Abstract
When epileptiform activity is acutely induced in vitro, transient partial blockade of N-methyl-D-aspartic acid (NMDA) receptor-mediated calcium influx leads to selective long-term depotentiation of the synapses involved in the epileptic activity as well as a reduction in the probability of further epileptiform activity. If such selective depotentiation occurred within foci of epileptic activity in vivo, the corresponding long-term reduction in seizure probability could form the basis for a novel treatment of epilepsy. Continuous radiotelemetric EEG monitoring demonstrated modest acute anticonvulsant effects but no long-term reductions in the probability of spontaneous seizures after transient partial blockade of NMDA receptors (NMDAR) during ictal and interictal activity in the kainate animal model of chronic epilepsy. In vitro, depotentiation was induced when NMDAR were partially blocked during epileptiform activity in hippocampal slices from control animals, but not in slices from chronically epileptic rats. However in slices from epileptic animals, depotentiation during epileptiform activity was induced by partial block of NMDAR using NR2B but not NR2A selective antagonists. These results suggest that chronic epileptic activity is associated with changes in NMDA receptor-mediated signaling that is reflected in the pharmacology of activity- and NMDA receptor-dependent depotentiation.
Keywords: kainate, hippocampus, depotentiation, seizures
Introduction
Synchronous activation of pre and postsynaptic neurons is a necessary condition for long-term modification of synaptic strength (Sjostrom and Nelson 2002). The necessity of synchronous activation has been supported by a variety of experiments, perhaps most specifically by the observation of spike timing-dependent long-term synaptic plasticity (Caporale and Dan 2008). Pathologically high levels of neuronal synchronization occur during epileptic activity, including both seizures and interictal electroencephalographic (EEG) spikes. The synchronization during epileptiform activity suggests that interictal spikes and seizures may be a robust means of inducing long-term synaptic plasticity. In brain slice preparations, long-term potentiation of active synapses can be readily induced by seizures (Ben-Ari and Gho 1988) as well as by interictal spikes (Bains et al. 1999; Abegg et al. 2004; Debanne et al. 2006; Behrens et al. 2005). Strengthening of the synaptic connections between neurons in epileptic foci as a consequence of ongoing interictal activation of the neurons in the focus has been proposed as a means of maintaining the increased probability of seizure activity and thereby stabilizing the epileptic state (Staley and Dudek 2006).
Because long-term synaptic plasticity is bidirectional, it should also be possible to weaken synapses in epileptic foci. Such weakening reduces the probability of synchronous activity (Chamberlin et al. 1990; Traub and Miles 1991; Bains et al. 1999; Staley et al. 2001;) including seizures, which would be of significant therapeutic interest. The calcium hypothesis proposes that large increases in postsynaptic calcium lead to long-term synaptic potentiation (LTP), but smaller increases lead either to de novo long-term depression (LTD) or reversal of previously established LTP (depotentiation) (Lisman 2001). Reduction in postsynaptic calcium influx by partial blockade of NMDA receptors with competitive antagonists induces robust depotentiation when synapses are activated by either tetanic stimuli (Cummings et al. 1996) or epileptiform activity in acute slice preparations (Bains et al. 1999; Hellier et al. 2007). This depotentiation is specific for synapses that participate in the epileptiform activity (Bains et al. 1999), making it an attractive potential mechanism to selectively reduce the strength of synapses in epileptic foci in order to prevent further seizures.
Although the data from acute slice experiments support the possibility that synapse-specific reductions in the strength of synaptic connections in epileptic foci could lead to long-term reductions in seizure probability, there are several possible complications. First, depotentiation of recently potentiated synapses is much more robust than LTD of synapses that presumably were potentiated in the more distant past (Montgomery and Madison 2004). Supporting this observation, LTD induction is accompanied by anatomical alterations manifest primarily in the smallest synapses (Bastrikova et al. 2008) that are presumably the weakest (Baude et al. 1995; Zhou et al. 2004). This suggests that in long-standing epilepsy, where synapses have been repeatedly potentiated by interictal and seizure activity, depotentiation or long-term depression of synaptic strength may be less robust. Second, the mechanisms of synaptic plasticity in vivo may represent a superset of the mechanisms observed to date in vitro. For example in contrast to what has been observed in vitro, NMDA antagonists have increased the strength of active synapses in vivo (Clem et al. 2008). Third, activity-dependent changes in NMDA receptor subunit expression (Galvan et al. 2003; Yang et al. 2006) or the linkage of NMDAR to second messenger systems (Chen et al. 2007) may alter the pharmacology of activity-dependent depotentiation in chronic epilepsy. Finally, activity and NMDAR-dependent synaptic weakening is accompanied by reductions in the fraction of membranous NMDA receptors (Hellier et al. 2007), which may limit further depotentiation.
In this study, we find that transient partial antagonism of NMDA receptors with the nonselective competitive NMDA antagonist SDZ 220-581 (Urwyler et al. 1996) in chronically epileptic animals does not lead to long-term reductions in seizure activity. In vitro slice experiments demonstrate that in contrast to acute slices from naïve animals, partial NMDA antagonism with nonselective competitive antagonists does not induce depotentiation during synchronous network activation in slices from epileptic animals. However, in slices from epileptic animals, partial blockade of NMDA receptors using an NR2B (but not an NR2A) specific antagonist during synchronous CA3 network activity does reduce the probability of further epileptiform activity in a pattern, consistent with depotentiation of the active synapses.
Methods
Surgery: freely-behaving rats
The methods have been described previously (for a detailed description see Williams et al. 2006). Briefly, male Sprague-Dawley rats (180–200g; Harlan, Indianapolis, IN) were injected subcutaneously (SC) with atropine (2.0 mg/kg), dexamethasone (4.0 mg/ml), and 0.2 ml penicillin (300,000 IU); anesthesia was induced and maintained with isoflurane. The head was placed in a stereotaxic apparatus, a mid-sagittal incision made on the scalp and the skin reflected with hemostats. Using bregma as a reference, holes were bored through the skull with a Dremel (#105 drill bit) for implantation of a single dural and bilateral intrahippocampal recording electrodes (rostral-caudal −4.0 mm, medial-lateral ±2.5 mm, dorsal-ventral −3.3 mm), a ground electrode, and three support screws placed behind the hippocampal sites. The intrahippocampal electrodes were placed in the granule cell layer of the dentate gyrus and confirmed by increased spike activity with an audio monitor. All electrodes where permanently fixed by dental cement. The radiotelemetry unit (Transoma Medical, Arden Hills, MN) was placed subcutaneously in the flank region through a 2-cm skin incision behind the scapula.
Both incisions were closed using 4–0 Dermalon (American Cyanamid Co., Danbury, CT). The animal was given 4–6 ml of warmed Ringer’s solution (SC), buprenorphine (0.01 mg/kg, SC), removed from anesthesia and placed back in its cage under a heat lamp for 30 min. All animals were given buprenorphine (0.01 mg/kg, SC) and 0.2 ml penicillin (300,000 IU, SC) for 3 days following surgery. The toenails of each rat were cut while the rat was anesthetized, and if necessary were trimmed once per week to decrease scratching and irritation at the incision sites.
Kainate treatment
The kainate-treatment protocol has been previously described (Hellier et al. 1998; Hellier and Dudek 2005). If the animal was implanted with electrodes, kainate treatment was performed 1–2 weeks after surgery. Rats (n = 20 for slice experiments and n = 9 for implantation) were given hourly injections of kainate (5 mg/kg, intraperitoneally, IP; Sigma, St. Louis, MO or Ocean Produce International, Nova Scotia, Canada) diluted in sterile 0.9% saline at 2.5 mg/ml. Motor seizure activity was rated according to a modified Racine’s scale (i.e., class III, IV, and V seizures; Ben-Ari 1985; Racine 1972). To minimize mortality, injections were reduced (2.5 mg/kg) or eliminated if an animal showed excessive inactivity or activity (for details see Hellier and Dudek 2005). Kainate treatment was continued until class IV and V seizures were elicited for ≥ 3 h. Implanted rats were continuously monitored for both electrographic and motor seizures throughout kainate treatment. Control rats (n = 10 for slice experiments) were treated with an equivalent volume and number of injections of sterile saline. All rats were given 3–6 ml warmed lactated Ringer’s (SC) and apple slices following treatment.
Radiotelemetry and video monitoring: freely-behaving rats
This study used the Dataquest A.R.T. Analog software provided by Transoma Medical (Arden Hills, MN). The radiotelemetry unit was specifically designed to transmit physiological data from conscious, freely behaving animals to the analog system. Rats were housed individually after surgery, given food and water ad libitum, and exposed to a 12-h light/dark cycle. Each cage was placed on an individual radio receiving plate (RPC-1; Transoma Medical, Arden Hills, MN), and captured data signals from the transmitter was sent to an input exchange matrix and then to a computer. Custom-made software (KS and AW) was used to acquire the data with routines written in Visual Basic 6.0 (Microsoft, Seattle, WA), and the data were written to DVD for analysis offline (for a detailed description see White et al. 2006).
Two Color Quad Observation Systems (SOD14C4LN; Samsung, Korea) were used to continuously videotape eight individually-housed rats, and the time stamp for each system was synchronized to the digitizing computer. Night recordings were performed with a Kodak 1A filter (Eastman Kodak, Rochester, NY) over a safelight and daytime recordings with a diffuse fluorescent light. The behavioral data were used in this study for identifying motor seizures from non-motor seizures and for differentiating EEG seizure activity from electrical noise generated by jaw artifact and grooming.
EEG analysis: freely-behaving rats
The seizure-detection analysis was performed in an automated manner with custom-written software that greatly minimizes the potential for bias (White et al. 2006), and also visually analyzed (AW and PAW). The investigators were not blinded to treatment or time after kainate-induced status epilepticus. Electroencephalographic seizures were differentiated from background noise by the appearance of large-amplitude (> three times baseline noise), high-frequency ( ≥ 5 Hz) EEG activity, with a sufficiently high temporal correlation and progression of spike frequency (White et al. 2006).
Chronic seizures
To ensure that kainate-treated rats used for slice experiments were epileptic, both kainate- and saline-treated rats were viewed directly for seizure activity during random 1–2 h intervals, totaling 6–8 h/wk (Hellier et al 1998). These observational periods were initiated approximately 3 months after treatment until euthanasia and occurred during the 12-h interval when lights were on. Behavioral observations of seizure activity were recorded, applying the same modified Racine scale used during kainate treatment. When a kainate-treated rat was observed to have at least two spontaneous motor seizures, the animal was defined as epileptic. Only rats with kainate-induced epilepsy were used for slice experiments. No saline-treated rats were observed to have motor seizures and were used as controls for slice experiments.
Acute Whole-Animal LTP Surgery and Electrophysiological Recordings
Adult male Sprague-Dawley rats (200–350 g, Harlan) were initially injected with atropine (1 mg/kg, IP) to prevent cardiorespiratory complications associated with surgery and general anesthesia. Subsequently, rats are anesthetized with urethane (1.8–2.0 mg/kg, SC) and animal body temperature was maintained between 37–40 °C. Once a level plane of anesthesia was obtained, the head was placed in a stereotaxic apparatus (bite bar = − 3.0 mm), a midsagittal incision made on the scalp, and the skin reflected with hemostats. Using bregma as a reference, 3 holes are burred with a Dremel (#105 bit) in the skull for implantation of recording (rostral-caudal −4.0 mm, medial-lateral −2.0 mm, dorsal-ventral −2.4 mm; 3 MΩ, Fredrick Haer, Co), stimulating (rostral-caudal −4.5 mm, medial-lateral −3.1 to −3.5 mm, dorsal-ventral −2.5 to −3.0 mm; wire size = 0.0045”, Teflon-coated, A-M Systems, Inc.), and grounding electrodes (rostral-caudal +2.0 mm, medial-lateral ±2.0 mm, on dura; wire size = 0.013”, Teflon-coated, A-M Systems, Inc.). Grounding electrodes were glued in position (Instant Krazy Glue, Advanced Formula Gel) prior to placing recording and stimulating electrodes. The stimulating electrode was lowered until a maximal response was observed while stimulating at 50 volts (0.1 Hz).
Spontaneous activity and responses to single stimulation of the Schaffer collaterals (intensity = 30–90 V; duration = 0.020 ms; frequency = 0.05 Hz) were studied in all rats. A baseline input-output relationship from single stimulation was performed at 10 V increments from 30–90 V prior to tetanic stimulation. LTP was produced by three tetanic stimuli 10 min apart (intensity = 60 V; duration = 1 s; frequency = 100 Hz) and measured by a second input-output protocol (30 min after the last tetanic stimulation). Once LTP was established, depotentiation was tested following 2–6 h after administering low doses of SDZ 220-581 (SDZ, IP). The same protocol as stated above was performed at a specified time after administration of SDZ.
All data analyses were performed online using routines written in VisualBasic 6.0 (KJS and AMW). Traces used in the analyses were an average of 10 responses and the maximal slope calculated to determine LTP and depotentiation.
Hippocampal Slice Preparation and Electrophysiological Recordings
Rats were anesthetized (sodium pentobarbital, 50 mg/kg, IP) and brains were quickly removed and sliced in ice-cold, high-sucrose solution (composition in mM: 87 NaCl, 2.5 KCl, 26 NaHCO3, 0.5 CaCl2, 7 MgCl2, 1.25 NaH2PO4, 25 glucose, 75 sucrose). Horizontal slices of the hippocampus (350–400 μm) were prepared with a vibrating microtome (Leica, Bannockburn, IL) and stored at 32–34°C in artificial cerebrospinal solution (ACSF, in mM: 106.5 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 CaCl2, 4.5 MgCl2, 1.25 NaH2PO4, 17.5 glucose, 37.5 sucrose), humidified with 95% O2-5% CO2, and recovered for 1.5–2 h before initiating experiments.
Extracellular field potentials were recorded from the stratum pyramidale of the CA3 region using glass pipette electrodes filled with 150 mM NaCl. To induce CA3 bursting, slices were bathed in a modified ACSF (changes in mM: 3.3 KCl, 1.3 CaCl2 and 0.9 MgCl2; Stasheff et al. 1989) containing 100 μM picrotoxin (Sigma, St. Louis, MO) and 1 μM CGP-55845 (Tocris, Ellisville, MO) to block GABAA and GABAB-mediated synaptic activity and unmask CA3-CA3 recurrent collateral synapses. As previously described (Bains et al. 1999; Hellier et al. 2007), depotentiation of recurrent CA3 synapses was induced by transient blockade of NMDA receptors through application of 2.5–10 μM D-(−)-2-Amino-5-phosphonopentanoic acid (D-APV; Tocris, Ellisville, MO) or 20–80 μM SDZ (Tocris, Ellisville, MO), both competitive NMDA antagonists. To partially block selective NMDA subunits, 0.125–0.5 μM NVP-AAM07 (selective NR2A antagonist; a generous gift from Novartis, Switzerland) or 0.25–1 μM Ro 25-6981 (selective NR2B antagonist; Tocris, Ellisville, MO) were added to the bath ACSF. Decreasing concentrations of antagonists were applied in 30 min intervals until wash. Extracellular events were recorded with an Axoclamp-2B amplifier (Axon Instruments, Union City, CA), digitized at 2–10 kHz, and analyzed using routines written in VisualBasic 6.0 (Microsoft, Seattle, WA).
Slice Electrophysiological Analysis
Interburst intervals were measured from the start of one burst to the beginning of the next burst. Burst durations were calculated as the time during which the absolute value of the burst amplitude remained greater than three times baseline noise (Hellier et al. 2007; Staley et al. 1998). Although partial blockade of NMDA receptors with either SDZ or D-APV significantly decreased burst durations by 15–20% (data not shown), we observed a more significant change in interburst intervals. Therefore, we focused on interburst intervals in our analyses.
Statistical Analysis
Analysis of variance (ANOVA, multiple-comparison test using Newman-Keuls) was performed to determine if significant differences existed between: 1) each animal’s baseline seizure frequency and the experimental conditions shown in Figures 2 – 4, and 2) between baseline bursting and bursts following depotentiation (e.g., transient application of D-APV, SDZ, NVP-AAM07, or Ro 25-6981) in the experiments illustrated in Figure 5. Significance for all statistical analyses was accepted when P < 0.05.
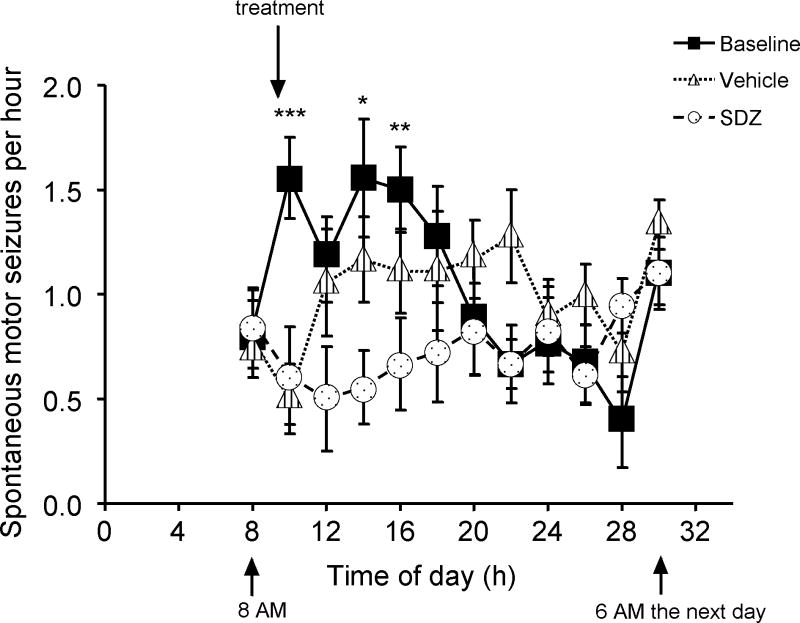
Figure 2.
Acute anticonvulsant effects of the NMDA antagonist SDZ. Animals were on a 6AM-6PM light/6PM-6AM dark cycle; X axis is labelled in hours from midnight, and data are presented as 2-hour averages beginning at 8AM. In control conditions, seizure frequency increased shortly after 6AM consistent with prior observations of diurnal seizure frequency (Bertram and Cornett, 1994; Hellier et al. 1999) A: The average and standard deviation of the behavioural seizure frequencies of 9 animals each observed for 3 different 24-h periods (baseline, and after injection of either saline vehicle, or SDZ 3.2 mg/kg IP) by an observer blinded to treatment. Saline and SDZ injections were separated by 24 hours. This figure shows the time course of the acute anticonvulsant effects of NMDAR antagonist administration. Anticonvulsant effects were significant for approximately 6 h. * = SDZ is significantly different from both vehicle and baseline. ** = SDZ is significantly different from baseline only. *** = SDZ is significantly different from baseline, and vehicle is different from baseline (i.e., injection effect).
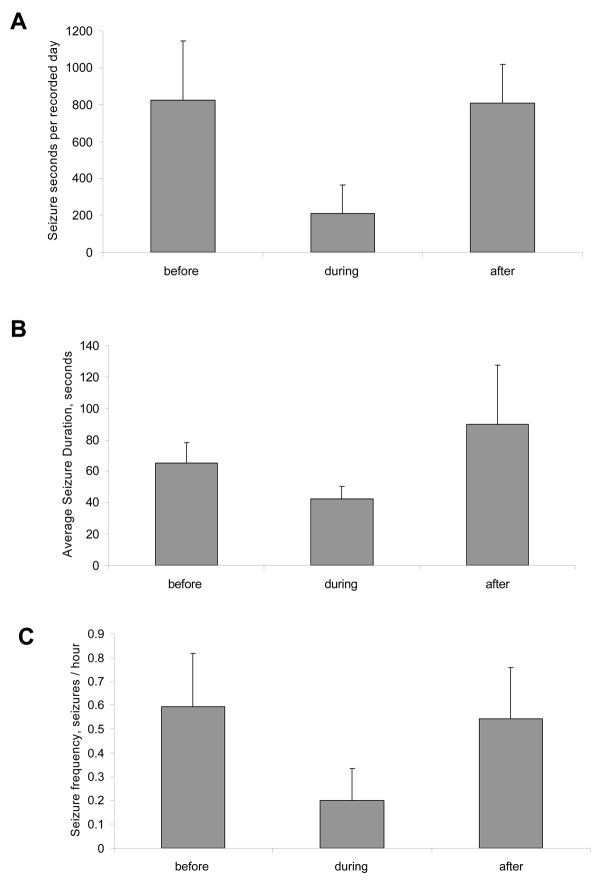
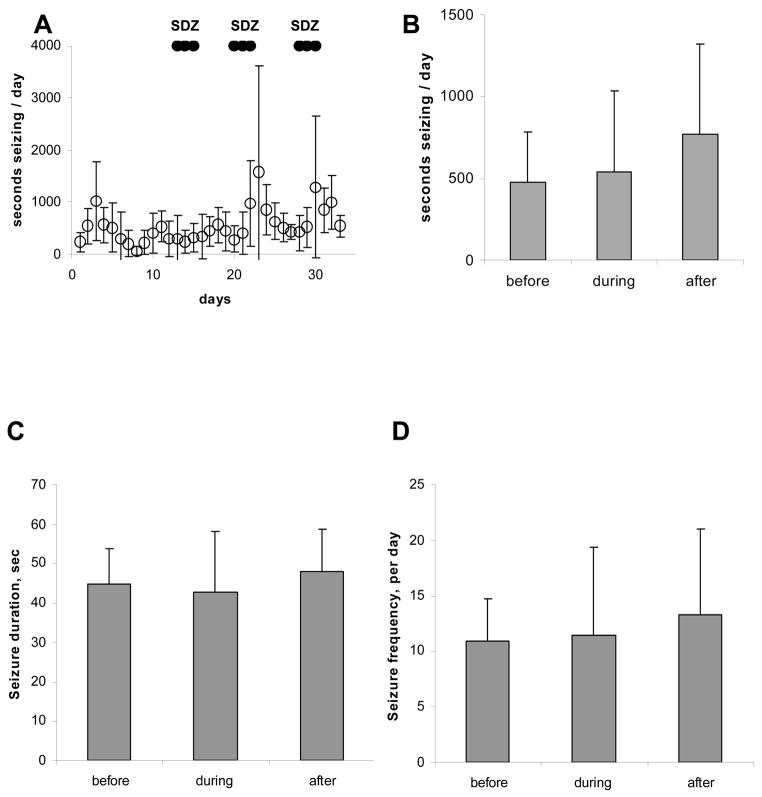
Figure 4.
Effects of intraperitoneal administration of SDZ on chronic EEG seizures in rats with kainate-induced epilepsy. Each bar in panels A–C represents a 3-day recording period either before, during or after the 3-day period of SDZ administration. The only statistically significant change is the number of seconds seizing during drug administration (P=0.04), which is consistent with the findings illustrated in Figure 2.
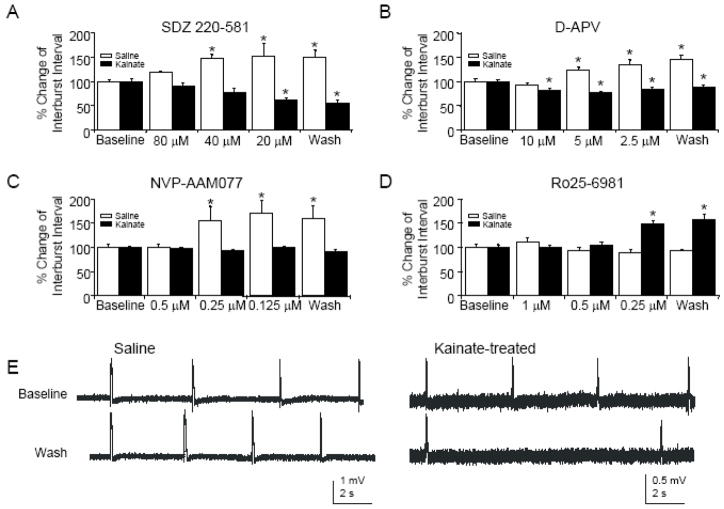
Figure 5.
In hippocampal slices prepared from chronically epileptic animals, only an NR2B-selective NMDAR antagonist induces activity-dependent depotentiation. A: Following bath application of SDZ, there are significant increases in intervals between spontaneous CA3 population bursts induced by pharmacological disinhibition in controls (n=6, ANOVA, P<0.01) but significant decreases in interburst intervals in kainite-treated rats (n=5, ANOVA, P<0.001). B: Similarly, following D-APV application, there are significant increases in CA3 interburst intervals in controls (n=7, ANOVA, P<0.01) and significant decreases in CA3 interburst intervals in kainite-treated rats (n=8, ANOVA, P<0.01). C: Partial blockade of NMDAR using an NR2A-subunit-selective antagonist significantly increases interburst intervals in saline-treated rats (n=6, ANOVA, P<0.01), while no change in interburst interval is observed in rats with kainite-induced epilepsy (n=6, ANOVA, P=0.18). D: Partial blockade of NMDAR using an NR2B-subunit-selective antagonist does not change interburst intervals in saline-treated rats (n=5, ANOVA, P=0.12). However, the same application produces significant increases in interburst intervals in chronically epileptic rats (n=5, ANOVA, P<0.001). E: Sample CA3 bursts from hippocampal slices prepared from saline- (left) and kainite- (right) treated rats before and after application of decreasing concentrations of the NR2B-selective NMDAR antagonist Ro25-6981. Asterisks represent significance compared to baseline.
Results
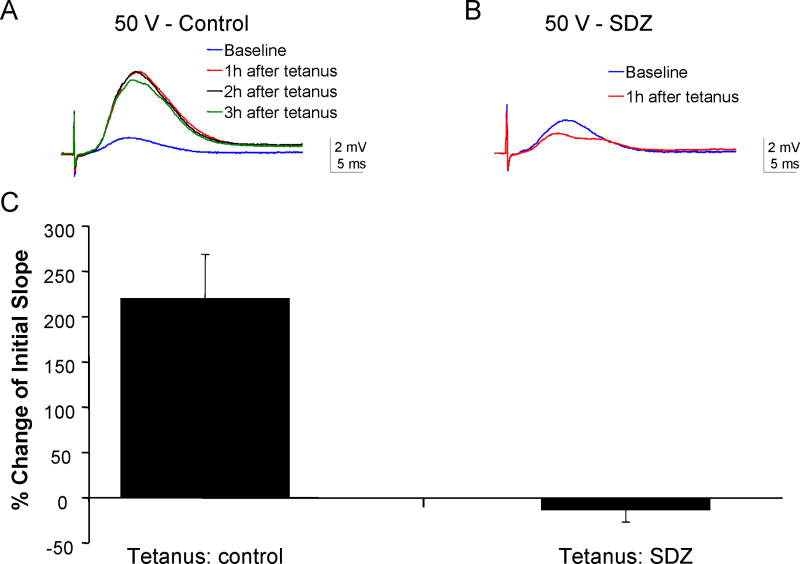
To ascertain the dose of NMDA antagonist needed to reduce postsynaptic calcium influx sufficient to induce depotentiation instead of LTP, we studied LTP induction at CA1 Schaffer collateral synapses in adult rats acutely anesthetized with urethane. Stable field excitatory evoked potentials (fEPSPs) could be recorded from the CA1 pyramidal cell layer following stimulation of the Schaffer collaterals (Figure 1A). Following a 30 min baseline, three 100-Hz stimuli delivered for 1 second increased the maximum initial slope of the fEPSP for 2 and 3 hours post-tetanus (n=3 animals). Intraperitoneal administration of the nonselective competitive NMDA antagonist SDZ (Urwyler et al. 1996) at a dosage of 3.2 mg/kg prior to tetanic stimulation blocked LTP in 2 of 2 rats and instead produced a long-term reduction in the fEPSP slope (Figure 1B), suggesting that doses in this range should partially block the NMDA receptor (Cummings et al. 1996). Because it was not feasible to directly stimulate the synapses in the epileptic focus, these experiments were not repeated in epileptic animals.
Figure 1.
Administration of the nonselective competitive NMDA antagonist SDZ prior to tetanic stimulation induces depotentiation in vivo in naïve rats anesthetized with urethane. A: In acute whole-animal recordings from the CA1, tetanic stimuli induced long-term increases in the slope of the fEPSP evoked at 50 V (220 ± 90 % of baseline, n=3). This increase persisted for 2 h after the tetanic stimuli. B: Subsequent administration of SDZ (3.2 mg/kg, IP) 2 h prior to a second tetanic stimuli caused the maximal slope of fEPSPs evoked at 50 V to decrease by 13 ± 7 % for at least 2 h compared to the response prior to the second tetanus in SDZ, consistent with induction of depotentiation (n=2). C: The bar chart illustrates the summary data for 50 V stimuli 2 h post-tetanus.
A previous study showed that SDZ acutely blocks electroshock convulsions in rodents at an oral dose of 10 mg/kg (Urwyler et al. 1996). To evaluate the effective dosage of NMDA receptor antagonists in chronically epileptic animals, we administered SDZ at 3.2 mg/kg IP and evaluated the acute anticonvulsant effects. Chronic epilepsy was induced by systemic kainate injections in 6 week old rats implanted with radiotelemetry transmitters to continuously record EEG activity (Williams et al. 2006; White et al. 2006). Animals were studied ≥ 100 days after kainate injection and after the onset of spontaneous seizures (Figure 2). Saline IP injection transiently reduced seizures compared to non-injected controls, but this effect lasted less than an hour and was likely related to an increase in the state of arousal of the animal after the injections, which took place at the start of the animal’s sleep cycle; this corresponds to the diurnal peak of their seizure frequency (Bertram and Cornett, 1994; Hellier and Dudek 1999). SDZ (3.2 mg/kg, IP) produced a modest but more sustained (compared to saline) and statistically significant acute anticonvulsant effect (Figure 2). These data indicate that SDZ crosses the blood brain barrier and effectively modulates NMDA receptor function in vivo and in chronically epileptic animals.
To test whether transient partial blockade of NMDA receptors in chronically epileptic animals produced a long-term alteration in seizure probability, the nonselective NMDA receptor antagonist SDZ was administered daily for three consecutive days at 1 week intervals. Animals were administered drug by gavage (PO) on three consecutive days: 20 mg/kg on day 1, 10 mg/kg on day 2, and 5 mg/kg on day 3. The seizure frequencies before, during and following administration of NMDA antagonist are illustrated in Figure 3A (n = 4 chronically epileptic rats). Although there was a transient reduction in seizure frequency during drug treatment, as seen in Figure 2, there were no significant differences in seizure duration, frequency, or total seizure time/day observed following antagonist treatment. To ascertain that the negative results were not due to altered availability of SDZ by PO administration, a separate group of chronically epileptic rats (n=5) were treated with SDZ using the same three-day dosage regimen administered IP. No differences in seizure frequency, duration, or total time seizing per day were observed following treatment (Figure 4).
Figure 3.
In vivo administration of the nonselective NMDA antagonist SDZ to chronically epileptic animals did not alter subsequent seizure probability. A: Total EEG seizure time per day during the 32 day experiment. Periods of administration of SDZ by gavage are marked. Dosage regimen (PO) was 20 mg/kg for the first day of treatment, 10 mg/kg for the second day of treatment, and 5 mg/kg for the last day of treatment. The 3-day treatment regimen was repeated with 5 days of recovery between treatments. B–D: Summary of seizures for 3-day blocks before, during, and after treatment with SDZ. Panel B demonstrates no significant change in total seizure time per day. Panel C demonstrates no long-term change in seizure duration, and panel D demonstrates no long-term change in seizure frequency. Note that all panels demonstrate a nonsignificant trend toward increased seizure severity after treatment.
These results suggest that dosages of nonselective competitive NMDA antagonists that induce activity-dependent depotentiation instead of LTP following tetanic stimulation in naïve animals and acute anticonvulsant effects in chronically epileptic animals do not induce significant long-term reductions in seizure frequency in chronically epileptic rats. To investigate the possibility that chronic epilepsy alters the expression or second messenger linkage of NMDA receptors in a manner that makes activity- and NMDAR-dependent depotentiation less likely to occur, we examined depotentiation in slices prepared from age-matched saline-treated (controls) and kainate-treated rats (chronically epileptic).
In the presence of GABAA and GABAB antagonists, spontaneous epileptiform population bursts were observed in CA3 networks of hippocampal slices prepared from naïve and epileptic animals (Traub and Miles 1991). There were no significant differences in the baseline frequency of spontaneous population bursts in control and epileptic rats. Slices from saline-treated animals had an interburst interval of 5.8 seconds (SEM = 0.168), while slices from kainate-treated rats had an interburst interval of 6.3 seconds (SEM = 0.162; T-test: p = 0.08).
In previous studies, we demonstrated that sequentially decreasing concentrations of NMDAR antagonists induced much more substantial depotentiation of CA3-CA3 synapses than either increasing or stable concentrations of antagonist due to endocytosis of NMDAR associated with depotentiation under these conditions (Hellier et al. 2007). Depotentiation of CA3-CA3 recurrent collateral synapses was monitored by the frequency of spontaneous population bursts (Bains et al. 1999; Staley et al. 2001). Application of sequentially decreasing concentrations of SDZ or D-APV applied by bath to slices prepared from naïve animals induced a robust increase in the time intervals between spontaneous CA3 population bursts (Figures 5A,B; Hellier et al. 2007). In slices prepared from kainate-treated rats, however, the time intervals between spontaneous CA3 population bursts significantly decreased following bath application of the same sequence of concentrations of SDZ or D-APV (Fig 5B), suggesting that recurrent collateral CA3 synapses had been potentiated (Bains et al. 1999; Behrens et al. 2005) by exposure to NMDA antagonists. These effects are consistent with the pharmacology of long-term potentiation in vivo, in which blockade of NMDA receptors at synapses potentiated in vivo is permissive for further activity-dependent LTP (Clem et al. 2008). This alteration in NMDA pharmacology in the slices from epileptic animals supports the idea that epileptiform activity induces LTP of active synapses in vivo as well as in vitro (Ben-Ari and Gho 1988; Schneiderman et al. 1994; Bains et al. 1999; Behrens et al. 2005).
To investigate whether the change in NMDA pharmacology involved a change in NMDA receptor subunits, NR2A- or NR2B-selective antagonists were applied by bath in sequentially decreasing concentrations to slices under the same conditions in which SDZ and D-APV had been applied (Figures 5C,D). Following application of NR2A-selective antagonists, control slices exhibited significantly increased interburst intervals while there was no change in epileptic slices. However, the opposite effect was observed with application of NR2B-selective antagonists, where interburst intervals increased in epileptic slices vs. no change in controls. These data suggest that NR2B-selective antagonists may be more effective in inducing activity-dependent depotentiation in epileptic hippocampal slices compared to nonselective NMDAR antagonists.
Discussion
NMDAR blockade using a variety of administration techniques and dosages produced no permanent reduction in seizure probability in chronically epileptic rats (Figures 3 and 4). Consistent with this finding, in hippocampal slices prepared from chronically epileptic animals, partial nonselective NMDAR blockade increased rather than decreased the probability of further epileptiform activity (Figure 5). Further, partial blockade of NMDAR using the NR2B-selective antagonist Ro 25-6981 but not the NR2A antagonist NVP-AAM07 resulted in long-term reductions in the probability of epileptiform activity in vitro. These results support a change in the role of NMDAR subunits in synaptic plasticity in a chronic epilepsy model and suggest that NR2B-selective antagonists are likely to be more effective than nonselective NMDAR antagonists as a means to induce long-term decreases in the probability of epileptiform activity. More generally, the results support the increased complexity of rules governing long-term synaptic plasticity in vivo.
Experimental limitations
In this study, we did not determine the concentrations of NMDAR antagonist in the blood or brains of the epileptic rats. In vitro, the magnitude of NMDAR antagonist-induced depotentiation at active synapses is largest when the synapses are exposed to sequentially decreasing concentrations NMDAR antagonists, due at least in part to activity-dependent internalization of NMDAR (Hellier et al. 2007). Thus the lack of effect of NMDAR antagonists in vivo could have resulted from an unexpectedly low level of NMDAR antagonist in the brain. This is unlikely because a similar dosage of NMDAR antagonist blocked LTP in vivo (Figure 1), equivalent dosages of antagonists with similar NMDAR affinities (Urwyler et al. 1996) also blocked LTP in vivo (Kentros et al. 1998), and similar doses of SDZ had an acute anticonvulsant effect (Fig 2). If on the other hand the dose of NMDAR antagonist was too high, so that NMDAR were completely blocked, no depotentiation would be induced (Cummings et al. 1996; Lisman 2001; Hellier et al. 2007). However, at the dosage schedule used here the concentration should fall between doses (Fig 2), and fall to zero after the last dose, so that synapses were exposed to sequentially decreasing concentrations of antagonist; some of those concentrations should have been effective at inducing depotentiation. Further, NMDAR expression is increased in chronic experimental epilepsy (Mathern et al. 1999), so that it is unlikely that the dose was too high. We used the frequency of interictal epileptiform activity as a proxy for synaptic strength in the experiments utilizing depotentiation induction protocols in vivo and in vitro (Figures 3–5). Depotentiation is reliably induced at synapses actively involved in epileptiform activity when NMDAR are partially blocked (Bains et al. 1999; Hellier et al. 2007), however in future studies it should be ascertained that depotentiation is the mechanism underlying the persistent reduction in frequency of epileptiform activity induced by transient exposure to NR2B-selective NMDAR antagonists (Figure 5).
Finally we did not attempt to induce long-term decreases in seizure frequency by administering NR2B-selective NMDAR antagonists to chronically epileptic rats. These experiments are difficult and the water-solubility of Ro 25-6981 is an additional complication that necessitates intracerebral or intraventricular administration. Osmotic pumps are not a good means of drug delivery for such an experiment, because stable concentrations of NMDAR antagonists are not optimal for induction of depotentiation; rather, an intermittent delivery system that allows inter-dosing decreases in antagonist concentration is required. This experiment will become feasible with the continued development of NR2B-selective antagonists (Kawai et al. 2007).
Long-term synaptic plasticity in vivo
Our results illustrate the complexity of the induction of long-term synaptic plasticity in vivo. Such complexity is necessary to ensure the stability of formed memories. These results suggest that regulation of synaptic plasticity induction based on the prior history of synaptic activity, or metaplasticity (MacDonald et al. 2007; Thiagarajan et al. 2007), stabilizes not only physiological but also pathological patterns of synaptic efficacy. For example, although NMDAR antagonists induced weakening of recently potentiated synapses in control tissue (Figure 5), they did not induce weakening of synapses in chronically epileptic tissue (Figures 3 and 5). Another example of such metaplasticity is the endocytosis of membrane-bound NMDAR during depotentiation induced by partial blockade of NMDAR during CA3 population bursts in slices prepared from naïve animals (Hellier et al. 2007). Endocytosis of the NMDAR changed the concentration-response curve for the depotentiation induced by the NMDAR antagonists such that reductions in concentration of antagonists were needed to induce further depotentiation; this is why the NMDAR antagonist concnentration was sequentially decreased in the experiments illustrated in Figure 5.
It has been known for over a decade that epileptiform activity induces long-term potentiation of the synapses that participate in that activity (Ben-Ari and Gho 1988; Schneiderman et al 1994; Bains et al. 1999; Behrens et al. 2005). This phenomenon may underlie the long-held clinical suspicion that “seizures beget seizures” (Berg and Shinnar 1997), and the more recent hypothesis that interictal EEG spike activity stabilizes the seizure probability (Staley and Dudek 2006). LTP of the synapses that participate in epileptiform activity increases the probability of future epileptiform activity (Bains et al. 1999; Behrens et al. 2005; Debanne et al. 2006). While this LTP has been demonstrated to be NMDAR-dependent in vitro (Bains et al. 1999), the role of NMDAR in induction of long-term synaptic plasticity in vivo is more complex. Clem et al. (2008) recently demonstrated that although initial LTP is mediated by NMDAR in vivo, subsequent activation of metabotropic receptors was necessary to permanently alter synaptic strength. Further, following in vivo LTP induction, blockade of NMDAR in vitro resulted in further LTP rather than no change as would be expected based on in vitro experiments (Cummings et al. 1996; Malenka and Bear 2004; Hellier et al. 2007). Another example of the complexity of synaptic plasticity in vivo is the block of early epileptogenesis by nonselective NMDA antagonists or NR2A selective antagonists (Chen et al. 2007). These results suggest that the early physiological and proconvulsant synaptic plasticity is NMDAR dependent, as in acute naïve slices, but later plasticity is not. These ideas support our findings that in chronic epilepsy, epileptiform activity is not reduced by transient nonselective blockade of NDMAR. If NMDAR-dependent LTP was induced early in the course of chronic epilepsy, subsequent long-term synaptic plasticity may no longer be NMDAR-dependent. After NMDAR-mediated synaptic strengthening, complete or perhaps even partial blockade of NMDAR may result in further potentiation (Clem et al. 2008). This could explain why epileptiform activity increased rather than decreased after exposure to nonspecific NMDA antagonists in vivo (Figures 3 and 4) and in vitro (Figure 5A,B). The mechanisms for such alterations in the role of NMDAR in plasticity induction are unknown, although our findings parallel what has been found in learning paradigms in vivo (Clem et al. 2008). Future calcium imaging experiments may resolve whether calcium influx becomes altered in vivo, or whether it is the response to calcium influx that becomes altered.
Role of NR2B receptors
Mechanisms of induction of depotentiation and LTD have not been extensively studied in vivo to date. However, our results suggest that synaptic weakening is unlikely to be induced by partial blockade of NMDAR during epileptiform activity. Rather, synaptic weakening may be induced by partial blockade of NR2B-containing NMDAR (Figure 5), which is substantially different from what has been observed in acute slices (Morishita et al. 2007, but see Massey et al. 2004). The NR2B dependence may arise as a result of several mechanisms: selective expression and/or trafficking of NMDAR subunits during epilepsy resulting in increased expression of NR2B subunits in hippocampus (Galvan et al. 2003), or by selective linking of NR2B to second messenger systems subserving LTD (Chen et al. 2007), or reductions in glutamate release due to antagonism of presynaptic NR2B receptors whose expression is increased in epilepsy (Yang 2006). An increased role of NR2B-containing NMDAR in chronic epilepsy is also suggested by the efficacy of NR2B selective antagonists in vitro in both experimental and human epilepsy (Moddel et al. 2005; Bandyopadhyay and Hablitz 2006). The effects of NR2B-slective antagonists on CA3 bursts, which are considered a model of interictal spiking, suggests that future in vivo studies of these agents in epilepsy should include an analysis of interictal spike frequencies in addition to seizure frequencies.
Implications for epilepsy therapy
If transient exposure to NMDAR antagonists during interictal activity resulted in depotentiation of synapses in epileptic foci and subsequent reductions in seizure probability (Staley and Dudek 2006, Dudek and Staley 2007), then epileptic patients exposed to NMDAR antagonists such as the anesthetic ketamine (Celesia et al. 1975) or experimental anticonvulsants (Kohl and Dannhardt 2001), should have experienced long-term reductions in seizure probability. This has not been reported, but there are several reasons why NMDA antagonists may not have induced long-term depression at synapses generating periodic epileptic discharges. First, uncompetitive NMDAR antagonists such as ketamine may not reduce postsynaptic calcium influx in the manner necessary to induce depotentiation. Second, seizures in the generalized epilepsies may not be reduced by this strategy. Third, NR2B-selective antagonists may be required to induce depotentiation of chronically active synapses (Galvan et al. 2003; Yang 2006; Chen et al. 2007; Figure 5). These results suggest that treatment with NR2B antagonists may be a more effective means of induction of depotentiation at synapses in epileptic foci, and consequent long-term reductions in seizure probability.
It is also possible that metabotropic glutamate receptor (mGluR) activation is necessary for depotentiation of synapses in epileptic foci. MGluR-mediated synaptic plasticity is well-established in vitro (Huber et al. 2001) and in vivo (Clem et al. 2008). MGluR are activated by epileptiform activity (Lee et al. 2002), and mGluR subserve a variety of epilepsy-related long-term changes in neuronal excitability (Chuang et al. 2000; Sayin and Rutecki 2003). Although long-term changes induced by exogenous mGluR agonists have been primarily proconvulsant in vitro (Lee et al. 2002; Sayin and Rutecki 2003), activation of Group I mGluR are necessary for long-term increases and decreases in synaptic strength in vivo (Neyman and Manahan-Vaughan 2008). Further, exogenous activation of Group III mGluR receptors has been demonstrated to reduce the probability of epileptiform activity (Ngomba et al. 2005). This anticonvulsant activity of Group III mGluR is not likely to be due to long-term synaptic plasticity (Altinbilek and Manahan-Vaughan 2007), however, because mGluR-mediated long-term synaptic plasticity is substantially reduced during epileptogenesis (Kirschstein et al. 2007). These findings suggest that the persistence of the chronically epileptic state is likely to involve multiple mechanisms of metaplasticity. Pharmacological manipulation of activity-dependent long-term determinants of synaptic strength is a challenging but potentially powerful means of interrupting or reversing the synaptic mechanisms that stabilize the epileptic state.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer L. Hellier, Neuroscience Program, University of Colorado Health Sciences Center.
Andrew White, Pediatrics Department, University of Colorado Health Sciences Center.
Philip A. Williams, Physiology Department, Case Western University
F. Edward Dudek, Department of Physiology, University of Utah School of Medicine.
Kevin J Staley, Neurology Department, Harvard University School of Medicine.
References
- Abegg MH, Savic N, Ehrengruber MU, McKinney RA, Gähwiler BH. Epileptiform activity in rat hippocampus strengthens excitatory synapses. J Physiol. 2004 Jan 15;554(Pt 2):439–48. doi: 10.1113/jphysiol.2003.052662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altinbilek B, Manahan-Vaughan D. Antagonism of group III metabotropic glutamate receptors results in impairment of LTD but not LTP in the hippocampal CA1 region, and prevents long-term spatial memory. Eur J Neurosci. 2007 Sep;26(5):1166–72. doi: 10.1111/j.1460-9568.2007.05742.x. [DOI] [PubMed] [Google Scholar]
- Bains JS, Longacher JM, Staley KJ. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nat Neurosci. 1999 Aug;2(8):720–6. doi: 10.1038/11184. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Hablitz JJ. NR2B antagonists restrict spatiotemporal spread of activity in a rat model of cortical dysplasia. Epilepsy Res. 2006 Dec;72(2–3):127–39. doi: 10.1016/j.eplepsyres.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc Natl Acad Sci USA. 2008 Feb 26;105(8):3123–7. doi: 10.1073/pnas.0800027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Molnár E, McIlhinney RA, Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995 Dec;69(4):1031–55. doi: 10.1016/0306-4522(95)00350-r. [DOI] [PubMed] [Google Scholar]
- Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci. 2005;8:1560–1567. doi: 10.1038/nn1571. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy [Review] [200 refs] Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gho M. Long-lasting modification of the synaptic properties of rat CA3 hippocampal neurones induced by kainic acid. J Physiol (Lond) 1988;404:365–384. doi: 10.1113/jphysiol.1988.sp017294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT, Shinnar S. Do seizures beget seizures? An assessment of the clinical evidence in humans. J Clin Neurophysiol. 1997;14:102–10. doi: 10.1097/00004691-199703000-00003. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Cornett JF. The evolution of a rat model of chronic spontaneous limbic seizures. Brain Res. 1994 Oct 24;661(1–2):157–62. doi: 10.1016/0006-8993(94)91192-4. [DOI] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike Timing-Dependent Plasticity: A Hebbian Learning Rule. Annu Rev Neurosci. 2008 Feb 14; doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Celesia GG, Chen RC, Bamforth BJ. Effects of ketamine in epilepsy. Neurology. 1975 Feb;25(2):169–72. doi: 10.1212/wnl.25.2.169. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Traub RD, Dingledine R. Role of EPSPs in initiation of spontaneous synchronized burst firing in rat hippocampal neurons bathed in high potassium. J Neurophysiol. 1990;64:1000–1008. doi: 10.1152/jn.1990.64.3.1000. [DOI] [PubMed] [Google Scholar]
- Chen Q, He S, Hu XL, Yu J, Zhou Y, Zheng J, Zhang S, Zhang C, Duan WH, Xiong ZQ. Differential roles of NR2A- and NR2B-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J Neurosci. 2007 Jan 17;27(3):542–52. doi: 10.1523/JNEUROSCI.3607-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SC, Bianchi R, Wong RK. Group I mGluR activation turns on a voltage-gated inward current in hippocampal pyramidal cells. J Neurophysiol. 2000;83:2844–2853. doi: 10.1152/jn.2000.83.5.2844. [DOI] [PubMed] [Google Scholar]
- Clem RL, Celikel T, Barth AL. Ongoing in Vivo Experience Triggers Synaptic Metaplasticity in the Neocortex. Science. 2008;319:101. doi: 10.1126/science.1143808. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron. 1996 Apr;16(4):825–33. doi: 10.1016/s0896-6273(00)80102-6. [DOI] [PubMed] [Google Scholar]
- Debanne D, Thompson SM, Gahwiler BH. A brief period of epileptiform activity strengthens excitatory synapses in the rat hippocampus in vitro. Epilepsia. 2006;47:247–256. doi: 10.1111/j.1528-1167.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Staley KJ. How Does the Balance of Excitation and Inhibition Shift during Epileptogenesis? Epilepsy Curr. 2007 May–Jun;7(3):86–8. doi: 10.1111/j.1535-7511.2007.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan CD, Wenzel JH, Dineley KT, Lam TT, Schwartzkroin PA, Sweatt JD, Swann JW. Postsynaptic contributions to hippocampal network hyperexcitability induced by chronic activity blockade in vivo. Eur J Neurosci. 2003 Oct;18(7):1861–72. doi: 10.1046/j.1460-9568.2003.02920.x. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Dudek FE. Spontaneous motor seizures of rats with kainate-induced epilepsy: effect of time of day and activity state. Epilepsy Res. 1999 May;35(1):47–57. doi: 10.1016/s0920-1211(98)00127-2. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Dudek FE. Chemoconvulsant Model of Chronic Spontaneous Seizures. In: Crawley J, editor. Current Protocols in Neuroscience: Models of Chronic Spontaneous Seizures. Wiley & Sons; 2005. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Grosshans DR, Coultrap SJ, Jones JP, Dobelis P, Browning MD, Staley KJ. NMDA receptor trafficking at recurrent synapses stabilizes the state of the CA3 network. J Neurophys. 2007;98(5):2818–2826. doi: 10.1152/jn.00346.2007. [DOI] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis--dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001 Jul;86(1):321–5. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- Kawai M, Nakamura H, Sakurada I, Shimokawa H, Tanaka H, Matsumizu M, Ando K, Hattori K, Ohta A, Nukui S, Omura A, Kawamura M. Discovery of novel and orally active NR2B-selective N-methyl-D-aspartate (NMDA) antagonists, pyridinol derivatives with reduced HERG binding affinity. Bioorg Med Chem Lett. 2007 Oct 15;17(20):5533–6. doi: 10.1016/j.bmcl.2007.08.039. [DOI] [PubMed] [Google Scholar]
- Kentros C, Hargreaves E, Hawkins RD, Kandel ER, Shapiro M, Muller RV. Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science. 1998 Jun 26;280(5372):2121–6. doi: 10.1126/science.280.5372.2121. [DOI] [PubMed] [Google Scholar]
- Kirschstein T, Bauer M, Müller L, Rüschenschmidt C, Reitze M, Becker AJ, Schoch S, Beck H. Loss of metabotropic glutamate receptor-dependent long-term depression via downregulation of mGluR5 after status epilepticus. J Neurosci. 2007 Jul 18;27(29):7696–704. doi: 10.1523/JNEUROSCI.4572-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl BK, Dannhardt G. The NMDA receptor complex: a promising target for novel antiepileptic strategies. Curr Med Chem. 2001 Sep;8(11):1275–89. doi: 10.2174/0929867013372328. [DOI] [PubMed] [Google Scholar]
- Lee AC, Wong RK, Chuang SC, Shin HS, Bianchi R. Role of synaptic metabotropic glutamate receptors in epileptiform discharges in hippocampal slices. J Neurophysiol. 2002;88:1625–1633. doi: 10.1152/jn.2002.88.4.1625. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Three Ca2+ levels affect plasticity differently: the LTP zone, the LTD zone and no man’s land. J Physiol. 2001 Apr 15;532(Pt 2):285. doi: 10.1111/j.1469-7793.2001.0285f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JF, Jackson MF, Beazely MA. G protein-coupled receptors control NMDARs and metaplasticity in the hippocampus. Biochim Biophys Acta. 2007 Apr;1768(4):941–51. doi: 10.1016/j.bbamem.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004 Sep 8;24(36):7821–8. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, Pretorius JK, Mendoza D, Leite JP, Chimelli L, Born DE, Fried I, Assirati JA, Ojemann GA, Adelson PD, Cahan LD, Kornblum HI. Hippocampal N-methyl-D-aspartate receptor subunit mRNA levels in temporal lobe epilepsy patients. Ann Neurol. 1999 Sep;46(3):343–58. doi: 10.1002/1531-8249(199909)46:3<343::aid-ana10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Möddel G, Jacobson B, Ying Z, Janigro D, Bingaman W, González-Martínez J, Kellinghaus C, Prayson RA, Najm IM. The NMDA receptor NR2B subunit contributes to epileptogenesis in human cortical dysplasia. Brain Res. 2005 Jun 7;1046(1–2):10–23. doi: 10.1016/j.brainres.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Madison DV. Discrete synaptic states define a major mechanism of synapse plasticity. Trends Neurosci. 2004;27(12):744–750. doi: 10.1016/j.tins.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology. 2007 Jan;52(1):71–6. doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Neyman S, Manahan-Vaughan D. Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur J Neurosci. 2008 Mar;27(6):1345–52. doi: 10.1111/j.1460-9568.2008.06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngomba RT, Biagioni F, Casciato S, Willems-van Bree E, Battaglia G, Bruno V, Nicoletti F, van Luijtelaar EL. The preferential mGlu2/3 receptor antagonist, LY341495, reduces the frequency of spike-wave discharges in the WAG/Rij rat model of absence epilepsy. Neuropharmacology. 2005;49( Suppl 1):89–103. doi: 10.1016/j.neuropharm.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalography & Clinical Neurophysiology. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Sayin U, Rutecki PA. Group I metabotropic glutamate receptor activation produces prolonged epileptiform neuronal synchronization and alters evoked population responses in the hippocampus. Epilepsy Res. 2003;53:186–195. doi: 10.1016/s0920-1211(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Schneiderman JH, Sterling CA, Luo R. Hippocampal plasticity following epileptiform bursting produced by GABAA antagonists. Neuroscience. 1994;59:2259–273. doi: 10.1016/0306-4522(94)90594-0. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Nelson SB. Spike timing, calcium signals and synaptic plasticity. Curr Opin Neurobiol. 2002 Jun;12(3):305–14. doi: 10.1016/s0959-4388(02)00325-2. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Bains JS, Yee A, Hellier J, Longacher JM. Statistical model relating CA3 burst probability to recovery from burst-induced depression at recurrent collateral synapses. J Neurophysiol. 2001 Dec;86(6):2736–47. doi: 10.1152/jn.2001.86.6.2736. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Dudek FE. Interictal spikes and epileptogensis. Epilepsy Currents. 2006;6:1–4. doi: 10.1111/j.1535-7511.2006.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley KJ, Longacher M, Bains JS, Yee A. Presynaptic modulation of CA3 network activity. Nat Neurosci. 1998 Jul;1(3):201–9. doi: 10.1038/651. [DOI] [PubMed] [Google Scholar]
- Stasheff SF, Anderson WW, Clark S, Wilson WA. NMDA antagonists differentiate in epileptogenesis from seizure expression in an in vitro model. Science. 1989;24:648–651. doi: 10.1126/science.2569762. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Malgaroli A, Tsien RW. LTP and adaptation to inactivity: overlapping mechanisms and implications for metaplasticity. Neuropharmacology. 2007 Jan;52(1):156–75. doi: 10.1016/j.neuropharm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Traub RD, Miles R. Neuronal Networks Of The Hippocampus. Ch 6. Cambridge Univ. Press; Cambridge, UK: 1991. [Google Scholar]
- Urwyler S, Campbell E, Fricker G, Jenner P, Lemaire M, McAllister KH, Neijt HC, Park CK, Perkins M, Rudin M, Sauter A, Smith L, Wiederhold KH, Muller W. Biphenyl-derivatives of 2-amino-7-phosphono-heptanoic acid, a novel class of potent competitive N-methyl-D-aspartate receptor antagonists--II. Pharmacological characterization in vivo. Neuropharmacology. 1996 Jun;35(6):655–69. doi: 10.1016/0028-3908(96)84637-5. [DOI] [PubMed] [Google Scholar]
- White AM, Williams PA, Ferraro DJ, Clark S, Kadam SD, Dudek FE, Staley KJ. Efficient unsupervised algorithms for the detection of seizures in continuous EEG recordings from rats after brain injury. J Neurosci Methods. 2006;152:255–266. doi: 10.1016/j.jneumeth.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Williams P, White A, Ferraro D, Clark S, Staley K, Dudek FE. The use of radiotelemetry to evaluate electrographic seizures in rats with kainate-induced epilepsy. J Neurosci Methods. 2006;155:39–48. doi: 10.1016/j.jneumeth.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Yang J, Woodhall GL, Jones RSG. Tonic facilitation of glutamate release by presynaptic NR2B-containing NMDA receptors is increased in the entorhinal cortex of chronically epileptic rats. J Neurosci. 2006;26:406–410. doi: 10.1523/JNEUROSCI.4413-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004 Dec 2;44(5):749–57. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]