Abstract
The second messenger, cGMP, mediates a host of cellular responses to various stimuli, resulting in the regulation of many critical physiologic functions. The existence of specific cGMP transporters on the plasma membrane that participate in the regulation of cGMP levels has been suggested in a large number of studies. In this study, we identified a novel plasma membrane transporter for cGMP. In particular, we showed that hOAT2 (SLC22A7), a member of the solute carrier (SLC) superfamily, was a facilitative transporter for cGMP and other guanine nucleotides. hOAT2, which is ubiquitously expressed at high levels in many cell types, was previously thought to primarily transport organic anions. Among purine and pyrimidine nucleobases, nucleosides, and nucleotides, hOAT2 showed the greatest preference for cGMP, which transported cGMP with a Km value of 88 ± 11 μM and exhibited between 50-and 100-fold enhanced uptake over control cells. Our data revealed that hOAT2 is a bidirectional facilitative transporter that can control both intracellular and extracellular levels of cGMP. In addition, we observed that a common alternatively spliced variant of hOAT2 demonstrated a complete loss of transport function as a result of a low expression level on the plasma membrane. We conclude that hOAT2 is a highly efficient, facilitative transporter of cGMP and may be involved in cGMP signaling in many tissues. Our study suggests that hOAT2 represents a potential new drug target for regulating cGMP levels.
Introduction
The cyclic nucleotide cGMP is a second messenger involved in mediating cellular response to various stimuli in numerous cell types. cGMP signaling through the activation of cGMP-dependent protein kinases regulates a wide variety of intracellular functions, and the potential involvement of extracellular cGMP in a number of biological processes has been suggested (Poulopoulou and Nowak, 1998; Sager, 2004). Because of its critical roles, proteins that regulate intracellular cGMP levels have been the subject of many studies. Such proteins include guanylate cyclases (GC), which play key roles in cGMP production, and phosphodiesterases (PDEs), involved in cGMP degradation.
The existence of plasma membrane transporters capable of transporting cGMP into as well as out of cells has been suggested in a large number of studies in a variety of cell types (Sager, 2004), although the molecular identities of such transporters have remained unclear. Three members of the multidrug resistance protein (MRP) family (part of the ATP binding cassette superfamily of transporters), MRP4, MRP5, and MRP8 have been shown to transport cGMP (Jedlitschky et al., 2000; Chen et al., 2001; Guo et al., 2003). These transporters may play a role in the extrusion of cGMP from cells; however, these transporters do not participate in the transmembrane influx of cGMP because they are exclusively efflux pumps.
The solute carrier (SLC) superfamily is a major class of transporters responsible for the cellular influx and efflux of a great variety of endogenous substances, including amino acids, peptides, sugars, organic ions, and nucleosides as well as a multitude of xenobiotic drugs. Although nucleoside transporter families exist within the SLC superfamily [i.e., the concentrative nucleoside transporter (CNT) family (SLC28A) and the equilibrative nucleoside transporter (ENT) family (SLC29A)], none of these have been shown to transport cGMP (Baldwin et al., 2004; Gray et al., 2004).
In humans, four organic anion transporters, OAT1 (SLC22A6) (Cihlar et al., 1999; Koepsell and Endou, 2004), OAT2 (SLC22A7) (Sun et al., 2001), OAT3 (SLC22A8) (Cha et al., 2001), and OAT4 (SLC22A11) (Cha et al., 2000) have been functionally characterized and have been shown to mediate the facilitative transport of various structurally diverse organic anions with partly overlapping substrate specificities (Burckhardt and Burckhardt, 2003). Among the OAT family, only hOAT2 has ubiquitous expression pattern with abundant expression in many tissues, whereas the other OATs are primarily expressed in the kidney. The specificity of hOAT2 has not been studied in great detail, although it has been thought to be an organic anion-preferring transporter with substrate specificity overlapping that of the other OATs. While performing a routine screen to identify substrates of hOAT2, we discovered that the guanine nucleoside analog acyclovir vigorously interacted with hOAT2 (C. D. Cropp, T. Komori, K. M. Giacomini, unpublished data). This finding led us to explore the interaction of other guanine nucleotides with hOAT2.
The data presented in this study suggest that an important function of hOAT2 is to transport naturally occurring nucleobases, nucleosides, and nucleotides, with a particular preference for guanine analogs. The transport of cGMP is the most avid of the substrates yet identified for hOAT2 and our data indicate that hOAT2 functions as a bidirectional facilitative cGMP transporter.
Materials and Methods
Materials
All standard chemicals were purchased from Sigma (St, Louis, MO). [3H]PAH and [3H]estrone sulfate were obtained from American Radiolabeled Chemicals (St. Louis, MO), and all radiolabeled nucleobases, nucleosides, and nucleotides were from Moravek Biochemicals (Brea, CA). Cell culture materials were supplied from UCSF Cell Culture Facility. Human total RNA and cDNA were purchased from Clontech (Mountain View, CA). All primers were obtained from Invitrogen (Carlsbad, CA), and probes for Taq-Man assays were from Applied Biosystems (Foster City, CA).
Bioinformatics
Full-length reference sequences for all proteins (OAT1-7, ENT 1-4, and CNT1-3) were obtained from the National Center for Biotechnology Information. A multiple sequence alignment and neighbor-joining tree were created using ClustalX 1.83 using the default parameters (Thompson et al., 1997). The resulting dendrogram was created using TreeView 1.6.6 (Page, 1996).
Cloning and Expression of Human OATs
cDNAs coding human OAT2 (hOAT2 or hOAT2-546aa; GenBank accession number NM_006672 [GenBank]) and its splice variant (hOAT2-548aa; GenBank accession number NM_153320 [GenBank]) were cloned by reverse transcription-PCR from human liver and kidney cDNA (Clontech), respectively. Primers used were 5′-CCAGAGTCCAAGGGTCTATGT-3′ (sense) and 5′-ATCAAGGATGGATGAGCAGAG-3′ (antisense). Human OAT4 cDNA (GenBank accession number NM_018484 [GenBank]) was cloned from kidney. cDNA clones for human OAT1 (GenBank accession number NM_004790 [GenBank]) and OAT3 (GenBank accession number NM_004254 [GenBank]) were obtained similarly. Each cDNA was subcloned into pcDNA5/FRT (Invitrogen) and used to generate transient or stable human embryonic kidney (HEK) 293 cell lines using the Flp-In system (Invitrogen) as described previously (Erdman et al., 2006). To generate stable cell lines, Flp-In-293 cells were plated at a density of 6×105 cells per well in six-well poly-D-lysine-treated tissue culture plates using antibiotic-free media and incubated overnight. Cells reached 95% confluence 24 h after seeding, at which point cells were transfected with 0.4 μg of hOAT2 cDNA or pcDNA5/FRT (empty vector), 3.6 μg of pOG44 DNA, and 20 μg of Lipofectamine 2000. Two days after transfection, cells were trypsinized and split 1:4 into new six-well plates and selected for stable transfectants by addition of hygromycin B (75 μg/ml) to the growth medium (Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml strep-tomycin). After 10 to 14 days under hygromycin B selection, colonies were pooled and expanded in 25-cm2 flasks.
Functional Assays
HEK293 cells were cultured in the growth media described under Cloning and Expression of Human OATS at 37°C, 95% humidity, and 5% CO2. Cells were transiently transfected with 1 μg of hOAT2 or other transporter and 3 μg of Lipofectamine 2000 (Invitrogen) in each well according to the manufacturer's protocol. Cells were seeded at a density of 4×105 cells per well in poly-D-lysine-coated 24-well plates (BD Biosciences, San Jose, CA) and were grown overnight. Uptake assays with transiently transfected cells were conducted 18 to 24 h after transfection. For uptake studies, the cells were rinsed with prewarmed uptake buffer (128 mM NaCl, 4.73 mM KCl, 1.25 mM CaCl2, 1.25 mM MgSO4, and 5 mM HEPES, pH 7.4), and then incubated in 0.3 ml of prewarmed buffer containing radiolabeled test compounds in the presence or absence of 10 μM nitrobenzylthioinosine (NBMPR), which was added to inhibit background ENT-mediated uptake of nucleosides and nucleobases. At the time points indicated in the figure legends, the reaction was terminated by washing with ice-cold buffer. Test substrates were quantified by liquid scintillation counting, and the uptake amounts were normalized to total protein in each well. cGMP levels were measured radiometrically (Figs. 1, 2A, 3, and 5A) or enzymatically (Fig. 4). For the enzymatic measurement of cGMP, stably transfected empty vector and hOAT2 cells were rinsed with 0.5 ml of uptake buffer and then incubated with 0.25 ml of test solution. After a 1-h incubation, an aliquot of an extracellular sample was immediately aspirated. The remaining portion was subsequently washed twice with ice-cold buffer. Afterward, 0.5 ml of lysis reagent was added and shaken for 1 h to retrieve the intracellular cGMP sample according to the manufacturer's protocol [cGMP Enzymeimmunoassay Biotrak (EIA) System; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK]. The determined concentration of intracellular and extracellular cGMP was corrected as a cGMP level per cells.
Fig. 1.
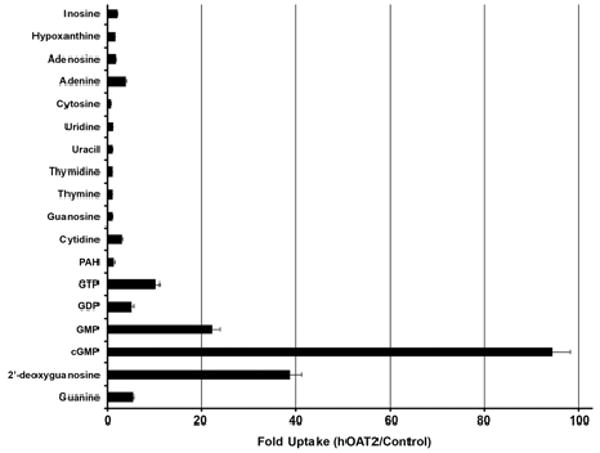
Uptake of naturally occurring nucleotides, nucleobases, and nucleosides by hOAT2. hOAT2 (solid bars) transiently transfected HEK293 cells were incubated for 1.5 min with each radiolabeled compound (2 μM) in the presence of 10 μM NBMPR. Uptake of PAH, a typical substrate of organic anion transporters, is shown for comparison. Each value represents the mean uptake via hOAT2 divided by the mean respective uptake in empty vector transfected cells. Data are from three independent experiments performed on separate days. The uptake of each compound was tested in triplicate samples in each independent experiment.
Fig. 2.
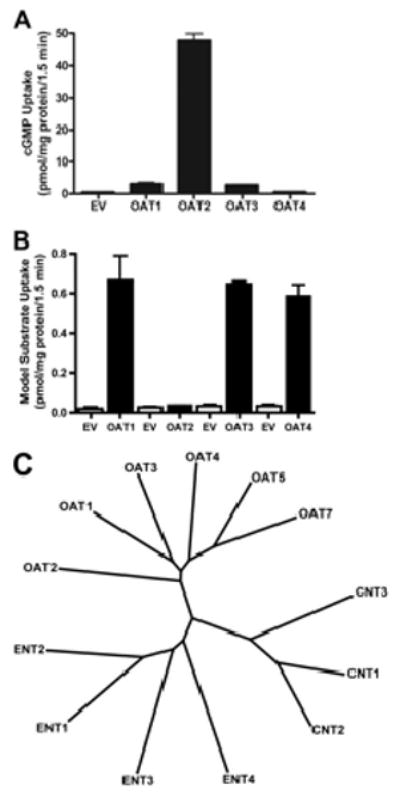
Specificity of cGMP and model substrate transport among hOAT family members (A and B) and dendrogram of the human OAT, ENT, and CNT transporter families (C). A, HEK cells transiently transfected with each hOAT isoform were incubated for 1.5 min with 2 μM cGMP. Only hOAT2-transfected cells showed substantially increased rate of uptake of cGMP above EV-transfected controls. cGMP transport by hOAT1 and hOAT3 was 6- and 5-fold greater than control, respectively, and was not transported by hOAT4 (<2 fold). hOAT2 was the most selective for cGMP (50-fold enhanced uptake). Each bar represents the mean ± S.D. from triplicate samples in a representative experiment. B, a 1.5-min uptake after a transient transfection of each hOATs' respective model substrate (black bars) tested simultaneously with EV (white bars). The model substrates are as follows: adefovir for hOAT1, PAH for hOAT2, and estrone sulfate for hOAT3 and hOAT4. C, a multiple sequence alignment using reference sequences for each transporter was performed using ClustalX (ftp://ftp.ebi.ac.uk/pub/software/clustalw2); the dendrogram was generated from the ClustalX alignment output.
Fig. 3.
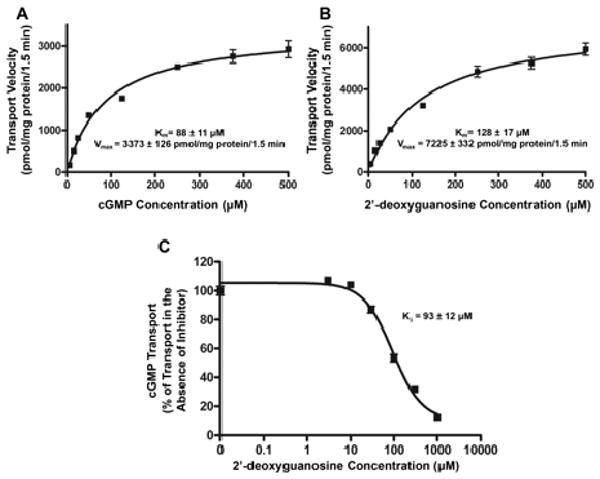
Concentration-dependent transport of cGMP (A) and 2′-deoxyguanosine (B) by hOAT2 and 2′-deoxyguanosine inhibition of hOAT2-mediated cGMP transport (C). Empty vector-stably transfected and hOAT2-stably transfected HEK293 cells were incubated for 1.5 min with varying concentrations (5, 15, 25, 50, 125, 250, 375, and 500 μM) of cGMP (A), or 2′-deoxyguanosine (B). 2′-Deoxyguanosine transport rate was measured in the presence of 10 μM NBMPR to inhibit background uptake. hOAT2-specific uptake was determined by subtracting uptake in empty vector-transfected cells from that in hOAT2-transfected cells after correcting for total protein. Kinetic parameters were estimated by fitting hOAT2-specific uptake rates to a Michaelis-Menten equation by nonlinear regression. Data are shown as the mean ± S.D. from triplicate samples in a representative experiment. C, empty vector-transfected and hOAT2-transfected HEK293 cells were incubated for 1.5 min with 2 μM radiolabeled cGMP and varying concentrations (0, 3, 30, 100, 300, and 1000 μM) of 2′-deoxyguanosine. NBMPR (10 μM) was added to all of the 2′-deoxyguanosine reaction mixes to inhibit ENT1 transport. hOAT2-specific uptake was determined by subtracting uptake by EV-transfected cells from that in hOAT2-transfected cells after correcting for total protein. IC50 and Ki parameters were estimated by nonlinear regression. Uptake results shown in the graphs are from a representative experiment. Each point represents the mean ± S.D. of triplicate samples.
Fig. 5.
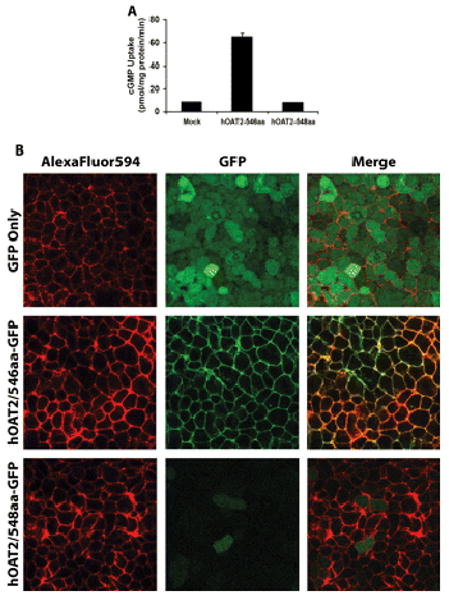
cGMP uptake (A) and intracellular localization in HEK293 cells expressing two alternatively spliced variants of hOAT2 (B). A, HEK293 cells were transiently transfected with empty vector, hOAT2-546aa-GFP, or hOAT2-548aa-GFP. Cells were incubated for 1.5 min with 2 μM cGMP. Each bar represents the mean ± S.D. from triplicate samples in a representative experiment. Results were confirmed in two independent experiments. B, cells stably expressing GFP, hOAT2-546aa-GFP, or hOAT2-548aa-GFP were visualized by confocal microscopy. Plasma membrane was stained using AlexaFluor594 wheat germ agglutinin. Cells transfected with GFP alone show diffuse expression throughout the intracellular space. hOAT2-546aa-GFP localized specifically to the plasma membrane, whereas hOAT2-548aa-GFP showed diffuse localization throughout the cytoplasm with lower fluorescence intensity.
Fig. 4.
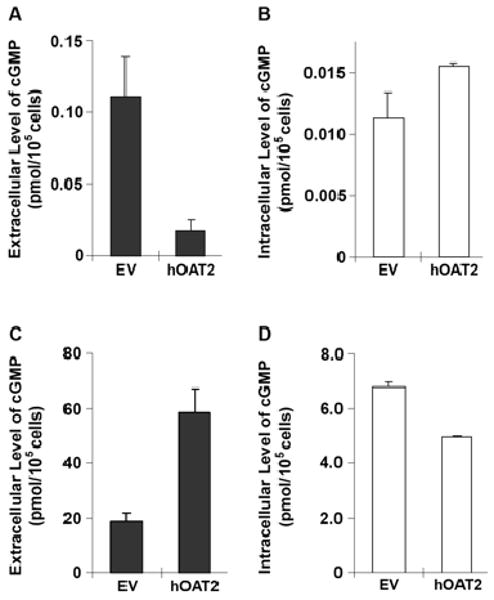
Extracellular and intracellular cGMP levels in HEK293 cells stably transfected with hOAT2. Extracellular (A and C; solid bar) and intracellular (B and D; open bar) cGMP levels in cells stably transfected with empty vector (EV) or hOAT2 were determined after 1 h incubation in the absence (A and B) or presence (C and D) of sodium nitroprusside (750 μM) and IBMX (750 μM). Data are the mean ± S.D. of three independent experiments. Each experiment was conducted in duplicate samples.
RNA Extraction, Reverse Transcription, and Real-Time PCR Assay
The expression of the organic anion transporters (OAT1, OAT2, OAT3, and OAT4) was quantified by real-time reverse transcription-PCR, using TaqMan Gene Expression Assays and an ABI 7500 Fast sequence detection system (Applied Biosystems). Human GAPDH was used as an endogenous control to normalize expression. Total RNA was extracted 48 h after transfection using the RNAqueous system (Ambion Inc., Austin, TX). One microgram of each RNA preparation was reverse-transcribed by random priming using high-capacity cDNA synthesis kit (Applied Biosystems, Foster City). Realtime PCR was performed using a 4.5-μl aliquot of the total cDNA sample using the TaqMan Gene Expression Assays Hs00537914_m1 for human OAT1, Hs00198527_m1 for human OAT2, Hs00188599_m1 for human OAT3, and Hs00218486_m1 for human OAT4. Human GAPDH endogenous control (Hs99999905_m1) was used as an internal standard for sample normalization. Relative levels of the human OAT1, OAT2, OAT3, and OAT4 mRNAs were calculated using the ΔΔCT (comparative threshold cycle) method. Each test was performed as a duplicate, and all experiments were repeated three times. The levels of the human OAT1, OAT2, OAT3, and OAT4 mRNAs were expressed relative to the HEK293 Flp-In cell lines transfected with pcDNA5FRT vector only, which was normalized to 1.
Tissue distribution of an alternative splice form of hOAT2 was detected using a TaqMan real-time PCR custom assay was performed with an ABI Prism 7700 Sequence Detector system.
Thin-Layer Chromatography
After allowing uptake to proceed for 1.5 min in OAT2-transfected and corresponding untransfected HEK cell lines in a 24-well plate, cells were lysed with 1% pentadecafluorooctanoic acid/ammonium salt buffer for an hour. The lysate was diluted to twice its volume with acetonitrile to precipitate all protein content. An aliquot of supernatant was evaporated and redissolved in 10 μl of water. Silica gel TLC of each uptake sample processed as described above was carried out with CHCl3/MeOH/H2O (6:6:1) as the mobile phase and compared with a standard sample of cGMP. The identity of the compound was confirmed by analysis using a Mariner electrospray ionization-time-of-flight mass spectrometer (Applied Biosystems). In addition, the amount of cGMP present in uptake samples was determined by measuring the radioactivity of the cGMP spot on the TLC plate and comparing it with the measured uptake.
Construction for GFP-Tagged Proteins and Microscopic Studies
Plasmids containing GFP-fused proteins were constructed as described previously (Erdman et al., 2006). The resulting fusion constructs were used to generate stable HEK293 cell lines using the Flp-In System (Invitrogen) and analyzed by confocal microscopy as described previously (Urban et al., 2006).
Results
hOAT2 Transported Nucleobases and Nucleosides
hOAT2 was capable of transporting a wide range of purine and pyrimidine nucleobases, nucleosides, and nucleotides (Fig. 1, Table 1). Among the nucleobases and the nucleosides, cGMP was the preferred hOAT2 substrate [approximately 60-fold uptake over empty vector (EV) transfected cell lines in the absence of NBMPR; 4.11 ± 1.65 pmol/mg protein/min for hOAT2 versus 0.0710 ± 0.0315 pmol/mg protein/min for EV]. When ENT-mediated background transport was inhibited with 10 μM NBMPR, hOAT2-mediated transport of cGMP was approximately 86-fold uptake over EV-transfected cell lines; 3.26 ± 0.819 pmol/mg protein/min for hOAT2 versus 0.0380 ± 0.0196 pmol/mg protein/min for EV. 2′-Deoxyguanosine was also shown to be a hOAT2 substrate in the presence of NBMPR (approximately 24-fold uptake over EV; 74.0 ± 26.2 fmol/mg protein/min for hOAT2 versus 3.13 ± 0.745 fmol/mg protein/min for EV). To determine whether the compounds tested were also substrates of hENT1 and hENT2, we compared their relative uptake in EV-transfected HEK cells in the presence and absence of NBMPR (Table 1). Gemcitabine, a known substrate of ENTs, served as a positive control. As shown, cGMP was not transported by ENTs, whereas some of the nucleobases, nucleosides, and nucleotides were. Additional experiments with TLC and mass spectrometry confirmed that >90% of the intracellular [3H]cGMP in hOAT2 stably transfected cells at 1.5 min was intact (data not shown).
Table 1.
Uptake of naturally-occurring nucleotides, nucleobases and nucleosides in empty vector and hOAT2 transfected cells
The mean uptake values of nucleotides, nucleobases, and nuclesides in the absence and presence of NBMPR (10 μM) are shown. The uptake of PAH (a known hOAT2 substrate) and gemcitabine (a known ENT substrate), are shown for comparison. Each value represents the mean ± S.D. ENT -fold transport was calculated by dividing empty vector transport in the absence of NBMPR by empty vector transport in the presence of NBMPR.
| Uptake Rate in the Absence of NBMPR | Uptake Rate in the Presence of NBMPR | ||||
|---|---|---|---|---|---|
| Compound | EV | hOAT2 | EV | hOAT2 | ENT Transport |
| pmol/mg protein/min | -fold | ||||
| cGMP | 0.0710 ± 0.0315 | 4.11 ± 1.65 | 0.0380 ± 0.0196 | 3.26 ± 0.819 | 1.87 |
| Guanine | 0.263 ± 0.119 | 0.804 ± 0.513 | 0.0859 ± 0.0292 | 0.604 ± 0.313 | 3.06 |
| Adenine | 0.412 ± 0.0578 | 1.30 ± 0.239 | 0.262 ± 0.0530 | 1.10 ± 0.212 | 1.57 |
| Guanosine | 0.109 ± 0.0575 | 0.226 ± 0.169 | 0.0775 ± 0.0110 | 0.225 ± 0.163 | 1.41 |
| GMP | 0.00935 ± 0.00813 | 0.0196 ± 0.0190 | 0.00113 ± 0.000545 | 0.0185 ± 0.0185 | 8.27 |
| GDP | 0.00638 ± 0.000838 | 0.0110 ± 0.00328 | 0.00218 ± 0.0000522 | 0.00892 ± 0.00271 | 2.93 |
| GTP | 0.00724 ± 0.000970 | 0.0113 ± 0.00135 | 0.00143 ± 0.000502 | 0.00901 ± 0.00216 | 5.06 |
| PAH | 0.0228 ± 0.00326 | 0.0344 ± 0.00699 | 0.0265 ± 0.00684 | 0.0312 ± 0.00568 | 0.860 |
| 2′-Deoxyguanosine | 0.0551 ± 0.0234 | 0.0790 ± 0.0314 | 0.00313 ± 0.000745 | 0.0740 ± 0.0262 | 17.6 |
| Uracil | 0.0280 ± 0.00870 | 0.0411 ± 0.00825 | 0.0198 ± 0.00725 | 0.0258 ± 0.00333 | 1.41 |
| Uridine | 1.14 ± 0.242 | 1.34 ± 0.102 | 0.115 ± 0.0305 | 0.151 ± 0.0224 | 9.91 |
| Hypoxanthine | 0.351 ± 0.0523 | 0.403 ± 0.0351 | 0.123 ± 0.0171 | 0.203 ± 0.0304 | 2.85 |
| Thymine | 0.00774 ± 0.00451 | 0.00845 ± 0.00544 | 0.00585 ± 0.00296 | 0.00707 ± 0.00394 | 1.32 |
| Adenosine | 0.108 ± 0.0353 | 0.126 ± 0.0133 | 0.0295 ± 0.00581 | 0.0521 ± 0.0132 | 3.66 |
| Thymidine | 0.306 ± 0.0694 | 0.331 ± 0.0306 | 0.0338 ± 0.00415 | 0.0391 ± 0.00254 | 9.05 |
| Cytidine | 0.0548 ± 0.0177 | 0.0571 ± 0.00903 | 0.00194 ± 0.000239 | 0.00463 ± 0.00145 | 28.2 |
| Cytosine | 0.0101 ± 0.00552 | 0.0112 ± 0.00124 | 0.00768 ± 0.00512 | 0.00704 ± 0.00176 | 1.32 |
| Inosine | 0.306 ± 0.0904 | 0.282 ± 0.0174 | 0.0193 ± 0.00159 | 0.0354 ± 0.00524 | 15.8 |
| Gemcitabine | 0.699 ± 0.0958 | N/A | 0.0998 ± 0.0343 | N/A | 7.00 |
hOAT2 Was Distinct from Other hOATs in Substrate Selectivity and Structure
Although the other hOAT transporters showed efficient uptake of model substrates (61-fold uptake of adefovir by hOAT1, 25-fold uptake of estrone sulfate by hOAT3, and 21-fold uptake of estrone sulfate by hOAT4), they exhibited less uptake of cGMP compared with hOAT2 (Fig. 2B). In contrast, hOAT2 exhibited a great ability to transport cGMP (50-fold enhanced uptake) compared with its model substrate PAH (Fig. 2B). However, surface expression of these proteins is not known, and differences in surface expression may have influenced the comparative uptake values of cGMP. Multiple sequence analysis indicated that hOAT2 is distinct from other organic anion transporters and despite its functional similarities compared with nucleobase transporters, it does not seem to be related to ENT and CNT transporters at the amino acid level to any greater extent than the other members of the organic anion transporter family (Fig. 2C).
hOAT2 Facilitated the Transport of cGMP and 2′-Deoxyguanosine
Multiple time course studies of cGMP and 2′-deoxyguanosine confirmed that 1.5 min was in the linear range of transport (data not shown); therefore, 1.5 min was used for cGMP and 2′-deoxyguanosine kinetics studies. The transport kinetics of cGMP and 2′-deoxyguanosine were saturable, with Km estimates of 88 ± 11 μM for cGMP and 128 ± 17 μM for 2′-deoxyguanosine, and Vmax values of 3373 ± 126 pmol/mg protein/1.5 min for cGMP and 7225 ± 332 pmol/mg protein/1.5 min for 2′-deoxyguanosine (Fig. 3, A and B). cGMP was shown to have the lowest Km among the guanine derivatives tested.
hOAT2-mediated transport of cGMP was inhibited by 2′-deoxyguanosine with a Ki value of 93 ± 12 μM, which was approximately equal to the Km value for hOAT2-mediated 2′-deoxyguanosine transport in the presence of NBMPR (Fig. 3C).
Intracellular and Extracellular Levels of cGMP Were Modulated by hOAT2
Because our preliminary data indicated that hOAT2 could mediate either uptake or efflux, we were interested in whether the transporter served primarily in the influx or efflux of cGMP. We determined the extracellular levels of endogenously produced cGMP extruded from EV- and hOAT2-transfected HEK293 cells treated with or without 750 μM sodium nitroprusside (SNP, a guanylate cyclase stimulator) plus 750 μM 3-isobutyl-1-methylxanthine (IBMX, a phosphodiesterase inhibitor). Figure 4A shows that basal extracellular levels of cGMP in hOAT2-transfected cells were 6-fold lower than those in EV-transfected cells, suggesting that hOAT2 mediates cGMP influx under these conditions. That is, the net effect of hOAT2 under basal conditions is in the influx direction. In contrast, when intracellular cGMP levels were increased by 1-h treatment with SNP and IBMX, extracellular cGMP levels were 3-fold higher in hOAT2-transfected cells compared with EV-transfected cells (Fig. 4C). In this case, when a steep outwardly directed cGMP concentration gradient was generated, the net effect of hOAT2 was in the efflux direction. In both EV and hOAT2 expressing cells, the intracellular pools of cGMP were a small fraction of the total, suggesting endogenous cGMP efflux activity in both cell lines. However, the intracellular cGMP amounts qualitatively and inversely reflected extracellular cGMP levels (Fig. 4, B and D). This is consistent with hOAT2 acting in the influx of cGMP (in opposition to the efflux transporters) under basal conditions, and in the efflux of cGMP when its concentration gradient was directed outwardly by SNP and IBMX. Taken together, the data suggest that hOAT2, as a bidirectional facilitative transporter, can modulate cellular levels of cGMP. In addition, timed efflux studies in hOAT2 and EV transiently transfected HEK cells preloaded with [3H]cGMP (2 μM) demonstrated that the extracellular concentrations of [3H]cGMP increased with time in the hOAT2-expressing cells (16.4 ± 3.20 pmol/mg protein at 10 min versus 21.8 ± 0.988 pmol/mg protein at 30 min), indicating that the transporter mediated [3H]cGMP efflux. Concentrations of [3H]cGMP in the extracellular media of empty vector transfected cells did not increase with time (data not shown). Mass spectrometric analysis of the extracellular media indicated that cGMP was transported in the efflux direction by hOAT2; i.e., we detected substantial [3H]cGMP concentrations in the extracellular media at 10 min.
hOAT2 Had Two Splice Variants That Differ in Terms of Two Amino Acids (hOAT2-546 and hOAT2-548)
The hOAT2 cDNA used in the previous experiments (hOAT2-546aa) contained a 1638-bp open reading frame encoding a 546-amino acid protein, which corresponds to the NCBI-registered reference sequence for SLC22A7 (NM_06672). During our initial procedures, we cloned another hOAT2 cDNA (hOAT2-548aa), which has been listed as a related sequence to SLC22A7 in the NCBI data base (NM_153320 [GenBank]) from a human kidney cDNA library. The sequence analysis revealed that the only difference between the two clones is a 6-bp insertion (TCCCAG) between exons 1 and 2 of the reference SLC22A7 gene. This additional nucleotide sequence is found in intron 1 of the reference SLC22A7 gene and is flanked by the consensus sequence for intron/exon boundaries, suggesting that the two cDNAs of hOAT2 are alternatively spliced variants.
Our studies indicated that hOAT2-546aa was capable of transporting cGMP, whereas the hOAT2-548aa isoform exhibited a complete loss of cGMP uptake (Fig. 5A). Mutagenesis analysis demonstrated that deletion of two amino acid residues from hOAT2-548aa restored function, whereas insertion of two amino acid residues into hOAT2-546aa caused a complete loss of function (data not shown). In addition, cellular localization via fluorescence and confocal microscopy of GFP (control), hOAT2-546aa-GFP (functional isoform), and hOAT2-548aa-GFP (nonfunctional isoform) revealed that hOAT2-546aa-GFP was localized to the plasma membrane. In contrast, hOAT2-548aa-GFP remained exclusively in the intracellular compartment with markedly lower fluorescence (Fig. 5B). The lack of surface expression of the hOAT2-548aa isoform most likely results in the observed loss of its transport function. Quantitative reverse transcription-PCR assays specific for each isoform showed that each hOAT2-expressing tissue contained approximately equal levels of the mRNA species of the two splice forms (Fig. 6).
Fig. 6.
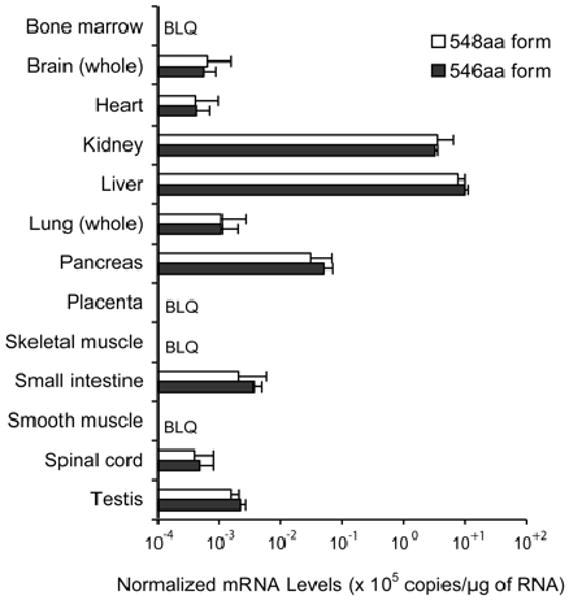
Tissue distribution of alternative splice forms of hOAT2. mRNA expression of hOAT2-546aa (solid bars) and hOAT2-548aa (open bars) was determined by real-time PCR using TaqMan primers and probes specific to each isoform. Sensitivity of each assay for its target sequence was >10,000-fold greater than for the alternative target. Expression level of each isoform was normalized to GAPDH expression. Each bar represents the mean ± S.D. from quadruplicate assays of two independent cDNA sources. BLQ, below the limit of quantification.
Discussion
Our studies indicate that hOAT2 exhibits a robust transport function for a wide array of naturally occurring nucleobases, nucleosides, and nucleotides (Fig. 1 and Table 1) with a particular role for cGMP. cGMP is a second messenger involved in the intracellular signal transduction of a variety of extracellular stimuli in many tissues. Intracellular levels of cGMP are thought to be determined primarily by synthesizing and catabolizing enzymes, in the GC and PDE families. cGMP signaling can be modulated pharmacologically by nitric oxide (GC activator) and sildenafil (PDE5-specific inhibitor) (Dousa, 1999; Friebe and Koesling, 2003). Our data suggest that hOAT2 works in concert with these enzymes in regulating intracellular cGMP levels. The interaction of cGMP with PDE5 has a lower Km (2-5 μM) compared with its interaction with hOAT2 determined in this study (Km = 88 μM). These interaction kinetics support the notion that both hOAT2 and PDE5 are determinants of intracellular levels of cGMP. Although speculative, it is possible that hOAT2 acts to eliminate excess intracellular cGMP that is not enzymatically inactivated by PDE5. Intracellular cGMP levels vary widely depending upon the expression levels and activities of transporters and enzymes involved in its production and breakdown. Some reports include values as low as 1 μM and as high as 300 μM (Andric et al., 2006). The Km for OAT2 obtained in our study is consistent with reported intracellular concentrations of cGMP. In particular, we observed that under control conditions, hOAT2 mediates the influx of cGMP, whereas under conditions in which GC is stimulated and PDE is inhibited, hOAT2 functions in the efflux of cGMP from cells (Fig. 4, A-D). Additional experiments in our lab reveal that ENTs do not transport cGMP. In addition, CNTs have not been demonstrated to accept nucleotide monophosphates of any kind. Sequence comparisons indicate that OAT2 is approximately 40% identical to other OATs and only 15 to 20% identical to the ENTs and CNTs. Such sequence comparisons may not provide much information on the substrate specificities of various transporters because small differences in sequence can result in noticeable differences in protein structure.
To our knowledge, only one other influx transporter has been shown to transport cGMP (Sekine et al., 1997). This transporter, OAT1, is expressed almost exclusively in the kidney and takes up cGMP much less avidly than hOAT2 (Fig. 2A). Because hOAT2 is expressed at high levels in many tissues, our data suggest that hOAT2 plays a critical role in transmembrane influx of cGMP in many cell types.
Three efflux transporters for cGMP in the ATP binding cassette superfamily have been identified and are members of the multidrug resistance protein family with Km values between 2 and 10 μM (MRP4, MRP5, and MRP8) (Jedlitschky et al., 2000; Chen et al., 2001; Guo et al., 2003; Andric et al., 2006). Because hOAT2 is a facilitative transporter, it will have a distinct role from the MRPs, which, as efflux transporters, will function to actively eliminate cGMP from cells in tissues in which these transporters are expressed. In contrast, hOAT2 may facilitate both the uptake and efflux of cGMP in hOAT2-expressing tissues. Based on its wide tissue distribution, we speculate that hOAT2 is a key transporter for modulating intracellular cGMP levels in many tissues. In addition, several studies suggest a role for cGMP as an extracellular effector molecule (Poulopoulou et al., 1998), particularly in brain (Montoliu et al., 1999; Wang et al., 2004; Erceg et al., 2006) and kidney (Neant and Bailly, 1993; Chevalier et al., 1996), tissues in which hOAT2 is abundantly expressed. hOAT2 may be involved in modulating the effects of extracellular cGMP in these tissues.
Structure activity studies indicated that the addition of a monophosphate, rather than a cyclic monophosphate, at the 5′-position reduced both the affinity and the capacity of hOAT2-mediated transport (Fig. 1B). Addition of one (GDP) or two (GTP) phosphate groups caused a further reduction in hOAT2 transport activity. These results suggest that cyclic 5′,3′ monophosphate increases substrate-hOAT2 interaction and further suggest that the primary substrate of hOAT2 is cGMP.
To date, alternatively spliced variants in the OAT family have been identified for hOAT1 (Bahn et al., 2004). Two of four hOAT1 isoforms are nonfunctional because of the deletion of transmembrane domain 11 and a portion of transmembrane 12. The OAT2 splice variant, hOAT2-548aa, is unusual because it contains such a small exon, and does not result in a frameshift. Nevertheless, the small difference between the two isoforms of hOAT2 produced a clear distinction in the activity of hOAT2, with hOAT2-548aa resulting in complete loss of transport function. Furthermore, mRNA expression studies demonstrating that the 6-bp insertion did not affect mRNA stability suggest that alternative splicing of this small exon is a stochastic event.
We determined that the mechanism for the loss of function of hOAT2-548aa was related to a reduced expression level of the protein on the plasma membrane (Fig. 5A). Similar to other OATs, the predicted secondary structure of hOAT2 consists of 12 TMDs with a large hydrophilic loop between TMD1 and TMD2. hOAT2-548aa contains a two-amino acid insertion, Ser and Gln, between Glu131 and Trp132 in the large extracellular loop 1 of hOAT2-546aa. It is likely that addition of these two amino acids results in reduced stability of the 548 form. In particular, hOAT2-548aa-GFP-transfected cells displayed a much lower GFP-derived signal compared with those transfected with hOAT2-546aa-GFP (Fig. 5B). This suggests that the lower levels of hOAT2-548aa protein on the plasma membrane are the result of a generalized increased rate of degradation of the 548-aa form. In fact, the 548-aa form was not present in Western blots using a GFP-specific antibody; whereas the 546-aa form was present in abundance (data not shown). It is also possible that these two amino acids disrupt a motif responsible for proper trafficking to the plasma membrane. It is noteworthy that the amino acid sequence near Glu131 is highly conserved among members of the OAT family, suggesting that this region may contain a motif or motifs that are essential for stability or trafficking of the protein.
In summary, our study revealed a novel functionality of hOAT2 to transport guanine nucleosides and in particular, cGMP in a bidirectional manner. This activity was present in one splice form, hOAT2-546aa but not in the alternatively spliced form hOAT2-548aa, which seemed to exhibit reduced stability in cells. By regulating intracellular as well as extracellular levels of cGMP, hOAT2, together with guanylate cyclases and phosphodiesterases, may play a key role in cGMP mediated signaling. Small molecule inhibitors may be used as tools to understand the role of hOAT2 in the context of regulation of intracellular and extracellular cGMP levels.
Acknowledgments
This work was supported by National Institutes of Health grants GM36780 and GM61390. Dr. Cropp is a recipient of a NIGMS Predoctoral Fellowship (1R25-GM56847).
Abbreviations
- GC
guanylate cyclase
- PDE
phosphodiesterase
- MRP
multidrug resistance protein
- SLC
solute carrier
- CNT
concentrative nucleoside transporter
- ENT
equilibrative nucleoside transporter
- hOAT
human organic anion transporter
- PAH
p-aminohippurate
- PCR
polymerase chain reaction
- HEK
human embryonic kidney
- NBMPR
nitrobenzylthioinosine
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- TLC
thin-layer chromatography
- GFP
green fluorescent protein
- EV
empty vector
- SNP
sodium nitroprusside
- IBMX
3-isobutyl-1-methylxanthine
- bp
base pair(s)
- aa
amino acid(s)
References
- Andric SA, Kostic TS, Stojilkovic SS. Contribution of multidrug resistance protein MRP5 in control of cyclic guanosine 5′-monophosphate intracellular signaling in anterior pituitary cells. Endocrinology. 2006;147:3435–3445. doi: 10.1210/en.2006-0091. [DOI] [PubMed] [Google Scholar]
- Bahn A, Ebbinghaus C, Ebbinghaus D, Ponimaskin EG, Fuzesi L, Burckhardt G, Hagos Y. Expression studies and functional characterization of renal human organic anion transporter 1 isoforms. Drug Metab Dispos. 2004;32:424–430. doi: 10.1124/dmd.32.4.424. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–743. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- Burckhardt BC, Burckhardt G. Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev Physiol Biochem Pharmacol. 2003;146:95–158. doi: 10.1007/s10254-002-0003-8. [DOI] [PubMed] [Google Scholar]
- Cha SH, Sekine T, Fukushima JI, Kanai Y, Kobayashi Y, Goya T, Endou H. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59:1277–1286. doi: 10.1124/mol.59.5.1277. [DOI] [PubMed] [Google Scholar]
- Cha SK, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem. 2000;275:4507–4512. doi: 10.1074/jbc.275.6.4507. [DOI] [PubMed] [Google Scholar]
- Chen ZS, Lee K, Kruh GD. Transport of cyclic nucleotides and estradiol 17-β-D-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem. 2001;276:33747–33754. doi: 10.1074/jbc.M104833200. [DOI] [PubMed] [Google Scholar]
- Chevalier RL, Fang GD, Garmey M. Extracellular cGMP inhibits transepithelial sodium transport by LLC-PK1 renal tubular cells. Am J Physiol. 1996;270:F283–F288. doi: 10.1152/ajprenal.1996.270.2.F283. [DOI] [PubMed] [Google Scholar]
- Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, Sweet DH. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol. 1999;56:570–580. doi: 10.1124/mol.56.3.570. [DOI] [PubMed] [Google Scholar]
- Dousa TP. Cyclic-3′,5′-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int. 1999;55:29–62. doi: 10.1046/j.1523-1755.1999.00233.x. [DOI] [PubMed] [Google Scholar]
- Erceg S, Monfort P, Cauli O, Montoliu C, Llansola M, Piedrafita B, Felipo V. Role of extracellular cGMP and of hyperammonemia in the impairment of learning in rats with chronic hepatic failure. Therapeutic implications. Neurochem Int. 2006;48:441–446. doi: 10.1016/j.neuint.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Erdman AR, Mangravite LM, Urban TJ, Lagpacan LL, Castro RA, de la Cruz M, Chan W, Huang CC, Johns SJ, Kawamoto M, et al. The human organic anion transporter 3 (OAT3; SLC22A8): genetic variation and functional genomics. Am J Physiol Renal Physiol. 2006;290:F905–F912. doi: 10.1152/ajprenal.00272.2005. [DOI] [PubMed] [Google Scholar]
- Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- Gray JH, Owen RP, Giacomini KM. The concentrative nucleoside transporter family, SLC28. Pflugers Arch. 2004;447:728–734. doi: 10.1007/s00424-003-1107-y. [DOI] [PubMed] [Google Scholar]
- Guo Y, Kotova E, Chen ZS, Lee K, Hopper-Borge E, Belinsky MG, Kruh GD. MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2′,3′-dideoxycytidine and 9′-(2′-phosphonylmethoxyethyl)adenine. J Biol Chem. 2003;278:29509–29514. doi: 10.1074/jbc.M304059200. [DOI] [PubMed] [Google Scholar]
- Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275:30069–30074. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- Koepsell H, Endou H. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. Pflugers Arch. 2004;447:666–676. [Google Scholar]
- Montoliu C, Llansola M, Kosenko E, Corbalan R, Felipo V. Role of cyclic GMP in glutamate neurotoxicity in primary cultures of cerebellar neurons. Neuropharmacology. 1999;38:1883–1891. doi: 10.1016/s0028-3908(99)00071-4. [DOI] [PubMed] [Google Scholar]
- Neant F, Bailly C. Luminal and intracellular cGMP inhibit the mTAL reabsorptive capacity through different pathways. Kidney Int. 1993;44:741–746. doi: 10.1038/ki.1993.308. [DOI] [PubMed] [Google Scholar]
- Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Poulopoulou C, Nowak LM. Extracellular 3′,5′ cyclic guanosine monophosphate inhibits kainate-activated responses in cultured mouse cerebellar neurons. J Pharmacol Exp Ther. 1998;286:99–109. [PubMed] [Google Scholar]
- Sager G. Cyclic GMP transporters. Neurochem Int. 2004;45:865–873. doi: 10.1016/j.neuint.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. Expression cloning and characterization of a novel multispecific organic anion transporter. J Biol Chem. 1997;272:18526–18529. doi: 10.1074/jbc.272.30.18526. [DOI] [PubMed] [Google Scholar]
- Sun W, Wu RR, van Poelje PD, Erion MD. Isolation of a family of organic anion transporters from human liver and kidney. Biochem Biophys Res Commun. 2001;283:417–422. doi: 10.1006/bbrc.2001.4774. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson JT, Plewniak F, Jeanmougin F, Higgins JD. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban TJ, Gallagher RC, Brown C, Castro RA, Lagpacan LL, Brett CM, Taylor TR, Carlson EJ, Ferrin TE, Burchard EG, et al. Functional genetic diversity in the high-affinity carnitine transporter OCTN2 (SLC22A5) Mol Pharmacol. 2006;70:1602–1611. doi: 10.1124/mol.106.028126. [DOI] [PubMed] [Google Scholar]
- Wang M, Urenjak J, Fedele E, Obrenovitch TP. Effects of phosphodiesterase inhibition on cortical spreading depression and associated changes in extracellular cyclic GMP. Biochem Pharmacol. 2004;67:1619–1627. doi: 10.1016/j.bcp.2003.12.029. [DOI] [PubMed] [Google Scholar]


