMed] [Google Scholar]
- 8.Choi, H. C., P. Song, Z. Xie, Y. Wu, J. Xu, M. Zhang, Y. Dong, S. Wang, K. Lau, and M. H. Zou. 2008. Reactive nitrogen species is required for the activation of the AMP-activated protein kinase by statin in vivo. J. Biol. Chem. 28320186-20197. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Collins, S. P., J. L. Reoma, D. M. Gamm, and M. D. Uhler. 2000. LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem. J. 345673-680. [PMC free article] [PubMed] [Google Scholar]
- 10.Davis, B. J., Z. Xie, B. Viollet, and M. H. Zou. 2006. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55496-505. [DOI] [PubMed] [Google Scholar]
- 11.Denison, F. C., N. J. Hiscock, D. Carling, and A. Woods. 2009. Characterisation of an alternative splice variant of LKB1. J. Biol. Chem. 28467-76. [DOI] [PubMed] [Google Scholar]
- 12.Dolinsky, V. W., A. Y. Chan, I. Robillard Frayne, P. E. Light, C. Des Rosiers, and J. R. Dyck. 2009. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation 1191643-1652. [DOI] [PubMed] [Google Scholar]
- 13.Dorfman, J., and I. G. Macara. 2008. STRADalpha regulates LKB1 localization by blocking access to importin-alpha, and by association with Crm1 and exportin-7. Mol. Biol. Cell 191614-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esteve-Puig, R., F. Canals, N. Colome, G. Merlino, and J. A. Recio. 2009. Uncoupling of the LKB1-AMPKalpha energy sensor pathway by growth factors and oncogenic BRAF. PLoS. ONE 4e4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogarty, S., and D. G. Hardie. 2009. C-terminal phosphorylation of LKB1 is not required for regulation of AMP-activated protein kinase, BRSK1, BRSK2, or cell cycle arrest. J. Biol. Chem. 28477-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forcet, C., S. Etienne-Manneville, H. Gaude, L. Fournier, S. Debilly, M. Salmi, A. Baas, S. Olschwang, H. Clevers, and M. Billaud. 2005. Functional analysis of Peutz-Jeghers mutations reveals that the LKB1 C-terminal region exerts a crucial role in regulating both the AMPK pathway and the cell polarity. Hum. Mol. Genet. 141283-1292. [DOI] [PubMed] [Google Scholar]
- 17.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 901051-1060. [DOI] [PubMed] [Google Scholar]
- 18.Hardie, D. G., D. Carling, and M. Carlson. 1998. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67821-855. [DOI] [PubMed] [Google Scholar]
- 19.Hardie, D. G., J. W. Scott, D. A. Pan, and E. R. Hudson. 2003. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 546113-120. [DOI] [PubMed] [Google Scholar]
- 20.Hemminki, A. 1999. The molecular basis and clinical aspects of Peutz-Jeghers syndrome. Cell. Mol. Life Sci. 55735-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemminki, A., D. Markie, I. Tomlinson, E. Avizienyte, S. Roth, A. Loukola, G. Bignell, W. Warren, M. Aminoff, P. Hoglund, H. Jarvinen, P. Kristo, K. Pelin, M. Ridanpaa, R. Salovaara, T. Toro, W. Bodmer, S. Olschwang, A. S. Olsen, M. R. Stratton, A. de la Chapelle, and L. A. Aaltonen. 1998. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 391184-187. [DOI] [PubMed] [Google Scholar]
- 22.Hirai, T., and K. Chida. 2003. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J. Biochem. 1331-7. [DOI] [PubMed] [Google Scholar]
- 23.Iakoubov, R., A. Izzo, A. Yeung, C. I. Whiteside, and P. L. Brubaker. 2007. Protein kinase Czeta is required for oleic acid-induced secretion of glucagon-like peptide-1 by intestinal endocrine L cells. Endocrinology 1481089-1098. [DOI] [PubMed] [Google Scholar]
- 24.Jenne, D. E., H. Reimann, J. Nezu, W. Friedel, S. Loff, R. Jeschke, O. Muller, W. Back, and M. Zimmer. 1998. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 1838-43. [DOI] [PubMed] [Google Scholar]
- 25.Jishage, K., J. Nezu, Y. Kawase, T. Iwata, M. Watanabe, A. Miyoshi, A. Ose, K. Habu, T. Kake, N. Kamada, O. Ueda, M. Kinoshita, D. E. Jenne, M. Shimane, and H. Suzuki. 2002. Role of Lkb1, the causative gene of Peutz-Jegher's syndrome, in embryogenesis and polyposis. Proc. Natl. Acad. Sci. USA 998903-8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joberty, G., C. Petersen, L. Gao, and I. G. Macara. 2000. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2531-539. [DOI] [PubMed] [Google Scholar]
- 27.Katajisto, P., T. Vallenius, K. Vaahtomeri, N. Ekman, L. Udd, M. Tiainen, and T. P. Makela. 2007. The LKB1 tumor suppressor kinase in human disease. Biochim. Biophys. Acta 177563-75. [DOI] [PubMed] [Google Scholar]
- 28.Kemp, B. E., D. Stapleton, D. J. Campbell, Z. P. Chen, S. Murthy, M. Walter, A. Gupta, J. J. Adams, F. Katsis, B. van Denderen, I. G. Jennings, T. Iseli, B. J. Michell, and L. A. Witters. 2003. AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 31162-168. [DOI] [PubMed] [Google Scholar]
- 29.Liu, L. Z., H. L. Zhao, J. Zuo, S. K. Ho, J. C. Chan, Y. Meng, F. D. Fang, and P. C. Tong. 2006. Protein kinase Czeta mediates insulin-induced glucose transport through actin remodeling in L6 muscle cells. Mol. Biol. Cell 172322-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lizcano, J. M., O. Goransson, R. Toth, M. Deak, N. A. Morrice, J. Boudeau, S. A. Hawley, L. Udd, T. P. Makela, D. G. Hardie, and D. R. Alessi. 2004. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moscat, J., P. Rennert, and M. T. az-Meco. 2006. PKCzeta at the crossroad of NF-kappaB and Jak1/Stat6 signaling pathways. Cell Death Differ. 13702-711. [DOI] [PubMed] [Google Scholar]
- 32.Nagata, D., M. Mogi, and K. Walsh. 2003. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J. Biol. Chem. 27831000-31006. [DOI] [PubMed] [Google Scholar]
- 33.Nakau, M., H. Miyoshi, M. F. Seldin, M. Imamura, M. Oshima, and M. M. Taketo. 2002. Hepatocellular carcinoma caused by loss of heterozygosity in Lkb1 gene knockout mice. Cancer Res. 624549-4553. [PubMed] [Google Scholar]
- 34.Neumann, D., M. Suter, R. Tuerk, U. Riek, and T. Wallimann. 2007. Co-expression of LKB1, MO25alpha and STRADalpha in bacteria yield the functional and active heterotrimeric complex. Mol. Biotechnol. 36220-231. [DOI] [PubMed] [Google Scholar]
- 35.Neumann, D., A. Woods, D. Carling, T. Wallimann, and U. Schlattner. 2003. Mammalian AMP-activated protein kinase: functional, heterotrimeric complexes by co-expression of subunits in Escherichia coli. Protein Expr. Purif. 30230-237. [DOI] [PubMed] [Google Scholar]
- 36.Nezu, J., A. Oku, and M. Shimane. 1999. Loss of cytoplasmic retention ability of mutant LKB1 found in Peutz-Jeghers syndrome patients. Biochem. Biophys. Res. Commun. 261750-755. [DOI] [PubMed] [Google Scholar]
- 37.Ohno, S. 2001. Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr. Opin. Cell Biol. 13641-648. [DOI] [PubMed] [Google Scholar]
- 38.Ono, Y., T. Fujii, K. Ogita, U. Kikkawa, K. Igarashi, and Y. Nishizuka. 1989. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc. Natl. Acad. Sci. USA 863099-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ossareh-Nazari, B., F. Bachelerie, and C. Dargemont. 1997. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278141-144. [DOI] [PubMed] [Google Scholar]
- 40.Rattan, R., S. Giri, A. K. Singh, and I. Singh. 2005. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J. Biol. Chem. 28039582-39593. [DOI] [PubMed] [Google Scholar]
- 41.Redig, A. J., and L. C. Platanias. 2007. The protein kinase C (PKC) family of proteins in cytokine signaling in hematopoiesis. J. Interferon Cytokine Res. 27623-636. [DOI] [PubMed] [Google Scholar]
- 42.Rossi, D. J., A. Ylikorkala, N. Korsisaari, R. Salovaara, K. Luukko, V. Launonen, M. Henkemeyer, A. Ristimaki, L. A. Aaltonen, and T. P. Makela. 2002. Induction of cyclooxygenase-2 in a mouse model of Peutz-Jeghers polyposis. Proc. Natl. Acad. Sci. USA 9912327-12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowan, A., M. Churchman, R. Jefferey, A. Hanby, R. Poulsom, and I. Tomlinson. 2000. In situ analysis of LKB1/STK11 mRNA expression in human normal tissues and tumours. J. Pathol. 192203-206. [DOI] [PubMed] [Google Scholar]
- 44.Sapkota, G. P., J. Boudeau, M. Deak, A. Kieloch, N. Morrice, and D. R. Alessi. 2002. Identification and characterization of four novel phosphorylation sites (Ser31, Ser325, Thr336 and Thr366) on LKB1/STK11, the protein kinase mutated in Peutz-Jeghers cancer syndrome. Biochem. J. 362481-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sapkota, G. P., A. Kieloch, J. M. Lizcano, S. Lain, J. S. Arthur, M. R. Williams, N. Morrice, M. Deak, and D. R. Alessi. 2001. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90RSK and cAMP-dependent protein kinase, but not its farnesylation at Cys433, is essential for LKB1 to suppress cell growth. J. Biol. Chem. 27619469-19482. [DOI] [PubMed] [Google Scholar]
- 46.Scott, K. D., S. Nath-Sain, M. D. Agnew, and P. A. Marignani. 2007. LKB1 catalytically deficient mutants enhance cyclin D1 expression. Cancer Res. 675622-5627. [DOI] [PubMed] [Google Scholar]
- 47.Shaw, R. J., M. Kosmatka, N. Bardeesy, R. L. Hurley, L. A. Witters, R. A. DePinho, and L. C. Cantley. 2004. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 1013329-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaw, R. J., K. A. Lamia, D. Vasquez, S. H. Koo, N. Bardeesy, R. A. DePinho, M. Montminy, and L. C. Cantley. 2005. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 3101642-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song, P., Z. Xie, Y. Wu, J. Xu, Y. Dong, and M. H. Zou. 2008. Protein kinase Czeta-dependent LKB1 serine 428 phosphorylation increases LKB1 nucleus export and apoptosis in endothelial cells. J. Biol. Chem. 28312446-12455. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Sun, Y., K. E. Connors, and D. Q. Yang. 2007. AICAR induces phosphorylation of AMPK in an ATM-dependent, LKB1-independent manner. Mol. Cell. Biochem. 306239-245. [DOI] [PubMed] [Google Scholar]
- 51.Suter, M., U. Riek, R. Tuerk, U. Schlattner, T. Wallimann, and D. Neumann. 2006. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J. Biol. Chem. 28132207-32216. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki, A., T. Yamanaka, T. Hirose, N. Manabe, K. Mizuno, M. Shimizu, K. Akimoto, Y. Izumi, T. Ohnishi, and S. Ohno. 2001. Atypical protein kinase C is involved in the evolutionarily conserved PAR protein complex and plays a critical role in establishing epithelia-specific junctional structures. J. Cell Biol. 1521183-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tiainen, M., K. Vaahtomeri, A. Ylikorkala, and T. P. Makela. 2002. Growth arrest by the LKB1 tumor suppressor: induction of p21(WAF1/CIP1). Hum. Mol. Genet. 111497-1504. [DOI] [PubMed] [Google Scholar]
- 54.Towler, M. C., S. Fogarty, S. A. Hawley, D. A. Pan, D. M. Martin, N. A. Morrice, A. McCarthy, M. N. Galardo, S. B. Meroni, S. B. Cigorraga, A. Ashworth, K. Sakamoto, and D. G. Hardie. 2008. A novel short splice variant of the tumour suppressor LKB1 is required for spermiogenesis. Biochem. J. 4161-14. [DOI] [PubMed] [Google Scholar]
- 55.Woods, A., S. R. Johnstone, K. Dickerson, F. C. Leiper, L. G. Fryer, D. Neumann, U. Schlattner, T. Wallimann, M. Carlson, and D. Carling. 2003. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 132004-2008. [DOI] [PubMed] [Google Scholar]
- 56.Xie, Z., Y. Dong, R. Scholz, D. Neumann, and M. H. Zou. 2008. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation 117952-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie, Z., Y. Dong, M. Zhang, M. Z. Cui, R. A. Cohen, U. Riek, D. Neumann, U. Schlattner, and M. H. Zou. 2006. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J. Biol. Chem. 2816366-6375. [DOI] [PubMed] [Google Scholar]
- 58.Xie, Z., M. Singh, D. A. Siwik, W. L. Joyner, and K. Singh. 2003. Osteopontin inhibits interleukin-1beta-stimulated increases in matrix metalloproteinase activity in adult rat cardiac fibroblasts: role of protein kinase C-zeta. J. Biol. Chem. 27848546-48552. [DOI] [PubMed] [Google Scholar]
- 59.Ylikorkala, A., E. Avizienyte, I. P. Tomlinson, M. Tiainen, S. Roth, A. Loukola, A. Hemminki, M. Johansson, P. Sistonen, D. Markie, K. Neale, R. Phillips, P. Zauber, T. Twama, J. Sampson, H. Jarvinen, T. P. Makela, and L. A. Aaltonen. 1999. Mutations and impaired function of LKB1 in familial and non-familial Peutz-Jeghers syndrome and a sporadic testicular cancer. Hum. Mol. Genet. 845-51. [DOI] [PubMed] [Google Scholar]
- 60.Ylikorkala, A., D. J. Rossi, N. Korsisaari, K. Luukko, K. Alitalo, M. Henkemeyer, and T. P. Makela. 2001. Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science 2931323-1326. [DOI] [PubMed] [Google Scholar]
- 61.Zheng, B., J. H. Jeong, J. M. Asara, Y. Y. Yuan, S. R. Granter, L. Chin, and L. C. Cantley. 2009. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol. Cell 33237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zou, M. H., X. Y. Hou, C. M. Shi, S. Kirkpatrick, F. Liu, M. H. Goldman, and R. A. Cohen. 2003. Activation of 5′-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J. Biol. Chem. 27834003-34010. [DOI] [PubMed] [Google Scholar]
- 63.Zou, M. H., S. S. Kirkpatrick, B. J. Davis, J. S. Nelson, W. G. Wiles, U. Schlattner, D. Neumann, M. Brownlee, M. B. Freeman, and M. H. Goldman. 2004. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J. Biol. Chem. 27943940-43951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.