Abstract
Dioxygen (O2) and other gas molecules have a fundamental role in a variety of enzymatic reactions. However, it is only poorly understood which O2 uptake mechanism enzymes employ to promote efficient catalysis and how general this is. We investigated O2 diffusion pathways into monooxygenase and oxidase flavoenzymes, using an integrated computational and experimental approach. Enhanced-statistics molecular dynamics simulations reveal spontaneous protein-guided O2 diffusion from the bulk solvent to preorganized protein cavities. The predicted protein-guided diffusion paths and the importance of key cavity residues for oxygen diffusion were verified by combining site-directed mutagenesis, rapid kinetics experiments, and high-resolution X-ray structures. This study indicates that monooxygenase and oxidase flavoenzymes employ multiple funnel-shaped diffusion pathways to absorb O2 from the solvent and direct it to the reacting C4a atom of the flavin cofactor. The difference in O2 reactivity among dehydrogenases, monooxygenases, and oxidases ultimately resides in the fine modulation of the local environment embedding the reactive locus of the flavin.
Keywords: computational biochemistry, enzymology, flavin, oxygen reactivity
Dioxygen (O2) and other gas molecules have a fundamental role in a variety of enzymatic reactions carried out in living organisms. Oxygen-using enzymes usually have kinetic rates many folds higher than those attained by artificial catalysts (1–4). One crucial unsolved question concerns how these small gas molecules reach their active sites in an effective manner. Former studies supported the general model of passive diffusion through proteins (5, 6). However, it was more recently proposed that O2 preferentially follows highly specific tunnels to reach the core of protein matrices (7–13). Is it possible to generalize these features for different classes of enzymes? Do proteins preferentially employ single specific pathways or a multitude of routes to optimize the O2 uptake process?
Pioneering studies on gas-diffusion in biomolecular systems focused on single diffusion pathways in proteins of the globin family (2, 14). For example, myoglobin served as an ideal test system for the first computational studies on gaseous reactants (14). Fluorescence-quenching experiments were used to correlate the diffusion behavior with solvent properties, e.g., viscosity (15, 16). Reaction intermediates of small gas molecules can be trapped in X-ray enzyme structures in limited favorable cases (17, 18) whereas time-resolved X-ray crystallography provides single-molecule-averaged insight (19). A general observation emerging from recent engineering studies of an O2 transport protein is that design efforts based on static-structure models fail to address the molecular complexity critical for function (20). Thus, complementing experimental and computational approaches can improve our understanding of these dynamic processes at the atomic-level scale (7, 8, 21–25). However, no molecular dynamics (MD) study to date reported on the spontaneous, nonbiased diffusion of O2 molecules from the bulk solvent to the active site of a flavoenzyme.
Flavoenzymes catalyze a wide range of reactions essential for maintaining cellular processes. Their striking chemical versatility is largely based on their ability to control the reaction of a flavin cofactor with O2. Three major groups of flavoenzymes can be distinguished (3, 26, 27). First, the dehydrogenases, that are characterized by poor or no reactivity with oxygen. Second, the monooxygenases, that react very fast with O2, forming a flavin adduct intermediate (C4a-hydroperoxyflavin; Scheme 1 and Fig. S1), which subsequently inserts an oxygen atom into the substrate molecule. Third, the oxidases, that catalyze a rapid 2-electron transfer to O2 to produce H2O2, typically—but not always (28)—with no detectable catalytic intermediates. The reaction of reduced flavins with O2 proceeds through an electron-transfer step that generates a caged radical pair, which is generally thought to be rate limiting. Reduced flavoprotein monooxygenases and oxidases typically react very rapidly with O2, exhibiting bimolecular rate constants (up to 106 M−1·s−1) that are close to a diffusion-controlled rate.
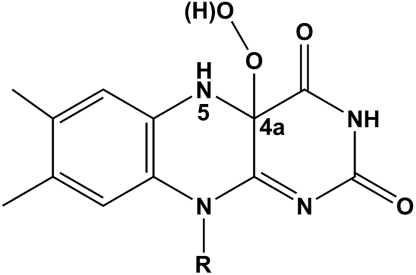
Scheme 1.
Chemical formula of the C4a-hydroperoxyflavin molecule. The N5 and C4a atoms are labeled. The flavin is facing the viewer with its re-side (the si-side is on the opposite side).
The biochemical and structural basis of the ability of flavoenzymes to react with O2 remains to a large extent poorly understood, representing a most fascinating issue in flavoenzymology (3, 26). A key requirement for monooxygenases is that O2 reaches the flavin to promote the catalyzed reaction through a C4a covalent adduct (3, 26, 27) (Scheme 1). However, only little is known about how oxidases react with O2. A direct contact between the oxidase flavin and O2 may not be necessary, leaving open the possibility that the enzymes of this class may react by tunneling pathways (24, 29) or electron transfer processes similar to what was reported for heme-containing enzymes (30).
Here, we investigate O2 diffusion in the monooxygenase component (C2) of p-hydroxyphenylacetate hydroxylase from Acinetobacter baumannii (31) and alditol oxidase (AldO) from Streptomyces coelicolor A3 (2) (32) using an integrated computational and experimental procedure. Enhanced-statistics MD simulations capture the spontaneous (unbiased) diffusion of O2, filling the gap between current sampling times in protein simulations with explicit solvent (≈101 ns scale) (33, 34) and measured diffusion times (>10 ns scale). Our theoretical predictions were followed by experimental verification through site-directed mutagenesis, rapid kinetics measurements, and structural analysis of the C2 and AldO enzymes. This integrated approach indicates that O2 diffusion into both the monooxygenase and the oxidase flavoenzymes is effectively guided by multiple-pathways funnels that lead to defined O2 entry points of active sites. Our data provide structural insight into the mechanism of O2 uptake by flavoenzymes, emphasizing the importance of the protein microenvironment of the flavin in modulating oxygen reactivity.
Results
Oxygen Diffusion into C2.
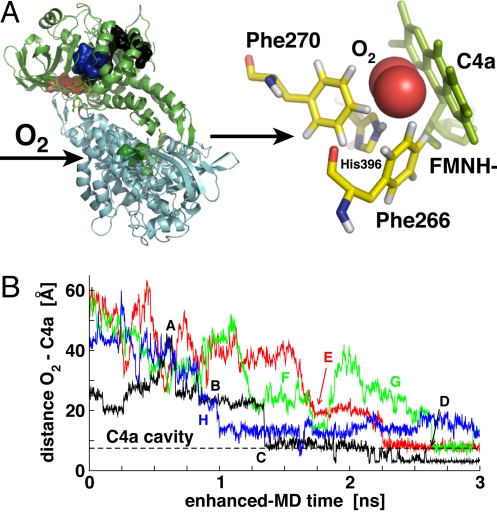
We investigated the diffusion of O2 molecules into the active site of C2. Enhanced-statistics MD simulations were initialized based on the X-ray structure (2.8 Å resolution) of the enzyme bound to reduced FMN (FMNH−) (31) and extended for 30 ns of overall enhanced time (Table S1). Out of a total of 500 O2 trajectories at 300 K, we observe 4 complete spontaneous diffusion pathways that bring O2 molecules in front of the re-side of FMNH− (Fig. 1) starting from random configurations in the bulk solvent. Successful paths typically cover overall distances of ≈40–60 Å from the protein surface to the flavin C4a carbon and display a stepwise behavior from the time of entrance through the C2 protein surface (Fig. 1B). In fact, O2 molecules may temporarily reside on the surface (e.g., Fig. 1B, step A) and generally visit several cavities along each of these paths (e.g., Fig. 1B, steps B and C). An example path is depicted in Fig. 2 and available as Movie S1 video online. Additionally, trajectories show O2 molecules residing transiently in cavities and niches on the protein surface characterized by the presence of Ala and/or Ile hydrophobic residues, an ideal physicochemical environment to host O2 molecules. These additional paths carrying O2 molecules inside the protein, but not in proximity of the C4a flavin atom (distance >6 Å) were therefore distinguished from the above mentioned “complete paths” (see also SI Text). 350 K simulations confirm the location of these paths (15 and 25 corresponding events, respectively; data not shown). The relatively limited number of complete paths observed might be, in part, dependent on the 30-ns overall sampling considered (13% of O2 molecules are inside the protein matrix at the end of our runs at 300 K; 27% at 350 K). However, we stress that this sampling time was sufficient to capture a multitude of diffusion paths.
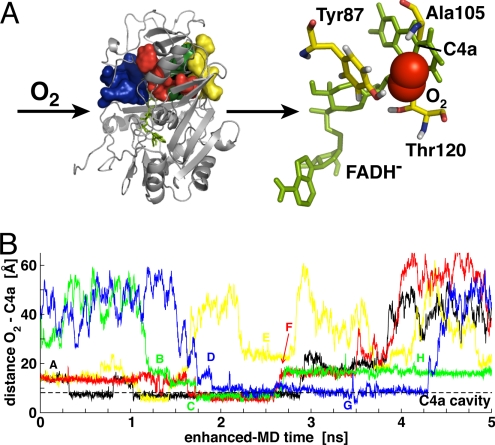
Fig. 1.
O2 spontaneous diffusion into C2. (A) Four complete paths are observed from simulations at 300 K (volume isosurfaces) (Left). All paths conduct O2 molecules (red spheres) (Right) to the re-side of the FMNH− (reduced flavin cofactor, green sticks) in close contact with its C4a carbon (Right). (B) Time series of the O2 – C4a distance for the successful diffusion paths and key steps (see text). The dashed horizontal line defines the C4a flavin oxygen cavity.
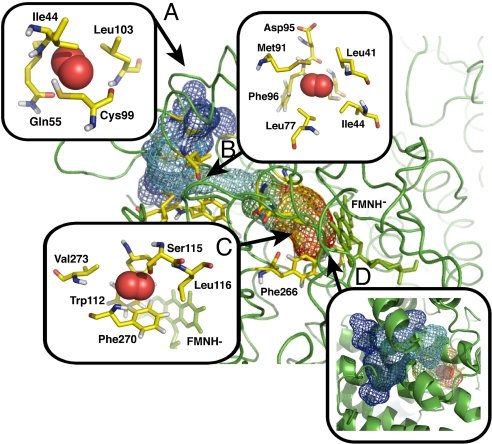
Fig. 2.
Side-chain flexibility assists O2 diffusion. Key steps along an example diffusion path for C2. (A) Entrance into surface cavity. (B) Transport to an internal cavity. (C) Side-chain fluctuation mechanism. (D) Final entrance into the C4a preorganized cavity [overall tunnel view; volume isosurface is colored depending on simulation time from blue (entrance into the protein) to red (end of successful path)]. Snapshots refer to Fig. 1B, black line. See Movie S1 for a corresponding video clip.
The observed spontaneous O2 diffusion matches a model consisting of multiple diffusion paths converging toward residues Phe-270 and Phe-266, in front of the re-side of the reduced FMNH− cofactor (Fig. 3 and Scheme 1). Once O2 molecules reach Phe-266 and Phe-270 (Fig. 1B, step C), transient fluctuations of these aromatic side chains allow O2 to further diffuse into a smaller cavity defined by flavin C4a, His-396, Phe-266, and Phe-270 (Fig. 1B, step D). The side chain of His-396 was proposed to facilitate formation of the C4a-hydroperoxide intermediate (31) and our MD simulations are consistent with the hypothesized role of this preorganized oxygen cavity for C4a-intermediate stabilization. Moreover, they also show that this preorganized cavity is characterized by remarkable side chain flexibility, a prerequisite of O2 diffusion toward the active site (Fig. 2).
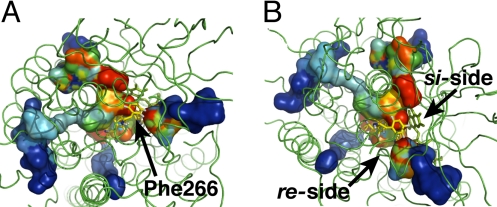
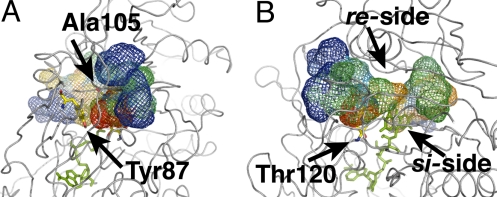
Fig. 3.
Funnel-shaped multiple pathways for C2. (A) Time-dependent representation of the 4 complete paths observed from simulations at 300 K converging in front of Phe-266 (top view). (B) All pathways conduct O2 molecules to the flavin re-side (side view). Volume isosurfaces are colored depending on simulation time from blue (entrance into the protein) to red (end of successful path).
Computational predictions and our model for protein-guided O2 diffusion were validated by combining site-directed mutagenesis and rapid kinetics experiments. Reactions of C2-FMNH− with O2 to form a C4a-hydroperoxyflavin intermediate were monitored using a stopped-flow spectrophotometer (Figs. S2–S4). The kinetics were comparatively investigated in the wild-type protein (35) and in 4 mutants that target Phe-266, the residue suggested by the MD simulations to represent the entry point for the O2 access to the reactive C4a locus of the flavin ring. The main results of this kinetic analysis were the following (Table 1): (i) Binding of the p-hydroxyphenylacetate (HPA) substrate to the wild-type C2-FMNH− complex causes a 20-fold reduction in the rate of formation of the C4a-hydroperoxyflavin intermediate; (ii) mutation of Phe-266 with a bulky Trp side chain causes a very similar drop in rate of the oxygen reaction; and (iii) replacement of Phe-266 with smaller side chains (Phe266Pro, Phe266Gly, and Phe266Ala) has little (<5-fold) effect on C4a-hydroperoxyflavin formation, indicating that these mutants retain the ability to efficiently react with oxygen.
Table 1.
Reoxidation rates for reduced C2(mutant)-FMNH− with O2 in presence/absence of HPA
| Mutants | Rate constants of formation of C4a-hydroperoxy FMN, 104 M−1·s−1 |
|
|---|---|---|
| −HPA | +HPA | |
| Wild-type | 110 | 4.8 |
| Phe266Gly* | 15 | 16 |
| Phe266Ala* | 20 | 19 |
| Phe266Pro* | 14 | 17 |
| Phe266Trp* | 4.8 | 4.6 |
| Tyr296Phe | 120 | 4.0 |
+HPA, reaction of C2(mutant)-FMNH− with O2 in presence of HPA. −HPA, reaction of C2(mutant)-FMNH− with O2 in absence of HPA.
*Differently from the wild-type, HPA does not affect the oxygen reactivity of the four Phe266 mutants which bind HPA only after reaction with O2 and formation of C4a-hydroperoxyflavin (see Figs. S3 and S4).
Correlating the kinetics with the mechanism of oxygen diffusion is especially difficult because the measured rates of the oxygen reaction and C4a-hydroperoxyflavin formation are composite parameters that result from the rates of oxygen diffusion through the protein matrix and of the chemical steps along the electron-transfer reaction between the oxygen and reduced flavin. Bearing in mind this inherent difficulty in data interpretation, we envision the following working model. O2 diffusion trajectories converge to Phe-266, at the entrance of the oxygen cavity in front of the flavin C4a atom (Figs. 1 and 2). As shown by the crystal structures, HPA binding involves a conformational change of Phe-266, which must rotate away to make room for the substrate (see Fig. S1 and ref. 31). As a result, substrate binding makes the protein matrix more tightly packed, limiting the conformational fluctuations in the active site. A similar effect is likely to be exerted by the Trp side chain of the Phe266Trp mutant. Consistently, binding of HPA in the wild-type C2-FMNH− enzyme and the Trp side chain of the Phe266Trp enzyme decrease the rate of O2 diffusion, slowing down the overall rate of C4a-hydroperoxyflavin formation (Table 1) (35). This limiting effect is not observed in the proteins in which Phe-266 is replaced by smaller side chains (Phe266Pro, Phe266Gly, and Phe266Ala). The differences between the rate constants of these mutants and of the wild-type enzyme are likely to reflect the increased solvent accessibility of the oxygen cavity as indicated by the lower hydroxylation ratio (i.e., product formed/FMNH− used) exhibited by the Phe266Gly protein compared with the wild type (62 ± 5% vs. 89 ± 3%) (see SI Text) (3, 31, 36). Moreover, it must be noted that the mutants can bind HPA only after formation of the hydroperoxyflavin intermediate and, therefore, their oxygen reaction rates are not affected by HPA binding, differently from the wild-type enzyme (Table 1).
To further validate the O2 diffusion model obtained from MD simulations, we constructed the C2(Tyr296Phe) mutant that targets a residue close to the reactive site of the flavin whereas it is not part of the predicted diffusion paths. This mutant reacts with O2 identically to the wild-type enzyme; the C4a-hydroperoxyflavin is formed with rate constants of 1.2 × 106 and 4.0 × 104 M−1·s−1 in absence and presence of HPA, respectively (Table 1). These values are essentially the same as those of the wild-type enzyme and confirm that Tyr-296 is not part of any O2 diffusion path as predicted by our simulations.
Oxygen Diffusion into AldO.
O2 diffusion into an oxidase was addressed for AldO, a soluble monomeric flavoprotein of the class of vanillyl-alcohol oxidase, which contains a covalently bound FAD cofactor (32, 37). AldO catalyzes a typical 2-electron flavin-mediated oxidation of a terminal C-OH moiety of a polyol substrate to the corresponding aldehyde, with the concomitant reduction of the flavin cofactor. The reduced flavin (FADH−) then reacts with O2 to form H2O2, which completes the catalytic cycle. Because its crystal structure has been solved at high resolution (1.1 Å) (32) and the kinetic mechanism has been established by presteady state kinetic analyses (37), AldO serves as an excellent oxidase prototype.
Enhanced-statistics MD simulations were initialized based on the 3-dimensional structure of FADH−-complexed AldO and extended for 50 ns of overall enhanced-time (Table S1). These simulations were set-up to capture O2 diffusion before FADH− oxidation. Out of a total of 500 O2 trajectories at 300 K, we observe 5 complete spontaneous diffusion pathways that bring O2 molecules in front of the isoalloxazine ring of the FADH− cofactor (Fig. 4), starting from random configurations in the bulk solvent. Simulations at 350 K confirm the location of these successful paths (22 events; data not shown). Several O2 molecules that initially reside in cavities on the protein surface subsequently diffuse into the AldO interior (e.g., Fig. 4B, steps A, B, D, F, and H). As observed in the C2 monooxygenase system, these surface cavities typically display at least one hydrophobic residue, such as Ala and/or Ile. A remarkable example is the all-Ala pocket of Ala-88, Ala-91, and Ala-277 used to capture O2 molecules from the bulk solvent (Fig. S5).
Fig. 4.
O2 spontaneous diffusion into alditol oxidase. (A) Five paths are observed at 300 K (volume isosurfaces) (Left). All paths conduct O2 molecules (red spheres) (Right) to the re-side of the FADH− reduced flavin cofactor (green sticks). (B) Time series of the O2 – C4a distance for the successful diffusion paths and key steps (see Oxygen Diffusion into AldO). The dashed horizontal line defines the C4a flavin cavity.
The spontaneous O2 diffusion into AldO matches a model consisting of multiple diffusion paths converging toward a few key residues neighboring the reactive moiety of the flavin cofactor, similarly to what observed for C2. All protein-guided diffusion pathways converge into a site defined by Ala-105, Tyr-87, and Thr-120 at ≈6 Å from the re-side of FADH− (Figs. 4 and 5). Once O2 molecules reach Tyr-87 or Thr-120, they (3 paths out of 5) eventually proceed to a preorganized cavity defined by flavin C4a and Ala-105, which coincides with the cavity previously proposed based on AldO X-ray structures (32). Thus, the side chain of Ala-105 creates a favorable hydrophobic environment to stabilize the presence of an O2 molecule next to the reduced flavin (Fig. 4 A and B, steps C and G). Clearly, protein dynamics is required for the successful diffusion of O2 molecules to the active site. It is striking to observe that, similar to C2, all complete diffusion pathways (i.e., all pathways carrying oxygen from the bulk solvent to the flavin cofactor) converge to only one side of the reduced flavin (re-side) (Fig. 5 and Scheme 1). Moreover, they all cross only a limited part of the AldO protein structure, i.e., the substrate-domain.
Fig. 5.
Funnel-shaped multiple pathways for alditol oxidase. (A) Time-dependent representation of 3 complete paths observed from simulations at 300 K converging in close contact with Ala-105 (volume surfaces, top view). (B) All pathways conduct O2 molecules to the flavin re-side (side view). For graphical purposes only 3 paths are displayed. Volume isosurfaces are colored depending on simulation time from blue (entrance into the protein) to red (end of successful path).
Table 2 summarizes the rapid kinetics experiments for oxygen reactivity of reduced AldO and its Ala-105 mutants. The data show that the rate constant of AldO reoxidation (13 × 104 M−1·s−1) is hardly affected by the Ala105Ser mutation (14 × 104 M−1·s−1) whereas the Ala105Gly mutant is somewhat faster (20 × 104 M−1·s−1) (Fig. S6). Apparently, small changes in the size of the side chain at position 105 have little effect on oxygen reactivity. In this regard, we notice that one would not expect a drastic increase in reactivity with oxygen in the Ala105Gly mutant, because the reaction rate of wild-type AldO is already high and is also limited by other funnel residues and the actual electron-transfer step from O2 to the flavin (3, 32). All attempts to replace Ala-105 with larger side chains failed because the mutants were extremely unstable and/or unable to bind FAD. However, strong support for the role of Ala-105 as O2 entry point to the active site is provided by recently reported mutagenesis data on plant l-galactono-1,4-lactone dehydrogenase (GALDH) (38), which shares 25% sequence identity with AldO. As a typical dehydrogenase, this enzyme reacts poorly with O2. However, its oxygen reactivity could be substantially increased (400-fold) to a level comparable to that of typical oxidases by simply mutating Ala-113 (homologous to Ala-105 of AldO) to Gly (38). Apparently, Ala-105 in AldO already allows O2 access to the reduced FAD whereas in GALDH—due to its specific active site—it needs to be replaced by a smaller residue to allow O2 to pass through. This finding indicates that, consistently with our MD predictions for AldO, also in GALDH O2 diffuses and approaches the flavin from the position corresponding to that of AldO Ala-105.
Table 2.
Kinetic parameters for reaction of AldO(mutant)-FADH− with O2
| Mutants | KM, mM | kcat, s−1 | kcat/KM, mM−1·s−1 | kox, 104 M−1·s−1 |
|---|---|---|---|---|
| Wild-type | 0.33 | 11.4 | 34.5 | 13 |
| Ala105Gly | 1.2 | 10.2 | 8.5 | 20 |
| Ala105Ser | 1.8 | 8.7 | 4.8 | 14 |
The steady state parameters were determined using xylitol as substrate. The kox reoxidation rate values denote the rate at which FADH− in AldO is reacting with O2 evidenced by flavin reoxidation.
Discussion
We used an integrated computational and experimental procedure, combining X-ray crystallography, enhanced-statistics MD simulations, rapid kinetic experiments, and site-directed mutagenesis to shed light on how O2 molecules diffuse into the active site of flavoenzymes with different catalytic activity (monooxygenase and oxidase) and folding topologies. In both simulated systems, distinct and multiple protein-guided O2 diffusion pathways converge toward defined active site entry points, forming funnel architectures. The observation that a specific set of residues form entry sites for the access of oxygen to the flavin cofactor—as Phe-266 in C2 and Ala-105 in AldO—is consistent with what was recently reported for cholesterol oxidase (12) and sarcosine oxidase (39). However, this does not strictly imply the presence of individual diffusion pathways. Instead, our results suggest that multiple diffusion pathways, converging to a few key residues, are operative and represent a more effective structural model to combine high specificity and high kinetic efficiency in oxygen-using flavoenzymes. From a computational standpoint, we emphasize that in this work, O2 diffusion was only solvent- or protein-guided—i.e., no biasing force was used to direct the reactants to the protein active sites. We stress, therefore, that a direct picture of the diffusion process at the atomic scale was obtained a priori and only subsequently used as a prediction tool to design the experiments addressing oxygen reactivity.
Our observations are also relevant to the understanding of the reaction mechanism of oxygen with reduced flavin (3, 26). MD simulations indicate that O2 is able to reach specifically the flavin C4a atom both in the monooxygenase and the oxidase (Figs. 1 and 4). This implies that—independently of the nature of the enzymatic reaction—O2 would react with the flavin by direct contact with the C4a atom of the cofactor. In this scenario, the C4a atom appears to be the locus directly involved in the electron transfer from the reduced cofactor to O2, which is consistent with isotope effect studies performed on glucose oxidase (29). The combination of our data with the results obtained by site-directed mutagenesis on various flavoenzymes (38, 39) indicates that the difference among dehydrogenases, monooxygenases, and oxidases ultimately resides in the fine modulation of the local environment embedding the C4a atom; its accessibility, the distribution of charged and polar groups to favor electron transfer between reduced flavin and O2, and the extent to which the oxygen cavity is capable of stabilizing the C4a-hydroperoxyflavin.
Methods
Molecular Model and Enhanced-Statistics MD Simulations.
Ten MD trajectories of the C2 homodimer (chain A and D; 798 residues; FMNH− cofactor; initial model PDB entry 2JBS; 2.8 Å resolution) (31) and 10 trajectories of the AldO monomer (418 residues; FADH− cofactor; initial model PDB entry 2VFR; 1.1 Å resolution) (32) at 300 and 350 K were generated using the GROMOS05 software for biomolecular simulation (40), the GROMOS 53A6 parameter set (41), and compatible ion parameters (42) and SPC water model (43). A summary is reported in SI Text and Table S1. A procedure for enhanced-statistics MD simulation was used for O2 spontaneous diffusion. It involves (i) reduced masses (1.6 u) for the oxygen atoms in the O2 molecules (21); (ii) enhanced statistics from 100 (independent and noninteracting) O2 molecules in the system, kept beyond a minimum pair distance by a network of repulsive half-harmonic distance-restraining potentials during the equilibration phase (to avoid biasing their initial location before diffusion); (iii) a fast grid-based pairlist-construction algorithm (44) as implemented in the GROMOS05 MD++ module (40); (iv) enhanced statistics from 10 independent MD runs at 300 and 350 K. O2 diffusion statistics were collected over a total of 10 independent trajectories of systems with 100 O2 molecules each, monitored for a total enhanced-MD time of 30 (C2) or 50 (AldO) ns. The rather high O2 apparent concentrations (C2: ≈0.14 M or AldO: ≈0.3 M; for comparison, an O2 saturated buffer is ≈1.2 mM) allow capturing a sufficient statistics of O2-protein encounter events without perturbing protein overall structure at both 300 and 350 K. RMSD time series of C2 and AldO backbone Cα-atoms are reported in Figs. S7 and S8, together with computational details. The term “enhanced-MD time” points out that, the kinetic properties of simulated O2 molecules differ from standard MD simulation. Computer simulations were used to predict the location of oxygen diffusion pathways along C2 and AldO configurational distributions; instead, kinetic properties were measured by experiments. The term “enhanced-statistics” emphasizes that an improved statistics is achieved compared with standard MD simulation. No biasing force or potential is used to bring O2 molecules inside the enzyme active sites. Instead, O2 freely diffuses on the free-energy landscape starting from a nonarbitrary configuration and spontaneously reaches the preorganized cavities described in the text. The Pymol (45) and VMD (46) software packages were used for graphical representations.
Rapid Kinetics and Oxygen Reactivity.
C2.
Rapid kinetics measurements were performed at 277 K with a Hi-Tech Scientific Model SF-61DX stopped-flow spectrophotometer in single- or double-mixing mode (observation cell optical path-length of 1 cm). The stopped-flow instrument was maintained under anaerobic conditions by flushing the flow system with an O2 scrubbing solution consisting of 400 μM glucose, 1 mg·ml−1 glucose oxidase (15.5 units per ml), and 4.8 μg·ml−1 catalase in 50 mM sodium phosphate buffer pH 7.0. The O2 scrubbing solution was left in the flow system overnight and was thoroughly rinsed with anaerobic buffer before experiments. To study the reaction of C2 mutants with O2, the reduced enzyme in presence and absence of substrate (HPA) in 50 mM sodium phosphate buffer pH 7.0 was prepared according to the protocol used for the wild-type enzyme (35), followed by mixing with buffers of different O2 concentrations. Apparent rate constants (kobs) from kinetic traces were calculated from exponential fits using the KineAsyst3 (Hi-Tech Scientific) or Program A (R. Chang, J.-y. Chiu, J. Dinverno, and D.P. Ballou, University of Michigan, Ann Arbor, MI) software packages. Rate constants were obtained by a Levenberg-Marquardt nonlinear fit of kobs versus O2 concentrations, as implemented in the KaleidaGraph software (35). SI Text reports experimental details.
AldO.
Purified mutant and wild-type AldO were obtained as described in ref. 37. Rapid kinetics measurements were performed using an Applied Photophysics stopped-flow apparatus model SX17MV following methods reported in ref. 37. Reduced AldO was obtained by anaerobic dithionite reduction. All experiments were performed at 300 K and in a 50 mM potassium phosphate buffer pH 7.5. Experimental details are reported in SI Text.
Supplementary Material
Acknowledgments.
This work was supported by the National Science Foundation Grant PHY-0822283; the National Institutes of Health and National Science Foundation (R.B. and J.A.M.); the Howard Hughes Medical Institute (J.A.M.); the American Chemical Society Petroleum Research Fund Grant 46271-C4 and Ministero dell'Istruzione, dell'Università e della Ricerca (A.M.); the EU-FP7 “Oxygreen” project (A.M. and M.W.F.); the Carbohydrate Research Center Wageningen (to W.J.H.v.B.); the Thailand Research Fund grant BRG5180002 and the Faculty of Science, Mahidol University (P. Chaiyen); the Institute for the Promotion of Teaching Science and Technology (P. Chenprakhon); Royal Golden Jubilee PhD Program Grant PHD/0008/2549 (to K.T.); the Dutch Technology Foundation Stichting Technische Wetenschappen Grant 7726, the Nederlandse Organisatie voor Wetenschappelijk Onderzoek applied science division, and the Technology Program of the Ministry of Economic Affairs (R.T.W.) We thank the Center for Theoretical Biological Physics for the computing resources.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903809106/DCSupplemental.
References
- 1.Que L, Jr, Tolman WB. Biologically inspired oxidation catalysis. Nature. 2008;455:333–340. doi: 10.1038/nature07371. [DOI] [PubMed] [Google Scholar]
- 2.Perutz MF. Regulation of oxygen affinity of hemoglobin: Influence of structure of the globin on the heme iron. Annu Rev Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- 3.Massey V. Activation of molecular oxygen by flavins and flavoproteins. J Biol Chem. 1994;269:22459–22462. [PubMed] [Google Scholar]
- 4.Zou S, Baskin JS, Zewail AH. Molecular recognition of oxygen by protein mimics: Dynamics on the femtosecond to microsecond time scale. Proc Natl Acad Sci USA. 2002;99:9625–9630. doi: 10.1073/pnas.152333399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calhoun DB, Vanderkooi JM, Woodrow GV, III, Englander SW. Penetration of dioxygen into proteins studied by quenching of phosphorescence and fluorescence. Biochemistry. 1983;22:1526–1532. doi: 10.1021/bi00276a002. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun DB, Vanderkooi JM, Englander SW. Penetration of small molecules into proteins studied by quenching of phosphorescence and fluorescence. Biochemistry. 1983;22:1533–1539. doi: 10.1021/bi00276a003. [DOI] [PubMed] [Google Scholar]
- 7.Johnson BJ, et al. Exploring molecular oxygen pathways in Hansenula polymorpha copper-containing amine oxidase. J Biol Chem. 2007;282:17767–17776. doi: 10.1074/jbc.M701308200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saam J, Ivanov I, Walther M, Holzhutter HG, Kuhn H. Molecular dioxygen enters the active site of 12/15-lipoxygenase via dynamic oxygen access channels. Proc Natl Acad Sci USA. 2007:13319–13324. doi: 10.1073/pnas.0702401104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Lyubimov AY, Brammer L, Vrielink A, Sampson NS. The Binding and release of oxygen and hydrogen peroxide are directed by a hydrophobic tunnel in cholesterol oxidase. Biochemistry. 2008:5368–5377. doi: 10.1021/bi800228w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koutsoupakis K, Stavrakis S, Soulimane T, Varotsis C. Oxygen-linked equilibrium CuB-CO species in cytochrome ba3 oxidase from Thermus thermophilus. Implications for an oxygen channel ar the CuB site. J Biol Chem. 2003;278:14893–14896. doi: 10.1074/jbc.M210293200. [DOI] [PubMed] [Google Scholar]
- 11.Lario PI, Sampson N, Vrielink A. Sub-atomic resolution crystal structure of cholesterol oxidase: What atomic resolution crystallography reveals about enzyme mechanism and the role of the FAD cofactor in redox activity. J Mol Biol. 2003;326:1635–1650. doi: 10.1016/s0022-2836(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 12.Piubelli L, et al. On the oxygen reactivity of flavoprotein oxidases: An oxygen access tunnel and gate in Brevibacterium sterolicum cholesterol oxidase. J Biol Chem. 2008:24738–24747. doi: 10.1074/jbc.M802321200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riistama S, et al. Channelling of dioxygen into the respiratory enzyme. Biochim Biophys Acta. 1996;1275(1–2):1–4. doi: 10.1016/0005-2728(96)00040-0. [DOI] [PubMed] [Google Scholar]
- 14.Elber R, Karplus M. Enhanced sampling in molecular dynamics: Use of the time-dependent hartree approximation for a simulation of carbon monoxide diffusion through myoglobin. J Am Chem Soc. 1990;112:9161–9175. [Google Scholar]
- 15.Lakowicz JR, Weber G. Quenching of fluorescence by oxygen. A probe for structural fluctuations in macromolecules. Biochemistry. 1973;12:4161–4170. doi: 10.1021/bi00745a020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jameson DM, Gratton E, Weber G, Alpert B. Oxygen distribution and migration within Mbdes Fe and Hbdes Fe. Multifrequency phase and modulation fluorometry study. Biophys J. 1984;45:795–803. doi: 10.1016/S0006-3495(84)84224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu K, et al. Structure of a ligand-binding intermediate in wild-type carbonmonoxy myoglobin. Nature. 2000;403:921–923. doi: 10.1038/35002641. [DOI] [PubMed] [Google Scholar]
- 18.Widboom PF, Fielding EN, Liu Y, Bruner SD. Structural basis for cofactor-independent dioxygenation in vancomycin biosynthesis. Nature. 2007;447:342–345. doi: 10.1038/nature05702. [DOI] [PubMed] [Google Scholar]
- 19.Schotte F, et al. Watching a protein as it functions with 150-ps time-resolved X-ray crystallography. Science. 2003;300:1944–1947. doi: 10.1126/science.1078797. [DOI] [PubMed] [Google Scholar]
- 20.Koder RL, et al. Design and engineering of an O(2) transport protein. Nature. 2009;458:305–309. doi: 10.1038/nature07841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofacker I, Schulten K. Oxygen and proton pathways in cytochrome c oxidase. Proteins. 1998;30:100–107. [PubMed] [Google Scholar]
- 22.Cohen J, et al. Molecular dynamics and experimental investigation of H2 and O2 diffusion in [Fe]-hydrogenase. Biochem Soc Trans. 2005;33(Pt 1):80–82. doi: 10.1042/BST0330080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J, Kim K, King P, Seibert M, Schulten K. Finding gas diffusion pathways in proteins: Application to O2 and H2 transport in CpI [FeFe]-hydrogenase and the role of packing defects. Structure. 2005;13:1321–1329. doi: 10.1016/j.str.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Masgrau L, et al. Atomic description of an enzyme reaction dominated by proton tunneling. Science. 2006;312:237–241. doi: 10.1126/science.1126002. [DOI] [PubMed] [Google Scholar]
- 25.Hummer G, Schotte F, Anfinrud PA. Unveiling functional protein motions with picosecond X-ray crystallography and molecular dynamics simulations. Proc Natl Acad Sci USA. 2004;101:15330–15334. doi: 10.1073/pnas.0405295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattevi A. To be or not to be an oxidase: Challenging the oxygen reactivity of flavoenzymes. Trends Biochem Sci. 2006;31:276–283. doi: 10.1016/j.tibs.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Massey V. Introduction: Flavoprotein structure and mechanism. FASEB J. 1995;9:473–475. doi: 10.1096/fasebj.9.7.7737454. [DOI] [PubMed] [Google Scholar]
- 28.Sucharitakul J, Prongjit M, Haltrich D, Chaiyen P. Detection of a C4a-hydroperoxyflavin intermediate in the reaction of a flavoprotein oxidase. Biochemistry. 2008;47:8485–8490. doi: 10.1021/bi801039d. [DOI] [PubMed] [Google Scholar]
- 29.Roth JP, et al. Oxygen isotope effects on electron transfer to O2 probed using chemically modified flavins bound to glucose oxidase. J Am Chem Soc. 2004;126:15120–15131. doi: 10.1021/ja047050e. [DOI] [PubMed] [Google Scholar]
- 30.Pau MY, Lipscomb JD, Solomon EI. Substrate activation for O2 reactions by oxidized metal centers in biology. Proc Natl Acad Sci USA. 2007;104:18355–18362. doi: 10.1073/pnas.0704191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alfieri A, et al. Structure of the monooxygenase component of a two-component flavoprotein monooxygenase. Proc Natl Acad Sci USA. 2007;104:1177–1182. doi: 10.1073/pnas.0608381104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forneris F, et al. Structural analysis of the catalytic mechanism and stereoselectivity in Streptomyces coelicolor alditol oxidase. Biochemistry. 2008;47:978–985. doi: 10.1021/bi701886t. [DOI] [PubMed] [Google Scholar]
- 33.Adcock SA, McCammon JA. Molecular dynamics: Survey of methods for simulating the activity of proteins. Chem Rev. 2006;106:1589–1615. doi: 10.1021/cr040426m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Gunsteren WF, et al. Biomolecular modeling: Goals, problems, perspectives. Angew Chem Int Ed Engl. 2006;45:4064–4092. doi: 10.1002/anie.200502655. [DOI] [PubMed] [Google Scholar]
- 35.Sucharitakul J, Chaiyen P, Entsch B, Ballou DP. Kinetic mechanisms of the oxygenase from a two-component enzyme, p-hydroxyphenylacetate 3-hydroxylase from Acinetobacter baumannii. J Biol Chem. 2006;281:17044–17053. doi: 10.1074/jbc.M512385200. [DOI] [PubMed] [Google Scholar]
- 36.Entsch B, Cole LJ, Ballou DP. Protein dynamics and electrostatics in the function of p-hydroxybenzoate hydroxylase. Arch Biochem Biophys. 2005;433:297–311. doi: 10.1016/j.abb.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Heuts DP, van Hellemond EW, Janssen DB, Fraaije MW. Discovery, characterization, and kinetic analysis of an alditol oxidase from Streptomyces coelicolor. J Biol Chem. 2007;282:20283–20291. doi: 10.1074/jbc.M610849200. [DOI] [PubMed] [Google Scholar]
- 38.Leferink NG, et al. Identification of a gatekeeper residue that prevents dehydrogenases from acting as oxidases. J Biol Chem. 2009;284:4392–4397. doi: 10.1074/jbc.M808202200. [DOI] [PubMed] [Google Scholar]
- 39.Zhao G, Bruckner RC, Jorns MS. Identification of the oxygen activation site in monomeric sarcosine oxidase: Role of Lys265 in catalysis. Biochemistry. 2008;47:9124–9135. doi: 10.1021/bi8008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christen M, et al. The GROMOS software for biomolecular simulation: GROMOS05. J Comput Chem. 2005;26:1719–1751. doi: 10.1002/jcc.20303. [DOI] [PubMed] [Google Scholar]
- 41.Oostenbrink C, Villa A, Mark AE, van Gunsteren WF. A biomolecular force field based on the free enthalpy of hydration and solvation: The GROMOS force-field parameter sets 53A5 and 53A6. J Comput Chem. 2004;25:1656–1676. doi: 10.1002/jcc.20090. [DOI] [PubMed] [Google Scholar]
- 42.Åqvist J. Ion Water Interaction Potentials Derived from Free-Energy Perturbation Simulations. J Phys Chem. 1990;94:8021–8024. [Google Scholar]
- 43.Berendsen HJC. Interaction Models for Water in Relation to Protein Hydration. Dordrecht, The Netherlands: Pullman; 1981. [Google Scholar]
- 44.Heinz TN, Hünenberger PH. A fast pairlist-construction algorithm for molecular simulations under periodic boundary conditions. J Comput Chem. 2004;25:1474–1486. doi: 10.1002/jcc.20071. [DOI] [PubMed] [Google Scholar]
- 45.De Lano WL. Pymol. Palo Alto, CA: DeLano Scientific; 2002. [Google Scholar]
- 46.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graphics. 1996;14:33-38:27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.