Abstract
At the onset of agonistic social challenge, individuals must assess the degree of threat the opponent represents in order to react appropriately. We aimed to characterize the neuroendocrine changes accompanying this period of initial social assessment using the lizard Anolis carolinensis. Conveyance of aggressive intent by male A. carolinensis is facilitated by rapid post-orbital skin darkening (eyespot), whereas eyespot presence inhibits opponent aggression. By manipulating this visual signal, we also investigated whether differing neuroendocrine changes were evoked by initial presentation of varying levels of social threat. Subjects were painted postorbitally either with black paint (high threat level), green paint (low threat level) or water (controls). Painted animals were presented with a mirror and sampled immediately upon exhibiting aggressive intent towards the reflected simulated opponent, but before producing behaviors such as motor pattern-based displays. Control animals (blank surface presented) were sampled at times derived from averaging response times of painted subjects. Brains and plasma were analyzed for monoamine activity and catecholamine levels using electrochemical HPLC. Social threat evoked increases in plasma catecholamine levels indistinguishable from those caused by brief environmental disturbance. However, brief social challenge caused distinct rapid increases in amygdala and nucleus accumbens (NAc) dopamine and serotonin levels. Amygdalar changes were associated with general social threat presence, but NAc monoamines were affected by both threat level and subject motivation to engage in confrontation. This suggests that specific rapid activity changes in key forebrain limbic nuclei differ according to the degree of social threat perceived at the start of the interaction.
Keywords: social stress, dopamine, serotonin, accumbens, amygdala, catecholamine, lizard
1. Introduction
Upon presentation with an agonistic social challenge, individuals must first assess the level of threat posed by the opponent, so that adaptive behavioral responses appropriate to that particular situation can be produced [1]. Such behavioral expression is often associated with physiological stress responses, which help to restore homeostasis and allow individuals to cope with the aversive context [1,2]. While a number of studies have demonstrated alterations to neurochemical and hormonal states during and following stressful agonistic encounters [3–5], little is known about the neuroendocrine changes that may accompany initial social threat assessment and manifest prior to the production of aggressive or submissive behaviors.
Peripheral neuroendocrine responses to stressful agonistic encounters include sympathetically-mediated release of adrenal catecholamines and activation of the hypothalamic-pituitary-adrenal (HPA) axis, while central responses are evidenced by changes in limbic monoaminergic activity [5–7]. In particular, alterations to dopamine (DA) and serotonin (5-HT) function within discrete basal ganglia and mesolimbic nuclei, including key forebrain regions such as the nucleus accumbens (NAc) and amygdala, occur during or immediately following production of agonistic social behavior [4,8–10]. Enhanced DA activity in the NAc normally mediates expression of motivated or goal-directed behaviors during both rewarding and aversive situations [11,12], while increased amygdalar 5-HT activity is associated with stress, fear and anxiety [13–17]. To better understand the temporal nature of limbic monoamine and peripheral hormone activation during social challenge, it is important to quantify the specific neuroendocrine changes elicited by discrete stages of a social interaction, such as during initial perception of social threat or following production of behavioral responses as conflict ensues.
The lizard Anolis carolinensis has become well established as a model for examining the effects of agonistic social stress on neuroendocrine activity [5,9,10]. During the early stages of interaction between A. carolinensis males, conveyance of aggressive intent is facilitated by the darkening of postorbital skin (known as the “eyespot”), caused by sympathetic catecholamine activation of β-adrenergic receptors [6,18]. Males that most rapidly manifest the eyespot win at least 95% of social contests [2,19], and eyespot presence will inhibit opponent aggression [20,21]. Prior manipulation of the eyespot will also regulate the amount of aggression expressed towards a mirrored image [4,22]. Subjects that have had eyespots hidden appear to perceive the reflected opponent as less threatening, and exhibit high amounts of aggression in the form of stereotyped motor-pattern based displays. In contrast, males viewing a mirror image with artificially darkened eyespots show greater latency to respond and are far less aggressive, possibly indicating that the reflected opponent has been perceived as highly threatening [4,22]. However, in these studies and others conducted with live opponents, subjects were exposed to social stress for 10 min or more [2,4,5,22], with measurements of physiological activity taken at the conclusion of the interaction. Thus, the relative contributions of social threat perception, threat level assessment and behavioral reaction on the temporal induction of neuroendocrine responses could not be adequately separated.
The aim of the current study was to characterize the neuroendocrine changes likely to occur at the start of an interaction, when individuals must perceive both the presence of the opponent and the degree of challenge it represents before producing an appropriate behavioral response [1]. To achieve this, we assessed whether brief presentation of a simulated (mirrored) opponent induces rapid neuroendocrine stress responses prior to subject production of motor pattern-based aggressive displays. Specifically, we examined if acute social challenge would elicit changes both in systemic catecholamine release and in DA and 5-HT activity within the NAc and amygdala. We chose these limbic regions based on their known association with aggression and stress responses, their homology with mammalian systems [23–25], and our previous data demonstrating robust changes in DA and 5-HT activity in response to a range of stressors, including social stress [4,5,26–28]. We also determined whether these neuroendocrine responses differed with the level of social threat, i.e., with the absence or presence of eyespots on the reflected opponent. Finally, we investigated whether neuroendocrine effects of social threat could be separated from those of equally brief environmental disturbance.
2. Methods
2.1. Animals
Adult male lizards from a commercial supplier (Marabella’s, Gonzales, LA, USA) were housed individually for three weeks in 5 gallon aquaria fitted with a diagonal wooden perch, given free access to water and fed 2 live crickets every other day. All subjects were seen to feed reliably (minimum one cricket per feeding) for 10 days prior to the start of experiments, and there was no difference among treatment groups in mass at the time of testing (mean±SE all subjects = 4.62±0.11 g). Environmental parameters (14L33°C:10D21°C, relative humidity 55–85%) were regulated to maintain gonadal activity [29]. Only males that indicated territoriality by producing stereotyped motor displays in the presence of a live female were used in this study. The following experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. In addition, all efforts were made to minimize the number of animals used and to ensure high standards of welfare during experimentation.
2.2. Behavioral treatments and testing
The level of social threat was manipulated by altering appearance of the eyespot 3 days prior to exposing the subjects to their own reflection. A low level of social threat was created by covering the postorbital regions of subjects with green paint (n = 16) to hide formation and appearance of the eyespot, such that the reflected opponent would appear less threatening upon initial presentation [4,22]. In contrast, a high social stress level was generated by treating a separate group of subject males with black paint (n = 17) to darken the eyespot permanently and thus mimic a highly aggressive opponent in the mirror image. Subjects were caught and restrained gently by hand (mean restraint time = 68.3 + 0.46 s) while the eyespot was painted. Three days later, a mirror was introduced into each subject’s enclosure. Subject perception of social threat (reflected opponent, i.e., the mirror image) was indicated by ventrolateral swelling of the throat, produced by extension of the basi-hyal component of the hyoid apparatus [30]. This visual signal is commonly expressed by contesting males in the very opening stages of social interaction [30, Watt, pers. obs.], and appears also to indicate individual willingness to respond aggressively to the challenge, as it precedes production of motor pattern-based displays and other directed offensive behaviors [30, 31, Watt, pers. obs.]. This throat swelling differs markedly from the full dewlap or gular flap extensions as generated by erection of the long retrobasal process of the hyoid apparatus [30], which causes the loose skin around the throat to be extended to its ventral extreme while being flattened laterally, creating a large brightly colored dewlap or throat fan. Throat swelling and dewlap extension also differ in their contextual use, with throat swelling only expressed by males when directly challenged by an opponent [30]. In contrast, dewlap extension is not produced as a lone signal but is normally added to a sequence of head-bobbing and pushup movements to form complex stereotyped displays that are produced under a wider range of social conditions, including territorial advertisement, courtship and social challenge [30–32].
Immediately upon exhibiting throat swelling towards the reflected opponent, subjects were caught and decapitated (mean±SE latency from response onset to capture and decapitation = 9.07±0.58 s). All subjects responding with throat swelling were caught before producing any motor-pattern based stereotyped aggressive displays. Trials were otherwise terminated at 300 s if the subject failed to express the swollen throat. It was noted that such lack of throat swelling only occurred among a subset of the high social threat treatment group. These subjects were included as a separate group for data analyses (for further details, see Section 2.6 below). We also recorded any change in body color over the course of the trial [20], as darkening of the skin in A. carolinensis is characteristic of elevated adrenergic activity and subordinate social behavior, while retention of normal bright green coloration is indicative of aggression and social dominance [6].
Two other subject groups were also included as experimental controls. Environmentally disturbed controls (n = 16) had eyespot regions painted with water, and were exposed 3 days later to a blank white screen before being caught and decapitated. Exposure time for each subject in this group was generated using a sliding scale, which was based on continually updated collection times averaged from the response latencies of all social threat subjects. Undisturbed controls (n = 13) comprised animals collected from their home cages, with no prior restraint, eyespot manipulation or environmental disturbance, i.e., mirror/blank screen insertion. Like experimental groups, controls were rapidly caught and decapitated (mean±SE time for capture and decapitation across all groups = 9.62±0.39 s). Blood was collected using heparinized microcapillary tubes, centrifuged and plasma drawn off. Brains were left in situ, and both heads and plasma were stored at −80°C immediately after collection.
2.3. Plasma catecholamine analysis
The details of this assay have been published elsewhere [33]. Briefly, 70 μl plasma, 20 μl 2,3-dihydroxybenzoic acid (DHBA, internal standard) and 1 ml 1.86M Tris buffer (pH 8.65) were added to 50 mg of acid-washed aluminum oxide and samples rotated for 10 min. The alumina was then washed four times with 1 ml dH2O (pH 7.0) and catecholamines extracted by adding 100 μl 0.1N perchloric acid (HClO4). Sixty μl of the HClO4 extract was injected into an HPLC system (Waters 717 Plus Autosampler) and levels of epinephrine (Epi) and norepinephrine (NE) analyzed electrochemically with an LC-4C amperometric detector (BioAnalytical Systems, Inc., IN). The electrode potential was set at +0.6 V with respect to an Ag/AgCl reference electrode. The mobile phase consisted of 14 g citric acid, 8.6 g sodium acetate, 110 mg 1-octanesulfonic acid (sodium salt), 150 mg EDTA disodim salt and 100 ml methanol in 1 L deionized water.
2.5. Measurement of accumbal and amygdalar monoamines
Details of this assay have also been published elsewhere [26,33]. Brains were sliced in situ at −12°C in 300 μm coronal sections and thaw-mounted on glass slides and refrozen for microdissection. The amygdala and NAc were identified using a stereotaxic atlas [34] and a map of central catecholamine staining [35] for A. carolinensis, and microdissected using a 300 μm id. cannula. These two regions have been shown previously to be involved in responses to different stress types [4,5,26–28]. Accumbal samples comprised tissue homologous to mammalian NAc shell [35–38] while amygdalar samples correspond to the combined central, lateral and medial regions of the mammalian amygdaloid complex without inclusion of the basolateral nucleus homologue [23–25,39].
Brain samples were expelled into 60 μl sodium acetate buffer (pH 5.0; 1 sample per vial) containing 9.42 pg/μl DHBA (internal standard). Each sample was frozen to cause cell lysis, which allows the release of soluble monoamines and their metabolites into the buffer solution upon thawing [40]. Each thawed sample had 2 ml of 1 mg/ml ascorbate oxidase added, and was then centrifuged at 15 000 × g for 3 min. Supernatant (45 μl) was injected into the same HPLC system used for plasma catecholamine measurement, and levels of dopamine (DA), the dopamine metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), serotonin (5-HT) and the serotonin metabolite 5-hyroxyindoleacetic acid (5-HIAA) were detected electrochemically. The tissue pellet remaining from each sample was dissolved in 110 ml 0.1 N NaOH and protein content assayed by coulometric absorbance using the Bradford method [41].
2.6. Data analysis
Latency to exhibit the throat swelling response to the mirror image was compared between responding subjects in the low and high social stress groups using unpaired t-test. All subjects in the low threat group exhibited throat swelling. However, it was noted that distinct behavioral differences occurred in the high social threat group, such that 12 subjects responded with throat swelling to the image while 5 did not produce any signals indicative of potential aggressive intent, implying individual variation in stress level perception. The proportion of individuals exhibiting throat swelling was compared statistically between high and low stress groups using Fisher’s exact test. Based on exhibition or not of the throat swelling response, subjects were allocated into separate groups (low threat responders, high stress responders and high stress non-responders) for analyses of plasma catecholamines and central monoamines.
Levels of plasma catecholamines and central monoamines were obtained and corrected for recovery using CSW32 v1.4 Chromatography Station for Windows (DataApex, Prague, Czech Republic). Plasma catecholamines were expressed as pg amine/μl plasma. For central monoamines, neurotransmitter and metabolite levels were expressed as pg amine/μg protein. We chose to use separate neurotransmitter and metabolite concentrations for estimates of monoaminergic activity, as previously justified by Korzan et al. [21] and Waters et al. [28], rather than the commonly used ratios of metabolite to transmitter. Briefly, when rapid synthesis or reuptake of transmitter coincides with extensive release and catabolism, both monoamine transmitter and metabolite concentrations are likely to rise. While this clearly indicates an increase in system activity, the ratio remains unchanged and is not valuable for determining monoaminergic activity. Second, measurements closely following stimuli, during a period when transmitter synthesis is rising, may reflect increased concentrations of transmitter from synthesis and non-metabolized contemporary release [21,28], as may occur with presentation of a brief stress such as used in the current study.
Levels of limbic monoamines and metabolites were compared across treatment groups (low stress responders, high stress responders, high stress non-responders, environmentally disturbed, and undisturbed controls) by separate one way analysis of variance (ANOVA), followed by Student-Newman-Keuls tests for all pairwise comparisons. Since data for levels of plasma catecholamines did not meet assumptions of equal variance and normality, non-parametric Kruskal-Wallis one way ANOVA on ranks with Dunn’s multiple comparison procedure were used. All analyses were performed using SigmaStat v.2.03, with the alpha level set at 0.05.
3. Results
3.1. Subject responses to brief social challenge
The majority of subjects exposed to a mirrored opponent rapidly expressed throat swelling, with no difference in latency to exhibit this signal between low threat (mean±SE latency = 77.3±18.2 s) and high threat (mean±SE latency = 102.5±23.7 s) treatments (t = −0.859, df = 26, P = 0.398). However, when compared to the low threat group, a significantly lower proportion of subjects in the high threat group expressed throat swelling towards the mirror image (low stress group 100%, high stress group 70.6%, χ2 = 5.54, P < 0.05). Subjects that did not respond with throat swelling in the high threat group (29.4%) all remained completely still with gaze fixed on the mirrored opponent for the duration of the trial, often compressing dorsoventrally to the substrate, and did not produce any signals conveying aggressive intent. This response was markedly distinct from that observed for environmentally disturbed controls (no social stimulus). All environmentally disturbed controls showed a slight startle response to introduction of the blank screen, but very quickly recovered from this and resumed normal basking and exploratory behaviors. Subjects in all groups maintained their normal body coloration of bright green, with no darkening noted.
3.2. Plasma catecholamines
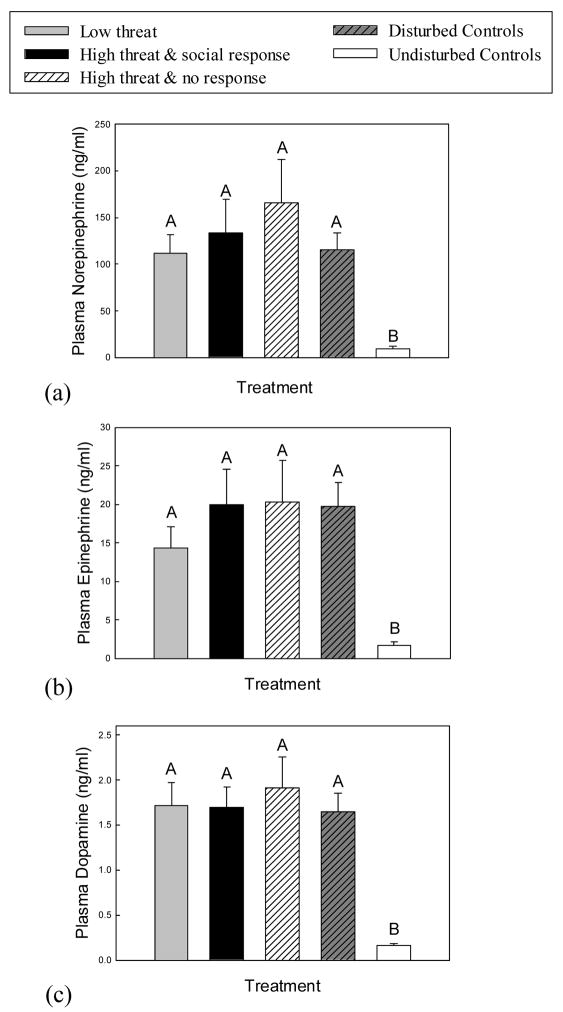
Plasma NE levels differed according to experimental treatment (H = 26.04, df = 4, P < 0.001), with all subjects experiencing either social threat or environmental disturbance showing increased NE relative to the undisturbed control group (P < 0.05, Fig 1A). Plasma Epi levels (H = 27.83, df = 4, P < 0.001) and DA levels (H = 26.97, df = 4, P < 0.001) also differed across experimental groups, with Epi and DA levels increased in social threat and environmentally disturbed groups compared to undisturbed animals (P < 0.05, Figs 1B and C). However, there were no differences in either NE, Epi or DA levels among environmentally disturbed, low social threat, high social threat/responder and high social threat/non-responder groups (P > 0.05).
Fig. 1.
Mean (+SE) plasma catecholamine levels in response to experimental treatment. (a) Plasma norepinephrine (NE), (b) epinephrine (Epi) and (c) dopamine (DA) increased in all subjects experiencing either social threat or environmental disturbance. Means with no common superscript letters (e.g., A, B) are significantly different (P < 0.05). Social response refers to expression of throat swelling, which indicates aggressive intent; no response refers to lack of throat swelling during the trial. All subjects in the low threat treatment responded with throat swelling.
3.3. Limbic Monoamines
3.3.1. Nucleus accumbens (NAc)
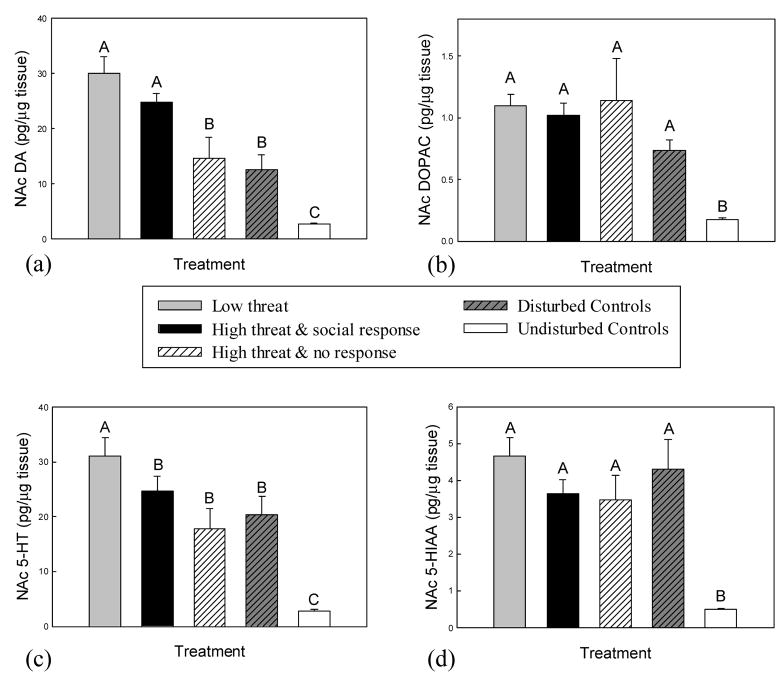
Levels of NAc DA differed significantly with treatment (F(4, 59) = 21.52, P < 0.001, Fig 2A). Subjects that responded with throat swelling in the low and the high threat treatments exhibited the greatest increases in DA (P < 0.03), with comparable levels in both groups (P = 0.12). Levels of NAc DA did not differ between high threat non-responders and environmentally disturbed controls (P = 0.64), but both these groups exhibited higher DA levels than undisturbed controls (P < 0.03).
Fig. 2.
Mean (+SE) accumbal (NAc) levels of (a) DA, (b) DOPAC, (c) 5-HT and (d) 5-HIAA in response to experimental treatment. Levels of NAc DA and 5-HT were increased significantly in subjects that indicated motivation to engage in confrontation, especially within the low threat treatment. Means with no common superscript letters (e.g., A, B) are significantly different (P < 0.05). Social response refers to expression of throat swelling, which indicates aggressive intent; no response refers to lack of throat swelling during the trial. All subjects in the low threat treatment responded with throat swelling.
There was a significant effect of experimental treatment on NAc DOPAC levels (F(4, 55) = 15.07, P < 0.001, Fig. 2B). Levels of NAc DOPAC in all socially threatened and environmentally disturbed subjects were increased relative to undisturbed controls (P < 0.003). However, there were no differences in NAc DOPAC levels among environmentally disturbed, low social threat, high social threat/responder and high social threat/non-responder groups (P > 0.07).
Accumbal 5-HT levels also showed significant differences with treatment (F(4, 52) = 21.35, P < 0.001, Fig 2C). While all subjects experiencing either social challenge or environmental disturbance exhibited increased NAc 5-HT compared to undisturbed controls (P < 0.002), the greatest increases were seen in the low social threat group (P < 0.001). No differences in NAc 5-HT were measured among environmentally disturbed, high social threat/responder and high social threat/non-responder groups (P > 0.072).
Experimental treatment had a significant effect on NAc 5-HIAA levels (F(4, 58) = 12.4, P < 0.001, Fig 2D). Similar to DOPAC, NAc 5-HIAA levels were increased relative to undisturbed controls in socially threatened and environmentally disturbed subjects (P < 0.003), but there were no differences in NAc 5-HIAA among these four treatment groups (P > 0.115).
3.3.2. Amygdala
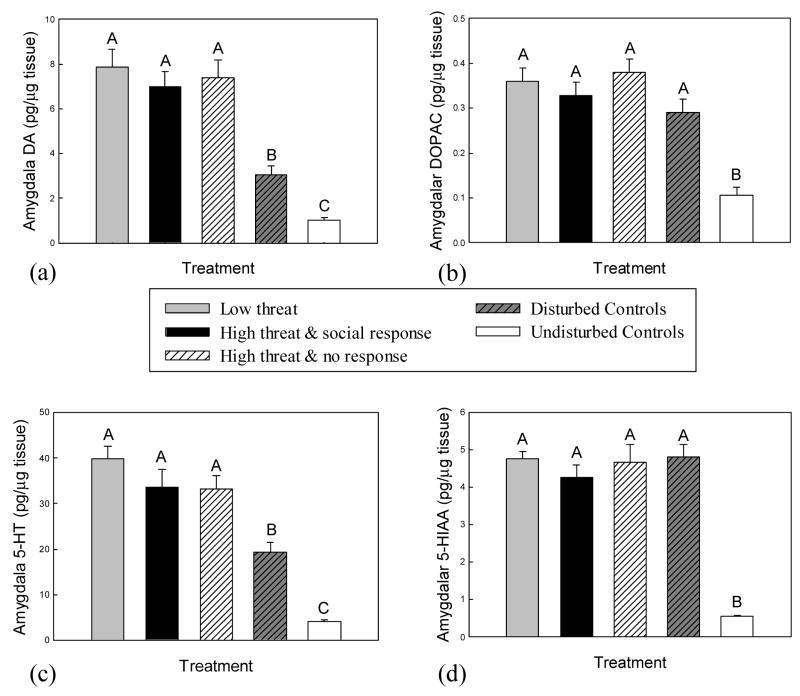
Levels of amygdalar DA differed significantly with treatment (F(4, 53) = 24.3, P < 0.001, Fig. 3A). All subjects experiencing social threat exhibited significantly higher amygdalar DA levels than environmentally disturbed controls and undisturbed controls (P < 0.001), but DA levels did not vary among the three social threat groups (P > 0.131). Environmentally disturbed subjects showed significant increases in DA levels compared to undisturbed controls (P = 0.02).
Fig. 3.
Mean (+SE) amygdalar levels of (a) DA, (b) DOPAC, (c) 5-HT and (d) 5-HIAA in response to experimental treatment. Social threat elicited significantly higher amygdalar DA and 5-HT levels than environmental disturbance, but there was no difference according to either level of perceived threat or expression of social response. Means with no common superscript letters (e.g., A, B) are significantly different (P < 0.05). Social response refers to expression of throat swelling, which indicates aggressive intent; no response refers to lack of throat swelling during the trial. All subjects in the low threat treatment responded with throat swelling.
There was a significant effect of experimental treatment on amygdalar DOPAC levels (F(4, 56) = 15.48, P < 0.001, Fig 3B). Amygdalar DOPAC levels were increased relative to undisturbed controls in socially threatened and environmentally disturbed subjects (P < 0.001), but there were no differences in amygdalar DOPAC among these latter treatment groups (P > 0.09).
Amygdalar 5-HT levels also differed significantly with treatment (F(4, 57) = 39.6, P < 0.001; Fig 3C), showing a similar pattern to amygdalar DA. While all subjects exposed to social threat exhibited higher 5-HT than either environmentally disturbed or undisturbed controls (P < 0.005), there were no differences among the three social threat groups according to level of threat or type of subject response (P > 0.11). Amygdalar 5-HT was also increased in environmentally disturbed controls relative to undisturbed controls (P < 0.001).
Experimental treatment had a significant effect on amygdalar 5-HIAA levels (F(4, 56) = 45.59, P < 0.001; Fig 3D). Amygdalar 5-HIAA levels among socially threatened and environmentally disturbed subjects did not differ (P > 0.38) but were increased relative to undisturbed controls (P < 0.001).
4. Discussion
Manipulation of eyespots alone was effective in generating differing levels of social threat, as significantly fewer subjects indicated potential intent to respond aggressively when briefly exposed to a non-displaying mirrored opponent bearing darkened eyespots. This result highlights the importance of initial eyespot perception by contesting male A. carolinensis in directing the course and outcome of social interactions, and further validates the artificial manipulation of this signal as a reliable means of altering the degree of social threat experienced by subjects [4,20–22,42]. While increases in plasma catecholamine levels did not differ with the level of social threat, distinct differences in accumbal DA and 5-HT levels were generated according to perceived threat level and individual variance in social response. In contrast, amygdalar DA and 5-HT levels increased independent of either threat level or type of social response.
4.1. Plasma catecholamines and sympathetic response to brief stress exposure
Brief presentation of social threat effectively increased plasma levels of NE, Epi and DA. However, these increases in plasma catecholamines were indistinguishable from those induced by equally brief environmental disturbance. Furthermore, there were no detectable differences in plasma catecholamines according to the level of perceived social threat. In previous studies, male A. carolinensis viewing a reflected opponent with pre-darkened eyespots exhibited decreased aggression and plasma catecholamine levels, while increased aggression and catecholamine levels were elicited by a simulated opponent with hidden eyespots [22]. However, the duration of mirror exposure was greater than that used in the current study, and subjects had the opportunity to produce dynamic and sustained behavioral responses [22]. Results of the current experiment suggest that perception of eyespots alone and awareness of threat level conveyed at the start of an interaction does not manifest immediately and directly in sympathetic activity variance. Rather, the increased plasma catecholamine levels elicited by brief social threat and environmental disturbance appear to represent initiation of the sympathetic fight or flight response, allowing maintenance and restoration of homeostasis following stress-induced metabolic disruption [43–45]. Capture and handling stress of comparable duration to the disturbance time used in our study generates increased plasma catecholamine levels in A. carolinensis and other lizards [7,27], with similar increases being elicited by equally brief social interaction [7,46]. Differences in sympathetic activity induced by more prolonged social confrontation [6,20,22] may only become apparent following a combination of social threat level establishment and production of behavioral responses.
4.2. Effects of social threat on limbic monoamines
Monoaminergic activity in forebrain limbic regions showed changes with presentation of social threat that were distinct from those induced by brief environmental disturbance. In both the NAc and amygdala, differences in monoaminergic activity resulting from either social threat level or type of subject response were evidenced by increases in levels of DA and 5-HT rather than their catabolites. This may be indicative of rapid pathway priming through increased synthesis, storage, terminal release and/or enhanced reuptake of neurotransmitter following initial threat perception, prior to increases in catabolite levels that may occur as behavioral responses start being produced [21,28].
Subjects demonstrating threat perception and potential willingness to fight regardless of threat level exhibited higher NAc DA levels than those that indicated lack of aggression in the high threat treatment. This rapid increase in NAc DA may reflect individual motivation to start escalating the contest through production of stereotyped displays and other aggressive behavior, similar to the NAc DA increases that occur prior to expression of goal-directed behaviors in mammalian models [3,8,11]. While all subjects indicating aggressive intent exhibited comparable NAc DA increases, there were differences in NAc 5-HT levels according to degree of social threat, with subjects in the low threat treatment showing the greatest increases in NAc 5-HT. Accumbal 5-HT levels were increased in all treatments relative to undisturbed controls. These rapid increases in NAc 5-HT levels following brief social threat or environmental disturbance may either facilitate NAc DA-mediated motivational behaviors [47,48], or enhance survival by reducing expression of inappropriate behavior in aversive contexts [49,50].
Brief presentation of a simulated opponent elicited increased levels of amygdalar DA and 5-HT, which were greater than those caused by environmental disturbance. However, there were no differences in amygdalar DA and 5-HT level increases according to degree of social threat. Similarly, DA and 5-HT increases did not differ with expression of aggressive intent by subjects. Previous studies have noted differences in amygdalar monoamine levels as a result of difference in social threat level, but these alterations were only evident following prolonged social interaction and establishment of social hierarchy [4,42]. For example, prolonged exposure to a mirror image simulating a low threat level elicited higher amygdalar monoaminergic activity than exposure to a high threat level, but subjects had the opportunity to produce sustained behavioral responses [4]. Similarly, amygdalar serotonergic activity was elevated in animals that achieved dominant status after interacting with a live opponent that had been manipulated to appear less threatening [42]. Combined, our results and those of previous studies suggest that initial presentation of social threat alone is sufficient in eliciting increases in amygdalar monoamine concentrations independent of perceived threat level, but both sustained behavioral activity and establishment of social hierarchy may be necessary for differences in amygdala monoamine concentrations to correlate with social threat level.
Increased amygdalar serotonergic activity is associated with social stress across vertebrate taxa [10], and is known to enhance mammalian hypothalamic-pituitary-adrenal (HPA) axis activity and glucocorticoid release [51,52]. The rapid increases in A. carolinensis amygdalar DA levels with social threat occurred in areas homologous with the central, lateral and medial nuclei of the mammalian amygdala [23–25]. Exposure to aversive conditioned stimuli is associated with increased DA levels in the central and lateral nuclei of the mammalian amygdala [53,54], and DA activity in the lateral amygdala is essential for inducing synaptic plasticity underlying acquisition of conditioned fear memories [55,56]. Results of the current study indicate that brief social threat is more effective than environmental disturbance at inducing the changes in amygdalar monoaminergic activity associated with exposure to stress, and perhaps facilitates changes associated with conditioned learning about potential social conflict. However, unlike in the NAc, variance in individual assessment of the degree of social threat is not revealed by differences in amygdalar monoamine levels.
4.3. Conclusions
Brief presentation of a simulated opponent was effective in evoking rapid changes in neuroendocrine activity. Although sympathetically-mediated plasma catecholamine increases elicited by social threat were not different from those caused by environmental disturbance, social challenge caused distinct changes in NAc and amygdala DA and 5-HT levels. Changes in the amygdala were associated with general social threat presence, but NAc monoamines were affected by the level of social threat presented along with individual variation in threat level perception and motivation to convey aggressiveness. This suggests that initial awareness of an opponent and assessment of the degree of challenge it represents combine to induce specific rapid activity changes in key forebrain limbic nuclei, which may initiate appropriate behavioral responses to allow the individual to cope with a potentially aversive situation.
Acknowledgments
This research was funded by National Institutes of Health (NIH) grants P20 RR15567 (Center for Biomedical Research Excellence [COBRE]), 1 R03 MH068364 (MJW), 1 R03 MH068303 (GLF) and 1 F31 MH64983 (WJK). Experiments were conducted under protocol 01-Anolis-08, approved by University of South Dakota Institutional Animal Care and Use Committee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenan CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999:925–35. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 2.Summers CH. Social interaction over time, implications for stress responsiveness. Integr Comp Biol. 2002;42:591–9. doi: 10.1093/icb/42.3.591. [DOI] [PubMed] [Google Scholar]
- 3.Miczek KA, Weerts E, Haney M, Tidey J. Neurobiological mechanisms controlling aggression: Preclinical developments for pharmacotherapeutic interventions. Neurosci Biobehav Rev. 1994;18:97–110. doi: 10.1016/0149-7634(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 4.Korzan WJ, Summers TR, Summers CH. Monoaminergic activities of limbic regions are elevated during aggression: influence of sympathetic social signaling. Brain Res. 2000;870:170–8. doi: 10.1016/s0006-8993(00)02420-3. [DOI] [PubMed] [Google Scholar]
- 5.Summers CH, Summers TR, Moore MC, Korzan WJ, Woodley SK, Ronan PJ, Höglund E, Watt MJ, Greenberg N. Temporal patterns of limbic monoamine and plasma corticosterone response during social stress. Neurosci. 2003;116:553–63. doi: 10.1016/s0306-4522(02)00708-x. [DOI] [PubMed] [Google Scholar]
- 6.Summers CH, Greenberg N. Somatic correlates of adrenergic activity during aggression in the lizard, Anolis carolinensis. Horm Behav. 1994;28:29–40. doi: 10.1006/hbeh.1994.1003. [DOI] [PubMed] [Google Scholar]
- 7.Matt KS, Moore MC, Knapp R, Moore IT. Sympathetic mediation of stress and aggressive competition: plasma catecholamines in free-living male tree lizards. Physiol Behav. 1997;61:639–47. doi: 10.1016/s0031-9384(96)00500-8. [DOI] [PubMed] [Google Scholar]
- 8.Miczek KA, Fish EW, De Bold JF, De Almeida RM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacol. 2002;163:434–58. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- 9.Summers CH, Forster GL, Korzan WJ, Watt MJ, Larson ET, Øverli Ø, Höglund E, Ronan PJ, Summers TR, Renner KJ, Greenberg N. Dynamics and mechanics of social rank reversal. J Comp Physiol A. 2005;191:241–52. doi: 10.1007/s00359-004-0554-z. [DOI] [PubMed] [Google Scholar]
- 10.Summers CH, Korzan WJ, Lukkes JL, Øverli Ø, Höglund E, Watt MJ, Larson ET, Forster GL, Ronan PJ, Summers TR, Renner KJ, Greenberg N. Does serotonin influence aggression? Comparing regional activity before and during social interaction. Physiol Biochem Zool. 2005;78:679–94. doi: 10.1086/432139. [DOI] [PubMed] [Google Scholar]
- 11.Blaha CD, Phillips AG. A critical assessment of electrochemical procedures applied to the measurement of dopamine and its metabolites during drug-induced and species-typical behaviors. Behav Pharmacol. 1996;7:675–708. [PubMed] [Google Scholar]
- 12.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 13.Reuters LE, Jacobs BL. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res. 1996;739:57–69. doi: 10.1016/s0006-8993(96)00809-8. [DOI] [PubMed] [Google Scholar]
- 14.Graeff FG, Viana MB, Mora PO. Dual role of 5-HT in defense and anxiety. Neurosci Biobehav Rev. 1997;21:791–9. doi: 10.1016/s0149-7634(96)00059-0. [DOI] [PubMed] [Google Scholar]
- 15.Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–20. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- 16.Konstandi M, Johnson E, Lang MA, Malamas M, Marselos M. Noradrenaline, dopamine, serotonin: different effects of psychological stress on brain biogenic amines in mice and rats. Pharmacol Res. 2000;41:341–6. doi: 10.1006/phrs.1999.0597. [DOI] [PubMed] [Google Scholar]
- 17.Zangrossi H, Jr, Viana MB, Zanoveli J, Bueno C, Nogueira RL, Graeff FG. Serotonergic regulation of inhibitory avoidance and one-way escape in the rat elevated T-maze. Neurosci Biobehav Rev. 2001;25:637–45. doi: 10.1016/s0149-7634(01)00047-1. [DOI] [PubMed] [Google Scholar]
- 18.Hadley ME, Goldman JM. Physiological color changes in reptiles. Am Zool. 1969;9:489–504. doi: 10.1093/icb/9.2.489. [DOI] [PubMed] [Google Scholar]
- 19.Larson ET, Summers CH. Serotonin reverses dominant social status. Behav Brain Res. 2001;121:95–102. doi: 10.1016/s0166-4328(00)00393-4. [DOI] [PubMed] [Google Scholar]
- 20.Korzan WJ, Summers TR, Summers CH. Manipulation of visual sympathetic sign stimulus modifies social status and plasma catecholamines. Gen Comp Endocrinol. 2002;128:153–61. doi: 10.1016/s0016-6480(02)00077-1. [DOI] [PubMed] [Google Scholar]
- 21.Korzan WJ, Forster GL, Watt MJ, Summers CH. Dopaminergic activity modulation via aggression, status and a visual social signal. Behav Neurosci. 2006;120:93–102. doi: 10.1037/0735-7044.120.1.93. [DOI] [PubMed] [Google Scholar]
- 22.Korzan WJ, Summers TR, Ronan PJ, Summers CH. Visible sympathetic activity as a social signal in Anolis carolinensis: changes in aggression and plasma catecholamines. Horm Behav. 2000;38:193–9. doi: 10.1006/hbeh.2000.1619. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Garcia F, Olucha FE, Teruel V, Lorente MJ. Fiber connections of the amygdaloid formation of the lizard Podarcis hispanica. Brain Behav Evol. 1993;41:156–62. doi: 10.1159/000113833. [DOI] [PubMed] [Google Scholar]
- 24.Bruce LL, Neary TJ. The limbic system of tetrapods: a comparative analysis of cortical and amygdalar populations. Brain Behav Evol. 1995;46:224–34. doi: 10.1159/000113276. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Garcia F, Martinez-Marcos A, Lanuza E. The pallial amygdala of amniote vertebrates: Evolution of the concept, evolution of the structure. Brain Res Bull. 2002;57:463–9. doi: 10.1016/s0361-9230(01)00665-7. [DOI] [PubMed] [Google Scholar]
- 26.Emerson AJ, Kappenman DP, Ronan PJ, Renner KJ, Summers CH. Stress induces rapid changes in serotonergic activity: restraint and exertion. Behav Brain Res. 2000;111:83–92. doi: 10.1016/s0166-4328(00)00143-1. [DOI] [PubMed] [Google Scholar]
- 27.Forster GL, Watt MJ, Korzan WJ, Renner KJ, Summers CH. Differential activation and recovery of central monoamines and plasma hormones following brief restraint stress. Horm Behav. 2004;46:111. [Google Scholar]
- 28.Waters RP, Emerson AJ, Watt MJ, Forster GL, Swallow JG, Summers CH. Stress induces rapid changes in central catecholaminergic activity in Anolis carolinensis: restraint and forced physical activity. Brain Res Bull. 2005;67:210–8. doi: 10.1016/j.brainresbull.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 29.Licht P. Regulation of the annual testis cycle by photoperiod and temperature in the lizard, Anolis carolinensis. Ecology. 1971;52:240–52. [Google Scholar]
- 30.Greenberg N. Sociality, stress, and the corpus striatum of the green anolis lizard. Physiol Behav. 2003;79:429–40. doi: 10.1016/s0031-9384(03)00162-8. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg N. A neuroethological study of display behavior in the lizard, Anolis carolinensis (Reptilia, Lacertilia, Iguanidae) Amer Zool. 1977;17:191–201. [Google Scholar]
- 32.DeCourcy KR, Jenssen TA. Structure and use of male territorial headbob signals by the lizard Anolis carolinensis. Anim Behav. 1994;47:251–62. [Google Scholar]
- 33.Höglund E, Korzan WJ, Watt MJ, Forster GL, Summers TR, Johannessen HF, Renner KJ, Summers CH. L-DOPA on aggressive behavior and central monoaminergic activity in the lizard Anolis carolinensis, using a new method for drug delivery. Behav Brain Res. 2005;156:53–64. doi: 10.1016/j.bbr.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg N. A forebrain atlas and stereotaxic technique for the lizard, Anolis carolinensis. J Morphol. 1982;174:217–36. doi: 10.1002/jmor.1051740210. [DOI] [PubMed] [Google Scholar]
- 35.Lopez KH, Jones RE, Seufert DW, Rand MS, Dores RM. Catecholaminergic cells and fibers in the brain of the lizard Anolis carolinensis identified by traditional as well as whole-mount immunohistochemistry. Cell Tissue Res. 1992;270:319–37. doi: 10.1007/BF00328017. [DOI] [PubMed] [Google Scholar]
- 36.Gonzales A, Russchen FT, Lohman AHM. Afferent connections of the striatum and the nucleus accumbens in the lizard Gekko gecko. Brain Behav Evol. 1990;36:39–58. doi: 10.1159/000115296. [DOI] [PubMed] [Google Scholar]
- 37.Reiner A, Medina L, Veenman CL. Structural and functional evolution of the basal ganglia in vertebrates. Brain Res Rev. 1998;28:235–85. doi: 10.1016/s0165-0173(98)00016-2. [DOI] [PubMed] [Google Scholar]
- 38.Guirado S, Davila JC, Real MA, Medina L. Nucleus accumbens in the lizard Psammodromus algirus: chemoarchitecture and cortical afferent connections. J Comp Neurol. 1999;405:15–31. doi: 10.1002/(sici)1096-9861(19990301)405:1<15::aid-cne2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 39.Lanuza E, Belekhova M, Martinez-Marcos A, Font C, Martinez-Garcia F. Identification of the reptilian basolateral amygdala: an anatomical investigation of the afferents to the posterior dorsal ventricular ridge of the lizard Podarcis hispanica. Eur J Neurosci. 1998;10:3517–34. doi: 10.1046/j.1460-9568.1998.00363.x. [DOI] [PubMed] [Google Scholar]
- 40.Renner KJ, Luine VN. Determination of monoamines in brain nuclei by high performance liquid chromatography with electrochemical detection: young vs. middle aged rats Life Sci. 1984;34:2193–9. doi: 10.1016/0024-3205(84)90320-5. [DOI] [PubMed] [Google Scholar]
- 41.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 42.Korzan WJ, Summers CH. Serotonergic response to social stress and artificial social sign stimuli during paired interactions between male Anolis carolinensis. Neurosci. 2004;123:835–45. doi: 10.1016/j.neuroscience.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Wilson MA, Gatten RE, Jr, Greenberg N. Glycolysis in Anolis carolinensis during agonistic encounters. Physiol Behav. 1990;48:139–42. doi: 10.1016/0031-9384(90)90274-8. [DOI] [PubMed] [Google Scholar]
- 44.Gleeson TT, Dalessio PM, Carr JA, Wickler SJ, Mazzeo RS. Plasma catecholamine and corticosterone and their in vitro effects on lizard skeletal muscle lactate metabolism. Am J Physiol. 1993;265:R632–9. doi: 10.1152/ajpregu.1993.265.3.R632. [DOI] [PubMed] [Google Scholar]
- 45.Nedrow JM, Scholnick DA, Gleeson TT. Roles of lactate and catecholamines in the energetics of brief locomotion in an ectothermic vertebrate. J Comp Physiol B. 2001;171:237–45. doi: 10.1007/s003600000168. [DOI] [PubMed] [Google Scholar]
- 46.Watt MJ, Forster GL, Korzan WJ, Renner KJ, Summers CH. Influences of behavioral state during brief social interactions on limbic monoamine activity and systemic hormone release. Horm Behav. 2004;46:124. [Google Scholar]
- 47.Parsons LH, Justice JB., Jr Perfusate serotonin increases extracellular dopamine in the nucleus accumbens as measured by in vivo microdialysis. Brain Res. 1993;606:195–9. doi: 10.1016/0006-8993(93)90984-u. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki-Adams DM, Kelley AE. Serotonin-dopamine interactions in the control of conditioned reinforcement and motor behavior. Neuropsychopharmacol. 2001;25:440–52. doi: 10.1016/S0893-133X(01)00240-8. [DOI] [PubMed] [Google Scholar]
- 49.Johansson AK, Bergvall AH, Hansen S. Behavioral disinhibition following basal forebrain excitotoxin lesions: alcohol consumption, defensive aggression, impulsivity and serotonin levels. Behav Brain Res. 1999;102:17–29. doi: 10.1016/s0166-4328(98)00159-4. [DOI] [PubMed] [Google Scholar]
- 50.King JA, Tenney J, Rossi V, Colamussi L, Burdick S. Neural substrates underlying impulsivity. Ann New York Acad Sci. 2003;1008:160–9. doi: 10.1196/annals.1301.017. [DOI] [PubMed] [Google Scholar]
- 51.Feldman S, Weidenfeld J. The excitatory effects of the amygdala on hypothalamo-pituitary-adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain Res Bull. 1998;45:389–93. doi: 10.1016/s0361-9230(97)00384-5. [DOI] [PubMed] [Google Scholar]
- 52.Feldman S, Newman ME, Gur E, Weidenfeld J. Role of serotonin in the amygdala in hypothalamo-pituitary-adrenocortical responses. Neuroreport. 1998;9:2007–9. doi: 10.1097/00001756-199806220-00017. [DOI] [PubMed] [Google Scholar]
- 53.Coco ML, Kuhn CM, Ely TD, Kilts CD. Selective activation of mesoamygdaloid dopamine neurons by conditioned stress: attenuation by diazepam. Brain Res. 1992;590:39–47. doi: 10.1016/0006-8993(92)91079-t. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki T, Ishigooka J, Watanabe S, Miyaoka H. Enhancement of delayed release of dopamine in the amygdala induced by conditioned fear stress in methamphetamine-sensitized rats. Eur J Pharmacol. 2002;435:59–65. doi: 10.1016/s0014-2999(01)01563-1. [DOI] [PubMed] [Google Scholar]
- 55.Rozenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–7. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- 56.Bissiere S, Humeau Y, Luthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–92. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]