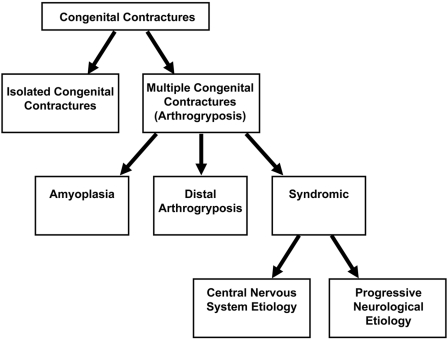
Congenital contractures can be divided into two groups: isolated contractures and multiple contractures (Fig. 1). Isolated congenital contractures affect only a single area of the body; the most common isolated contracture is congenital clubfoot, which occurs in one of every 500 live births1.
Fig. 1.
Types of congenital contractures.
The term arthrogryposis is often used as shorthand to describe multiple congenital contractures that affect two or more different areas of the body. Arthrogryposis is not a specific diagnosis, but rather a clinical finding, and it is a characteristic of more than 300 different disorders2,3. The overall prevalence of arthrogryposis is one in 3000 live births4. The inheritance, natural history, treatment guidelines, and outcomes of arthrogryposis vary among disorders, underscoring the importance of making a specific diagnosis in each child1,5-10. The purpose of this article is to present the current state of knowledge about the classification, etiology, and management of children with various types of arthrogryposis.
Classification of Arthrogryposis
To establish a differential diagnosis, it is important to first decide whether a child has normal neurological function. A normal neurological examination suggests that arthrogryposis is due to amyoplasia, a distal arthrogryposis, a generalized connective tissue disorder, or fetal crowding. In contrast, an abnormal neurological examination indicates that movement in utero was diminished as a result of an abnormality of the central or peripheral nervous system, the motor end plate, or muscle.
Amyoplasia
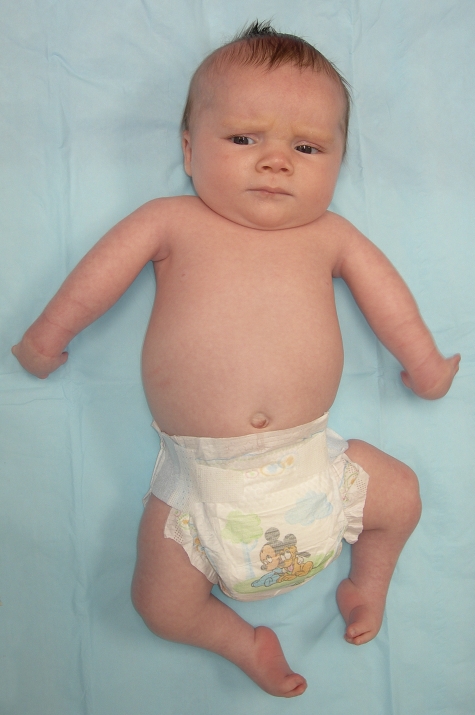
Amyoplasia (A = no; myo = muscle; plasia = growth) is a distinct form of arthrogryposis with characteristic clinical features as shown in Figure 2: the shoulders are usually internally rotated and adducted, the elbows are extended, the wrists are flexed and ulnarly deviated, the fingers are stiff, and the thumbs are positioned in the palm. In the lower limbs, the hips may be dislocated, the knees are usually extended, and the feet have severe equinovarus contractures. Many patients have a midfacial hemangioma. Most patients have normal intelligence. In one series, 10% of the patients had abdominal abnormalities such as gastroschisis or bowel atresia11. Clinical series10 have shown that 84% of the children have symmetric involvement of the upper and lower limbs; other variations of presentation include upper limb only, lower limb only, or asymmetric patterns of involvement5. In Hall's original description of 135 patients with amyoplasia12, all cases were sporadic. She also noted an increased prevalence in twins and in conditions that would lead to decreased limb movement, such as a bicornuate uterus, oligohydramnios, or intrauterine crowding.
Fig. 2.
Child with amyoplasia. In the upper extremity, the shoulders are internally rotated, the elbows are extended, the wrists are flexed and ulnarly deviated, the fingers are stiff, and the thumbs are positioned in the palm. In the lower limbs, the hips may be dislocated, the knees are extended, and the feet have severe equinovarus contractures.
The goals of initial treatment are to mobilize the joints, apply splints for improved position and function, and to provide physical and occupational therapy as well as instructions to the child's caregivers so that they may provide home therapy. Ongoing therapy services are part of most children's lives, with 80% of children with amyoplasia receiving therapy services into their teenage years5. Persistent limb deformities that restrict function are common and are often treated surgically. In one series of children with amyoplasia10, orthopaedic surgeries were performed on the feet in 76% of children; on the knees, in 39%; and on the hips, in 18%. The elbows were operated on in 24% of children; the wrists, in 16%; and the hands, in 8%; and 5% required spinal surgery.
The primary long-term goals of treatment of amyoplasia are increased joint mobility and muscle strength and the development of adaptive use patterns that allow for walking and independence with activities of daily living. Surgical intervention in the upper limb13-15 may be recommended for fixed joint contractures that preclude or interfere with upper-limb function. Surgery is rarely needed for shoulder contractures; however, if internal rotation of the shoulder specifically precludes functional positioning of the hand in space, an external humeral rotational osteotomy is indicated. An inability to reach the hand to the mouth due to an elbow extension contracture can be treated with a posterior capsulotomy of the elbow with triceps lengthening16,17. Multiple tendon transfers have been attempted to add active elbow flexion17-19. However, a recent study questioned the long-term result of elbow flexion transfers in this population20, and those authors recommended elbow capsulotomy without tendon transfer21. For the wrist, a dorsal carpal wedge osteotomy with a possible flexor carpi ulnaris tendon transfer to preserve the arc of motion but reposition the wrist in extension and neutral deviation has been reported12. Surgical correction of the thumb-in-palm deformity may improve thumb position for pincer function.
In summary, children with amyoplasia typically exhibit severe joint contractures with weakness of the muscles that are present. With multiple orthopaedic and rehabilitation interventions, the ability to walk and perform activities of daily living has been reported to be as high as 85%10. Nevertheless, children with amyoplasia usually require more surgical interventions than do children with any other type of arthrogryposis and, in adult life, the majority of individuals need assistance with activities of daily living5.
Distal Arthrogryposes
Distal arthrogryposes are a group of autosomal dominant disorders that mainly involve the distal parts of the limbs. Characterization of the genetic and molecular basis of the distal arthrogryposis syndromes has served as a valuable framework to identify genetic risk factors for congenital contractures.
The distal arthrogryposes are characterized by congenital contractures of two or more different body areas without a primary neurological and/or muscle disease22. Features shared among all distal arthrogryposes include a consistent pattern of hand and foot involvement, limited involvement of proximal joints, and variable expressivity. Ten different distal arthrogryposes have been described to date22-24 and are classified hierarchically according to the proportion of features they share with one another (Table I). For example, there is more overlap between the phenotypes of distal arthrogryposis type 1 (DA1) and distal arthrogryposis type 2 (DA2) than there is between DA1 and distal arthrogryposis type 3 (DA3).
TABLE I.
Current Labels and OMIM Numbers for the Distal Arthrogryposis Syndromes*
| Syndrome | New Label | OMIM Number |
|---|---|---|
| Distal arthrogryposis type 1 | DA1 | 108120 |
| Distal arthrogryposis type 2A (Freeman-Sheldon syndrome) | DA2A | 193700 |
| Distal arthrogryposis type 2B (Sheldon-Hall syndrome) | DA2B | 601680 |
| Distal arthrogryposis type 3 (Gordon syndrome) | DA3 | 114300 |
| Distal arthrogryposis type 4 (scoliosis) | DA4 | 609128 |
| Distal arthrogryposis type 5 (ophthalmoplegia, ptosis) | DA5 | 108145 |
| Distal arthrogryposis type 6 (sensorineural hearing loss) | DA6 | 108200 |
| Distal arthrogryposis type 7 (trismus-pseudocamptodactyly) | DA7 | 158300 |
| Distal arthrogryposis type 8 (autosomal dominant multiple pterygium syndrome) | DA8 | 178110 |
| Distal arthrogryposis type 9 (congenital contractural arachnodactyly) | DA9 | 121050 |
| Distal arthrogryposis type 10 (congenital plantar contractures) | DA10 | 187370 |
OMIM = Online Mendelian Inheritance in Man.
Major diagnostic criteria are used to make the diagnosis of a specific distal arthrogryposis. For the upper limb, major diagnostic criteria include camptodactyly or pseudocamptodactyly (limited passive proximal interphalangeal joint extension with hyperextension of the wrist), hypoplastic and/or absent flexion creases, overriding fingers, and ulnar deviation at the wrist. For the lower limb, major diagnostic criteria are talipes equinovarus, calcaneovalgus deformities, vertical talus, and/or metatarsus varus. To be considered affected, an individual must exhibit two or more of these major criteria, but when a first-degree family member (i.e., a parent or a sibling) meets these diagnostic criteria, other family members with at least one major diagnostic criterion are considered affected. Salient features of several distal arthrogryposes are described below. (For conditions referenced in this paper, the reader is encouraged to go to the human genetic database Online Mendelian Inheritance in Man [OMIM] at http://www.ncbi.nlm.nih.gov/sites/entrez?db=omim and enter the OMIM six-digit identifier number in the search field.)
Distal Arthrogryposis Type 1 (DA1)
The prototypic distal arthrogryposis is distal arthrogryposis type 1 (DA1, OMIM 108120)3. DA1 is characterized largely by camptodactyly and clubfoot. Hypoplasia and/or absence of some interphalangeal creases is common. The shoulders and hips are less frequently affected. While the pattern of affected joints is consistent, the degree to which the joints are affected is highly variable, with equinovarus deformities ranging from mild to severe and hand involvement ranging from isolated hypoplasia of the distal interphalangeal crease of the fifth digit to severely clenched fists and ulnar deviation of the wrist. With the mildest form of DA1, affected individuals have only hypoplasia of the gastrocnemius—although ascertainment of such cases requires that a family member meet the diagnostic criteria for distal arthrogryposis.
Distal Arthrogryposis Type 2 (DA2)
DA1 is phenotypically similar to a condition called Freeman-Sheldon syndrome (FSS or DA2A, OMIM 193700)25. In addition to contractures of the hands and feet, FSS is characterized by oropharyngeal abnormalities, scoliosis, and a distinctive face (Fig. 3) that includes a very small oral orifice (often only a few millimeters in diameter at birth), puckered lips, and an H-shaped dimple of the chin; hence, FSS has also been called “whistling-face syndrome.” Individuals with DA1 and FSS may have such similar limb phenotypes that they can only be distinguished by the differences in their facial morphology. Reports of extended families in which some individuals were diagnosed with DA1 while others were diagnosed with FSS26 led to the delineation of a distinct disorder with overlapping phenotypes between DA1 and FSS23. The congenital contractures in this distinct condition were similar to those observed in DA1, but affected individuals tended to have more prominent nasolabial folds, downslanting palpebral fissures, and a small mouth. This disorder is now called DA2B or Sheldon-Hall syndrome (SHS, OMIM 601680), whereas FSS is considered as DA2A23. Sheldon-Hall syndrome is probably the most common of the distal arthrogryposis disorders.
Fig. 3.
Child with distal arthrogryposis type 2A (Freeman-Sheldon syndrome).
Distal Arthrogryposis Type 5 (DA5)
DA5 (OMIM 108145) is unique among distal arthrogryposes because, in addition to contractures of the skeletal muscles, affected individuals have ocular abnormalities. These typically include ptosis, restricted movement of the extraocular muscles, and/or strabismus27,28, findings that suggest that the extraocular as well as the skeletal muscles are involved in the pathogenesis of DA5. Recently, several unrelated individuals with DA5 have been reported as having pulmonary hypertension as a result of restrictive lung disease29. This is consistent with earlier anecdotal observations of abnormal chest-wall muscles in adults with DA5.
Distal Arthrogryposis Type 7 (DA7)
DA7, or trismus-pseudocamptodactyly syndrome (TPS, OMIM 158300), is an uncommon distal arthrogryposis characterized by an inability to fully open the mouth (trismus) and pseudocamptodactyly. Additional reported features of TPS include shortened hamstring muscles and short stature. The clinical characteristics of TPS vary widely within families, and no single feature, including either trismus or pseudocamptodactyly, is present in all affected individuals.
Distal Arthrogryposis Types 3, 4, and 6 (DA3, DA4, and DA6)
DA3, DA4, and DA6 are very rare. DA3, or Gordon syndrome (OMIM 114300), is distinguished from other distal arthrogryposes by short stature and cleft palate. However, the majority of individuals who are considered as being affected have neither of these defects but have been ascertained from large, multiplex families in which the index individual had a cleft palate in addition to congenital contractures.
The Molecular Basis of Distal Arthrogryposis Syndromes
Mutations in at least five genes (TNNI2, TNNT3, TPM2, MYH3, and MYH8) that encode components of the contractile apparatus of fast-twitch myofibers can cause distal arthrogryposis30-32. FSS and SHS are caused by mutations in MYH3, a gene that encodes embryonic myosin. Mutations in MYH3 explain approximately 90% of the cases of FSS and approximately 40% of the cases of SHS, making MYH3 mutation the most common known cause of distal arthrogryposis. No mutations overlap between those that cause FSS and SHS, suggesting an unambiguous genotype-phenotype relationship. SHS can also be caused by mutations in either of the genes that encode troponin I (TNNI2) or troponin T (TNNT3)30,31. More recently, in families with SHS, mutations have been found in TPM2, a gene that encodes tropomyosin 2 (Bamshad, unpublished data). This is of interest because mutations in TPM2 were first identified in families with DA1. Moreover, mutations in TNNI2 and TNNT3 have also recently been found in individuals with DA1. Thus, mutations in TNNI2, TNNT3, and TPM2 can be found in individuals diagnosed with DA1 or SHS. These results suggest that DA1 and SHS represent variable expressivity of the same syndrome. Mutation analysis of these genes is not available for clinical service as of yet but is commonly performed in research laboratories.
Two DA5 families have been found to have mutations in MYH2 and MYH13, both of which are expressed in the skeletal muscles of the limbs and the extraocular muscles. Each of these mutations is a missense mutation that is predicted to cause substitution of highly conserved amino acid residues. No other DA5 families were found to have mutations in either gene. Accordingly, mutation of either MYH2 or MYH13 appears to be a rare cause of DA5. In each of approximately a dozen families studied to date, DA7 is caused by a single missense mutation in MYH8 that is predicted to cause an arginine-to-glutamine substitution in perinatal myosin33.
The mechanism by which mutations in these genes cause contractures is unclear. TnI, TnT, β-tropomyosin, and myosins are part of the multimeric troponin-tropomyosin-myosin complex of the sarcomere. Mutations in genes that encode sarcomeric proteins in cardiac muscle cause defects in force production that can result in either hypocontractility or hypercontractility. Similarly, the results from in vitro contractility studies in which recombinant mutant TnI and β-tropomyosin34 molecules were used suggest that distal arthrogryposis syndromes are, in some cases, caused by increased myofiber contractility. However, the mechanism by which contractility is altered is not yet clear35. In contrast, direct measurement of the contractile properties of chemically skinned single muscle fibers sampled from affected muscles in individuals with MYH3 mutations suggests that maximal force normalized to fiber cross-sectional area is less than that observed in myofibers from unaffected individuals (Bamshad and Beck, unpublished data). These results suggest that there might be multiple mechanisms by which contractility can be altered to cause contractures. Understanding these mechanisms could provide a model to explore the pathogenesis of more common contractures, such as idiopathic clubfoot, and to facilitate the development of novel therapeutic approaches.
Arthrogryposis in Children Who Have Abnormal Results on Neurological Examination
This section will be restricted to central nervous system and neuromuscular diseases. These are thought to be a common cause of arthrogryposis and the most common cause of severe arthrogryposis36-38.
Central Nervous System Causes of Arthrogryposis
Developmental abnormalities affecting the forebrain (e.g., hydranencephaly, microcephaly, or forebrain neuronal migration disorders), whether due to primarily genetic factors or as a consequence of fetal central nervous system infection, are sometimes associated with arthrogryposis37,39-41. In most such cases, joint contractures are probably due to diminished corticospinal tract activation of spinal cord motor neurons. Sometimes, however, the underlying disease also directly injures spinal cord motor neurons, contributing to fetal hypomotility42. Such disorders can be suspected on clinical examination if hyperreflexia, unilateral arthrogryposis, or cognitive deficits are present and can be anatomically localized by magnetic resonance imaging of the brain.
Chromosomal deletions or rearrangements are an occasional cause of arthrogryposis43,44. Developmental loss of facial and other brainstem motor neurons (e.g., Moebius syndrome) is also sometimes associated with arthrogryposis. It is not known whether the deficient limb movement in these children is due to impaired corticospinal input to spinal cord motor neurons or because development of spinal cord motor neurons is also impaired. Arthrogryposis is a frequent feature of X-linked spinal muscular atrophy, a progressive motor neuron disease caused by mutations of the ubiquitin proteasome system gene, UBE145, and it also occurs in fetuses with spinal cord anterior horn atrophy owing to mutation of ERBB3, which encodes a protein that modulates the phosphatidylinositol-3-kinase/Akt pathway46. Arthrogryposis also occasionally occurs in the much more common, recessively inherited, infantile spinal muscular atrophy (Werdnig-Hoffmann disease), which is seen in infants with inactivating mutations of both copies of SMN1 who lack more than two copies of SMN247-49.
Neuromuscular Causes of Arthrogryposis
Genetic peripheral neuropathies with an onset during fetal life have been described, but they are a rare cause of arthrogryposis50,51. Neuromuscular junction blockade in fetuses carried by mothers with myasthenia gravis and autoantibodies that recognize fetal acetylcholine receptors can result in arthrogryposis52. Measures that suppress autoantibody formation, including maternal thymectomy prior to pregnancy, or intravenous gamma globulin administration during pregnancy, are likely to reduce the risk of arthrogryposis in such pregnancies. Repeated administration of botulinum toxin to pregnant women has the theoretical potential to inhibit acetylcholine release at neuromuscular junctions in the fetus and hence cause fetal hypomotility, but children with arthrogryposis arising from this etiology have not been reported. Arthrogryposis also occurs in infants with inherited mutations in genes that encode skeletal muscle acetylcholine receptor proteins, or proteins associated with these receptors (e.g., rapsyn [RAPSN])53-55.
Congenital myopathies that are sometimes associated with arthrogryposis can be caused by mutations of genes that encode fetal skeletal-muscle myosin heavy chains32,56, skeletal-muscle thin filament proteins57,58, and the ryanodine receptor protein59-61. Congenital myotonic dystrophy causes arthrogryposis62 because of the toxicity of RNA encoded by a triplet repeat expansion in the 3-prime untranslated region of the dystrophia myotonica protein kinase (DMPK) gene63. Congenital muscular dystrophies caused by mutations of the LMNA gene, which encodes A-type lamins, or of several genes that participate in the glycosylation of alpha-dystroglycan, can cause congenital muscle disease that progresses after birth (congenital muscular dystrophy) and joint contractures that increase in severity after birth64,65. Congenital neuropathy associated with LMNA mutations may also contribute to the genesis of joint contractures66.
Electromyography is useful in detecting and anatomically localizing neuromuscular disorders that have caused fetal immobility and led to arthrogryposis. Evidences of denervation (e.g., fibrillations, giant motor unit potentials, and diminished numbers of motor units) would indicate the presence of skeletal muscle denervation, as would occur with spinal cord motor neuronopathy or axonal neuropathy. Abnormal skeletal muscle responses to repetitive motor-nerve stimulation are helpful in diagnosing neuromuscular transmission disorders. Profoundly slowed nerve conduction velocities indicate the presence of a dysmyelinative neuropathy, whereas electrically inexcitable nerves suggest an axonal neuropathy. Studies helpful in diagnosing neuromuscular causes for arthrogryposis include assays for acetylcholine receptor antibodies67 and creatine kinase65. Because DNA diagnostic tests to distinguish the various congenital myopathies and/or dystrophies are not yet readily available to the clinician, skeletal muscle biopsy is sometimes useful. However, care should be taken in the choice of anesthesia for this procedure in order to minimize the risk of malignant hyperthermia61,68,69.
Conclusions
Congenital contractures are a common birth defect and are associated with substantial morbidity and economic burden. Attempts to identify the etiology and understand the pathogenesis of congenital contractures are an important area of pediatric health-care research.
Arthrogryposis describes the multiple congenital contractures that are part of more than 300 different disorders. Amyoplasia and the distal arthrogryposis syndromes, of which there are at least ten different types, are common causes of arthrogryposis when the results of neurological examination are normal. Amyoplasia is a sporadic disorder, whereas the distal arthrogryposes are autosomal dominant disorders. Central nervous system abnormalities, peripheral nervous system defects, or intrinsic muscle diseases can also cause arthrogryposis. These can be due to genetic or environmental factors.
Any factor that diminishes fetal movement can, in principle, cause congenital contractures, so identifying these factors would seem to be relatively straightforward. Indeed, some environmental factors (e.g., uterine crowding) and genetic factors (e.g., trisomy 18) that cause congenital contractures have been relatively easy to find. However, the underlying etiology of most congenital contractures remains an active area of investigation. 
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from Shriners Hospital and the National Institutes of Health (National Institute of Neurological Disorders and Stroke). Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Hall JG. Arthrogryposis multiplex congenita: etiology, genetics, classification, diagnostic approach, and general aspects. J Pediatr Orthop B. 1997;6:159-66. [PubMed] [Google Scholar]
- 2.Hall JG, Reed SD, Greene G. The distal arthrogryposes: delineation of new entities—review and nosologic discussion. Am J Med Genet. 1982;11:185-239. [DOI] [PubMed] [Google Scholar]
- 3.Johns Hopkins University. OMIM. Online Mendelian inheritance in man. http://www.ncbi.nlm.nih.gov/sites/entrez?db=omim. Accessed 2009 Mar 11.
- 4.Fahy MJ, Hall JG. A retrospective study of pregnancy complications among 828 cases of arthrogryposis. Genet Couns. 1990;1:3-11. [PubMed] [Google Scholar]
- 5.Vanpaemel L, Schoenmakers M, van Nesselrooij B, Pruijs H, Helders P. Multiple congenital contractures. J Pediatr Orthop B. 1997;6:172-8. [DOI] [PubMed] [Google Scholar]
- 6.Bevan WP, Hall JG, Bamshad M, Staheli LT, Jaffe KM, Song K. Arthrogryposis multiplex congenita (amyoplasia): an orthopaedic perspective. J Pediatr Orthop. 2007;27:594-600. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein RM. Arthrogryposis and amyoplasia. J Am Acad Orthop Surg. 2002;10:417-24. [DOI] [PubMed] [Google Scholar]
- 8.Hall JG. Don't use the term “amyoplasia” loosely. Am J Med Genet. 2002;111:344. [DOI] [PubMed] [Google Scholar]
- 9.Sarwark JF, MacEwen GD, Scott CI Jr. Amyoplasia (a common form of arthrogryposis). J Bone Joint Surg Am. 1990;72:465-9. [PubMed] [Google Scholar]
- 10.Sells JM, Jaffe KM, Hall JG. Amyoplasia, the most common type of arthrogryposis: the potential for good outcome. Pediatrics. 1996;97:225-31. [PubMed] [Google Scholar]
- 11.Hall JG, Reed SD, McGillivray BC, Herrmann J, Partington MW, Schinzel A, Shapiro J, Weaver DD. Part II. Amyoplasia: twinning in amyoplasia—a specific type of arthrogryposis with an apparent excess of discordantly affected identical twins. Am J Med Genet. 1983;15:591-9. [DOI] [PubMed] [Google Scholar]
- 12.Hall JG, Reed SD, Driscoll EP. Part 1. Amyoplasia: a common sporadic condition with congenital contractures. Am J Med Genet. 1983;15:571-90. [DOI] [PubMed] [Google Scholar]
- 13.Ezaki M. Treatment of the upper limb in the child with arthrogryposis. Hand Clin. 2000;16:703-11. [PubMed] [Google Scholar]
- 14.Bennett JB, Hansen PE, Granberry WM, Cain TE. Surgical management of arthrogryposis in the upper extremity. J Pediatr Orthop. 1985;5:281-6. [DOI] [PubMed] [Google Scholar]
- 15.Williams PF. Management of upper limb problems in arthrogryposis. Clin Orthop Relat Res. 1985;194:60-7. [PubMed] [Google Scholar]
- 16.Axt MW, Niethard FU, Doderlein L, Weber M. Principles of treatment of the upper extremity in arthrogryposis multiplex congenita type I. J Pediatr Orthop B. 1997;6:179-85. [DOI] [PubMed] [Google Scholar]
- 17.Van Heest A, Waters PM, Simmons BP. Surgical treatment of arthrogryposis of the elbow. J Hand Surg [Am]. 1998;23:1063-70. [DOI] [PubMed] [Google Scholar]
- 18.Atkins RM, Bell MJ, Sharrard WJ. Pectoralis major transfer for paralysis of elbow flexion in children. J Bone Joint Surg Br. 1985;67:640-4. [DOI] [PubMed] [Google Scholar]
- 19.Goldfarb CA, Burke MS, Strecker WB, Manske PR. The Steindler flexorplasty for the arthrogrypotic elbow. J Hand Surg [Am]. 2004;29:462-9. [DOI] [PubMed] [Google Scholar]
- 20.Lahoti O, Bell MJ. Transfer of pectoralis major in arthrogryposis to restore elbow flexion: deteriorating results in the long term. J Bone Joint Surg Br. 2005;87:858-60. [DOI] [PubMed] [Google Scholar]
- 21.Van Heest A, James MA, Lewica A, Anderson KA. Posterior elbow capsulotomy with triceps lengthening for treatment of elbow extension contracture in children with arthrogryposis. J Bone Joint Surg Am. 2008;90:1517-23. [DOI] [PubMed] [Google Scholar]
- 22.Bamshad M, Jorde LB, Carey JC. A revised and extended classification of the distal arthrogryposes. Am J Med Genet. 1996;65:277-81. [DOI] [PubMed] [Google Scholar]
- 23.Krakowiak PA, Bohnsack JF, Carey JC, Bamshad M. Clinical analysis of a variant of Freeman-Sheldon syndrome (DA2B). Am J Med Genet. 1998;76:93-8. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson DA, Carey JC, Palumbos J, Rutherford A, Dolcourt J, Bamshad MJ. Clinical characteristics and natural history of Freeman-Sheldon syndrome. Pediatrics. 2006;117:754-62. [DOI] [PubMed] [Google Scholar]
- 25.Freeman EA, Sheldon J. Cranio-carpotarsal dystrophy: undescribed congenital malformation. Arch Disease Child. 1938;13:277-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klemp P, Hall JG. Dominant distal arthrogryposis in a Maori family with marked variability of expression. Am J Med Genet. 1995;55:414-9. [DOI] [PubMed] [Google Scholar]
- 27.Pallotta R, Ehresmann T, Fusilli P. Occurrence of Dandy-Walker anomaly in a familial case of distal arthogryposis type IIB. Am J Med Genet. 2000;95:477-81. [DOI] [PubMed] [Google Scholar]
- 28.Pallotta R, Ehresmann T, Fusilli P. Ocular findings in distal arthrogryposis. Ophthalmic Genet. 2001;22:125-30. [DOI] [PubMed] [Google Scholar]
- 29.Williams MS, Elliott CG, Bamshad MJ. Pulmonary disease is a component of distal arthrogryposis type 5. Am J Med Genet A. 2007;143:752-6. [DOI] [PubMed] [Google Scholar]
- 30.Sung SS, Brassington AM, Grannatt K, Rutherford A, Whitby FG, Krakowiak PA, Jorde LB, Carey JC, Bamshad M. Mutations in genes encoding fast-twitch contractile proteins cause distal arthrogryposis syndromes. Am J Hum Genet. 2003;72:681-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung SS, Brassington AM, Krakowiak PA, Carey JC, Jorde LB, Bamshad M. Mutations in TNNT3 cause multiple congenital contractures: a second locus for distal arthrogryposis type 2B. Am J Hum Genet. 2003;73:212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toydemir RM, Rutherford A, Whitby FG, Jorde LB, Carey JC, Bamshad MJ. Mutations in embryonic myosin heavy chain (MYH3) cause Freeman-Sheldon syndrome and Sheldon-Hall syndrome. Nat Genet. 2006;38:561-5. [DOI] [PubMed] [Google Scholar]
- 33.Toydemir RM, Chen H, Proud VK, Martin R, van Bokhoven H, Hamel BC, Tuerlings JH, Stratakis CA, Jorde LB, Bamshad MJ. Trismus-pseudocamptodactyly syndrome is caused by recurrent mutation of MYH8. Am J Med Genet A. 2006;140:2387-93. [DOI] [PubMed] [Google Scholar]
- 34.Ashrafian H, Redwood C, Blair E, Watkins H. Hypertrophic cardiomyopathy: a paradigm for myocardial energy depletion. Trends Genet. 2003;19:263-8. [DOI] [PubMed] [Google Scholar]
- 35.Robinson P, Lipscomb S, Preston LC, Altin E, Watkins H, Ashley CC, Redwood CS. Mutations in fast skeletal troponin I, troponin T, and beta-tropomyosin that cause distal arthrogryposis all increase contractile function. FASEB J. 2007;21:896-905. [DOI] [PubMed] [Google Scholar]
- 36.Banker BQ. Arthrogryposis multiplex congenita: spectrum of pathologic changes. Hum Pathol. 1986;17:656-72. [DOI] [PubMed] [Google Scholar]
- 37.Hageman G, Ippel EP, Beemer FA, de Pater JM, Lindhout D, Willemse J. The diagnostic management of newborns with congenital contractures: a nosologic study of 75 cases. Am J Med Genet. 1988;30:883-904. [DOI] [PubMed] [Google Scholar]
- 38.Pakkasjarvi N, Ritvanen A, Herva R, Peltonen L, Kestila M, Ignatius J. Lethal congenital contracture syndrome (LCCS) and other lethal arthrogryposes in Finland—an epidemiological study. Am J Med Genet A. 2006;140:1834-9. [DOI] [PubMed] [Google Scholar]
- 39.Gropman AL, Barkovich AJ, Vezina LG, Conry JA, Dubovsky EC, Packer RJ. Pediatric congenital bilateral perisylvian syndrome: clinical and MRI features in 12 patients. Neuropediatrics. 1997;28:198-203. [DOI] [PubMed] [Google Scholar]
- 40.Konstantinidou A, Anninos H, Spanakis N, Kotsiakis X, Syridou G, Tsakris A, Patsouris E. Transplacental infection of Coxsackievirus B3 pathological findings in the fetus. J Med Virol. 2007;79:754-7. [DOI] [PubMed] [Google Scholar]
- 41.Laugel V, Dalloz C, Tobias ES, Tolmie JL, Martin-Coignard D, Drouin-Garraud V, Valayannopoulos V, Sarasin A, Dollfus H. Cerebro-oculo-facio-skeletal syndrome: three additional cases with CSB mutations, new diagnostic criteria and an approach to investigation. J Med Genet. 2008;45:564-71. [DOI] [PubMed] [Google Scholar]
- 42.Chow G, Padfield CJ. A case of infantile neuroaxonal dystrophy: connatal Seitelberger disease. J Child Neurol. 2008;23:418-20. [DOI] [PubMed] [Google Scholar]
- 43.Destree A, Fourneau C, Dugauquier C, Rombout S, Sartenaer D, Gillerot Y. Prenatal diagnosis of trisomy 6 mosaicism. Prenat Diagn. 2005;25:354-7. [DOI] [PubMed] [Google Scholar]
- 44.Grati FR, Lalatta F, Turolla L, Cavallari U, Gentilin B, Rossella F, Cetin I, Antonazzo P, Bellotti M, Dulcetti F, Baldo D, Tenconi R, Simoni G, Miozzo M. Three cases with de novo 6q imbalance and variable prenatal phenotype. Am J Med Genet A. 2005;136:254-8. [DOI] [PubMed] [Google Scholar]
- 45.Ramser J, Ahearn ME, Lenski C, Yariz KO, Hellebrand H, von Rhein M, Clark RD, Schmutzler RK, Lichtner P, Hoffman EP, Meindl A, Baumbach-Reardon L. Rare missense and synonymous variants in UBE1 are associated with X-linked infantile spinal muscular atrophy. Am J Hum Genet. 2008;82:188-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narkis G, Ofir R, Manor E, Landau D, Elbedour K, Birk OS. Lethal congenital contractural syndrome type 2 (LCCS2) is caused by a mutation in ERBB3 (Her3), a modulator of the phosphatidylinositol-3-kinase/Akt pathway. Am J Hum Genet. 2007;81:589-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burglen L, Amiel J, Viollet L, Lefebvre S, Burlet P, Clermont O, Raclin V, Landrieu P, Verloes A, Munnich A, Melki J. Survival motor neuron gene deletion in the arthrogryposis multiplex congenita-spinal muscular atrophy association. J Clin Invest. 1996;98:1130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogino S, Wilson RB. Genetic testing and risk assessment for spinal muscular atrophy (SMA). Hum Genet. 2002;111:477-500. [DOI] [PubMed] [Google Scholar]
- 49.Hauke J, Riessland M, Lunke S, Eyupoglu IY, Blumcke I, El-Osta A, Wirth B, Hahnen E. Survival motor neuron gene 2 silencing by DNA methylation correlates with spinal muscular atrophy disease severity and can be bypassed by histone deacetylase inhibition. Hum Mol Genet. 2009;18:304-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boylan KB, Ferriero DM, Greco CM, Sheldon RA, Dew M. Congenital hypomyelination neuropathy with arthrogryposis multiplex congenita. Ann Neurol. 1992;31:337-40. [DOI] [PubMed] [Google Scholar]
- 51.Shibasaki H, Hitomi T, Mezaki T, Kihara T, Tomimoto H, Ikeda A, Shimohama S, Ito M, Oka N. A new form of congenital proprioceptive sensory neuropathy associated with arthrogryposis multiplex. J Neurol. 2004;251:1340-4. [DOI] [PubMed] [Google Scholar]
- 52.Polizzi A, Huson SM, Vincent A. Teratogen update: maternal myasthenia gravis as a cause of congenital arthrogryposis. Teratology. 2000;62:332-41. [DOI] [PubMed] [Google Scholar]
- 53.Burke G, Cossins J, Maxwell S, Owens G, Vincent A, Robb S, Nicolle M, Hilton-Jones D, Newsom-Davis J, Palace J, Beeson D. Rapsyn mutations in hereditary myasthenia: distinct early- and late-onset phenotypes. Neurology. 2003;61:826-8. [DOI] [PubMed] [Google Scholar]
- 54.Morgan NV, Brueton LA, Cox P, Greally MT, Tolmie J, Pasha S, Aligianis IA, van Bokhoven H, Marton T, Al-Gazali L, Morton JE, Oley C, Johnson CA, Trembath RC, Brunner HG, Maher ER. Mutations in the embryonal subunit of the acetylcholine receptor (CHRNG) cause lethal and Escobar variants of multiple pterygium syndrome. Am J Hum Genet. 2006;79:390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michalk A, Stricker S, Becker J, Rupps R, Pantzar T, Miertus J, Botta G, Naretto VG, Janetzki C, Yaqoob N, Ott CE, Seelow D, Wieczorek D, Fiebig B, Wirth B, Hoopmann M, Walther M, Korber F, Blankenburg M, Mundlos S, Heller R, Hoffmann K. Acetylcholine receptor pathway mutations explain various fetal akinesia deformation sequence disorders. Am J Hum Genet. 2008;82:464-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tajsharghi H, Kimber E, Kroksmark AK, Jerre R, Tulinius M, Oldfors A. Embryonic myosin heavy-chain mutations cause distal arthrogryposis and developmental myosin myopathy that persists postnatally. Arch Neurol. 2008;65:1083-90. [DOI] [PubMed] [Google Scholar]
- 57.Ryan MM, Schnell C, Strickland CD, Shield LK, Morgan G, Iannaccone ST, Laing NG, Beggs AH, North KN. Nemaline myopathy: a clinical study of 143 cases. Ann Neurol. 2001;50:312-20. [DOI] [PubMed] [Google Scholar]
- 58.Ochala J. Thin filament proteins mutations associated with skeletal myopathies: defective regulation of muscle contraction. J Mol Med. 2008;86:1197-204. [DOI] [PubMed] [Google Scholar]
- 59.Romero NB, Monnier N, Viollet L, Cortey A, Chevallay M, Leroy JP, Lunardi J, Fardeau M. Dominant and recessive central core disease associated with RYR1 mutations and fetal akinesia. Brain. 2003;126:2341-9. [DOI] [PubMed] [Google Scholar]
- 60.Treves S, Jungbluth H, Muntoni F, Zorzato F. Congenital muscle disorders with cores: the ryanodine receptor calcium channel paradigm. Curr Opin Pharmacol. 2008;8:319-26. [DOI] [PubMed] [Google Scholar]
- 61.Stamm DS, Aylsworth AS, Stajich JM, Kahler SG, Thorne LB, Speer MC, Powell CM. Native American myopathy: congenital myopathy with cleft palate, skeletal anomalies, and susceptibility to malignant hyperthermia. Am J Med Genet A. 2008;146:1832-41. [DOI] [PubMed] [Google Scholar]
- 62.Sarnat HB, Silbert SW. Maturational arrest of fetal muscle in neonatal myotonic dystrophy. A pathologic study of four cases. Arch Neurol. 1976;33:466-74. [DOI] [PubMed] [Google Scholar]
- 63.Ranum LP, Cooper TA. RNA-mediated neuromuscular disorders. Annu Rev Neurosci. 2006;29:259-77. [DOI] [PubMed] [Google Scholar]
- 64.Muntoni F, Brockington M, Godfrey C, Ackroyd M, Robb S, Manzur A, Kinali M, Mercuri E, Kaluarachchi M, Feng L, Jimenez-Mallebrera C, Clement E, Torelli S, Sewry CA, Brown SC. Muscular dystrophies due to defective glycosylation of dystroglycan. Acta Myol. 2007;26:129-35. [PMC free article] [PubMed] [Google Scholar]
- 65.Quijano-Roy S, Mbieleu B, Bonnemann CG, Jeannet PY, Colomer J, Clarke NF, Cuisset JM, Roper H, De Meirleir L, D'Amico A, Ben Yaou R, Nascimento A, Barois A, Demay L, Bertini E, Ferreiro A, Sewry CA, Romero NB, Ryan M, Muntoni F, Guicheney P, Richard P, Bonne G, Estournet B. De novo LMNA mutations cause a new form of congenital muscular dystrophy. Ann Neurol. 2008;64:177-86. [DOI] [PubMed] [Google Scholar]
- 66.Rankin J, Auer-Grumbach M, Bagg W, Colclough K, Nguyen TD, Fenton-May J, Hattersley A, Hudson J, Jardine P, Josifova D, Longman C, McWilliam R, Owen K, Walker M, Wehnert M, Ellard S. Extreme phenotypic diversity and nonpenetrance in families with the LMNA gene mutation R644C. Am J Med Genet A. 2008;146:1530-42. [DOI] [PubMed] [Google Scholar]
- 67.Vincent A, McConville J, Farrugia ME, Bowen J, Plested P, Tang T, Evoli A, Matthews I, Sims G, Dalton P, Jacobson L, Polizzi A, Blaes F, Lang B, Beeson D, Willcox N, Newsom-Davis J, Hoch W. Antibodies in myasthenia gravis and related disorders. Ann N Y Acad Sci. 2003;998:324-35. [DOI] [PubMed] [Google Scholar]
- 68.Wappler F. Malignant hyperthermia. Eur J Anaesthesiol. 2001;18:632-52. [DOI] [PubMed] [Google Scholar]
- 69.Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene—a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59:364-73. [DOI] [PubMed] [Google Scholar]