Limb development is a well-coordinated three-dimensional process in which limb-bud outgrowth and patterning along the different axes of development are intimately linked through interactions of the signaling molecules that mediate the function of three key signaling centers. The vertebrate limb develops from a primordial embryonic limb bud, consisting of a homogeneous mesoderm core covered by an ectodermal jacket, and development of the limb bud is an autonomous process controlled by these signaling centers, which are formed through epithelial-mesenchymal interactions. The major signaling centers in the limb each direct limb development along one of the three axes: proximodistal (from shoulder to digit tip), anteroposterior (from digit 1, or thumb, to digit 5, or small finger) and dorsoventral (from the dorsum to the palm of the hand) (Table I).
TABLE I.
Signaling Pathways During Embryogenesis
| Signaling Center | Responsible Substance | Action | Anomaly |
|---|---|---|---|
| Apical ectodermal ridge | Fibroblast growth factors | Proximal-to-distal limb development, interdigital necrosis | Transverse deficiency |
| Zone of polarizing activity | Sonic hedgehog protein | Radioulnar limb formation | Mirror hand |
| Digit formation | Polydactyly | ||
| Syndactyly | |||
| Oligodactyly | |||
| Limb ectoderm | Wnts transcription factor, Lmx-1 | Ventral and dorsal limb axis, apical ectodermal ridge formation and maintenance | Nail patella syndrome, abnormal nail and pulp arrangement |
These signaling centers were identified by classic embryological experiments; recent molecular genetic studies in both the mouse and the chick have revealed signaling molecules that mediate the functions of these centers1. Although they only act early in the limb bud, these signaling centers have a profound influence on limb morphology by providing positional cues that determine the shape and spatial organization, as well as the temporal order of formation, of limb structures such as bones and tendons.
We will describe several of the known links between the different development axes and give examples of the ways in which genetic mutations or disrupted embryonic development can affect these signaling events and cause longitudinal deficiencies and other malformations in humans and in mouse genetic mutants.
Fibroblast Growth-Factor Signaling and Proximodistal Limb Outgrowth and Patterning
The first signaling center to appear is the apical ectodermal ridge (AER), which is a thickened epithelial structure at the distal tip of the limb bud. In human embryos, limb initiation occurs at twenty-six days post coitum; in the mouse, the limb bud forms as a bulge from the lateral plate mesoderm at nine days post coitum.
Removal of the AER from the limb bud leads to limb truncation; earlier AER removal results in limb truncation at a more proximal level2 (Fig. 1). Fibroblast growth factors (Fgfs) expressed in the AER are both necessary and sufficient to mediate the AER function in controlling proximodistal limb outgrowth and patterning3,4. Four Fgfs (Fgf 4, 8, 9, and 17) are expressed in the mouse AER. Among these, Fgf 8 is expressed earliest and is the only Fgf that is indispensable for normal limb development4-6. Fgf 4, 9, and 17 play redundant roles with Fgf 8 in regulating proximodistal limb outgrowth and patterning, and their relative contributions to limb development are revealed by double mutants that lack Fgf 8 and either Fgf 4, 9, or 174. These studies show that Fgf 8 makes the greatest contribution to the AER-Fgf signal, followed by Fgf 4, 9, and 17. Although each of the individual AER-Fgfs is functionally equivalent, they differ in the extent to which they contribute to the AER function because of their different temporal and spatial expression profiles, expression levels, and binding specificities to Fgf receptors in the limb bud mesenchyme. Fgfs from the AER appear to control limb development in a dose-dependent manner4, with complete removal of Fgf 8, 4, and 9 from the developing AER causing a limbless phenotype, whereas removal of Fgf 8, 4, and one copy of Fgf 9 causes severe limb truncation with the radius, ulna, and most of the digits missing.
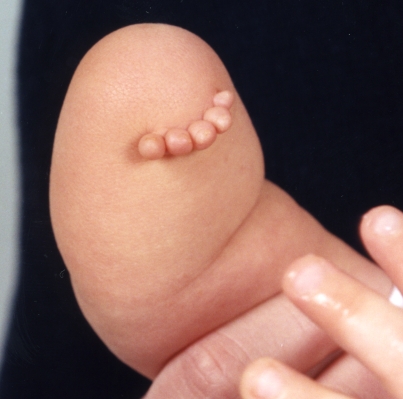
Fig. 1.
Right, short, below-the-elbow deficiency attributed to loss of AER and limb truncation (Courtesy of Shriners Hospital for Children, Philadelphia).
AER-derived Fgfs signal to the distal limb mesenchyme to maintain the expression of another Fgf family member, Fgf 10, which is required for limb development by signaling to the AER to maintain its integrity and activate AER-derived Fgf expression7-9. Such reciprocal Fgf signaling between AER and the distal limb mesenchyme is required for limb-bud outgrowth and patterning. Removal of Fgf 10 in the mouse limb bud also leads to a limbless phenotype8. The function of Fgfs in the limb is to maintain a pool of progenitor cells that are capable of forming later limb structures; this Fgf function is not only dose-dependent but also time-dependent10,11. Genetic removal of the AER later in limb development leads to distal limb truncation because only limb progenitor cells that are destined to form the digits are affected. Skeletal truncations or loss of skeletal elements are often observed in conditions of transverse and radial longitudinal deficiencies (Figs. 1 and 2), which resemble the mouse Fgf limb mutants. It is likely that loss of or reduced Fgf signaling is a critical molecular defect underlying these limb malformations.
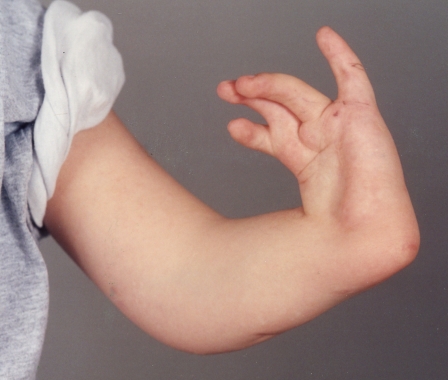
Fig. 2.
Radial longitudinal deficiency (Courtesy of Shriners Hospital for Children, Philadelphia).
Sonic Hedgehog (Shh) Signaling and Anteroposterior Growth and Patterning of the Limb
The second signaling center is the zone of polarizing activity (ZPA), which is a group of limb mesenchymal cells formed at the posterior margin of the limb bud. The polarizing activities of these cells were first discovered during grafting experiments that showed that ZPA cells, when grafted to the anterior margin of the limb under the AER, can induce mirror-image digit duplication12 (Fig. 3). Sonic hedgehog (Shh), a vertebrate hedgehog family member, is both necessary and sufficient to mediate the activity of ZPA13,14. Ectopic expression of Shh, just like ZPA grafting, leads to mirror-image digit duplication13,15. Such activity of Shh is also dose and time-dependent16. Higher Shh doses specify more posterior (ulnar) digits, whereas lower Shh doses specify anterior (radial) digits. The most anterior or radial digit, digit 1, does not need Shh to be specified. Conversely, absence of Shh leads to progressive loss of skeletal elements along the anteroposterior axis17,18. Reduced Shh activity in the early limb bud causes loss of posterior digits first. However, when Shh activity is reduced later in limb-bud development, progenitor-cell expansion is not supported; this also causes digit loss, but in this scenario, digit loss is a result of insufficient progenitor cells19. Therefore, the last digit to form is the first digit to fail to form when Shh is removed at the latest effective time point. The normal digit formation order is 4-2-5-3; when Shh is removed at progressively earlier time points, the digit loss order is 3-5-2-4. Apparently, digit specification and actual digit formation are two separate processes20. The former process occurs in a very short window of time at the beginning of limb-bud development, whereas the latter requires progenitor-cell expansion later in limb development.
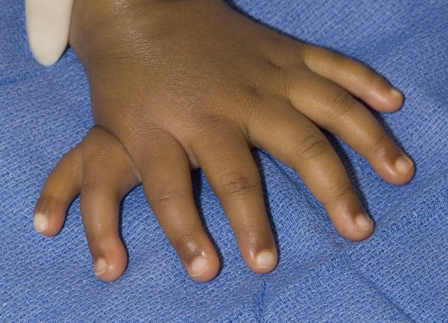
Fig. 3.
Mirror hand attributed to ZPA cells in the anterior limb margin and mirror-image digit duplication (Courtesy of Shriners Hospital for Children, Philadelphia).
All vertebrate Hedgehog (Hh) ligands (Shh, Indian hedgehog [Ihh], and Desert hedgehog [Dhh]) transduce their signals through the same pathway. Briefly, two transmembrane proteins (Patched 1 [Ptch1] and Smoothened [Smo]) receive the Hh ligand signals at the cell membrane. In the absence of the Hh ligand, Ptch1 suppresses the activity of Smo. When the Hh ligand is present, it binds to Ptch1, the inhibitory effect on Smo is relieved, and Smo transduces the Hh signal to downstream signaling components including Gli2 and Gli3, which activate expression of Hh downstream target genes including Hip1, Gli1 and Ptch121-23. Perturbation of the Hh signaling pathway has been found to cause limb malformations in both mouse and human. For instance, null mutations in Gli3 cause toe polydactyly in mice, and the Greig cephalopolysyndactyly syndrome in humans24-26.
Loss of Fgf and Shh signaling leads to loss of skeletal elements along both the proximodistal and anteroposterior axes. This is because Fgfs and Shh both regulate the size of the progenitor cell pool. In addition, limb outgrowth and patterning along proximodistal and anteroposterior axes are interconnected to each other by the epithelial-mesenchymal interaction that is mediated by Shh in the mesenchyme and Fgfs in the AER27,28. Fgf signaling from the AER is required for Shh expression, whereas Shh signaling is required to maintain the AER integrity.
Both Fgf and Shh signaling deficiency can lead to radius deficiency. Loss of the radius is observed in the Fgf 8 and Fgf 9 double mutant limb, in which there is also digit loss. In this case, the radius fails to form as a result of a reduction in the progenitor cell pool. Radius absence is also observed in the limb where Shh signaling has been ectopically upregulated at later stages in the developing limb bud, likely due to progenitor-cell redistribution. Progenitor cells in the presumptive radius area may have been distalized, because later upregulation of Shh predominantly affects the cells forming the distal limb. For instance, in the Gli3 and Alx4 double mutant, loss of the radius is observed together with polydactyly caused by ectopic Shh expression29.
Wnt Signaling and Dorsoventral Limb Patterning
The third signaling center is the non-AER limb ectoderm. In the early limb bud of a chick, if the limb bud ectoderm is removed from the limb and put back to the mesoderm core after reversing its dorsoventral polarity, the dorsoventral polarity of the limb mesoderm is also reversed accordingly30,31 (Fig. 4). The vertebrate Wnt family member Wnt7a is expressed specifically in the dorsal limb ectoderm and determines the dorsal limb identity32 by activating the expression of Lmx1b, which encodes a transcription factor both necessary and sufficient to determine dorsal limb-bud identity33-35. En-1, which encodes a transcription factor expressed in the ventral ectoderm, also regulates the dorsoventral polarity by inhibiting the expression of Wnt7a36. Loss of the ulna is observed in ventralized limbs in both Wnt7a and Lmx1b loss-of-function mutants32,35. Thus, dorsoventral patterning also affects anteroposterior patterning; indeed, Wnt7a is required to regulate Shh expression32,37. Wnt signaling in the ectoderm and AER also regulates proximodistal limb outgrowth and patterning by controlling the formation and maintenance of AER38-41.
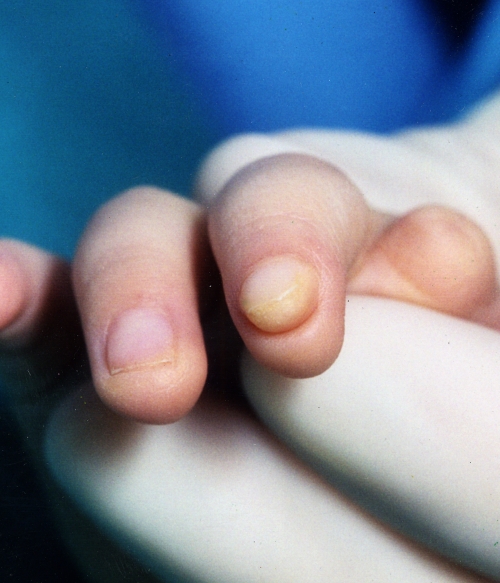
Fig. 4.
Atypical nail and pulp arrangement attributed to Wnt pathway abnormality (Courtesy of Shriners Hospital for Children, Philadelphia).
Summary
Molecular and genetic studies in vertebrates have demonstrated that disruption of signaling events leads to various limb malformations; many malformations of the human hand bear striking similarities to those seen in mouse genetic mutants. Cell signaling clearly plays a major role in regulating growth and patterning of the vertebrate limbs, and the three key signaling centers are linked by the interactions of their signaling molecules. Different dose-related, spatial, or timing errors in signaling can cause morphologically similar defects. Studies of the various causes of malformations in nonhuman vertebrates provide critical insights into the normal and abnormal development of the human limb. 
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institutes of Health. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Johnson RL, Tabin CJ. Molecular models for vertebrate limb development. Cell. 1997;90:979-90. [DOI] [PubMed] [Google Scholar]
- 2.Saunders JW Jr. The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J Exp Zool. 1948;108:363-403. [DOI] [PubMed] [Google Scholar]
- 3.Niswander L, Tickle C, Vogel A, Booth I, Martin GR. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell. 1993;75:579-87. [DOI] [PubMed] [Google Scholar]
- 4.Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature. 2008;453:401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat Genet. 2000;26:460-3. [DOI] [PubMed] [Google Scholar]
- 6.Sun X, Lewandoski M, Meyers EN, Liu YH, Maxson RE Jr, Martin GR. Conditional inactivation of Fgf4 reveals complexity of signalling during limb bud development. Nat Genet. 2000;25:83-6. [DOI] [PubMed] [Google Scholar]
- 7.Ohuchi H, Nakagawa T, Yamamoto A, Araga A, Ohata T, Ishimaru Y, Yoshioka H, Kuwana T, Nohno T, Yamasaki M, Itoh N, Noji S. The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development. 1997;124:2235-44. [DOI] [PubMed] [Google Scholar]
- 8.Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagashita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138-41. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Weinstein M, Li C, Naski M, Cohen RI, Ornitz DM, Leder P, Deng C. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development. 1998;125:753-65. [DOI] [PubMed] [Google Scholar]
- 10.Lu P, Yu Y, Perdue Y, Werb Z. The apical ectodermal ridge is a timer for generating distal limb progenitors. Development. 2008;135:1395-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu K, Ornitz DM. FGF signaling regulates mesenchymal differentiation and skeletal patterning along the limb bud proximodistal axis. Development. 2008;135:483-91. [DOI] [PubMed] [Google Scholar]
- 12.Saunders JWJ, Gasseling MT. Ectoderm-mesenchymal interaction in the origin of wing symmetry. In: Fleischmajer R, Billingham RE, editors. Epithelial-mesenchymal interactions. Baltimore: Williams and Wilkins; 1968. p 78-97.
- 13.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401-16. [DOI] [PubMed] [Google Scholar]
- 14.Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, Fallon JF. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol. 2001;236:421-35. [DOI] [PubMed] [Google Scholar]
- 15.Chan DC, Laufer E, Tabin C, Leder P. Polydactylous limbs in Strong's Luxoid mice result from ectopic polarizing activity. Development. 1995;121:1971-8. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Drossopoulou G, Chuang PT, Duprez D, Marti E, Bumcrot D, Vargesson N, Clarke J, Niswander L, McMahon A, Tickle C. Relationship between dose, distance and time in Sonic Hedgehog-mediated regulation of anteroposterior polarity in the chick limb. Development. 1997;124:4393-404. [DOI] [PubMed] [Google Scholar]
- 17.Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599-612. [DOI] [PubMed] [Google Scholar]
- 18.Scherz PJ, McGlinn E, Nissim S, Tabin CJ. Extended exposure to Sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev Biol. 2007;308:343-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Nakamura E, Nguyen MT, Bao X, Akiyama H, Mackem S. Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell. 2008;14:624-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Towers M, Mahood R, Yin Y, Tickle C. Integration of growth and specification in chick wing digit-patterning. Nature. 2008;452:882-6. [DOI] [PubMed] [Google Scholar]
- 21.Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3-14. [DOI] [PubMed] [Google Scholar]
- 22.Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755-9. [DOI] [PubMed] [Google Scholar]
- 23.Chen MH, Wilson CW, Chuang PT. SnapShot: hedgehog signaling pathway. Cell. 2007;130:386. [DOI] [PubMed] [Google Scholar]
- 24.Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet. 1993;3:241-6. Erratum in: Nat Genet. 1998;19:404. [DOI] [PubMed] [Google Scholar]
- 25.Vortkamp A, Franz T, Gessler M, Grzeschik KH. Deletion of GLI3 supports the homology of the human Greig cephalopolysyndactyly syndrome (GCPS) and the mouse mutant extra toes (Xt). Mamm Genome. 1992;3:461-3. [DOI] [PubMed] [Google Scholar]
- 26.Vortkamp A, Gessler M, Grzeschik KH. GLI3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature. 1991;352:539-40. [DOI] [PubMed] [Google Scholar]
- 27.Niswander L, Jeffrey S, Martin GR, Tickle C. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature. 1994;371:609-12. [DOI] [PubMed] [Google Scholar]
- 28.Laufer E, Nelson CE, Johnson RL, Morgan BA, Tabin C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell. 1994;79:993-1003. [DOI] [PubMed] [Google Scholar]
- 29.Panman L, Drenth T, Tewelscher P, Zuniga A, Zeller R. Genetic interaction of Gli3 and Alx4 during limb development. Int J Dev Biol. 2005;49:443-8. [DOI] [PubMed] [Google Scholar]
- 30.MacCabe JA, Errick J, Saunders JW Jr. Ectodermal control of the dorsoventral axis in the leg bud of the chick embryo. Dev Biol. 1974;39:69-82. [DOI] [PubMed] [Google Scholar]
- 31.Pautou MP. Dorso-ventral axis determination of chick limb bud development. In: Ede DA, Hinchliffe JR, Balls M, editors. Vertebrate limb and somite morphogenesis. Cambridge: Cambridge University Press; 1977. p 257-66.
- 32.Parr BA, McMahon AP. Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature. 1995;374:350-3. [DOI] [PubMed] [Google Scholar]
- 33.Riddle RD, Ensini M, Nelson C, Tsuchida T, Jessell TM, Tabin C. Induction of the LIM homeobox gene Lmx1 by WNT7a establishes dorsoventral pattern in the vertebrate limb. Cell. 1995;83:631-40. [DOI] [PubMed] [Google Scholar]
- 34.Vogel A, Rodriguez C, Warnken W, Izpiúa Belmonte JC. Dorsal cell fate specified by chick Lmx1 during vertebrate limb development. Nature. 1995;378:716-20. Erratum in: Nature. 1996;379:848. [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Lun Y, Ovchinnikov D, Kokubo H, Oberg KC, Pepicelli CV, Gen L, Lee B, Johnson RL. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51-5. [DOI] [PubMed] [Google Scholar]
- 36.Loomis CA, Harris E, Michaud J, Wurst W, Hanks M, Joyner AL. The mouse Engrailed-1 gene and ventral limb patterning. Nature. 1996;382:360-3. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Niswander L. Interaction between the signaling molecules WNT7a and SHH during vertebrate limb development: dorsal signals regulate anteroposterior patterning. Cell. 1995;80:939-47. [DOI] [PubMed] [Google Scholar]
- 38.Adamska M, MacDonald BT, Sarmast ZH, Oliver ER, Meisler MH. En1 and Wnt7a interact with Dkk1 during limb development in the mouse. Dev Biol. 2004;272:134-44. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald BT, Adamska M, Meisler MH. Hypomorphic expression of Dkk1 in the doubleridge mouse: dose dependence and compensatory interactions with Lrp6. Development. 2004;131:2543-52. [DOI] [PubMed] [Google Scholar]
- 40.Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, McMahon AP. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Belmonte JC, Westphal H. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423-34. [DOI] [PubMed] [Google Scholar]