Abstract
Scaffolds that better approximate the mechanical properties of cardiovascular and other soft tissues might provide a more appropriate mechanical environment for tissue development or healing in vivo. An ability to induce local angiogenesis by controlled release of an angiogenic factor, such as basic fibroblast growth factor (bFGF), from a biodegradable scaffold with mechanical properties more closely approximating soft tissue could find application in a variety of settings. Toward this end biodegradable poly(ester urethane)urea (PEUU) scaffolds loaded with bFGF were fabricated by thermally induced phase separation. Scaffold morphology, mechanical properties, release kinetics, hydrolytic degradation and bioactivity of the released bFGF were assessed. The scaffolds had interconnected pores with porosities of 90% or greater and pore sizes ranging from 34–173 μm. Scaffolds had tensile strengths of 0.25–2.8 MPa and elongations at break of 81–443%. Incorporation of heparin into the scaffold increased the initial burst release of bFGF, while the initial bFGF loading content did not change release kinetics significantly. The released bFGF remained bioactive over 21 days as assessed by smooth muscle mitogenicity. Scaffolds loaded with bFGF showed slightly higher degradation rates than unloaded control scaffolds. Smooth muscle cells seeded into the scaffolds with bFGF showed higher cell densities than for control scaffolds after 7 days of culture. The bFGF-releasing PEUU scaffolds thus exhibited a combination of mechanical properties and bioactivity that might be attractive for use in cardiovascular and other soft tissue applications.
Keywords: biodegradation, polyurethane, basic fibroblast growth factor, elastomer, smooth muscle cells
1. Introduction
A common approach in tissue engineering is to combine a highly porous scaffold, made from a biodegradable polymer, with cells having the potential to develop into the tissue of interest and then implanting this construct for development in situ. The importance of matching the biomechanical properties of the scaffold with the target or adjoining tissue is increasingly appreciated since such matching might provide appropriate stress transfer to the developing tissue. This should be the case for both tissues that will be dynamically cultured in vitro, or where constructs are placed for development in situ. [1–4] A number of research groups have thus been interested in developing biodegradable polymers and scaffolds with mechanical properties appropriate for application in the engineering of soft tissues.[5–8] In addition to appropriate mechanical properties other biofunctionality would be attractive for such scaffolds, for instance the controlled release of growth factors to stimulate local angiogenesis toward the developing tissue.
A major concern for implanted tissue constructs is that the implant will not develop adequate vascularization for long-term survival. Both mass transfer limitations and fibrosis associated with the foreign body response can contribute to implant necrosis. [9] Although certain features of the foreign body response to the implanted material could lead capillary ingrowth over time, this native angiogenic process is likely to be either too slow or result in a capillary density that is too low to provide sufficient nutrient transport for most cells in constructs approximately one mm thick and greater. [10–12] Stimulating the rapid formation of high density local vasculature is thus a topic of great interest in the tissue engineering community. Incorporation of angiogenic growth factors such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF), among others, into scaffolds for controlled release has been shown to promote local angiogenesis. [10, 13] There are many methods to incorporate growth factors into synthetic scaffolds, such as absorbing growth factor to the scaffold, [14] blending growth factor containing microspheres into the scaffold, [10, 15] or directly mixing growth factor containing protein powder into the scaffold during processing. [16, 17] Adsorbing growth factor onto the scaffold has the drawback of low loading efficiencies and rapid release. Loading growth factor into microspheres can be associated with a loss in bioactivity due to harsh solvents such as methylene chloride.[18] Incorporating growth factor directly into the scaffold can potentially avoid these shortcomings. In particular, a thermoplastic scaffold would be amenable to the mixing of growth factor during polymer processing provided an appropriately mild solvent or low processing temperature could be employed.
The objective of this work was to develop a biodegradable, elastomeric poly(ester urethane)urea (PEUU) scaffold capable of releasing bioactive bFGF over a period of several weeks. It is hypothesized that such a bFGF-loaded scaffold would be attractive for use in soft tissue engineering applications due to its mechanical properties combined with an ability to stimulate local angiogenesis. Thermally induced phase separation was used to prepare a highly porous scaffold and a bFGF-containing protein complex was directly loaded into the polymer during scaffold preparation. Heparin, known to stabilize bFGF by inducing a conformational change in the molecule and increasing its resistance to denaturation [19–21] was examined as an additive, while bovine serum albumin (BSA) was used as a stabilizing protein. Scaffold morphology, mechanical properties, bFGF release kinetics and bioactivity, scaffold degradation and in vitro cell culture within the scaffold were investigated.
2. Materials and methods
2.1. Polyurethane synthesis
PEUU, based on polycaprolactone diol (PCL, MW 2000, Aldrich), butyldiisocyanate (BDI, Fluka) and putrescine (Aldrich), was synthesized using a two-step solution polymerization. [22] The synthesis was carried out in a 250 mL three-necked round bottom flask. The stoichiometry of the reaction was 2:1:1 of BDI: PCL: putrescine. A 10 wt% solution of BDI in dimethyl sulfoxide (DMSO) was continuously stirred with a 10 wt% solution of diol in DMSO. Stannous octoate (Sigma) was then added. This mixture was allowed to react at 75°C for 3 h and cooled to room temperature. A solution of putrescine in DMSO was added to the prepolymer solution and the reaction was continued for 18 h. The polymer solutions were precipitated in distilled water. Polymers were dried under vacuum at 50°C for 24 h.
2.2. Preparation of bFGF loaded PEUU scaffolds
PEUU scaffolds were processed by a thermally induced phase separation method as described previously. [6] DMSO was used as the solvent porogen. PEUU was dissolved in DMSO at 80°C to form an 8 wt% solution that was injected into a 10 mm inner diameter glass cylinder mould equipped with two rubber stoppers. The mould was placed in a freezer at −80°C for 3 h. The mould was then placed in absolute alcohol at 4°C for 7 days to extract the DMSO. The resulting cylindrical scaffold was air dried at 4°C for 3 days. To prepare tubular scaffolds, a mould comprised of two glass tubes was used. The outer glass tube had a 10 mm inner diameter and the inner glass tube had a 5 mm outer diameter. The polymer solution was injected into the space between two glass tubes and processing proceeded as before.
To fabricate bFGF loaded scaffolds, bFGF stabilized with bovine serum albumin (BSA), and in some instances heparin, was added during the process. bFGF (50 or 200 μg, PeproTech Inc.) and BSA (2.5 mg), with or without heparin (200 μg, Sigma) and with or without replacement of a fraction (<0.11%) of the bFGF with 25 uCi I125-bFGF (Perkin-Elmer), were dissolved in 5 mL phosphate buffered saline (PBS, pH=7.4) and lyophilized. The powder was suspended in 1.25 mL DMSO at room temperature and vortexed for 2 min. The resulting particles had diameters of 0.5±0.4 μm and 0.3±0.2 μm for BSA with and without heparin respectively (p>0.10) as measured with scanning electron microscopy. The suspension was then mixed with the 5 mL 10 wt% polymer solution (containing 500 mg of PEUU) for 1 min at 80°C and injected into the appropriate mould for scaffold formation. The final bFGF concentrations were 100 and 400 ng/mg PEUU respectively. These concentrations were selected based on previous reports from the literature [23].
2.3 bFGF release kinetics
The release kinetics of bFGF were measured for I125-bFGF containing PEUU scaffolds. Dulbecco’s modified Eagle medium (DMEM) supplemented with 1% penicillin/streptomycin and 0.5% fetal bovine serum (FBS) was used as a release medium. [23] Scaffolds loaded with 100 ng bFGF/mg PEUU with or without heparin were cut into 50 mg pieces. Each sample was placed into a 15 mL conical tube and 10 mL release medium was added. These studies were conducted in a 37°C water bath. The release medium was collected at predetermined time intervals for assaying and replaced with fresh release medium. The concentration of I125-bFGF in the sample was measured by a βcounter (LS 1800, Beckman Coulter).
2.4 Bioactivity of released bFGF
The biological activity of released bFGF was assessed in terms of its ability to stimulate the growth of smooth muscle cells. [24] Scaffolds loaded with 100 ng bFGF /mg PEUU (without I125-bFGF) were cut into 50 mg pieces. Samples were placed into a 15 mL conical tube and 10 mL release medium was added. Release studies were conducted in a 37°C water bath. Release medium was collected at predetermined time intervals and frozen at −20°C. Fresh release medium was then added to the samples to continue the release study. Scaffolds loaded with heparin and BSA, but without bFGF, were created and used for control purposes in these studies.
Rat vascular smooth muscle cells were isolated according to the method of Ray et al. [25] Cells were cultured in culture medium (DMEM supplemented with 10% FBS) to the fifth passage and seeded into 96-well tissue culture plates at a density of 1.5 x 105/mL. After incubation for 24 h, the culture medium was removed and replaced with the release medium collected periodically from the incubating scaffold samples. The smooth muscle cells were then cultured in this release medium for 48 h. A colorimetric mitochondrial activity assay (MTT) was utilized to quantify relative cell viability. [6] For comparison purposes, release media with or without the addition of 1 ng/mL bFGF were used as controls, as was release medium that had been incubated with scaffolds identical to the bFGF-loaded scaffolds, but lacking only bFGF.
The stimulatory effect of bFGF in release medium on rat smooth muscle cell growth was calibrated by growing these cells in culture media containing different bFGF concentrations. The cells were seeded into the wells of a 96-well tissue culture plate at a density of 1.5x105/mL. After 24 h incubation, the culture medium was removed and replaced with culture media containing different concentrations of bFGF. The smooth muscle cells were then cultured for an additional 48 h and cell viability was evaluated by MTT assay.
2.5 Scaffold characterization
Scaffold surface and cross-sectional morphology were visualized with scanning electron microscopy (SEM). Pore sizes were calculated from SEM images by Image J software (National Institutes of Health). Scaffold porosity was determined by a liquid displacement method [26, 27]. Ethanol was used as the displacement liquid. A scaffold sample was placed in a 10 mL cylinder containing a defined volume of ethanol (V1). The sample was then pressed to force air out of the scaffold until no further air bubble release was possible. The total volume of ethanol and the ethanol impregnated scaffold was recorded as V2. The ethanol impregnated scaffold was removed from the cylinder and the residual ethanol volume was recorded as V3. The porosity of the scaffold was expressed as:
PEUU scaffold tensile properties were measured for cylindrical scaffolds cut in the transverse direction, to make a disc, and then cutting the disc into a rectangular shape. For tubular scaffolds strips were cut in the longitudinal direction. Testing was conducted in an ATS testing machine equipped with a 5 lb load cell. A cross-head speed of 10 mm/min was applied. Four samples were evaluated for each type of scaffold.
2.6 Scaffold degradation
Scaffold degradation was quantified in terms of dry weight change. Dry scaffolds were weighed (w1) and immersed in a conical tube containing 10 mL phosphate buffered saline (PBS, pH=7.4). The degradation was conducted at 37°C in a water bath. Samples were taken at intervals, rinsed with water, dried in a vacuum oven for 2 days at 50°C and weighed (w2), after which they were discarded. The weight remaining was calculated as:
2.7 Smooth muscle cell seeding
Rat smooth muscle cells were seeded in scaffolds with or without 100 ng bFGF/mg PEUU to evaluate the ability of the scaffolds to support cell growth. Cells at the fifth passage were used. Cylinder scaffolds were cut in the transverse direction to form discs with a thickness of 0.5 mm. The scaffolds were sterilized by UV irradiation for 30 min in a laminar flow hood. Cells at a density of 2 x106 cells/mL were seeded into scaffolds using the filtration cell seeding method [28]. The seeded samples were then cultured in a 24-well polystyrene tissue culture plate with DMEM supplemented with 10% FBS as culture medium. The culture medium was replaced every second day. Cell viability at 7 days in the construct was evaluated with the MTT mitochondrial assay (n =4). The cell morphology on the construct surface was examined by SEM. For histological analysis, samples were frozen in 2-methylbutane and sectioned (15 μm thickness), and stained with hematoxylin and eosin (H&E) for cell visualization.
2.8 Statistical methods
Data are expressed as mean ± standard deviation. Statistical analyses were performed by ANOVA with post hoc Neuman–Keuls testing of specific differences at times of interest. For scaffold degradation data, scaffolds with and without bFGF were compared with repeated measures ANOVA to evaluate the effect of time and bFGF on weight loss.
3. Results
3.1 Scaffold morphology
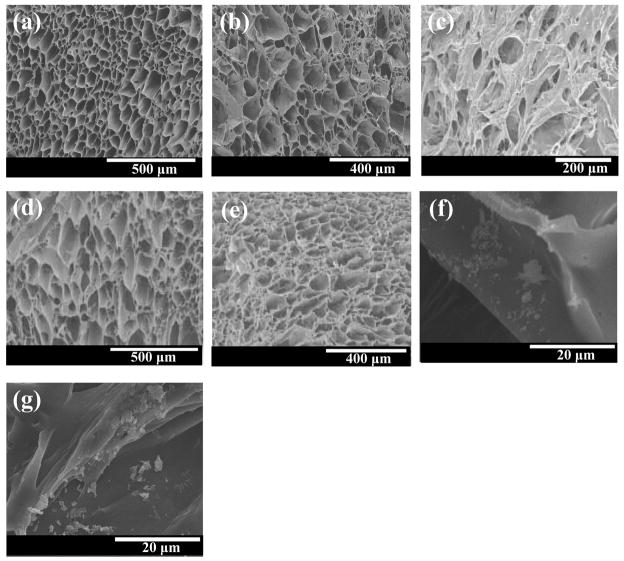
Both tubular and cylindrical PEUU scaffolds with and without bFGF were found to have porosities equal to or greater than 90% (Table I). Figure 1 presents cross-sectional and surface morphologies of the scaffolds, which were found to have open and inter-connected pores. Scaffolds without bFGF (Figure 1a and d) were seen to have more regular pores with the diameters ranging from 34 to 173 μm. The bFGF loaded scaffolds appeared to have slightly different morphologies (Figure 1b and e) with pores appearing to be less regular than the unloaded scaffolds. The tubular scaffold surface was relatively denser than the cross section and possessed smaller pores (Figure 1c). High magnification images of cylindrical scaffold pore walls (Figure 1f and g) show protein particles distributed on the surfaces.
Table I.
Scaffold porosities and pore sizes
| Scaffold | Porosity (%) |
Pore size (μm) |
Pore size range (μm) |
|---|---|---|---|
| PEUU(Tubular) | 90±5 | 87±20 | 60–126 |
| PEUU/BSA/bFGF (Tubular) | 90±3 | 101±31 | 45–159 |
| PEUU (Cylinder) | 93±1 | 95±81 | 34–173 |
| PEUU/BSA/Heparin/bFGF (Cylinder) | 95±2 | 97±27 | 49–163 |
Figure 1.
Electron micrographs of (a) tubular PEUU scaffold, cross-section; (b) tubular PEUU/bFGF scaffold, cross-section; (c) tubular PEUU/bFGF, surface; (d) cylindrical PEUU scaffold, cross-section; (e) cylindrical PEUU/bFGF scaffold, cross-section; (f) PEUU/bFGF scaffold without heparin, high magnification cross-section, (g) PEUU/bFGF scaffold with heparin, high magnification cross-section. Protein particles are seen in (f) and (g). Scale bars=500 μm in (a) and (d), 400 μm in (b) and (e), 200 μm in (c), 20 μm in (f) and (g).
3.2 Release of bFGF from PEUU scaffold
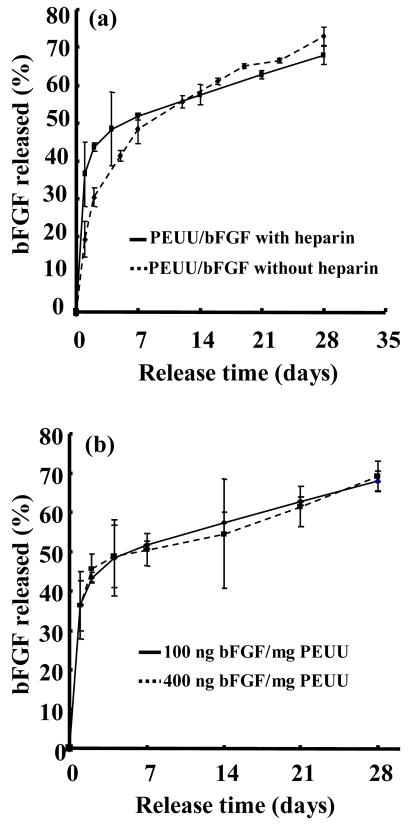
Figure 2a presents the bFGF release profile, as determined by radiolabeled bFGF, from scaffolds with or without heparin. For these experiments the bFGF amount was fixed at 100 ng/mg PEUU and the BSA amount was also constant. Both scaffolds showed two-stage release behavior, in that there was an initial fast release followed by slow release over a period of 4 weeks. Incorporation of heparin altered the kinetics of the initial release period. Scaffolds with heparin exhibited greater bFGF release over the first two days relative to scaffolds without heparin (p<0.04 for day 1 and p<0.01 for day 2). An initial release of 19% was seen for scaffolds without heparin during the first 24 hours, with an additional 11% between days 1 and 2. Scaffolds with heparin released 37% of the bFGF during the first 24 hours and an additional 6% between days 1 and 2. Both scaffolds had similar slow release kinetics after 7 days.
Figure 2.
Release profile of bFGF from PEUU scaffolds. (a) Effect of heparin content on the bFGF release kinetics. (b) Effect of bFGF content on the release kinetics.
Figure 2b presents the release behavior of scaffolds loaded with bFGF at two different concentrations, but with BSA and heparin concentrations held constant. No significant differences were found in the release curves between the two loading doses at any of the measured time points.
3.3 Bioactivity of released bFGF
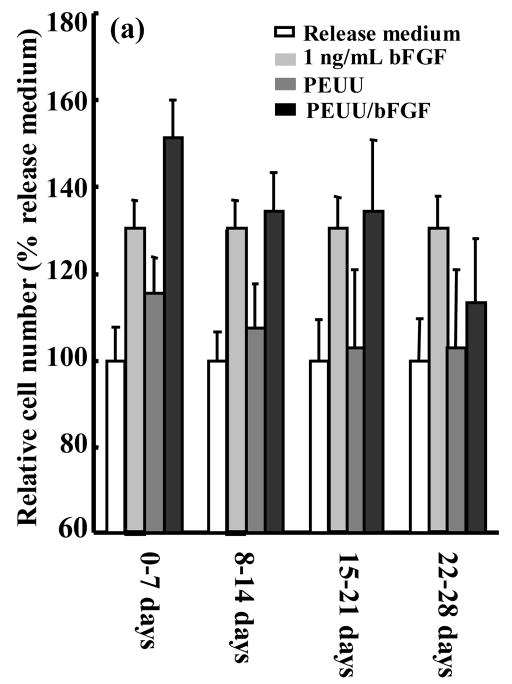
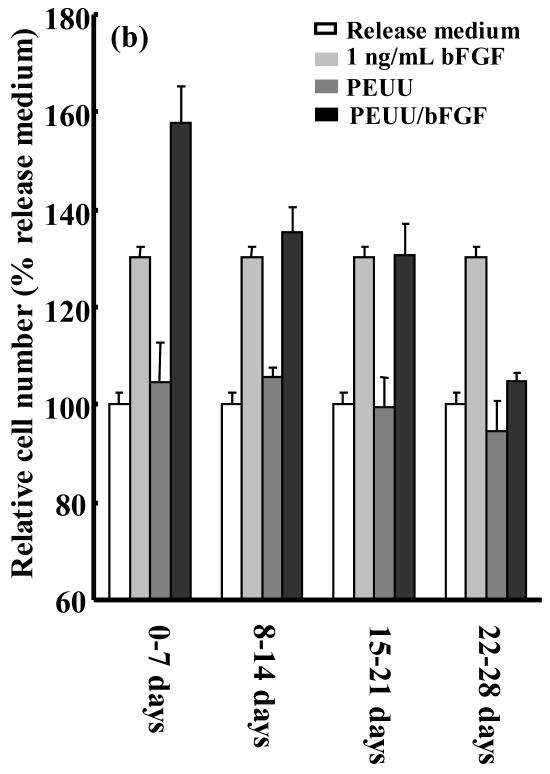
A bioactivity assay for released bFGF was employed that quantified rat smooth muscle cell proliferation after 48 hours incubation in the release medium. To examine the approximate dose response of this proliferation assay to a range of bFGF concentrations, a calibration curve (Figure 3) was generated. The bioactivity of bFGF released from scaffolds into the incubating medium was assessed after 1, 2, 3 and 4 weeks. Release medium (not incubated with a scaffold) to which 1 ng/mL bFGF was added served as a positive control since this concentration showed an effect in Figure 3. Release medium alone as well as release medium incubated with PEUU scaffolds not containing bFGF served as negative controls. Figure 4a and b present the temporal bioactivity data for bFGF released from scaffolds without and with heparin, respectively. The release media from both scaffolds loaded with bFGF showed significant (p<0.001) bioactivity over the first three weeks relative to PEUU scaffolds without bFGF and release medium alone. Release media from both bFGF scaffolds collected from the fourth week did not show significantly increased bioactivity relative to the negative control samples. The highest relative cell numbers resulted from release media incubated with bFGF loaded scaffolds in the first week, in agreement with the radiolabeled bFGF data. Cell numbers for release media incubated with bFGF loaded scaffolds with or without heparin showed no significant differences at any of the four time points.
Figure 3.
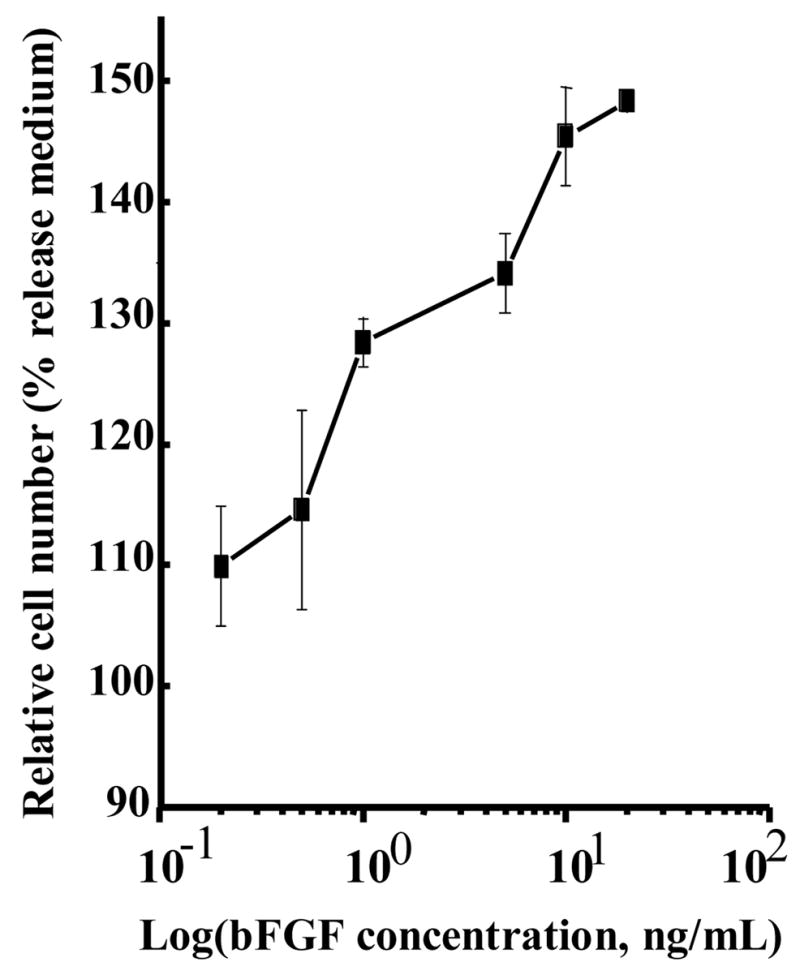
Effect of bFGF dose on the rat smooth muscle cell growth. The error bars for the final concentration point were not greater than the square used to denote that point.
Figure 4.
Bioactivity of bFGF released from (a) PEUU/bFGF scaffold without heparin and (b) PEUU/bFGF scaffold with heparin. Release medium alone, release medium with 1 ng/mL bFGF added and release medium from PEUU scaffolds without bFGF serve as controls.
3.4 Scaffold mechanical properties
Tubular PEUU scaffolds without and with bFGF had respective tensile strengths of 2.8 and 2.7 MPa and elongations at break of 443% and 272% (Table II). The reduction in breaking strain was significant (p<0.01) while the tensile strength differences were not. For cylindrical scaffolds, where disc cross-sections were cut for mechanical testing, the PEUU scaffolds without and with bFGF had respective tensile strengths of 0.28 and 0.25 MPa and elongations at break of 81% and 95%. Here again the tensile strengths were similar, but the breaking strain for the bFGF loaded scaffold in this case was significantly greater (p<0.05).
Table II.
Scaffold mechanical properties
| Scaffold | Cylindrical |
Tubular |
||
|---|---|---|---|---|
| w/o bFGF | w/bFGF | w/o bFGF | w/bFGF | |
| Tensile strength (MPa) | 0.28±0.02 | 0.25±0.03 | 2.83±0.25 | 2.66±0.36 |
| Elongation at break (%) | 81±14 | 95±15 | 443±38 | 272±36 |
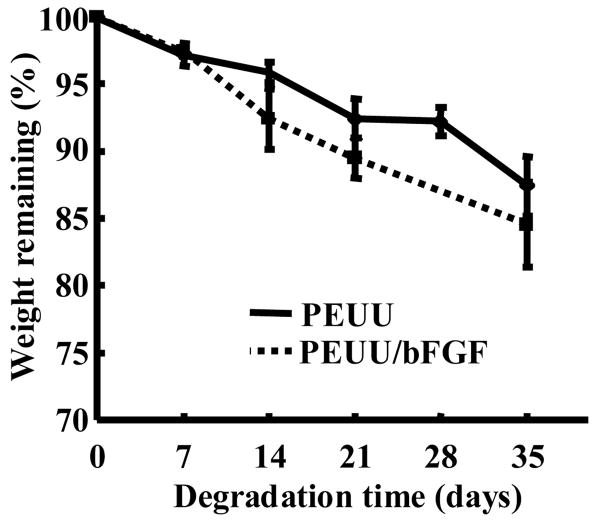
3.5 Scaffold degradation
Scaffold degradation curves in terms of mass loss are shown in Figure 5. PEUU and PEUU/bFGF scaffolds both exhibited progressive mass loss during the 5-week degradation period (p<0.01). There was a significant difference between scaffolds with and without bFGF in terms of weight loss over the 35 day period (p<0.05), with bFGF loaded scaffolds degrading significantly faster.
Figure 5.
Effect of biodegradation time on the weight remaining for PEUU and PEUU/bFGF scaffolds.
3.6 Smooth muscle cell growth in scaffolds
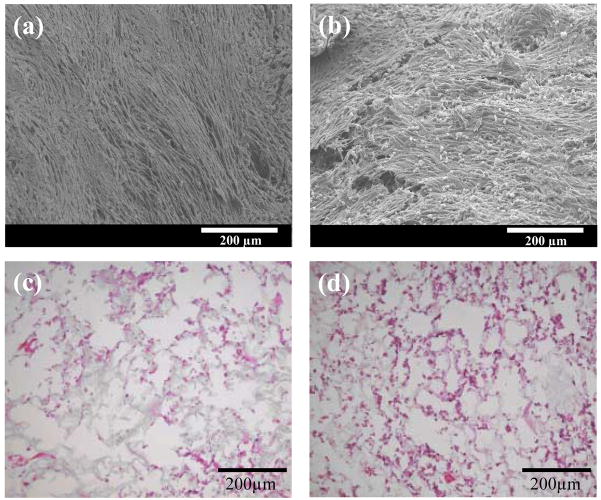
After 7 days of culture, the relative number of rat smooth muscle cells in the PEUU/bFGF scaffold was found to be significantly higher than in unloaded PEUU scaffolds (12±2 vs. 9.7±0.8; p<0.04). Electron micrographs (Figure 6a and b) showed that cells on both scaffold surfaces formed dense confluent layers after 7 days of culture. Qualitative examination of cells within both scaffolds at 7 days, as shown by H&E staining images from the scaffold interior sections (Figure 6c and d), appears to confirm a slightly higher smooth muscle cell density in the PEUU/bFGF scaffolds.
Figure 6.
Surface electron micrographs (a and b) and hematoxylin and eosin (H&E) staining (c and d) of vascular smooth muscle cells seeded in PEUU (a and c) and PEUU/bFGF (b and d) scaffolds after 7 days of culture. The H&E images are taken from the interior of the scaffolds. Scale bar in (a) and (b) are 200 μm.
4. Discussion
The angiogenic effect of bFGF has been widely utilized to stimulate blood vessel formation in vivo to improve the function of diseased tissue or increase the survival of transplanted cells by enhanced nutrient transport [5, 29, 30]. A drug delivery system is often employed to deliver bioactive bFGF over a prolonged period at the target site since bFGF has a short half-life in vivo. Various natural and synthetic matrices have been investigated as carriers for bFGF. Natural materials include gelatin [31, 32], collagen [33], fibrin [34], heparin [35] and alginate. [36] Synthetic matrices have been generated from poly(ethylene glycol)-g-poly(lactic acid) [37], poly(lactic acid) [38], poly(lactic-co-glycolic acid) [39,40], and methylidene malonate polymers. [41] From a mechanical perspective, these scaffolds have ranged from relatively weak materials (e.g. some of the natural and synthetic hydrogels) to relatively stiff polyester matrices. In this report we examined the ability of a thermoplastic elastomer system to provide controlled release of bFGF after being formed into a scaffold possessing open pores.
Applying the thermally induced phase separation technique allowed the thermoplastic PEUU to be processed into highly porous scaffolds and the blending of biomacromolecules. Incorporating BSA, heparin and bFGF did not substantially alter the scaffold morphology. High scaffold porosity, combined with good tensile strength, was deemed an attractive feature in that cell placement across the scaffold could readily be achieved and mass transport and cellular or vascular ingrowth might be facilitated by such a highly porous structure. With average pore sizes larger than 87 μm, vascular ingrowth should be facilitated since pore sizes greater than 50 μm have been considered to be essential for in vivo vascularization, although others have suggested that a pore size of 35 um is optimal.[10, 42]
Two scaffold shapes were prepared in this study, a tubular form that could be appropriate for tissue engineering blood vessels or other conduit structures, and a cylinder from which discs were cut that might be applied as patches in, for instance, cardiac applications. The tubular scaffolds were found to be flexible with tensile strengths higher than 2.7 MPa and breaking strains higher than 272%. The tensile strengths were comparable to that of the human coronary artery (1.4 MPa), [43] while the breaking strains were greater than that of human femoral and popliteal arteries (about 90%). [44] The patches from the cylindrical scaffolds had tensile strengths greater than 0.25 MPa and breaking strains exceeding 81%. The breaking strain was comparable to that of the rat heart (approximately 75%) while the tensile strength was six-fold higher than the rat heart (approximately 0.04 MPa). [45] The difference in mechanical properties for the tubular and cylindrical scaffolds can be attributed to differences in the surface structure. The tubular scaffolds had outer and inner surfaces with small pores (Figure 1c). This effectively decreased the void volume at the surfaces, increasing the mechanical properties. The discs derived from the cylindrical scaffold did not have this skin effect on their upper and lower surfaces and were thus weaker. The discs could be attractive for applications where higher surface porosity is valued to foster more rapid migration of cells into the scaffold, and a decrease in the tensile strength is acceptable.
The in vitro degradation studies demonstrated that the scaffolds degraded at rates that might be attractive for tissue engineering applications. Scaffolds loaded with BSA, heparin and bFGF degraded slightly faster than those without protein. The increased degradation rate probably resulted from higher water absorption of the scaffold. The biphasic bFGF release from the PEUU scaffolds was likely due to diffusion mechanisms. This is almost certainly true for the early burst phase. For the second phase, PEUU degradation is occurring concurrently and likely contributing to the diffusion of bFGF from the scaffold. The mass loss is relatively low in the first 5 weeks, but an earlier study has shown that the PEUU molecular weight is decreasing at a faster rate in this period. [22] Such PEUU degradation might thus be contributing to the bFGF release. Scaffolds with heparin experienced a higher initial burst release than those without heparin. This was attributed to the increased hydrophilicity associated with heparin incorporation and, perhaps also to the affinity of bFGF for more rapidly solubilizing heparin molecules. A similar trend has been reported for fibrin gels loaded with VEGF and heparin. [46] The initial bFGF loading amount did not change the release kinetics significantly at the concentrations studied. Since there was an excess of BSA and heparin employed to stabilize the bFGF, the increase in the bFGF content relative to the total protein/heparin added was small and thus a minimal effect on the protein release kinetics was observed.
The burst release observed for the PEUU scaffolds might be reduced by refining the drug loading method. For this study the protein solution was lyophilized and broken into a free-flowing powder. By generating smaller powder particles (e.g. with sonication) the burst might be reduced since smaller particles might be more readily encapsulated into the scaffold walls.[39] A second approach might be to form a bi-phasic system where the bFGF is encapsulated into nano- or micro-scale biodegradable particles and these particulates are incorporated into the scaffold. The release kinetics would then be mainly controlled by the degradation of the microsphere polymer. [10] One could theoretically tailor the polymer degradation rate, molecular weight, composition, particle size and other parameters to achieve desired release profiles. A second particulate phase might have implications for scaffold mechanical properties, however, and increasingly small particulate additives would be increasingly likely to exhibit quick protein release. The optimal bFGF release profile for in vivo application is not clear and it is likely that the shape of such a curve would vary depending upon the tissue bed under consideration and its status (i.e. ischemic, infarcted, healthy). The expression of bFGF receptors is a key consideration. In examining native bFGF expression profiles in response to traumatic injury in the central nervous system for example, it has been reported that bFGF expression in terms of immunoreactive bFGF and bFGF mRNA are maximal at about one week post-injury and the expression of bFGF receptors appears to peak within 1–2 weeks. [47]
The bFGF released from the PEUU scaffolds with or without heparin was shown to be bioactive for a period of 21 days as demonstrated by a smooth muscle cell proliferation assay. After 21 days no significant bioactivity was detected by the assay. The assay employed appeared to be appropriate to detect bioactivity differences in the ranges studied. There may have been further activity that was undetectable at lower levels due to the variability associated with this type of assay, or alternatively, the lack of bioactivity after 3 weeks was due to bFGF denaturation in the scaffold over time although the amount of bFGF released during days 22–28 is comparable to that released during days 15–21. bFGF has been shown to induce the proliferation of endothelial cells, fibroblasts and smooth muscle cells. [48–50] For angiogenesis stimulation, heparin is often added to induce endothelial cell growth. [51, 52] However, high concentrations of heparin inhibit the proliferation of smooth muscle cells. [53] The bFGF bioactivity from PEUU scaffolds without heparin indicated that commonly-used growth factor stabilizing protein BSA [20] alone could stabilize the bFGF and maintain smooth muscle cell mitogenicity. The incorporation of heparin may further stabilize the bFGF, but our data did not suggest such stabilization. Furthermore, there was no evidence that at the concentrations of heparin investigated smooth muscle cell growth was inhibited.
The release of bFGF was strongly dependent on the heparin incorporation. A 39% initial burst release was observed for the heparin containing release system whereas 19% was observed for the non-heparin containing system. The release profile of bFGF from heparin containing PEUU scaffolds was found to be similar to that from PLGA scaffolds and methylidene malonate polymer. A bFGF loaded PLGA scaffold fabricated using supercritical CO2 exhibited 45% initial burst release in one day. [39, 40] The release of bFGF from methylidene malonate polymer films had over 30% initial burst release in the first day. [41] In both of these studies the bioactivity of the released bFGF was verified with cellular mitogenicity assays similar to those employed in this study. Earlier work with bFGF loaded PLGA scaffolds suggested that an inappropriately harsh solvent could lead to substantial loss of growth factor bioactivity. [40] In this work, DMSO was used due to its relative high melting point (18°C) and lower toxicity relative to other solvents such as methylene chloride, a commonly used solvent for preparation of growth factor loaded microspheres. Moreover, DMSO is commonly used in cell preservation procedures.
Experiments where smooth muscle cells were filtration seeded and cultured on scaffolds with or without bFGF demonstrated the ability to seed cells relatively evenly across the scaffolds and also showed higher cell numbers for bFGF loaded scaffolds after a 7 day culture period. This effect was attributed to the release of bioactive bFGF. The MTT assay utilized was a metabolic assay, as opposed to a pure quantification of viable cell numbers; however, images of scaffold sections supported the conclusion of higher cell numbers. A primary reason for endowing a scaffold with the ability to release bFGF would be to impact local angiogenesis in vivo. Such an investigation would be necessary to further demonstrate the value of the current system. It is speculated that in vivo one might find that the bFGF release kinetics are accelerated due to an increased degradation rate, and that both the degradation and bFGF release kinetics would vary based on the internal location of scaffold placement and factors such as the local mechanical environment.
In summary we have demonstrated the development of biodegradable, porous polyurethane scaffolds that have the ability to release bioactive bFGF over a three week period in vitro. These scaffolds have mechanical properties attractive for future application in soft tissue engineering and might provide a means to deliver a cell-seeded construct that can stimulate local angiogenesis, although further studies will need to be performed to evaluate in vivo functionality.
Acknowledgments
This work was funded through the NIH, grant #HL069368.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 2.Hoerstrup SP, Zund G, Sodian R, Schnell AM, Grunenfelder J, Turina MI. Tissue engineering of small caliber vascular grafts. Eur J Cardiothorac Surg. 2001;20:164–169. doi: 10.1016/s1010-7940(01)00706-0. [DOI] [PubMed] [Google Scholar]
- 3.Tiwari A, Salacinski HJ, Punshon G, Hamilton G, Seifalian AM. Development of a hybrid cardiovascular graft using a tissue engineering approach. FASEB J. 2002;16:791–796. doi: 10.1096/fj.01-0826com. [DOI] [PubMed] [Google Scholar]
- 4.Opitz F, Schenke-Layland K, Richter W, Martin DP, Degenkolbe I, Wahlers T, Stock UA. Tissue engineering of ovine aortic blood vessel substitutes using applied shear stress and enzymatically derived vascular smooth muscle cells. Ann Biomed Eng. 2004;32:212–222. doi: 10.1023/b:abme.0000012741.85600.f1. [DOI] [PubMed] [Google Scholar]
- 5.Guan J, Wagner WR. Synthesis, characterization and cytocompatibility of polyurethaneurea elastomers with designed elastase sensitivity. Biomacromolecules. 2005;6:2833–2842. doi: 10.1021/bm0503322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan J, Fujimoto KL, Sacks MS, Wagner WR. Preparation and characterization of highly porous. biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26:3961–3971. doi: 10.1016/j.biomaterials.2004.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Ameer G, Sheppard B, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Webb A, Ameer GA. Novel citric acid-based biodegradable elastomers for tissue engineering. Advanced Materials. 2004;16:511–516. [Google Scholar]
- 9.Dee KC, Puleo DA, Bizios R. An introduction to tissue-biomaterial interactions. Hoboken: John Wiley & Sons; 2002. pp. 127–147. [Google Scholar]
- 10.Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S. Enhancing the vasularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J Biomed Mater Res. 2003;65A:489–497. doi: 10.1002/jbm.a.10542. [DOI] [PubMed] [Google Scholar]
- 11.Radisic M, Euloth M, Yang L, Langer R, Freed L, Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotech Bioeng. 2003;82:403–414. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 12.Mikos AG, Sarakinos G, Ingber DE, Vacanti JP, Langer R. Prevascularization of porous biodegradable polymers. Biotech Bioeng. 1993;42:716–723. doi: 10.1002/bit.260420606. [DOI] [PubMed] [Google Scholar]
- 13.Mooney DJ, Kaufmann PM, Sano K, McNamara KM, Vacanti JP, Langer R. Transplantation of hepatocytes using porous, biodegradable sponges. Transplant P. 1994;26:3425–3426. [PubMed] [Google Scholar]
- 14.Ziegler J, Mayr-Wohlfart U, Kessler S, Breitig D, Gunther KP. Adsorption and release properties of growth factors from biodegradable implants. J Biomed Mater Res. 2002;59:422–428. doi: 10.1002/jbm.1258. [DOI] [PubMed] [Google Scholar]
- 15.Wei GB, Jin QM, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J Control Release. 2006;112:103–110. doi: 10.1016/j.jconrel.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521–2527. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 17.Hile DD, Pishko MV. Solvent-free protein encapsulation within biodegradable polymer foams. Drug Delivery. 2004;11:287–293. doi: 10.1080/10717540490493961. [DOI] [PubMed] [Google Scholar]
- 18.King TW, Patrick CW. Development and in vitro characterization of vascular endothelial growth factor (VEGF)-loaded poly(DL-lactic-co-glycolic acid)/poly(ethylene glycol) microspheres using a solid encapsulation/single emulsion/solvent extraction technique. J Biomed Mater Res. 2000;51:383–390. doi: 10.1002/1097-4636(20000905)51:3<383::aid-jbm12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Gospodarowicz D, Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986;128:457–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- 20.Gospodarowicz D, Ferrara N, Schweigerer L, Neufeld G. Structural characterization and biological function of fibroblast growth factor. Endocr Rev. 1987;8:95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- 21.Jeon O, Ryu SH, Chung JH, Kim BS. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J Control Release. 2005;105:249–259. doi: 10.1016/j.jconrel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Guan J, Sacks MS, Beckman EJ, Wagner WR. Synthesis, characterization and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J Biomed Mater Res. 2002;61:493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 23.Tanihara M, Suzuki Y, Yamamoto E, Noguchi A, Mizushima Y. Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate. J Biomed Mater Res. 2001;56:216–221. doi: 10.1002/1097-4636(200108)56:2<216::aid-jbm1086>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Bono F, Rigon P, Lamarche I, Savi P, Salel V, Herbert JM. Heparin inhibits the binding of basic fibroblast growth factor to cultured human aortic smooth-muscle cells. Biochem J. 1997;326:661–668. doi: 10.1042/bj3260661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci. 2002;23:185–188. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- 26.Zhang RY, Ma PX. Poly(α-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering, I. Preparation and morphology. J Biomed Mater Res. 1999;44:446–455. doi: 10.1002/(sici)1097-4636(19990315)44:4<446::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 27.Hsu YY, Gresser JD, Trantolo DJ, Lyons CM, Gangadharam PR, Wise DL. Effect of polymer foam morphology and density on kinetics of in vitro controlled release of isoniazid from compressed foam matrices. J Biomed Mater Res. 1997;35:107–116. doi: 10.1002/(sici)1097-4636(199704)35:1<107::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Ma T, Kniss DA, Lasky LC, Yang ST. Effects of filtration seeding on cell density, spatial distribution, and proliferation in nonwoven fibrous matrices. Biotechnol Progress. 2001;17:935–944. doi: 10.1021/bp0100878. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa K, Asonuma K, Inamoto Y, Tabata Y, Tanaka K. The efficacy of prevascularization by basic FGF for hepatocyte transplantation using polymer devices in rats. Cell Transplant. 2001;10:723–729. [PubMed] [Google Scholar]
- 30.Sakakibara Y, Nishimura K, Tambara K, Yamamoto M, Lu F, Tabata Y, Komeda M. Prevascularization with gelatin microspheres containing basic fibroblast growth factor enhances the benefits of cardiomyocyte transplantation. J Thorac Cardiovasc Surg. 2001;124:50–56. doi: 10.1067/mtc.2002.121293. [DOI] [PubMed] [Google Scholar]
- 31.Kawai K, Suzuki S, Tabata Y, Ikada Y, Nishimura Y. Accelerated tissue regeneration through incorpoartion of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials. 2000;21:489–499. doi: 10.1016/s0142-9612(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 32.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 33.Cote MF, Laroche G, Gagnon E, Chevallier P, Doillon CJ. Denatured collagen as support for a FGF-2 delivery system: physicochemical characterizations and in vitro release kinetics and bioactivity. Biomaterials. 2004;25:3761–72. doi: 10.1016/j.biomaterials.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 34.DeBlois C, Cote MF, Doillon CJ. Heparin–fibroblast growth factor–fibrin complex: In vitro and in vivo applications to collagen-based materials. Biomaterials. 1994;15:665–672. doi: 10.1016/0142-9612(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 35.Tanihara M, Suzuki Y, Yamamoto E, Noguchi A, Mizushima Y. Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate. J Biomed Mater Res. 2001;56:216–21. doi: 10.1002/1097-4636(200108)56:2<216::aid-jbm1086>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 36.Edelman ER, Mathiowitz E, Langer R, Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991;12:619–626. doi: 10.1016/0142-9612(91)90107-l. [DOI] [PubMed] [Google Scholar]
- 37.Maquet V, Martin D, Scholtes F, Franzen R, Schoenen J, Moonen G, Jerome R. Poly(D,L-lactide) foams modified by poly(ethylene oxide)-block-poly(D,L-lactide) copolymers and a-FGF: in vitro and in vivo evaluation for spinal cord regeneration. Biomaterials. 2001;22:1137–1146. doi: 10.1016/s0142-9612(00)00357-4. [DOI] [PubMed] [Google Scholar]
- 38.Isogai N, Morotomi T, Hayakawa S, Munakata H, Tabata Y, Ikada Y, Kamiishi H. Combined chondrocyte-copolymer implantation with slow release of basic fibroblast growth factor for tissue engineering an auricular cartilage construct. J Biomed Mater Res A. 2005;74:408–418. doi: 10.1002/jbm.a.30343. [DOI] [PubMed] [Google Scholar]
- 39.Hile DD, Pishko MV. Solvent-free protein encapsulation within biodegradable polymer foams. Drug Deliv. 2004;11:287–293. doi: 10.1080/10717540490493961. [DOI] [PubMed] [Google Scholar]
- 40.Hile DD, Amirpour ML, Akgerman A, Pishko MV. Active growth factor delivery from poly(D,L-lactide-co-glycolide) foams prepared in supercritical CO2. J Control Release. 2000;66:177–185. doi: 10.1016/s0168-3659(99)00268-0. [DOI] [PubMed] [Google Scholar]
- 41.Desire L, Mysiakine E, Bonnafous D, Couvreur P, Sagodira B, Breton P, Fattal E. Sustained delivery of growth factors from methylidene malonate 2.1.2-based polymers. Biomaterials. 2006;27:2609–2620. doi: 10.1016/j.biomaterials.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 42.Marshall AJ, Barker T, Sage EH, Hauch KD, Ratner BD. 7th World Biomaterials Congress. Sidney: 2004. Pore Size Controls Angiogenesis in Subcutaneously Implanted Porous Matrices; p. 710. [Google Scholar]
- 43.Valenta J. In: Clinical aspects of biomedicine. Jaroslav V, editor. Vol. 2. Amsterdam: Elsevier Science; 1993. pp. 142–179. [Google Scholar]
- 44.Evans FG. In: Strength of biological materials. Yamada H, editor. Baltimore: Williams & Wilkins; 1980. pp. 115–129. [Google Scholar]
- 45.Boublik J, Park H, Radisic M, Tognana E, Chen F, Pei M, Vunjak-Novakovic G, Freed LE. Mechanical properties and remodeling of hybrid cardiac constructs made from heart cells, fibrin, and biodegradable, elastomeric knitted fabric. Tissue Engineering. 2005;11:1122–1132. doi: 10.1089/ten.2005.11.1122. [DOI] [PubMed] [Google Scholar]
- 46.Logan A, Frautschy SA, Gonzalez AM, Baird A. A time course for the focal elevation of synthesis of basic fibroblast growth factor and one of its high-affinity receptors (flg) following a localized cortical brain injury. J Neurosci. 1992;12:3828–3837. doi: 10.1523/JNEUROSCI.12-10-03828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shireman PK, Greisler HP. Mitogenicity and release of vascular endothelial growth factor with and without heparin from fibrin glue. J Vasc Surg. 2000;31:936–943. doi: 10.1067/mva.2000.106420. [DOI] [PubMed] [Google Scholar]
- 48.Baird A, Walicke PA. Fibroblast growth factors. Br Med Bull. 1989;45:438–452. doi: 10.1093/oxfordjournals.bmb.a072333. [DOI] [PubMed] [Google Scholar]
- 49.Burgess WH, Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Ann Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- 50.Ghiselli G, Chen J, Kaou M, Hallak H, Rubin R. Ethanol inhibits fibroblast growth factor–induced proliferation of aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:1808–1813. doi: 10.1161/01.ATV.0000090140.20291.CE. [DOI] [PubMed] [Google Scholar]
- 51.Li LY, Safran M, Aviezer D, Bohlen P, Seddon AP, Yayon A. Diminished heparin-binding of a basic fibroblast growth-factor mutant is associated with reduced receptor-binding, mitogenesis, plasminogen-activator induction, and in-vitro angiogenesis. Biochemistry. 1994;33:10999–11007. doi: 10.1021/bi00202a020. [DOI] [PubMed] [Google Scholar]
- 52.Faham S, Hileman RE, Fromm JR, Linhardt RJ, Rees DC. Heparin structure and interactions with basic fibroblast growth factor. Science. 1996;271:1116–1120. doi: 10.1126/science.271.5252.1116. [DOI] [PubMed] [Google Scholar]
- 53.Bono F, Rigon P, Lamarche L, Savi P, Salel V, Herbert JM. Heparin inhibits the binding of basic fibroblast growth factor to cultured juman aortic smooth-muscle cells. Biochem J. 1997;326:661–668. doi: 10.1042/bj3260661. [DOI] [PMC free article] [PubMed] [Google Scholar]