Abstract
Cell-based treatments for insulin-dependent diabetes (IDD) may provide more physiologic regulation of blood glucose levels than daily insulin injections, thereby reducing the occurrence of secondary complications associated with diabetes. An autologous cell source is especially attractive for regulatory and ethical reasons in addition to eliminating the need for immunosuppression. This study uses non-β-cells, genetically modified for physiologic insulin secretion. Enteroendocrine L-cells, exhibit regulated secretion in response to physiologic stimuli and their endogenous products are fully compatible with prandial metabolism. Murine GLUTag L-cells were transfected with a plasmid co-expressing human insulin and neomycin resistance and the stable cell line, GLUTag-INS, was established. Secretion properties of GLUTag-INS cells were investigated in vitro through induced secretion tests using meat hydrolysate or 3-isobutyl-1-methylxanthine and forskolin as secretagogues. GLUTag-INS cells rapidly co-secreted recombinant insulin and endogenous glucagon-like peptide in response to metabolic cues from the surrounding medium and demonstrated efficient processing of proinsulin to insulin.
Keywords: Diabetes, L-cells, GLUTag, Insulin, Enteroendocrine, Autologous, Non-Beta-Cell
INTRODUCTION
A current cell based therapy for insulin-dependent diabetes (IDD) consists of infusing isolated islets from cadeveric donors into the liver of selected recipients placed under immunosuppression. The Edmonton protocol greatly increased the success of this procedure, with 80% of patients being insulin independent one year post-transplantation [1]. A five year follow-up however, revealed that although 80% of patients still had detectable C-peptide, only 10% remained insulin independent, indicating that there was a progressive decline in graft function [1]. Widespread application of islet transplantation is also limited by the sparse supply of donor tissue. The use of autologous, non-β-cells would eliminate the need for immunosuppresion and relax the constraint of tissue supply.
Though studies have achieved insulin secretion in non-β-cells, attempts to restore normoglycemia have fallen short of reconstructing the complex sensory and regulatory machinery unique to β-cells. Pituitary cells were engineered for insulin production because they efficiently process proinsulin to insulin and can secrete insulin in a regulated manner upon expression of the glucose sensor, glucokinase (GK), and the glucose transporter, GLUT2 [2; 3]. Many pituitary products are not compatible with prandial metabolism, however, and over-secretion of the native hormones may upset the metabolic state of the patient, unless efforts are made to knock down endogenous hormone expression. Autologous neuroendocrine tissues are also difficult to obtain or transduce in vivo. More easily accessible targets, such as muscle [4] and skin [5] have also been used for insulin gene therapy. These cells, however, require the use of recombinant insulin that is either bioactive as a single chain [6] or able to be cleaved by a ubiquitous endopeptidase [7]. Additionally, these non-β-cell sources do not possess the elements necessary for nutrient-regulated secretion, and so, may only be used to fulfill the need for basally secreted insulin. Like β-cells, hepatocytes possess GK and GLUT2, making them glucose sensitive, but these cells possess no regulated secretion pathway, and attempts to reengineer regulated release have relied on transcriptional regulation. Although success has been achieved in animal models with genetically engineered hepatocytes expressing insulin under transcriptional regulation by a glucose and insulin-sensitive promoter [8; 9], this system cannot provide the acute post-meal insulin release considered necessary for glycemic normalization in higher animals and, eventually, humans.
Enteroendocrine cells, exhibit many useful properties that make them appropriate targets for recombinant insulin expression. Like other endocrine cells, they are capable of processing wild type proinsulin to produce C-peptide, which has many beneficial effects on complications normally associated with IDD. Enteroendocrine cells’ function is to secrete incretin hormones which serve to potentiate insulin secretion from β-cells in the presence of glucose, and the release of incretins is controlled in a tightly regulated manner that closely parallels the secretion of insulin by β-cells, following an oral glucose load [10; 11]. Because of the unique connection between incretins and insulin, engineering of enteroendocrine L-cells for insulin expression arises as an appealing approach for IDD treatment in terms of the dynamic release of insulin and compatibility of incretins and insulin in glycemic normalization. Furthermore, insulin produced and secreted by genetically modified enteroendocrine K cells in transgenic mice prevented the animals from becoming diabetic after injection with streptozotocin [12]. Similarly, transgenic mice which produced human insulin in gastric G cells, displayed meal-regulated increase in the level of transgenic insulin and corresponding decrease in blood glucose levels [13]. These are important proof-of-concept studies which showed that insulin-expressing enteroendocrine cells can provide regulation of blood glucose levels.
Previously, our lab engineered a human L-cell line to release insulin in response to nutrient administration [14] and showed preferential transduction by adeno-associated virus for L-cells over enterocytes in a co-culture model [15]. In advancing L-cell mediated insulin therapy to a tissue-engineered treatment for an adult mouse model for IDD, the murine L-cell line, GLUTag, was selected for this study, as it would represent a closer allograft model for mice and glucagon-like peptide (GLP-1) secretion from this cell line has been found similar to those of primary cell cultures and in vivo [16]. This study describes the genetic modification of GLUTag cells for the stable expression of insulin and the characterization of the newly developed cell line.
MATERIALS & METHODS
All reagents were purchased from Sigma (St Louis, MO) unless otherwise noted.
Cell Culture
GLUTag cells were obtained from the laboratory of Dr. P.L. Brubaker with the permission of Dr. D.J. Drucker (University of Toronto, Ontario, Canada). The cells were cultured in a 37°C/5% CO2 humidified incubator in T-flasks in complete medium consisting of L-glutamine-free Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Cellgro, Herndon, VA); cultures were split at a 1:5 ratio when 80% confluency was reached.
Antibody Staining & Microscopy
Cells were washed then fixed in 4% paraformaldehyde in phosphate buffered saline (PBS), permeabilized with 0.5% Triton X-100 in PBS, blocked using 10% horse serum in PBS before adding diluted primary antibodies (either rabbit antihuman prohormone convertase (PC) 1/3, PC 2, or mouse antihuman insulin). Cells were incubated overnight at 4°C. The following day, cells were rinsed twice with PBS and diluted secondary antibody (either anti-rabbit or anti-mouse IgG-TRITC-conjugate) was added and incubated for 1.25 hours in the dark at room temperature. Cells were rinsed twice in PBS, coverslipped, and imaged by confocal microscopy.
Transfection & Selection of Stable Clone
The transgene for stable insulin expression was constructed by inserting the human B10 mutated insulin gene (Genentech, San Francisco, CA) into the pcDNA3.1(+) vector (Invitrogen, Carlsbad, CA). The B10 mutation is a naturally occurring, single point substitution of aspartic acid for histidine at position 10 of the B chain of insulin which results in a superactive hormone [17]. The expression cassette directs simultaneous expression of human insulin from the cytomegalovirus (CMV) promoter and neomycin resistance from the simian virus 40 (SV40) promoter. GLUTag cells, seeded two days prior to transfection (half-a-million cells per well of a 12-well plate), were transfected using FugeneHD (Stratagene, La Jolla, CA) according to manufacturer’s protocol at a ratio of 8μl FugeneHD:2μg DNA. Selection of a stable clone was performed by replacing medium the day after transfection with complete medium, supplemented with 200μg/ml Geneticin (Invitrogen) and increasing the concentration of Geneticin to 600μg/ml by incremental steps for 2 days. Selective pressure was maintained for a month with medium changes every 1 to 3 days until colonies that were large enough to be seen with the unaided eye formed. Individual colonies were transferred to a well of a 24-well plate. Spent medium from these wells was assayed for insulin production, and upon confirmation of robust, stable expression of insulin, the cell clone with the highest expression was used in the remainder of the studies and is henceforth referred to as GLUTag-INS.
Secretion Tests
Secretion test were performed on GLUTag-INS cell monolayers in 6-well tissue culture plates. One million cells were seeded per well 2 to 4 days prior to induced secretion tests. On the evening prior to the secretion tests, the medium was changed to basal (DMEM with 5mM glucose, without L-glutamine, supplemented with 1% FBS). On the day of the secretion test, parallel cultures were briefly washed with PBS, and then subjected to 3 consecutive one-hour incubations in basal medium to stabilize basal insulin and GLP-1 secretion. The secretion test was then initiated by incubating the stabilized monolayers in basal medium for 2 hours to establish the basal secretion rate. Two washes with PBS were performed between medium changes and monolayers were either changed to fresh basal medium as non-induced controls or to basal medium supplemented with 2% (w/v) meat hydrolysate (MH) or 3-isobutyl-1-methylxanthine (IBMX) and forskolin at 10μM each to stimulate insulin and GLP-1 secretion. Samples were taken at the end of the 2-hour induction period.
Assays
Insulin and GLP-1 concentrations were determined using the human insulin and GLP-1 radioimmunoassay kits (Millipore, Billerica, MA) according to the manufacturer’s protocols.
RESULTS & DISCUSSION
PC 1/3 & PC 2 Expression
In pancreatic β-cells, both PC1/3 and PC2 are needed to process proinsulin to insulin. Human NCI-H716 L-cells and canine primary L-cell cultures have also been demonstrated to express both PC1/3 and PC2 [15; 18]. While in intestinal L-cells PC1/3 is responsible for processing the proglucagon transcript to produce glicentin, GLP-1 and GLP-2, in pancreatic alpha-cells proglucagon is alternately processed by PC2 to produce glucagon. Despite the presence of both PC1/3 and PC2, minimal amounts of glucagon are found in intestinal L-cells [18]. To confirm that GLUTag cells also possess these enzymes, antibody staining was performed. Indeed, both PC1/3 and PC2 are expressed in GLUTag cells (figures 1B and 1C) with no background staining observed when cells were treated with the secondary, TRITC-conjugated, antibody alone (figure 1A). The presence of both PC1/3 and PC2 confers L-cells the ability to properly process proinsulin, as evidenced by the presence of immuno-reactive insulin in GLUTag-INS cells and, although not demonstrated in this study, it is reasonably expected that the insulin produced in these cells is also bioactive. Given the growing understanding of C-peptide’s role in promoting vascular health [19], a cell type that expresses equimolar amounts of insulin and C-peptide (from proper processing of wild-type proinsulin) would offer a clear advantage in treating IDD.
Figure 1. Confocal images of immunofluorescent staining.
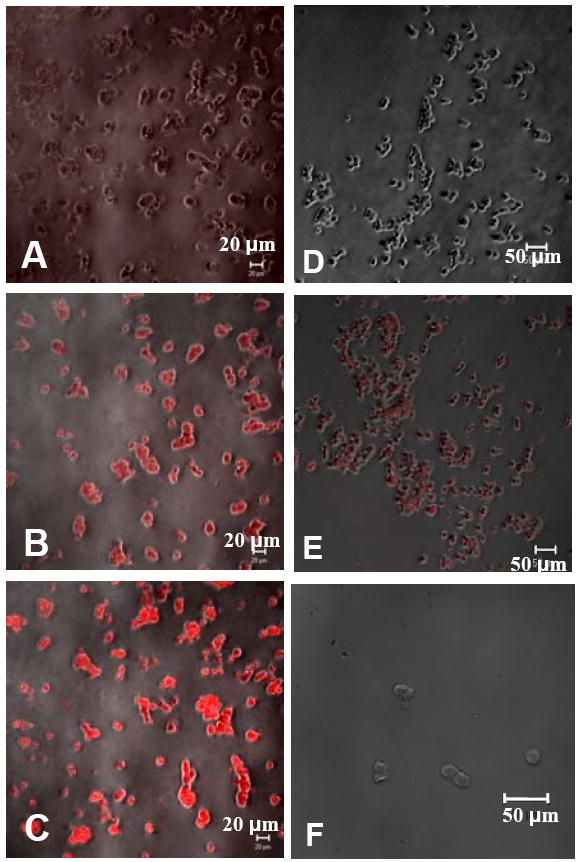
Parental GLUTag cells stained in the absence of primary antibody (A), with an antibody for PC 2 (B), PC 1/3 (C), and insulin (F). GLUTag-INS cells stained without primary antibody (D), and with an antibody for human insulin (E). Phase contrast images have been overlayed to show cell outlines.
Generating a Stable Clone
For tissue engineering purposes, establishing a cell line which permanently and stably expresses insulin is desirable, such that a homogeneous population of insulin-secreting cells can be characterized in vitro and transplanted, as such or in a three-dimensional construct, with a predictable outcome. In this work, this was achieved by transfection and continued culture under selection pressure. Following this selection period, 3 colonies were verified to produce insulin at significant levels and displayed good growth characteristics. The colony with the most robust insulin expression was used in all experiments and these cells are referred to as GLUTag-INS cells. Insulin production was verified through radioimmunoassay of spent medium and by immunofluorescent staining of GLUTag-INS cells (figure 1D-F). To ensure that the parental GLUTag cells do not express insulin, the concentrations of primary and secondary antibodies used to stain parental cells were 10-fold higher than those used to stain GLUTag-INS cells and the cells were observed under a higher magnification.
Induced Secretion of Insulin and GLP-1 from GLUTag-INS Cells
For in vitro characterization of GLUTag-INS cells, induced insulin secretion tests were performed, to investigate if these cells would regulate the secretion of insulin as they do GLP-1, despite the use of the constitutive CMV promoter to drive the production of insulin. GLUTag cells are commonly reported to be glucose sensitive [20; 21; 22; 23; 24], but induction occurs at a subphysiological level of 0.5mM, with no significant difference between 5mM and 25mM glucose concentrations [24]. In tests with GLUTag-INS cells using physiologically relevant glucose concentrations, no significant change in insulin secretion was measured when cells were exposed to a step change from 5 mM to 20 mM glucose (data not shown), likely due to the hypersensitivity inherent to the cell line.
For the experiments described here, nutrient stimulation came in the form of MH, a secretagogue known to induce secretion in a number of studies using enteroendocrine cell lines. Indeed, peptones, such as MH, are potent nutrient secretagogues for intestinal L-cells, and act not only by triggering secretion of accumulated hormone, but also by increasing gene transcription [14; 15; 25; 26; 27]. IBMX and forskolin, a pair frequently used as putative secretagogues [22; 24; 28], were also used as a positive control, while basal secretion was determined through parallel tests with basal medium. Insulin and GLP-1 induction were calculated by normalizing the amount of insulin and GLP-1, respectively, secreted during the 2 hours of induced secretion to the amount secreted during the prior 2 hour basal period. Results are shown in Figures 2A and B. Secretion of insulin for all groups during treatment with basal medium was 424.8 ± 86.4 fmole/(well·2hr) (n=27 from 3 independent experiments). Cells treated with MH and IBMX/forskolin exhibited 220.7% ± 32.4% and 423.7% ± 47.1% induction relative to basal secretion, respectively (Figure 2A). With approximately 2.4 million cells per well, this level of insulin expression is on par with values reported for other insulin-secreting non-β-cells, but about 5-fold lower than that reported for β-cell lines. The basal rates of insulin secretion for engineered AtT20 cells [32], engineered NCI-H716 cells [14], and GLUTag-INS cells are 60, 79, 86 fmole/(106 cells·hr) respectively, while the basal rate of secretion for βTC3 cells [29] is 384 fmole/(106 cells·hr). For GLP-1, the basal secretion rate was 1301.5 ± 245.1 fmole/(well·2hr). GLP-1 induction for cells exposed to MH, and IBMX/forskolin were 178.4% ± 10.3%, and 226.0% ± 50.8% of the basal rates, respectively (Figure 2B).
Figure 2. Induced secretion test.
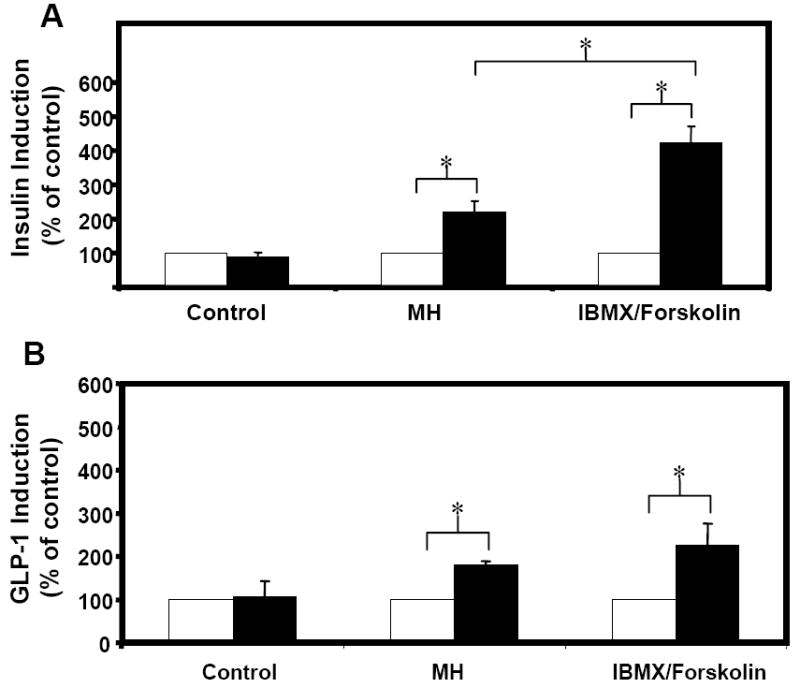
GLUTag-INS cells were exposed to basal medium for 2 hours (open bars) followed by another 2 hours in either basal medium (control), 2% MH, or 10μM IBMX and 10μM Forskolin (solid bars). Secreted insulin (A) and GLP-1 (B) are expressed as a percent of the amount secreted during the initial 2-hour period. Error bars indicate standard deviations. N=9 from 3 independent experiments. * paired t-test with unequal variances, p < 0.005.
As the basal medium used in this study was DMEM with 5mM glucose and 1% FBS, a higher rate of basal secretion was expected than that seen in electrophysiology studies in which the basal medium is typically a nutrient-free buffer. For instance, the basal rate of GLP-1 secretion from GLUTag-INS cells measured in this study is estimated to be 7.1-fold greater than that reported by Reimann and Gribble for GLUTag cells in Krebs Ringer Buffer, when corrected for differences in culture size [24]. Though there may be inherent differences in the amount of GLP-1 secreted from parental GLUTag cells and GLUTag-INS, much of the difference in basal GLP-1 secretion rates is expected to be due to medium differences. This trend has also been noted for insulinoma cells: insulin secretion rates in nutrient-free PBS, without or with the addition of 16mM glucose, were significantly lower compared to insulin secretion rates in nutrient-rich DMEM, again, without or with the addition of 16mM glucose [30].
While the induction fold of insulin secretion from GLUTag-INS cells in response to MH was 221%, the induction fold for GLP-1 from the same cells was slightly lower (p<0.05) at only 178%. The basal rate of GLP-1 secretion, however, is 3-fold greater than the basal insulin secretion, which may explain why the induction fold was not as high for GLP-1 as it was for insulin (as induction folds are determined through normalization to the basal secretion rate). It is interesting to note that the relative induction folds in response to IBMX and forskolin were 226.0% for GLP-1 and 423.7% for insulin, indicating that while MH caused nearly maximal secretion of GLP-1, quite a bit more insulin was secreted in response to IBMX and forskolin. It has been reported that forskolin results in strong activation of the CMV promoter [31] so the higher induction of insulin relative to GLP-1 during treatment with IBMX /forskolin is likely the result of additional activation of the CMV promoter controlling insulin expression in GLUTag-INS cells, while GLP-1 expression (controlled by the proglucagon promoter) is unaffected.
The secretory properties of GLUTag-INS cells demonstrate that the insulin secretion response of this engineered cell line to various nutrient and non-nutrient secretagogues is inline with what has been observed for secretion of GLP-1 from parental GLUTag cells. In previous studies in which an insulin-EGFP fusion protein was transiently expressed in human L-cells, engineered insulin-EGFP and endogenous GLP-1 co-localized in secretory granules [14]. The similarity in the secretion of insulin and GLP-1 from GLUTag-INS cells suggests that co-localization of insulin and GLP-1 occurs in GLUTag-INS cells as well.
Detailed Secretion Profile of Insulin from GLUTag-INS Cells
To explore how quickly GLUTag-INS cells respond to nutrient administration, another secretion test was performed to better resolve the time axis, with samples taken every 20 minutes. In the time course experiment (figure 3) engineered GLUTag-INS cells released insulin in response to 2% MH in an acute manner, with a significant difference in insulin secretion observed within the first 20 minutes. This behavior was expected, as parental GLUTag cells have been shown to respond rapidly to 10mM l-glutamine by enhanced secretion of GLP-1 [22].
Figure 3. Time course of insulin secretion from GLUTag-INS cells.
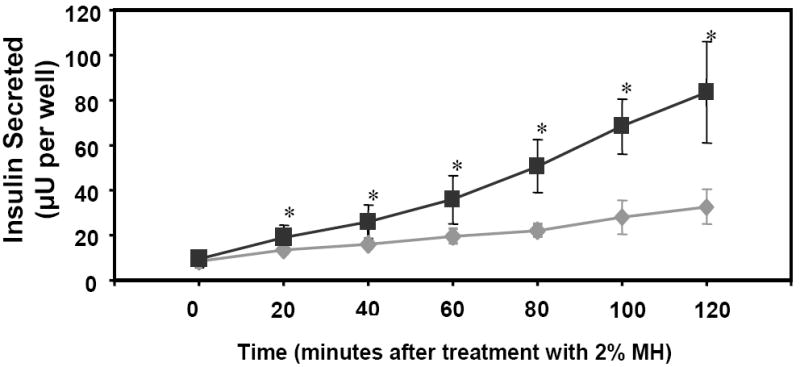
Grey diamonds and black squares indicate insulin secreted by GLUTag-INS cells in basal and induced secretion (with 2% MH, as secretagogue) medium, respectively. Error bars represent standard deviations. N=9 from 3 independent experiments. * Significant difference between basal and MH groups at these times, one-way ANOVA p < 0.05.
Proinsulin conversion
To evaluate the efficiency of proinsulin to insulin conversion, the percentage of insulin in culture medium, calculated as (insulin*100%)/(insulin + proinsulin), was evaluated for the experiment of figure 2. The percentage of insulin secreted from GLUTag-INS cells relative to total secreted insulin and proinsulin was about 70% when cells were exposed to basal medium. Incremental increases in proinsulin conversion were noted in groups treated with MH and IBMX/forskolin, 77.6% and 88.3% respectively (figure 4). These rises in proinsulin conversion, which were statistically significant (p<0.05), indicate that GLUTag-INS cells efficiently process proinsulin to insulin, especially in times of high demand.
Figure 4. Proinsulin conversion in basal and induced secretion medium.
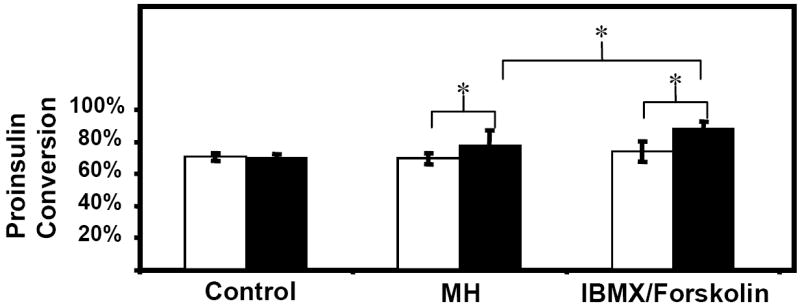
GLUTag-INS cells were exposed to basal medium for 2 hours (open bars) followed by another 2 hours in either basal medium (control), 2% MH, or 10μM IBMX and 10μM Forskolin (solid bars). Proinsulin conversion was calculated as (insulin*100%)/(insulin + proinsulin). Error bars indicate standard deviations. N=9 from 3 independent experiments. * paired t-test with unequal variances, p < 0.05.
Acknowledgments
This work was primarily supported by NIH R01DK076801 and by the ERC program of the National Science Foundation under award number EEC-9731643. Funding was also provided by Johnson & Johnson Healthcare Innovation Program. This work was also made possible through the generous donations of materials: the B10 human insulin gene by Genentech, SIS by Cook Biotech, GLUTag cells by Drs. Brubaker and Drucker (University of Toronto, Ontario, Canada).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bertuzzi F, Marzorati S, Secchi A. Islet Cell Transplantation. Curr Mol Med. 2006;6:369–74. doi: 10.2174/156652406777435453. [DOI] [PubMed] [Google Scholar]
- 2.Motoyoshi S, Shirotani T, Araki E, Sakai K, Kaneko K, Motoshima H, Yoshizato K, Shirakami A, Kishikawa H, Shichiri M. Cellular characterization of pituitary adenoma cell line (AtT20 cell) transfected with insulin, glucose transporter type 2 (GLUT2) and glucokinase genes: insulin secretion in response to physiological concentrations of glucose. Diabetologia. 1998;41:1492–501. doi: 10.1007/s001250051096. [DOI] [PubMed] [Google Scholar]
- 3.Hughes SD, Quaade C, Johnson JH, Ferber S, Newgard CB. Transfection of AtT-20ins cells with GLUT-2 but not GLUT-1 confers glucose-stimulated insulin secretion. Relationship to glucose metabolism. J Biol Chem. 1993;268:15205–12. [PubMed] [Google Scholar]
- 4.Yin D, Tang JG. Gene therapy for streptozotocin-induced diabetic mice by electroporational transfer of naked human insulin precursor DNA into skeletal muscle in vivo. FEBS Lett. 2001;495:16–20. doi: 10.1016/s0014-5793(01)02352-3. [DOI] [PubMed] [Google Scholar]
- 5.Lei P, Ogunade A, Kirkwood KL, Laychock SG, Andreadis ST. Efficient production of bioactive insulin from human epidermal keratinocytes and tissue-engineered skin substitutes: implications for treatment of diabetes. Tissue Eng. 2007;13:2119–31. doi: 10.1089/ten.2006.0210. [DOI] [PubMed] [Google Scholar]
- 6.Lee HC, Kim SJ, Kim KS, Shin HC, Yoon JW. Remission in models of type 1 diabetes by gene therapy using a single-chain insulin analogue. Nature. 2000;408:483–8. doi: 10.1038/35044106. [DOI] [PubMed] [Google Scholar]
- 7.Groskreutz DJ, Sliwkowski MX, Gorman CM. Genetically engineered proinsulin constitutively processed and secreted as mature, active insulin. J Biol Chem. 1994;269:6241–5. [PubMed] [Google Scholar]
- 8.Thule PM, Liu JM. Regulated hepatic insulin gene therapy of STZ-diabetic rats. Gene Ther. 2000;7:1744–52. doi: 10.1038/sj.gt.3301297. [DOI] [PubMed] [Google Scholar]
- 9.Olson DE, Paveglio SA, Huey PU, Porter MH, Thule PM. Glucose-responsive hepatic insulin gene therapy of spontaneously diabetic BB/Wor rats. Hum Gene Ther. 2003;14:1401–13. doi: 10.1089/104303403769211628. [DOI] [PubMed] [Google Scholar]
- 10.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 11.Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996;97:92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung AT, Dayanandan B, Lewis JT, Korbutt GS, Rajotte RV, Bryer-Ash M, Boylan MO, Wolfe M, Kieffer TJ. Glucose-Dependent Insulin Release from Genetically Engineered K cells. Science. 2000;290:1959–1963. doi: 10.1126/science.290.5498.1959. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y-C, Sternini C, Rozengurt E, Zhokova E. Release of Transgenic Human Insulin from Gastric G Cells: A Novel Approach for the Amelioration of Diabetes. Endocrinology. 2005;146:2610–2619. doi: 10.1210/en.2004-1109. [DOI] [PubMed] [Google Scholar]
- 14.Tang SC, Sambanis A. Development of genetically engineered human intestinal cells for regulated insulin secretion using rAAV-mediated gene transfer. Biochem Biophys Res Commun. 2003;303:645–52. doi: 10.1016/s0006-291x(03)00399-1. [DOI] [PubMed] [Google Scholar]
- 15.Tang SC, Sambanis A. Differential rAAV2 transduction efficiencies and insulin secretion profiles in pure and co-culture models of human enteroendocrine L-cells and enterocytes. J Gene Med. 2004;6:1003–13. doi: 10.1002/jgm.587. [DOI] [PubMed] [Google Scholar]
- 16.Brubaker PL, Schloos J, Drucker DJ. Regulation of glucagon-like peptide-1 synthesis and secretion in the GLUTag enteroendocrine cell line. Endocrinology. 1998;139:4108–14. doi: 10.1210/endo.139.10.6228. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GP, Burke GT, Katsoyannis PG. A superactive insulin: [B10-aspartic acid]insulin(human) Proc Natl Acad Sci U S A. 1987;84:6408–11. doi: 10.1073/pnas.84.18.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damholt AB, Buchan AM, Holst JJ, Kofod H. Proglucagon processing profile in canine L cells expressing endogenous prohormone convertase 1/3 and prohormone convertase 2. Endocrinology. 1999;140:4800–8. doi: 10.1210/endo.140.10.7068. [DOI] [PubMed] [Google Scholar]
- 19.Vague P, Coste TC, Jannot MF, Raccah D, Tsimaratos M. C-peptide, Na+,K(+)-ATPase, and diabetes. Exp Diabetes Res. 2004;5:37–50. doi: 10.1080/15438600490424514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reimann F, Ward PS, Gribble FM. Signaling mechanisms underlying the release of glucagon-like peptide 1. Diabetes. 2006;55:S78–S85. [Google Scholar]
- 21.Reimann F, Maziarz A, Flock G, Habib AM, Drucker DJ, Gribble EM. Characterization and functional role of voltage gated cation conductances in the glucagon-like peptide-1 secreting GLUTag cell line. Journal of Physiology-London. 2005;563:161–175. doi: 10.1113/jphysiol.2004.076414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47:1592–601. doi: 10.1007/s00125-004-1498-0. [DOI] [PubMed] [Google Scholar]
- 23.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–54. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]
- 24.Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes. 2002;51:2757–63. doi: 10.2337/diabetes.51.9.2757. [DOI] [PubMed] [Google Scholar]
- 25.Cordier-Bussat M, Bernard C, Haouche S, Roche C, Abello J, Chayvialle JA, Cuber JC. Peptones stimulate cholecystokinin secretion and gene transcription in the intestinal cell line STC-1. Endocrinology. 1997;138:1137–44. doi: 10.1210/endo.138.3.5023. [DOI] [PubMed] [Google Scholar]
- 26.Cordier-Bussat M, Bernard C, Levenez F, Klages N, Laser-Ritz B, Philippe J, Chayvialle JA, Cuber JC. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes. 1998;47:1038–45. doi: 10.2337/diabetes.47.7.1038. [DOI] [PubMed] [Google Scholar]
- 27.Gevrey JC, Malapel M, Philippe J, Mithieux G, Chayvialle JA, Abello J, Cordier-Bussat M. Protein hydrolysates stimulate proglucagon gene transcription in intestinal endocrine cells via two elements related to cyclic AMP response element. Diabetologia. 2004;47:926–36. doi: 10.1007/s00125-004-1380-0. [DOI] [PubMed] [Google Scholar]
- 28.Dhanvantari S, Izzo A, Jansen E, Brubaker PL. Coregulation of glucagon-like peptide-1 synthesis with proglucagon and prohormone convertase 1 gene expression in enteroendocrine GLUTag cells. Endocrinology. 2001;142:37–42. doi: 10.1210/endo.142.1.7870. [DOI] [PubMed] [Google Scholar]
- 29.Mukundan NE, Flanders PC, Constantinidis I, Papas KK, Sambanis A. Oxygen consumption rates of free and alginate entrapped bTC3 mouse insulinoma cells. Biochem Biophys Res Commun. 1995;210:113–118. doi: 10.1006/bbrc.1995.1634. [DOI] [PubMed] [Google Scholar]
- 30.Papas KK, Jarema MA. Glucose-stimulated insulin secretion is not obligatorily linked to an increase in O2 consumption in betaHC9 cells. Am J Physiol. 1998;275:E1100–6. doi: 10.1152/ajpendo.1998.275.6.E1100. [DOI] [PubMed] [Google Scholar]
- 31.Loser P, Jennings GS, Strauss M, Sandig V. Reactivation of the previously silenced cytomegalovirus major immediate-early promoter in the mouse liver: involvement of NFkappaB. J Virol. 1998;72:180–90. doi: 10.1128/jvi.72.1.180-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


