Abstract
The subjective experience of stress leads to reproductive dysfunction in many species, including rodents and humans. Stress effects on reproduction result from multilevel interactions between the hormonal stress response system, i.e., the hypothalamic–pituitary–adrenal (HPA) axis, and the hormonal reproductive system, i.e., the hypothalamic–pituitary–gonadal (HPG) axis. A novel negative regulator of the HPG axis known as gonadotropin-inhibitory hormone (GnIH) was recently discovered in quail, and orthologous neuropeptides known as RFamide-related peptides (RFRPs) have also been identified in rodents and primates. It is currently unknown, however, whether GnIH/RFRPs influence HPG axis activity in response to stress. We show here that both acute and chronic immobilization stress lead to an up-regulation of RFRP expression in the dorsomedial hypothalamus (DMH) of adult male rats and that this increase in RFRP is associated with inhibition of downstream HPG activity. We also show that adrenalectomy blocks the stress-induced increase in RFRP expression. Immunohistochemistry revealed that 53% of RFRP cells express receptors for glucocorticoids (GCs), indicating that adrenal GCs can mediate the stress effect through direct action on RFRP cells. It is thought that stress effects on central control of reproduction are largely mediated by direct or indirect effects on GnRH-secreting neurons. Our data show that stress-induced increases in adrenal GCs cause an increase in RFRP that contributes to hypothalamic suppression of reproductive function. This novel insight into HPA-HPG interaction provides a paradigm shift for work on stress-related reproductive dysfunction and infertility, and indicates that future work on stress and reproductive system interactions must include investigation of the role of GnIH/RFRP.
Keywords: GnIH, reproduction, RFRP
Stress has acute and chronic effects on many aspects of vertebrate physiology and behavior. Reproduction is a key life-history component that is often adversely affected by physical and psychological stressors. The negative impact of stress occurs at several levels of the vertebrate hypothalamic–pituitary–gonadal (HPG) axis. Centrally, it leads to activation of the hypothalamic–pituitary–adrenal (HPA) axis, which in turn leads to suppression of HPG activity through inhibition of gonadotropin-releasing hormone (GnRH) secretion (1, 2). Downstream of GnRH, the functional effects of stress on reproduction can be seen with suppression of luteinizing hormone (LH) release from the pituitary (3, 4) and suppression of sexual behavior (5, 6). The stress effect on HPG function appears to be mediated by the adrenal stress hormones glucocorticoids (GCs). In male mammals, systemic GC administration inhibits circulating gonadotropin levels (7), decreases seminal vesicle weight, and results in fewer implantation sites and viable fetuses in female mates (8). However, hypothalamic corticotropin-releasing hormone (CRH) has also been strongly implicated in stress effects on reproduction (2, 9, 10).
Gonadotropin inhibitory hormone (GnIH) is a recently-discovered hypothalamic RFamide peptide that inhibits gonadotropin synthesis and secretion (11). The inhibitory action of this neuropeptide raises the question of whether stress-mediated reproductive dysfunction could operate through the GnIH system. If so, this would provide a previously uninvestigated level of stress-regulated central HPG control. The mammalian ortholog of avian GnIH (RFamide-related peptide, RFRP) is expressed in neurons within the dorsomedial nucleus of the hypothalamus (DMH) of rats, with fibers extending both to the median eminence and to the preoptic area, making putative contact with GnRH neurons within the hypothalamus (12, 13). Mammalian RFRP is produced as a full-length precursor peptide that is cleaved into two active peptides in vivo, RFRP-1 and RFRP-3 (12). The amino acid sequences of rat RFRP peptides are highly homologous to GnIH-precursor derived peptides in quail and white-crowned sparrow (12). The receptor for RFRP (known as OT7T022) is also similar to the GnIH receptor in birds (14) and is found in the hypothalamus, pituitary, and testes (15, 16). In birds and mammals, RFRP plays a functional role in suppression of HPG function in vivo (13, 17–19). When RFRP-3 is administered systemically to male rats, it suppresses both LH release and sexual behavior (13), much as stress does. Moreover, RFRP expression is altered by stress in breeding house sparrows (20). Together, this body of data suggests that RFRP (21) may act as an influential mediator of stress effects upon mammalian reproduction.
The present experiments were designed to investigate whether stress could affect mammalian HPG function through increased expression of RFRP. Furthermore, we wished to determine whether any observed effect is mediated by stress hormones such as GCs or CRH. We found that both acute and chronic stress elevate RFRP expression and lead to down-stream HPG dysfunction. We also found that RFRP cells express stress hormone-responsive receptors. In addition, adrenalectomy (ADX) prevents the stress-induced increase in RFRP expression. Thus our data provide strong evidence that RFRP expression and HPG function are directly influenced by stress-induced adrenal hormone release.
Results
Acute Stress Increases Hypothalamic RFRP Expression.
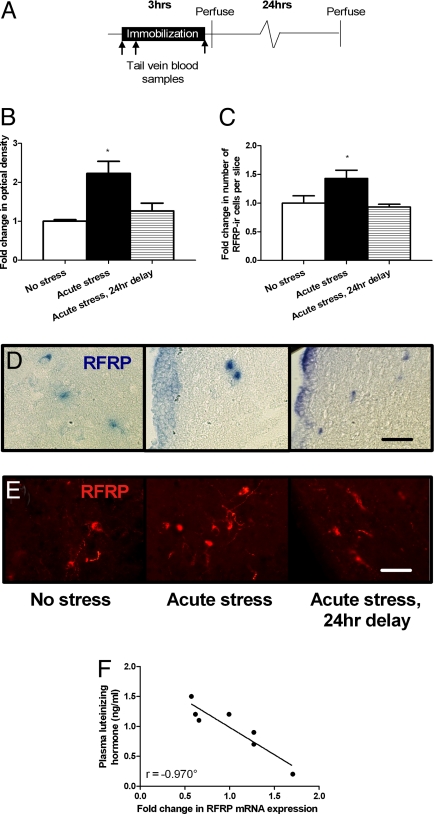
To determine whether RFRP could contribute to stress-induced suppression of the HPG axis, we investigated RFRP gene expression in response to acute stress in adult male rats (Fig. 1A). Three hours of immobilization stress led to an increase in RFRP mRNA and peptide in the hypothalamus when measured immediately after the immobilization ended (Fig. 1 B–E). Using in situ hybridization with a probe that identifies the full-length rat RFRP precursor mRNA (12), we found a 2.3 (±0.3 SEM)–fold increase in RFRP mRNA levels in the DMH immediately after immobilization, relative to no-stress controls. When measured 24 hours after the end of immobilization, RFRP mRNA levels in stressed animals did not differ from controls (Fig. 1 B and D). A similar pattern was found for RFRP precursor peptide (Fig. 1 C and E). Stressed rats showed a 1.4 (± 0.1 SEM)–fold increase in number of RFRP-ir cells immediately after immobilization. RFRP in rats stressed and then killed 24 hours later did not differ from that in nonstressed control rats.
Fig. 1.
Acute stress effects on RFRP expression. (A) Experimental time line. (B) Stressed rats showed higher RFRP mRNA expression levels (mean ± SEM) than controls immediately after stress (P = 0.002). (C) Stressed rats showed more RFRP peptide positive cells (mean ± SEM) than controls immediately after stress (P = 0.006). (D) Representative images of RFRP mRNA positive cells in the DMH. (E) Representative images of RFRP-ir cells in the DMH. (F) There was a significant negative correlation between fold change in RFRP mRNA and plasma-luteinizing hormone (r = −0.920, P = 0.003). *P < 0.05 compared with no-stress and acute stress, 24-hour-delay controls combined. °P < 0.05 correlation. Scale bar, 40 μM.
To investigate whether the stress-induced changes in RFRP expression might be important for HPG function, we used real-time reverse transcriptase PCR (RT-PCR) to determine hypothalamic RFRP mRNA transcript abundance, and correlated the mRNA expression level with circulating LH levels. RFRP mRNA expression levels showed a significant negative correlation with plasma LH levels (Fig. 1F), a finding that is consistent with previous work showing that RFRP lowers circulating LH levels (13) either through reduced GnRH neuron activity or through reduced responsiveness of pituitary cells to hypophysial GnRH (22–25). No effect of acute stress was found on RFRP (1.2 ± 0.1–fold change, P = 0.33) or OT7T022 (1.0 ± 0.1–fold change, P = 0.82) mRNA transcript expression immediately after stress as measured by real-time reverse transcription–polymerase chain reaction (RT-PCR) in the testes. These findings indicate that acute immobilization stress leads to a temporary increase in hypothalamic RFRP expression, coincident with suppression of downstream HPG activity.
Chronic Stress Increases Hypothalamic RFRP Expression.
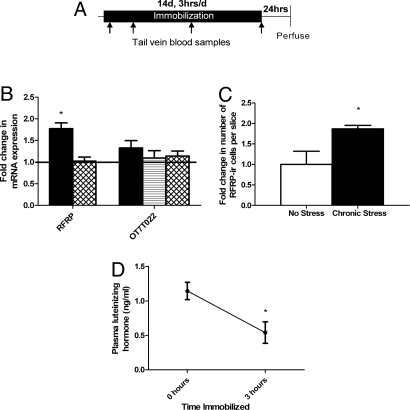
Because chronic stress is also known to suppress reproductive function, with effects possibly extending beyond the termination of the stress (6, 26), we next investigated whether RFRP expression in the rodent DMH is also altered by chronic stress and whether those effects might be more prolonged or greater than acute stress effects. Adult male rats were immobilized for 3 hours per day for 14 consecutive days and killed 24 hours after the last immobilization session (Fig. 2A). Chronic immobilization led to up-regulation of RFRP mRNA and peptide expression in the hypothalamus up to 24 hours after the end of immobilization. Using real time RT-PCR, we found that chronic immobilization stress led to a 1.8 (± 0.1 SEM)–fold increase in RFRP mRNA levels (Fig. 2B) compared with controls. Chronic stress also increased RFRP peptide levels. Immunohistochemistry revealed a 1.9 (± 0.1 SEM)–fold increase in number of RFRP-ir cells in the DMH in stressed rats compared with control rats (Fig. 2C). Stress did not alter RFRP mRNA levels in the testes. Similarly, there were no changes in RFRP receptor (OT7T022) expression in the hypothalamus, pituitary or testes (Fig. 2B). However, our chronic stressor did reduce plasma LH concentrations, confirming that HPG function was suppressed in stressed rats (Fig. 2D).
Fig. 2.
Chronic stress effects on RFRP expression. (A) Experimental time line. (B) Gene expression changes in the hypothalamus (solid bars), pituitary (lined bar), and testes (cross-hatched bars) after chronic immobilization, normalized to no-stress control levels. Immobilization led to an increase in hypothalamic RFRP mRNA expression (mean ± SEM, P = 0.007). No change was seen in hypothalamic, pituitary, or testicular RFRP receptor (OT7T022) or testicular RFRP expression (all P > 0.10). (C) Chronic immobilization led to an increase in hypothalamic RFRP-ir cell number in the DMH (mean ± SEM, P = 0.041). (D) Immobilization decreased luteinizing hormone (LH) on the last day of the stressor. Plasma LH levels after stress were significantly lower than those at baseline on day 14 (P = 0.023). *P < 0.05 no stress versus chronic stress or effect of time immobilized within day, as appropriate. †P < 0.10 effect of time immobilized within day. Scale bar, 40 μM.
Acute and Chronic Stress Activate the HPA Axis.
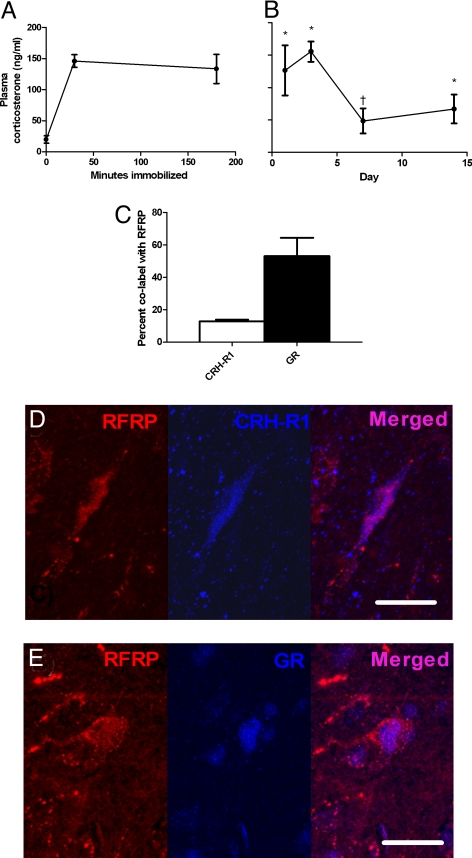
To verify HPA axis activation during the immobilization stress protocol, tail vein plasma samples were collected from acutely and chronically stressed animals. Acutely stressed animals showed a rapid and sustained rise in plasma corticosterone (Fig. 3A). Chronically stressed animals showed elevations of plasma corticosterone in response to restraint throughout all 14 days of restraint, indicating that they did not fully habituate to the stressor (Fig. 3B). These peak values of corticosterone are similar to those found in previous studies showing impaired HPG function after chronic or acute stress (27, 28).
Fig. 3.
HPA responsivity of RFRP cells. (A) Three hours of immobilization led to a rapid increase in plasma corticosterone (mean ± SEM; P = 0.001). (B) Poststress corticosterone difference from baseline. Daily immobilization led to an increase in plasma corticosterone (P < 0.001 effect of time immobilized within day; P < 0.001 time by day interaction). Immobilization led to a significant increase in corticosterone (mean difference from day 1 baseline) on days 1 (P = 0.022), 3 (P < 0.001), and 14 (P = 0.030). The increase on day 7 was not significant (P = 0.054). (C) Percentage of RFRP cells co-expressing CRH-R1 and GR. (D) An RFRP-ir cell (red, first panel) co-expressing CRH-R1 (blue, second panel) in the adult rat DMH. (E) An RFRP-ir cell (red, first panel) co-expressing GR (blue, second panel). *P < 0.05 effect of immobilization over baseline day 1. †P < 0.10 effect of time immobilized over baseline day 1. Scale bar, 20 μM.
Hypothalamic RFRP Cells Express Stress Hormone Receptors.
The stress paradigm reliably activated the HPA axis, and stress effects on hypothalamic RFRP were observed. Thus, we investigated whether RFRP cells in the hypothalamus express receptors that would allow them to respond directly to the two major HPA-regulated hormones, namely, corticotropin-releasing hormone (CRH) and corticosterone (the major rat GC). Using confocal microscopy and double immunohistochemical labeling for CRH receptor-1 (CRH-R1) or glucocorticoid receptor (GR) with RFRP, we found that RFRP-positive cells co-express both of these stress-responsive receptors (Fig. 3 C–E). Approximately 12.8% (±1.1%, SEM) of RFRP-positive cells were co-labeled with CRH-R1, whereas 53.1% (±11.4%, SEM) were co-labeled with GR (Fig. 3 D–E).
Adrenalectomy Prevents the Stress-Induced Increase in RFRP Expression.
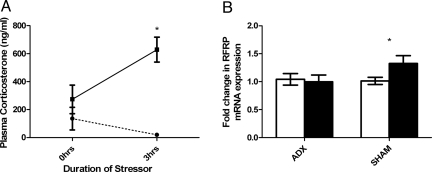
Because approximately half of hypothalamic RFRP cells expressed GR, we next investigated whether the stress-induced rise in RFRP was dependent on increased circulating GCs levels by surgically removing the GC-secreting tissue, i.e., the adrenal glands. Male rats were bilaterally adrenalectomized (ADX) and allowed to recover for 2 weeks. Sham operated animals served as controls. Rats were exposed to daily immobilization stress for 2 weeks as above. The day after the end of stress, rats were killed and RFRP mRNA expression was quantified. Corticosterone response to restraint on day 1 of stress verified that ADX effectively eliminated stress-related corticosterone release (Fig. 4A). In sham-operated rats, chronic immobilization stress led to a significant increase in hypothalamic RFRP mRNA expression, whereas in ADX rats there was no change in RFRP expression after stress (Fig. 4B), indicating that the stress-induced rise in RFRP expression is dependent on high circulating level of GCs.
Fig. 4.
Adrenalectomy prevents stress-induced increase in hypothalamic RFRP expression. (A) Stress led to a significant increase in plasma corticosterone in sham-operated solid line, (P = 0.001) but not ADX rats dashed line, (P = 0.891). (B) Two weeks of daily immobilization led to a significant increase in RFRP mRNA expression in the hypothalamus of sham operated (P = 0.050) but not ADX (P = 0.794) rats. Open bar = control; closed bar = stress. * P < 0.05 stress versus no stress or 0 hours versus 3 hours of stress as appropriate.
Discussion
Here we show that both acute and chronic stress stimulate expression of rat RFRP (considered a GnIH) in the adult male rat hypothalamus while suppressing reproductive function. Our data also indicate that the stress-induced increase in RFRP expression is dependent on adrenal hormones. Acute and chronic immobilization stress led to increases in hypothalamic RFRP at both the mRNA and protein levels. Acute stress effects on RFRP expression largely dissipated within 24 hours of removal of the stressor, whereas chronic stress effects lasted longer. This latter result is consistent with previous work showing that the effects of stress are often greater the longer the stressor is applied (6, 26).
Stress and RFRP are involved in the suppression of sexual behavior in adult male rats (6, 13). Moreover, administering RFRP to adult male rats suppresses plasma LH levels (13), as does exposure to stress (3, 4). In the present study, we show a direct correlation between stress-induced increases in RFRP expression and reduction in plasma LH. In addition, we demonstrate that receptors for stress hormones exist on RFRP neurons, thus providing the neuroanatomical architecture for stress-mediated effects on the RFRP system. Taken together, these data suggest that RFRP plays an influential role in stress-induced effects on gonadotropin release and thus HPG function.
The functional consequences of stress on reproductive success are found in many species and appear to be a common feature of mammalian and avian systems. Stress has been linked to infertility in adult men (29), lower sperm motility in medical students (30), reduced proceptive sexual behavior (5), and fewer successful impregnations (8) by male rats, as well as lower hatchling survival in free-living European starlings (31). In addition, the effects of malnutrition on reproduction may result from activation of the stress system (32, 33), suggesting that the importance of stress effects on the HPG may extend beyond traditional stressors. The HPA axis has also been implicated in aging and the increase in reproductive dysfunction with age (34).
The HPA axis interaction with the HPG axis occurs at multiple levels and appears to be conserved across many species. In response to stress, the hypothalamus releases CRH into the hypophysial portal system, after which anterior pituitary cells respond to CRH by releasing adrenocorticotropic hormone (ACTH). The hormone ACTH stimulates the adrenal cortex to release GCs into the bloodstream. The HPG axis runs in parallel to the HPA, with hypothalamic GnRH stimulating pituitary LH and FSH, which in turn stimulate testosterone-secreting Leydig cells and sperm-producing Sertoli cells. Both GCs and CRH affect multiple levels of the HPG axis in several species (Fig. 5A and [supporting information (SI) Fig. S1]) (1, 35, 36). Injections of GC can suppress plasma LH in rats (2), rhesus monkeys (7), and humans (37), and CRH receptor antagonists can block the effects of stress on plasma LH (2, 10). In vitro, GnRH neurons show changes in gene expression in response to GCs (38), but GnRH expression does not always change in response to a stressor that inhibits downstream reproductive function (3). Indeed, our data did not show changes in GnRH expression despite suppression of plasma LH secretion. These findings suggest that the stress effects on circulating gonadotrophin may act via suppression of GnRH release and/or via decreased pituitary sensitivity to GnRH. Previous studies have shown evidence for both of these mechanisms of stress-mediated suppression of HPG function (39, 40), and our present study provides strong evidence for a mechanistic basis for this response.
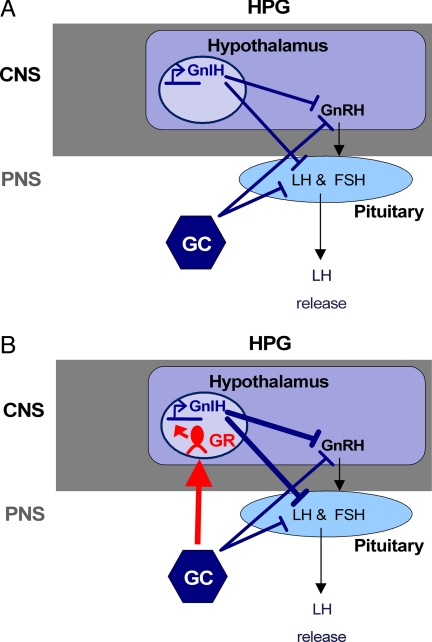
Fig. 5.
Model of how stress may affect the HPG axis through GnIH. (A) Previously, it was known that both GCs and GnIH inhibited the HPG axis independently. These interactions are shown in blue. (B) We propose that GCs released in response to stress act on GnIH via GR to increase the inhibitory actions of GnIH on GnRH secretion and/or pituitary sensitivity, resulting in decreased LH release. Our proposed pathway of GC–GnIH interaction is represented in red, joining the previously established independent effects of GCs and GnIH on reproduction. Arrows represent stimulation; Ts represent inhibition. CNS, central nervous system; PNS, peripheral nervous system.
In avian and rodent models, GnIH/RFRP fibers extend to the median eminence and come in close proximity to GnRH cells (12, 13, 41, 42). In addition, GnIH receptors are expressed on GnRH cells (41). Together, these results suggest that GnIH/RFRP may regulate the HPG axis by influencing hypothalamic GnRH release and/or pituitary sensitivity to GnRH. Currently, there is evidence for GnIH/RFRP influencing hypothalamic GnRH neuron excitability/release as well as pituitary gonadotropin release in birds (18, 21, 41) and mammals (12, 13, 24, 25, 43–45), suggesting that these mechanisms of regulating HPG function are conserved across species. For example, Rizwan et al. (22) found that systemic RFRP-3 administration in rats reduced LH response to GnRH (suggesting RFRP acts via pituitary mechanisms), whereas Ducret et al. (24) found that GnRH neurons in mice hyperpolarize in response to RFRP in vitro (suggesting that RFRP affects hypothalamic GnRH release). It is possible, then, that both pathways could play a role in the stress-reproduction interaction via GnIH/RFRP. Therefore, given the changes we found in RFRP in rats after acute and chronic stress, we propose that GnIH/RFRP acts as a common contributory mechanism by which stress inhibits GnRH release and/or gonadotropin secretion in response to GnRH (Fig. 5 A and B).
In addition to being present in the hypothalamus, GnIH/RFRP is also found in the testes of rats and birds (16). However, we found no change in RFRP expression in the testes of adult male rats following stress, indicating that the stress effect on RFRP expression is specifically a central, hypothalamic phenomenon. We also found no change in RFRP receptor expression at any level of the HPG axis. Therefore, it appears that stress largely acts on RFRP synthesis rather than altering the sensitivity of other cell types to RFRP via changes in RFRP receptor expression.
Within the hypothalamus, we found GR and CRH-R1 expressed in a large proportion of RFRP-expressing cells. Stress effects on the HPG have been linked to both GCs and CRH. Exogenous GC injection suppresses HPG activity and sexual behavior, whereas CRH receptor blockers (including those specific to CRH-R1) can block stress effects on reproductive function (2, 8, 10). Because our results indicated that a greater proportion of RFRP cells expressed GR than expressed CRH-R1, we investigated whether GCs might be the mediator of stress effects on RFRP expression by adrenalectomizing rats before stress. We found that ADX prevented the stress-induced increase in hypothalamic RFRP expression, indicating that RFRP changes depend on stress-induced elevations in GCs (Fig. 5B). Our results are therefore consistent with previous work showing that stress effects on HPG function are mediated by GCs (7, 8) and our data provide a mechanistic framework for those findings. In addition, a search of the rat RFRP promoter region reveals four potential glucocorticoid responsive elements (GREs) within 5000 base pairs (bp) upstream of the RFRP gene, the closest being ≈1240 bp upstream. Although more work is needed to show definitively that GCs act directly on GR in hypothalamic RFRP cells, our findings of GC-dependent changes in RFRP, co-expression of RFRP and GR and the existence of potential GREs in the RFRP promoter strongly imply that stress-induced increases in circulating GCs stimulate GR in hypothalamic RFRP cells, causing increased transcription of the RFRP gene (Fig. 5B).
Previous studies of HPA-HPG interactions have provided evidence for multiple, possibly overlapping mechanisms for stress effects on reproduction, including effects on dopaminergic, serotonergic, and cholinergic transmission at multiple levels (1, 35, 46, 47), in addition to direct effects on the HPG axis (1, 35). It seems possible that these mechanisms may have evolved in parallel to allow for HPG–HPA axis interaction in response to a variety of stimuli. The RFRP/HPA interaction that we have demonstrated here may therefore represent a pathway that provides a greater sensitivity to certain stressor stimuli than others. More research is necessary to determine whether different stressors activate different HPA–HPG interaction pathways, but such a phenomenon could help explain discrepant findings in the stress and reproduction literature.
In summary, our data indicate a novel mechanism by which HPA axis activation can influence reproductive function, namely through a GC-induced increase in RFRP signaling to GnRH neurons and/or the anterior pituitary. Moreover, given the common interaction of HPA and HPG axes across many species and the similarity of GnIH/RFRP actions in birds and mammals (48), the involvement of RFRP in the HPA-HPG interaction investigated here in rats is predicted to be pertinent to most vertebrates. HPA axis activation is implicated in a number of causes of infertility and reproductive dysfunction, including chronic stress and malnutrition (32, 33, 49), and may significantly influence the efficacy of assisted reproductive procedures in humans (36, 50). In addition, chronic stress is of primary concern in captive breeding programs as well as in agricultural breeding programs (51). The current findings, therefore, contribute a new key level of understanding to the mechanism underlying one of the most common suppressors of HPG activity.
Materials and Methods
Experimental Subjects.
Adult male Sprague-Dawley rats were pair-housed on a 12/12-hour light–dark cycle with lights on at 07:00 hours and ad libitum food and water. For the acute stress study, animals were immobilized and killed immediately after immobilization (n = 5) or 24 hours later (n = 4). Controls were left undisturbed in their home cages until perfused (n = 3). A separate group of rats was used for correlation of trunk blood LH and RFRP expression (n = 3 con, n = 4 str). For assessment of testes transcript expression by real-time RT-PCR after acute stress, con rats (n = 4) con and str rats (n = 4) were used. For the chronic stress experiment, rats were immobilized daily (n = 6 for PCR, n = 4 for IHC) or left undisturbed in their home cages (n = 6 for PCR, n = 4 for IHC). Confocal analysis was performed on tissue from three control rats. For the ADX experiment, 14 animals received ADX (n = 6 stress, n = 8 no stress) and 16 received sham surgery (n = 8 stress, n = 8 no stress). All animal care and procedures were approved by the University of California–Berkeley Animal Care and Use Committee.
Immobilization Stress.
Rats were immobilized in Decapicone bags (Braintree scientific) and placed in individual cages in a fume hood for 3 hours. For the chronic study, immobilization occurred daily for 14 days.
Plasma Hormone Sampling.
All blood samples were centrifuged at 2000 g for 15 minutes and plasma was extracted and stored at −20 °C until assayed. Corticosterone was measured using a Corticosterone EIA kit (Cayman Chemical). Plasma LH was assessed by the National Hormone and Peptide Program RIA. For chronic stress experiments, tail blood samples from two cagemates were pooled. For acute stress, individual samples were used for analysis. Sample values below the detection level of the assay were included as the lowest detectible value.
Adrenalectomy.
Adult male rats underwent either bilateral removal of the adrenal glands or bilateral sham surgery. After 1 week of recovery in isolation, rats were pair-housed with their original cage mate. One week later, immobilization stress began as described above. The ADX rats were maintained on water with 0.9% NaCl and 25 μg/ml corticosterone for the duration of the experiment. One ADX stress rat was removed from analysis because day 1 corticosterone response to restraint revealed that ADX was incomplete.
In Situ Hybridization and Immunohistochemical Staining for Stress Studies.
Rats were transcardially perfused with 4% paraformaldehyde. Brains were postfixed for 3–4 hours, equilibrated in 30% sucrose in 0.1M phosphate buffered saline (PBS), and then stored at −80 °C. In situ hybridization was performed similar to a previously reported procedure (12) with a DIG-labeled probe against full-length precursor rat RFRP (more detail is available in SI Methods). For immunohistochemical labeling of RFRP peptide, sections were then rinsed with PBS, incubated in blocking solution (2% normal goat serum, 0.3% tritonX-100 in PBS) for 1 hour and then transferred into primary antibody against GnIH (12) (PAC123/124, 1:5000 in PBS plus 0.3% Triton-100 [PBS-T]) overnight at 4 °C. The next day, slides were rinsed with PBS-T and incubated in goat anti-rabbit Alexafluor 568 (1:500, Molecular Probes Inc.) for 2 hours at room temperature. After rinsing in PBS-T, slides were coverslipped using DABCO antifading medium and stored in the dark at 4 °C.
Immunohistochemical Staining for Confocal Analysis.
Free-floating sections were rinsed in 0.1M PBS then incubated in Tris buffer, pH 9 at 60 °C for 20 minutes. After rinsing, tissue was blocked with 2% normal donkey serum, 0.3% Triton-X 100 in PBS. All antibodies were diluted in blocking solution. Primary (rabbit anti-RFRP with mouse anti-GR [1:200, Affinity BioReagents] or goat anti-CRHR1 [1:200, Santa Cruz]) was applied overnight, on rotation, at 4 °C. The next day, sections were rinsed in PBS and incubated in secondary for 2 hours at room temperature (Cy3 anti-mouse with FITC anti-rabbit or Cy5 anti-goat with Cy3 anti-rabbit, 1:500, Jackson ImmunoResearch). After rinsing in PBS, sections were mounted on gelatin-coated slides and coverslipped/stored as above.
Confocal Analysis.
Twenty-five RFRP-ir cells were located in the DMH for each animal and assessed in z-series of <1.0-μm slices to determine whether CRH-R1 or GR was co-labeled. Confocal images were captured on a Zeiss 510 META/NLO confocal microscope with a ×40 oil objective and adjusted for brightness and contrast using LSM Image Browser software. The wavelengths used were 488 nm, 543 nm, and 643 nm.
Real-Time Reverse Transcriptase PCR.
Rats were lightly anesthetized with isoflurane and rapidly decapitated before bilateral hypothalami, pituitary, and testes were dissected and flash-frozen in liquid nitrogen. Real-time reverse transcriptase PCR was run on TRIzol-extracted RNA with primers for rat RFRP and OT7T022. The Ct values were determined using PCR miner (52) and normalized to the reference gene, RPLP. Primer sequences were designed using Primer1 software and checked for specificity using BLAST. For all stress studies, the manufacturer's instructions for SYBR Green One-step PCR kit (BioRad) were followed. For the ADX study, extracted RNA was treated with DNase (DNA-free, Ambion), and two-step PCR was used, following the manufacturer's instructions for iScript cDNA synthesis kit (BioRad) and then iQ SYBR Green Supermix. Samples were run in a BioRad IQ5 real-time PCR machine. After the PCR was complete, specificity of each primer pair was confirmed using melt curve analysis. Primer sequences are given in SI Methods
Statistical Analysis.
Group differences in RFRP mRNA and peptide expression were detected using two tailed t tests. For the acute stress experiment, no-stress controls were found not to differ significantly from acute stress, 24-hour delay rats in mRNA (P = 0.444) or peptide expression (P = 0.604), so the latter two were combined for statistical comparison with acute stress, 0-hour delay rats. The effect of immobilization time on plasma corticosterone in the acute stress experiment was assessed using a repeated-measures analysis of variance (ANOVA). In the chronic stress experiment, the effect of immobilization on plasma corticosterone was tested using a 2 × 4 (time of immobilization by day) repeated measures (within-day) ANOVA followed by paired t tests to detect differences on each day from day 1 baseline. Stress effect on day 14 plasma LH was detected using a two-sample t test. In the ADX study, planned comparisons (one-sample t tests) were used to detect a stress effect in RFRP expression in sham and ADX rats. Similarly, planned comparisons (two-sample paired t tests) were used to detect a stress effect on plasma corticosterone in sham and ADX rats. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments.
We thank Al Parlow of the National Hormone and Peptide Program for conducting luteinizing hormone RIA on plasma samples. This work was supported by National Science Foundation grant IOS-0641188 and a Hellmann Foundation grant (to G.E.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901176106/DCSupplemental.
References
- 1.Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gondal axis: Peripheral and central mechanisms. Biol Reprod. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- 2.Rivier C, Rivier J, Vale W. Stress-induced inhibition of reproductive functions: Role of endogenous corticotropin-releasing factor. Science. 1986;231:607–609. doi: 10.1126/science.3003907. [DOI] [PubMed] [Google Scholar]
- 3.Du Ruisseau P, Tache Y, Brazeau P, Collu R. Effects of chronic immobilization stress on pituitary hormone secretion, on hypothalamic factor levels, and on pituitary responsiveness to LHRH and TRH in female rats. Neuroendocrinology. 1979;29:90–99. doi: 10.1159/000122910. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Quijano MI, Ariznavarreta C, Martin AI, Treguerres JAF, Lopez-Calderon A. Naltrexone does not reverse the inhibitory effect of chronic restraint on gonadotropin secretion in the intact male rat. Neuroendocrinology. 1991;54:447–453. doi: 10.1159/000125933. [DOI] [PubMed] [Google Scholar]
- 5.Sato Y, et al. Effects of long-term psychological stress on sexual behavior and brain catecholamine levels. J Androl. 1996;17:83–90. [PubMed] [Google Scholar]
- 6.Retana-Marquez S, Bonilla-Jaime H, Vazquez-Palacios G, Matrinez-Garcia R, Velazquez-Moctezuma J. Changes in masculine sexual behavior, corticosterone and testosterone in response to acute and chronic stress in male rats. Horm Behav. 2003;44:327–337. doi: 10.1016/j.yhbeh.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Dubey AK, Plant TM. A suppression of gonadotropin secretion by cortisol in castrated male rhesus monkeys (Macaca mulatta) mediated by the interruption of hypothalamic gonadotropin-releasing hormone release. Biol Reprod. 1985;33:423–431. doi: 10.1095/biolreprod33.2.423. [DOI] [PubMed] [Google Scholar]
- 8.Lerman SA, et al. Effects of corticosterone on reproduction in male Sprague-Dawley rats. Reprod Toxicol. 1997;11:799–805. doi: 10.1016/s0890-6238(97)00063-4. [DOI] [PubMed] [Google Scholar]
- 9.Kalantaridou SN, Makrigiannakis A, Zoumakis E, Chrousos GP. Reproductive functions of corticotropin-releasing hormone. Research and potential clinical utility of antalarmins (CRH receptor type 1 antagonists) Am J Reprod Immunol. 2004;51:269–274. doi: 10.1111/j.1600-0897.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 10.Li XF, et al. Differential role of corticotropin-releasing factor receptor types 1 and 2 in stress-induced suppression of pulsatile luteinizing hormone secretion in female rat. J Neuroendocrinol. 2006;18:602–610. doi: 10.1111/j.1365-2826.2006.01450.x. [DOI] [PubMed] [Google Scholar]
- 11.Tsutsui K, et al. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- 12.Kriegsfeld LJ, et al. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51:171–180. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin H, Ukena K, Ubuka T, Tsutsui K. A novel G protein-coupled receptor for gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica): Identification, expression and binding activity. J Endocrinol. 2005;184:257–266. doi: 10.1677/joe.1.05926. [DOI] [PubMed] [Google Scholar]
- 15.Hinuma S, et al. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol. 2000;2:703–708. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- 16.Bentley GE, et al. Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system. Gen Comp Endocrinol. 2008;156:34–43. doi: 10.1016/j.ygcen.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Murakami M, et al. Hypophysiotropic role of RFamide-related peptide-3 (RFRP-3) in the inhibition of LH secretion in female rats. J Endocrinol. 2008;199:105–112. doi: 10.1677/JOE-08-0197. [DOI] [PubMed] [Google Scholar]
- 18.Bentley GE, et al. Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH) Horm Behav. 2006;49:550–555. doi: 10.1016/j.yhbeh.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Osugi T, et al. Gonadotropin-inhibitory hormone in Gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. J Endocrinol. 2004;182:33–42. doi: 10.1677/joe.0.1820033. [DOI] [PubMed] [Google Scholar]
- 20.Calisi RM, Rizzo NO, Bentley GE. Seasonal differences in hypothalamic EGR-1 and GnIH expression following capture-handling stress in house sparrows (Passer domesticus) Gen Comp Endocrinol. 2008;157:283–287. doi: 10.1016/j.ygcen.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Ubuka T, Ukena K, Sharp PJ, Bentley GE, Tsutsui K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology. 2006;147:1187–1194. doi: 10.1210/en.2005-1178. [DOI] [PubMed] [Google Scholar]
- 22.Rizwan MZ, Porteous R, Herbison AE, Anderson GM. Cells expressing RFRP-1/3, the mammalian GnIH orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology. 2009;150:1413–1420. doi: 10.1210/en.2008-1287. [DOI] [PubMed] [Google Scholar]
- 23.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone (GnIH) innervates, inhibits basal forebrain vGluT2-GnRH neurons via a direct postsynaptic mechanism. J Physiol. 2009;587:1401–1411. doi: 10.1113/jphysiol.2008.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3 (RFRP-3), a mammalian gonadotropin-inhibitory ortholog, regulates gonadotropin-releasing hormone (GnRH) neuron firing in the mouse. Endocrinology. 2009;150:2799–2804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- 25.Anderson GM, Relf HL, Rizwan MZ, Evans JJ. Central and peripheral effects of RFamide-related peptide-3 on LH and prolactin secretion in rats. Endocrinology. 2009;150:1834–1840. doi: 10.1210/en.2008-1359. [DOI] [PubMed] [Google Scholar]
- 26.Suarez M, Fiol de Cuneo M, Vincenti L, Ruiz RD. Changes in corticosterone levels and sperm functional activity by chronic stress in rats. Arch Physiol Biochem. 1996;104:351–356. doi: 10.1076/apab.104.3.351.12903. [DOI] [PubMed] [Google Scholar]
- 27.Retana-Márquez S, Bonilla-Jaime H, Vázquez-Palacios G, Martínez-García R. Naltrexone effects on male sexual behavior, corticosterone, and testosterone in stressed male rats. Physiol Behav. 2009;96:333–342. doi: 10.1016/j.physbeh.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 28.Li XF, et al. Differential effects of repeated restraint stress on pulsatile lutenizing hormone secretion in female fischer, lewis and wistar rats. J Neuroendocrinol. 2004;16:620–627. doi: 10.1111/j.1365-2826.2004.01209.x. [DOI] [PubMed] [Google Scholar]
- 29.Sheiner EK, Sheiner E, Carel R, Potashnik G, Shoham-Vardi I. Potential association between male infertility and occupational psychological stress. J Occup Environ Med. 2002;44:1093–1099. doi: 10.1097/00043764-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Eskiocak S, et al. Glutathione and free sulphydryl content of seminal plasma in healthy medical students during and after exam stress. Hum Reprod. 2005;20:2595–2600. doi: 10.1093/humrep/dei062. [DOI] [PubMed] [Google Scholar]
- 31.Cyr NE, Romero LM. Chronic stress in free-living European starlings reduces corticosterone concentrations and reproductive success. Gen Comp Endocrinol. 2007;151:82–89. doi: 10.1016/j.ygcen.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Vulliemoz NR, Xiao E, Xia-Zhang L, Rivier J, Ferin M. Astressin B, a nonselective corticotropin-releasing hormone receptor antagonist, prevents the inhibitory effect of ghrelin on luteinizing hormone pulse frequency in the ovariectomized rhesus monkey. Endocrinology. 2008;149:869–874. doi: 10.1210/en.2007-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berga SL. Stress and reproduction: A tale of false dichotomy. Endocrinology. 2008;149:867–868. doi: 10.1210/en.2008-0004. [DOI] [PubMed] [Google Scholar]
- 34.Hafez B, Hafez ESE. Stress/aging: Endocrine profiles/reproductive dysfunction in men. Arch Androl. 2004;50:207–238. doi: 10.1080/01485010490448534. [DOI] [PubMed] [Google Scholar]
- 35.Tilbrook AJ, Turner AI, Clarke IJ. Effects of stress on reproduction in non-rodent mammals: The role of glucocorticoids and sex differences. Rev Reproduction. 2000;5:105–113. doi: 10.1530/ror.0.0050105. [DOI] [PubMed] [Google Scholar]
- 36.Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: When and how. J Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- 37.Sakakura M, Takebe K, Nakagawa S. Inhibition of luteinizing hormone secretion induced by synthetic LRH by long-term treatment with glucocorticoids in human subjects. J Clin Endocrinol Metab. 1975;40:774–779. doi: 10.1210/jcem-40-5-774. [DOI] [PubMed] [Google Scholar]
- 38.Attardi B, et al. Glucocorticoid repression of gonadotropin-releasing hormone gene expression and secretion in morphologically distinct subpopulations of GT1–7 cells. Mol Cell Endocrinol. 1997;131:241–255. doi: 10.1016/s0303-7207(97)00102-0. [DOI] [PubMed] [Google Scholar]
- 39.Suter DE, Schwartz NB. Effects of glucocorticoids on responsiveness of luteinizing hormone and follicle-stimulating hormone to gonadotropin-releasing hormone by male rat pituitary cells in vitro. Endocrinology. 1985;117:849–854. doi: 10.1210/endo-117-3-855. [DOI] [PubMed] [Google Scholar]
- 40.Gambacciani M, Yen SSC, Rasmussen DD. GnRH release from the mediobasal hypothalamus: In vitro inhibition by corticotropin-releasing factor. Neuroendocrinology. 1986;43:533–536. doi: 10.1159/000124578. [DOI] [PubMed] [Google Scholar]
- 41.Ubuka T, et al. Gonadotropin-inhibitory hormones neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in european starling brain. Endocrinology. 2008;149:268–278. doi: 10.1210/en.2007-0983. [DOI] [PubMed] [Google Scholar]
- 42.Bentley GE, et al. Interactions of gonadotropin-releasing hormone (GnRH) and gonadotropin-inhibtory hormone (GnIH) in birds and mammals. J Exp Zool. 2006;305A:807–814. doi: 10.1002/jez.a.306. [DOI] [PubMed] [Google Scholar]
- 43.Gibson EM, et al. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clarke IJ, et al. Potent action of RFRP-3 on pituitary gonadotropes indicative of an hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149:5811–5821. doi: 10.1210/en.2008-0575. [DOI] [PubMed] [Google Scholar]
- 45.Rizwan MZ, Porteous R, Herbison AE, Anderson GM. Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology. 2009;150:1413–1420. doi: 10.1210/en.2008-1287. [DOI] [PubMed] [Google Scholar]
- 46.Mor I, et al. Modified testicular expression of stress-associated “readthrough” acetylcholinesterase predicts male infertility. FASEB J. 2001;15:2039–2041. doi: 10.1096/fj.00-0814fje. [DOI] [PubMed] [Google Scholar]
- 47.Kaufer D, Friedman A, Seidman S, Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature. 1999;393:373–377. doi: 10.1038/30741. [DOI] [PubMed] [Google Scholar]
- 48.Bentley GE, et al. GnIH: A multifunctional neuropeptide. J Neuroendocrinol. 2009 doi: 10.1111/j.1365-2826.2009.01851.x. [DOI] [PubMed] [Google Scholar]
- 49.Ferin M. Clinical review 105: Stress and the reproductive cycle. J Clin Endocrinol Metab. 1999;84:1768–1774. doi: 10.1210/jcem.84.6.5367. [DOI] [PubMed] [Google Scholar]
- 50.Champagne DM. Should fertilization treatment start with reducing stress? Hum Reprod. 2006;21:1651–1658. doi: 10.1093/humrep/del078. [DOI] [PubMed] [Google Scholar]
- 51.Einarsson S, Brandt Y, Rodriguez-Martinez H, Madej A. Conference lecture: Influence of stress on estrus, gametes and early embryo development in the sow. Theriogenology. 2008;70:1197–1201. doi: 10.1016/j.theriogenology.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12:1045–1062. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.