Abstract
Background and Purpose
Cerebral amyloid angiopathy (CAA) is a major cause of lobar intracerebral hemorrhage and cognitive impairment and is associated with white matter hyperintensities and cerebral microbleeds. MRI diffusion tensor imaging detects microstructural tissue damage in advanced CAA even in areas that appear normal on conventional MRI. We hypothesized that higher global mean apparent diffusion coefficient (mean ADC), reflecting a higher amount of chronic tissue disruption caused by CAA, would be independently associated with CAA-related cognitive impairment.
Methods
Preintracerebral hemorrhage cognitive impairment was systematically assessed using a standardized questionnaire (IQCODE) in 49 patients. Volume of white matter hyperintensities, number of microbleeds, and mean ADC were determined from MRIs obtained within 14.0±22.5 days of intracerebral hemorrhage cognitive impairment. White matter hyperintensities and mean ADC were measured in the hemisphere uninvolved by intracerebral hemorrhage to avoid confounding.
Results
Preintracerebral hemorrhage cognitive impairment was identified in 10 of 49 subjects. Mean ADC was the only variable associated with preintracerebral hemorrhage cognitive impairment and was elevated in those with preintracerebral hemorrhage cognitive impairment compared with those without (12.4×10-4 versus 11.7×10-4 mm2/s; P=0.03). Mean ADC positively correlated with age but not white matter hyperintensities or number of microbleeds. In logistic regression controlling for age and visible cerebral atrophy, mean ADC was independently associated with preintracerebral hemorrhage cognitive impairment (OR per 1×10-4 mm2/s increase=2.45, 95% CI 1.11 to 5.40, P=0.04).
Conclusions
Mean ADC is independently associated with preintracerebral hemorrhage cognitive impairment in CAA. The lack of correlation with other MRI markers of CAA suggests that mean ADC may be sensitive to distinct aspects of CAA pathology and its tissue consequences. These results suggest that global MRI diffusion changes are sensitive to clinically relevant microstructural alterations and may be useful markers of CAA-related tissue damage.
Keywords: cerebral amyloid angiopathy, cerebral microhemorrhage, cognitive impairment, dementia, diffusion, microbleeds, white matter disease
Cerebral amyloid angiopathy (CAA) is a major cause of lobar intracerebral hemorrhage (ICH) and cognitive impairment in the elderly and is associated with a high prevalence of markers of small vessel disease, including white matter hyper-intensities (WMH) and cerebral microbleeds (MB).1-4
ICH in sporadic CAA is likely caused by vessel fragility and rupture due to the deposition of amyloid within the media and adventitia of small- to medium-sized cerebral arteries.5 The presence of WMH visualized on conventional MRI sequences4 suggests that vascular amyloid deposition may alter white matter perfusion by causing stenosis or vascular dysfunction.6 This hypothesis is supported by the observations of extensive white matter damage in families with rare hereditary forms of CAA due to point mutations within the amyloid precursor protein.7,8 WMH have been associated with cognitive impairment in sporadic CAA.4
MRI diffusion tensor imaging has been used in other cerebral small vessel diseases to detect microstructural changes in cerebral tissue, even in areas that appear normal on conventional MRI,9-11 and to detect significant differences between CAA and control populations.12 Global quantitative measures can be obtained with diffusion tensor imaging by using whole-brain histograms of mean diffusivity such as mean apparent diffusion coefficient (ADC).9,11,13 Two recent publications, by independent research groups, showed that whole-brain ADC histograms were closely correlated with clinical disability in the rare hereditary small vessel disease cerebral autosomal-dominant arteriopathy with subcortical ischemic leukoencephalopathy.9,11 CAA, like cerebral autosomal-dominant arteriopathy with subcortical ischemic leukoencephalopathy, is also a well-defined cerebral small vessel disease with a high prevalence of subcortical tissue lesions.1-4 We hypothesized that a similar relationship might be present between these global diffusion tensor imaging measures and CAA-related clinical disability. We therefore sought to determine whether higher global mean ADC would be associated with chronic cognitive impairment in CAA, independent of the effects of hemorrhagic stroke, possibly by reflecting a higher amount of chronic tissue disruption caused by the disease.
Methods
Subjects
The study subjects were recruited from an ongoing single-center prospective longitudinal cohort study of CAA.14,15 Subjects were consecutive patients aged ≥55 years who presented to the Massachusetts General Hospital between August 2001 and August 2005 with symptomatic lobar ICH and had MRI with diffusion-weighted sequences within 90 days of the index event. MRI was obtained at the discretion of the treating physician and was performed according to departmental protocol. Those with other potential causes of hemorrhage such as cavernous angioma or other vascular malformations, or hemorrhages in locations more typical of hypertensive ICH such as the basal ganglia and thalamus, were excluded. CAA was diagnosed according to the previously validated Boston Criteria16; there were 5 subjects (10%) with definite CAA by tissue diagnosis, 19 subjects (39%) with probable CAA (2 or more ICH, including microbleeds on MRI, restricted to lobar regions), and 25 subjects (51%) with possible CAA (a single lobar ICH without MRI evidence of other hemorrhages).
Baseline clinical and demographic information was collected blinded to imaging data. Hypertension was defined as previous diagnosis of hypertension (>140/90 mm Hg) or use of antihypertensive treatment for control of blood pressure.17 Like in prior studies,4 we measured cognitive impairment before ICH to avoid the major influence on cognition introduced by occurrence of the hemorrhagic stroke itself. Pre-ICH cognitive impairment (PICI) was defined as the presence of deficits in memory or other cognitive areas sufficient to interfere with tasks of daily living before ICH and was assessed based on interview with the subject and informants, review of the medical records, and results of a standardized questionnaire (IQCODE short form) that included items related to memory, praxis, calculation, and reasoning.18 The IQCODE short form is administered to a caregiver or other informant and includes 16 detailed questions regarding the subject's change in memory and cognitive status compared with 10 years prior.18 Each question is scored from 1 (much improved compared with 10 years prior) to 5 (much worse compared with 10 years prior) and the questions are averaged to generate a total score. IQCODE scores of >3.4 were interpreted as supportive of the presence of PICI based on previous validation studies.19,20 Subject and informant interview, with administration of the IQCODE, was done shortly after the index event during the hospitalization for ICH.
Informed consent was obtained from each subject or from a close relative if the subject was too severely disabled to provide written consent. This study was performed with approval and in accordance with the guidelines of the Institutional Review Boards of Massachusetts General Hospital.
Image Acquisition and Analysis
MR images were acquired on 1.5-Tesla Signa scanners (GE Medical Systems, Milwaukee, Wis) as previously described.14,21 Diffusion-weighted images (TR/TE 7500/97 to 99 ms, slice thickness 5 to 6 mm, interslice gap 1.0 mm, 128×128; b value=1000 s/mm2;23 slices) were performed. Diffusion-weighted imaging scans were acquired in orthogonal directions and then averaged to make ADC measurements largely independent of the effects of anisotropic diffusion.22,23 Fluid-attenuated inversion recovery images (TR/TE 10 000/140 ms, slice thickness 5 mm, interslice gap 1.0 mm, 256×256) and axial gradient-echo images were obtained as previously described.1,2,24 MRIs were performed an average of 14.0 days after ICH (range, 0 to 89 days).
Mean ADC was determined in the hemisphere contralateral to the hematoma using a previously described method.13 Because CAA is a microangiopathy that affects cerebral small vessels diffusely and symmetrically in the brain, measurement of mean ADC values in one hemisphere should be reflective of whole-brain tissue microstructural change.12 Briefly, histograms of ADC values from ADC maps were generated for each patient using a bin width equal to 0.1×10×4 mm2s×1. Voxels containing cerebrospinal fluid or areas of hemorrhage were excluded in all patients before calculation using a previously established superior threshold value of 27×10×4 mm2s×1.13 To correct for cross-subject differences in brain volume, each histogram was normalized to the total number of hemispheric tissue voxels. The mean ADC derived from each histogram was used for analysis.
The volume of WMH was determined on fluid-attenuated inversion recovery sequence according to our previously published method.21 Using MRIcro software (University of Nottingham School of Psychology, Nottingham, UK; www.mricro.com), a region-of-interest map of supratentorial WMH in the hemisphere contralateral to the hematoma was created by signal intensity thresholding followed by manual editing as necessary. To correct for head size, we used the sagittal midline cross-sectional intracranial area as a surrogate measure of the intracranial volume.25,26 WMH volume was normalized to head size (nWMH) by dividing the subject's WMH volume by the ratio of the subject's intracranial area to the mean intracranial area of the study population.21 The number of hemorrhages, including MB, was determined on the gradient-echo sequence.1,2 A global visible cerebral atrophy score was calculated on T2 or fluid-attenuated inversion recovery sequences by summing individual scores (range, 0 to 3) in 5 separate brain regions to give a total score of 0 to 15.4,27 ICH volume was determined by segmentation of the ICH on the baseline CT scan, at the time of hemorrhagic stroke, using Alice software (Parexel Corporation, Waltham, Mass).28 We have previously shown high interrater reliability, by intraclass correlation coefficient (ICC), for measurements of nWMH (ICC=0.98),21 midsagittal intracranial area (ICC=0.92),25 MRI hemorrhages (ICC=0.97),24 visible cerebral atrophy score (ICC=0.79), and ICH volume (ICC=0.99).28
All imaging analyses were performed by raters blinded to clinical information.
Statistical Analysis
Mean ADC was compared with the presence or absence of PICI by Student t test. The numbers of MRI microhemorrhages were divided into 3 categories (0, 1 to 4, or ≥5) to divide groups into tertiles1,29 and tested for association with PICI by Fisher exact test. Because the distribution of nWMH and MRI hemorrhages were right-skewed, Spearman's correlations were used for comparisons with other continuous variables and Wilcoxon's rank sum tests were used for comparisons with dichotomous variables.
Multivariate logistic regression models were used to find predictors of PICI; candidate covariates were those associated with PICI in univariate analysis (P<0.10). The final model variables were selected by the stepwise regression analysis with entry and removal values set to 0.05. Statistical analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC).
Results
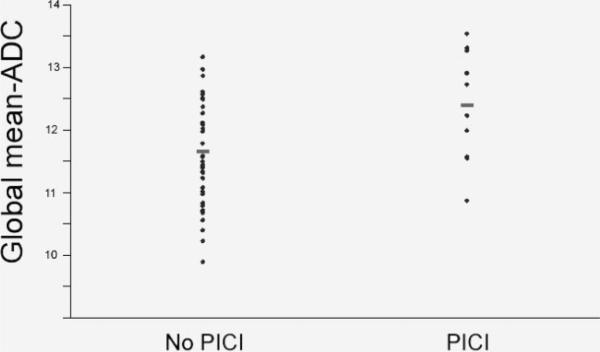
Baseline characteristics, according to the presence or absence of PICI, are presented in the Table. History of hypertension was more common in subjects with PICI, but this was not statistically significant (P=0.46). Seven of 10 of subjects with PICI had a diagnosis of probable CAA compared with 11 of 38 subjects without PICI (P=0.12). Subjects with PICI had higher whole-brain mean ADC values compared with individuals without pre-ICH cognitive impairment (Table; Figure). No differences in nWMH or number of MB were observed between subjects with or without PICI. Patients with PICI demonstrated a trend toward greater amount of visible cerebral atrophy, but this did not reach statistical significance (Table).
Table.
Baseline Characteristics of Subjects in the Cohort by the Presence or Absence of PICI
| Characteristic | PICI (n = 10, n; %) | No PICI (n = 39, n; %) | P Value |
|---|---|---|---|
| Sex | 0.99 | ||
| Male | 6 (60) | 21 (54) | |
| Female | 4 (40) | 18 (46) | |
| Age, years±SD | 75.8 ± 6.4 | 73.5 ± 8.3 | 0.44 |
| History of hypertension | 8 (80.0) | 25 (64.1) | 0.46 |
| Atrial fibrillation | 1 (10.0) | 7 (18.0) | 0.99 |
| Diabetes mellitus | 3 (30.0) | 3 (7.7) | 0.09 |
| History of coronary artery disease | 2 (20.0) | 4 (10.3) | 0.59 |
| History of ischemic stroke | 1 (10.0) | 1 (2.6) | 0.37 |
| History of ICH | 2 (20.0) | 1 (2.6) | 0.10 |
| ICH volume, mL±SD | 31.2 ± 35.6 | 43.9 ± 24.0 | 0.19 |
| nWMH | 17.3 [9.3, 31.6] | 15.9 [2.8, 33.8] | 0.67 |
| No. of MB | 1 [0, 4] | 0.5 [0, 3] | 0.76 |
| Mean ADC, ×10-4 mm2/s | 12.4 ± 0.89 | 11.7 ± 0.91 | 0.03 |
| Visible cerebral atrophy score | 5.1 ± 3.6 | 3.0 ± 3.1 | 0.08 |
Values are displayed as mean±SD, median [25th and 75th quartile], or n (%).
Figure.
Box-and-whisker plots of global mean ADC in subjects with and without PICI. ADC values are expressed in as mean ADC×10-4 mm2/s. Box borders represent the 25th and 75th percentiles, the middle bar is at the median, and the whiskers extend to 1.5 interquartile ranges. Statistical testing is by Student t test. *P=0.03.
Mean ADC was not associated with nWMH (P=0.44) or the number of MB (P=0.38). The presence or absence of a history of hypertension was not significantly associated with mean ADC (P=0.13). We found no association between ICH volume and mean ADC values in the non-ICH hemisphere. The side of the hematoma did not have a significant effect on mean ADC values in the non-ICH hemisphere. Patients with higher mean ADC values were older (r=0.51, P<0.001) and had a higher visible cerebral atrophy score (r=0.48, P<0.001).
Clinical variables and MRI measures that were correlated with PICI in univariate analyses (P<0.10) were entered into a stepwise multivariate logistic regression model to determine independent predictors of PICI in CAA. In the final maximally adjusted model controlling for age and amount of visible cerebral atrophy, only mean ADC was independently associated with PICI (OR per 1×10-4 mm2/s increase=2.45, 95% CI 1.11 to 5.40, P=0.04). The effect of visible cerebral atrophy on PICI was not significant in the final model (P=0.33). Other MRI markers such as nWMH and number of MB also did not have an independent effect on PICI. Other clinical variables, including history of hypertension and coronary artery disease, were not associated with PICI in multivariate models. Excluding patients with a history of ICH (n=3) from these analyses did not significantly change our results. In all of these analyses, there was no scanner effect that modified the interpretation of the results (data not shown).
Discussion
The major finding from this cohort study of patients with possible or probable CAA is that, among MRI markers of CAA, global mean ADC (reflecting microstructural tissue organization) is most strongly related to the presence of pre-ICH cognitive impairment. The association of mean ADC with PICI was independent of age, clinical variables, amount of visible cerebral atrophy, and other MRI markers. By contrast, we failed to detect a relationship between PICI and WMH volume, number of MB, or visible cerebral atrophy.
Decreased cognitive performance has previously been associated with CAA. The prevalence of cognitive dysfunction before CAA-related ICH is reported to be 20% to 40%4,5,17,30 Autopsy-based studies of stroke-free individuals show that CAA is a risk factor for decreased antemortem cognitive performance while simultaneously controlling for the pathology of Alzheimer disease.31,32 This suggests that the association between CAA and cognitive dysfunction is not entirely mediated by concomitant Alzheimer disease. Another plausible mechanism by which CAA might cause cognitive dysfunction is impaired vascular dysfunction with intermittent ischemia.3 White matter damage, a marker of vascular disease,33-35 is seen in CAA4,14 and has been associated with PICI.4 The lack of association between nWMH volume and PICI in the current study likely reflects the small sample size and highlights the robustness of the association detected between mean ADC and PICI.
Global mean ADC measures the degree of chronic tissue disruption and has been shown, in other cerebral small vessel diseases, to be a very sensitive measure of cerebral tissue damage.9,11,13 The strong independent association between mean ADC and PICI that we observed in this study suggests that global MRI diffusion changes may be a useful marker of CAA-related cognitive impairment by detecting clinically relevant tissue microstructural alterations. These microstructural alterations could be caused by direct ischemic injury or decreased white matter organization from neurodegeneration with neuronal loss.9,11,13
In a previous study, we found an association between nWMH volume and subcortical regional fractional anisotropy measures,12 whereas in this work, we did not find an association between nWMH volume and mean ADC. This may be related to our use of global measures, instead of region of interest-based measures of fractional anisotropy from subcortical regions, or may be related to the use of ADC rather than fractional anisotropy. Interestingly, in our previous study comparing subjects with CAA and control subjects, fractional anisotropy differences were greatest in regions of white matter and gray matter not typically involved by WMH.12 These observations are consistent with previous studies in sporadic leukoaraiosis36,37 and cerebral autosomal-dominant arteriopathy with subcortical ischemic leukoencephalopathy9 that show that diffusion tensor imaging measures are sensitive to tissue changes in apparently nonlesional areas on T2 sequences.
The main limitation of this study is the relatively small sample size, which may have precluded the detection of independent associations of MRI markers, other than global mean ADC, with PICI. The fact that global mean ADC was the only MRI marker associated with PICI attests to the strength of the observed association, however. Other vascular risk factors may act synergistically to cause tissue microstructural changes in patients with CAA, although we were unable to detect any significant associations between these variables and mean ADC in this small cohort. Further studies are necessary to confirm the findings seen in the current work. Second, because data from clinical MRI images were analyzed in this study, the spatial resolution was insufficient to perform quantitative volumetric analyses of brain atrophy. Our semiquantitative measure may not fully account for the impact on cerebral atrophy on PICI. Further studies using serial high-resolution quantitative MRI are ongoing. We cannot definitively exclude that some subjects had ICH from non-CAA-related pathology; however, such subjects likely represent a small portion of the cohort. The likelihood of underlying CAA was assessed using the validated, highly specific Boston criteria.16,24 The majority of subjects with PICI (7 of 10) had a diagnosis of probable CAA according to these criteria; this diagnosis has near 100% specificity for the pathological presence of CAA.16
In summary, these results show that the degree of tissue microstructural alteration, quantified by global mean ADC, is strongly associated with pre-ICH cognitive impairment independent of age and other MRI markers, including a measure of brain atrophy. The lack of correlation with most other MRI markers suggests that mean ADC may be sensitive to distinct aspects of CAA pathology and its tissue consequences. Further prospective studies in CAA will attempt to define the relationship among changes in mean ADC, other MRI markers, and clinical impairment. More generally, these results as well as other studies in cerebral autosomal-dominant arteriopathy with subcortical ischemic leukoencephalopathy11,13 suggest that mean ADC should be evaluated as a potential marker of cognitive dysfunction in MRI studies of cerebral small vessel diseases and cognition.
Acknowledgments
We thank Dr Ona Wu for her assistance with the MRI data.
Sources of Funding This work was supported by National Institutes of Health grants 5K23NS046327-04 and 5R01AG026484-02 (Massachusetts General Hospital) and with help from ARNEVA (Association de Recherche en Neurologie Vasculaire), Hopital Lariboisière, France.
Footnotes
Disclosures None.
References
- Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Finklestein SP, Schaefer PW. Petechial hemorrhages accompanying lobar hemorrhage: detection by gradient-echo MRI. Neurology. 1996;46:1751–1754. doi: 10.1212/wnl.46.6.1751. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Gurol ME, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke. 2004;35:2616–2619. doi: 10.1161/01.STR.0000143224.36527.44. [DOI] [PubMed] [Google Scholar]
- Smith EE, Gurol ME, Eng JA, Engel CR, Nguyen TN, Rosand J, Greenberg SM. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606–1612. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- Greenberg SM. Cerebral amyloid angiopathy and vessel dysfunction. Cerebrovasc Dis. 2002;13(suppl 2):42–47. doi: 10.1159/000049149. [DOI] [PubMed] [Google Scholar]
- Haan J, Roos RA, Algra PR, Lanser JB, Bots GT, Vegter-Van der Vlis M. Hereditary cerebral haemorrhage with amyloidosis-dutch type. Magnetic resonance imaging findings in 7 cases. Brain. 1990;113:1251–1267. doi: 10.1093/brain/113.5.1251. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Cho HS, Vonsattel JP, Rebeck GW, Greenberg SM. Novel amyloid precursor protein mutation in an iowa family with dementia and severe cerebral amyloid angiopathy. Ann Neurol. 2001;49:697–705. doi: 10.1002/ana.1009. [DOI] [PubMed] [Google Scholar]
- Chabriat H, Pappata S, Poupon C, Clark CA, Vahedi K, Poupon F, Mangin JF, Pachot-Clouard M, Jobert A, Le Bihan D, Bousser MG. Clinical severity in CADASIL related to ultrastructural damage in white matter: in vivo study with diffusion tensor MRI. Stroke. 1999;30:2637–2643. doi: 10.1161/01.str.30.12.2637. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Holtmannspotter M, Peters N, Opherk C, Martin D, Herzog J, Bruckmann H, Samann P, Gschwendtner A, Dichgans M. Diffusion magnetic resonance histograms as a surrogate marker and predictor of disease progression in CADASIL: a two-year follow-up study. Stroke. 2005;36:2559–2565. doi: 10.1161/01.STR.0000189696.70989.a4. [DOI] [PubMed] [Google Scholar]
- Salat DH, Smith EE, Tuch DS, Benner T, Pappu V, Schwab KM, Gurol ME, Rosas HD, Rosand J, Greenberg SM. White matter alterations in cerebral amyloid angiopathy measured by diffusion tensor imaging. Stroke. 2006;37:1759–1764. doi: 10.1161/01.STR.0000227328.86353.a7. [DOI] [PubMed] [Google Scholar]
- Jouvent E, Viswanathan A, Mangin JF, O'Sullivan M, Guichard JP, Gschwendtner A, Cumurciuc R, Buffon F, Peters N, Pachai C, Bousser MG, Dichgans M, Chabriat H. Brain atrophy is related to lacunar lesions and tissue microstructural changes in CADASIL. Stroke. 2007;38:1786–1790. doi: 10.1161/STROKEAHA.106.478263. [DOI] [PubMed] [Google Scholar]
- Chen YW, Gurol ME, Rosand J, Viswanathan A, Rakich SM, Groover TR, Greenberg SM, Smith EE. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006;67:83–87. doi: 10.1212/01.wnl.0000223613.57229.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell HC, Rosand J, Knudsen KA, Furie KL, Segal AZ, Chiu RI, Ikeda D, Greenberg SM. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342:240–245. doi: 10.1056/NEJM200001273420403. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Briggs ME, Hyman BT, Kokoris GJ, Takis C, Kanter DS, Kase CS, Pessin MS. Apolipoprotein E epsilon 4 is associated with the presence and earlier onset of hemorrhage in cerebral amyloid angiopathy. Stroke. 1996;27:1333–1337. doi: 10.1161/01.str.27.8.1333. [DOI] [PubMed] [Google Scholar]
- Jorm AF. A short form of the informant questionnaire on cognitive decline in the elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- Jorm AF. The informant questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004;16:275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- Fuh JL, Teng EL, Lin KN, Larson EB, Wang SJ, Liu CY, Chou P, Kuo BI, Liu HC. The informant questionnaire on cognitive decline in the elderly (IQCODE) as a screening tool for dementia for a predominantly illiterate Chinese population. Neurology. 1995;45:92–96. doi: 10.1212/wnl.45.1.92. [DOI] [PubMed] [Google Scholar]
- Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, Rosand J, Growdon JH, Greenberg SM. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- Sorensen AG, Wu O, Copen WA, Davis TL, Gonzalez RG, Koroshetz WJ, Reese TG, Rosen BR, Wedeen VJ, Weisskoff RM. Human acute cerebral ischemia: detection of changes in water diffusion anisotropy by using MR imaging. Radiology. 1999;212:785–792. doi: 10.1148/radiology.212.3.r99se24785. [DOI] [PubMed] [Google Scholar]
- Roberts TP, Rowley HA. Diffusion weighted magnetic resonance imaging in stroke. Eur J Radiol. 2003;45:185–194. doi: 10.1016/s0720-048x(02)00305-4. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, O'Donnell HC, Schaefer PW, Kraft E. MRI detection of new hemorrhages: potential marker of progression in cerebral amyloid angiopathy. Neurology. 1999;53:1135–1138. doi: 10.1212/wnl.53.5.1135. [DOI] [PubMed] [Google Scholar]
- Nandigam RN, Chen YW, Gurol ME, Rosand J, Greenberg SM, Smith EE. Validation of intracranial area as a surrogate measure of intracranial volume when using clinical MRI. J Neuroimaging. 2007;17:74–77. doi: 10.1111/j.1552-6569.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- Ferguson KJ, Wardlaw JM, Edmond CL, Deary IJ, Maclullich AM. Intracranial area: a validated method for estimating intracranial volume. J Neuroimaging. 2005;15:76–78. doi: 10.1177/1051228404270243. [DOI] [PubMed] [Google Scholar]
- Heijer T, Skoog I, Oudkerk M, de Leeuw FE, de Groot JC, Hofman A, Breteler MM. Association between blood pressure levels over time and brain atrophy in the elderly. Neurobiol Aging. 2003;24:307–313. doi: 10.1016/s0197-4580(02)00088-x. [DOI] [PubMed] [Google Scholar]
- Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–1064. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- Viswanathan A, Guichard JP, Gschwendtner A, Buffon F, Cumurcuic R, Boutron C, Vicaut E, Holtmannspotter M, Pachai C, Bousser MG, Dichgans M, Chabriat H. Blood pressure and haemoglobin A1C are associated with microhaemorrhage in CADASIL: a two-centre cohort study. Brain. 2006;129:2375–2383. doi: 10.1093/brain/awl177. [DOI] [PubMed] [Google Scholar]
- Mandybur TI. Cerebral amyloid angiopathy: the vascular pathology and complications. J Neuropathol Exp Neurol. 1986;45:79–90. [PubMed] [Google Scholar]
- Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. Cerebral amyloid angiopathy and cognitive function: the Haas Autopsy Study. Neurology. 2002;58:1629–1634. doi: 10.1212/wnl.58.11.1629. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O'Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- De Groot JC, De Leeuw FE, Oudkerk M, Van Gijn J, Hofman A, Jolles J, Breteler MM. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002;52:335–341. doi: 10.1002/ana.10294. [DOI] [PubMed] [Google Scholar]
- Chabriat H, Levy C, Taillia H, Iba-Zizen MT, Vahedi K, Joutel A, Tournier-Lasserve E, Bousser MG. Patterns of MRI lesions in CADASIL. Neurology. 1998;51:452–457. doi: 10.1212/wnl.51.2.452. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry. 2004;75:441–447. doi: 10.1136/jnnp.2003.014910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, Markus HS. Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology. 2001;57:2307–2310. doi: 10.1212/wnl.57.12.2307. [DOI] [PubMed] [Google Scholar]