Abstract
3,4-Methylenedioxymethamphetamine (MDMA, Ecstasy) is a widely misused synthetic amphetamine derivative and a serotonergic neurotoxicant in animal models and possibly humans. The underlying mechanism of neurotoxicity involves the formation of reactive oxygen species although their source remains unclear. It has been postulated that MDMA-induced neurotoxicity is mediated via the formation of bioreactive metabolites. In particular, the primary catechol metabolites, 3,4-dihydroxymethamphetamine (HHMA) and 3,4-dihydroxyamphetamine (HHA), subsequently cause the formation of glutathione and N-acetylcysteine conjugates, which retain the ability to redox cycle and are serotonergic neurotoxicants in rats. Although the presence of such metabolites has been recently demonstrated in rat brain microdialysate, their formation in humans has not been reported. The present study describes the detection of 5-(N-acetylcystein-S-yl)-3,4-dihydroxymethamphetamine (N-Ac-5-Cys-HHMA) and 5-(N-acetylcystein-S-yl)-3,4-dihydroxyamphetamine (N-Ac-5-Cys-HHA) in human urine of 15 recreational users of MDMA (1.5 mg/kg) in a controlled setting. The results reveal that in the first 4 h after MDMA ingestion ∼0.002% of the administered dose was recovered as thioether adducts. Genetic polymorphisms in CYP2D6 and catechol-O-methyltransferase expression, the combination of which are major determinants of steady-state levels of HHMA and 4-hydroxy-3-methoxyamphetamine, probably explain the interindividual variability seen in the recovery of N-Ac-5-Cys-HHMA and N-Ac-5-Cys-HHA. In summary, the formation of neurotoxic thioether adducts of MDMA has been demonstrated for the first time in humans. The findings lend weight to the hypothesis that the bioactivation of MDMA to neurotoxic metabolites is a relevant pathway to neurotoxicity in humans.
3,4-Methylenedioxymethamphetamine (MDMA, Ecstasy) is a psychostimulant widely abused among young people. MDMA exhibits distinct pharmacological properties, collectively described as entactogenic, which differentiate it from classic amphetamines (Nichols, 1986). MDMA produces acute and long-term serotonergic neurotoxicity in rodents, primates, and, possibly, in humans, with the severity of toxicity dependent on the dose and frequency of administration (Green et al., 2003). Such neurotoxicity is demonstrated by a decrease in tryptophan hydroxylase activity (Stone et al., 1988), a reduction in serotonin content, a dose-dependent persistent decrease in the number of 5-HT transporter sites and 5-HT receptors in several regions of the brain (Aguirre et al., 1995; Ricaurte et al., 2000), and an impairment of central 5-HT function (Barrionuevo et al., 2000).
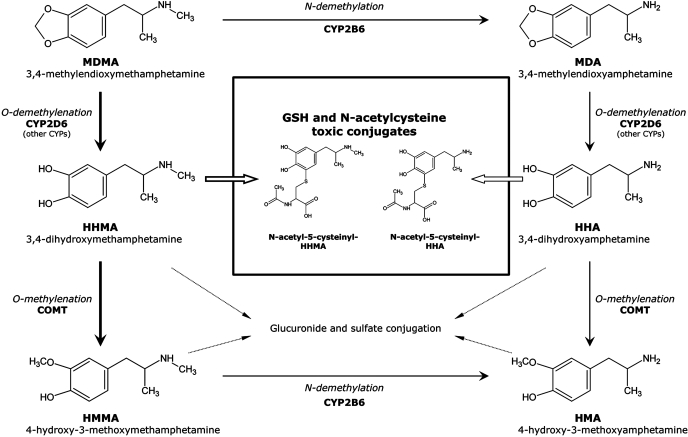
Oxidative stress, hyperthermia, excitotoxicity, and various apoptotic pathways have been invoked as the underlying mechanism(s) of MDMA-induced neurotoxicity (Cadet et al., 2007). With respect to oxidative stress, various sources of reactive oxygen species have been suggested, including the monoamine oxidase-mediated metabolism of tyrosine/dopamine, which generates hydrogen peroxide as a by-product, and the oxidative metabolism of MDMA to redox active catechols. However, until recently, evidence supporting a link between MDMA metabolism and neurotoxicity has been primarily indirect (Monks et al., 2004; Jones et al., 2005). MDMA is metabolized via cytochrome P450-mediated N-demethylation to the active metabolite 3,4-methylenedioxyamphetamine (MDA). MDMA and MDA are both O-demethylenated, again via cytochrome P450, to 3,4-dihydroxymethamphetamine (HHMA, N-methyl-α-methyldopamine) and 3,4-dihydroxyamphetamine (HHA, α-methyldopamine), respectively (Fig. 1) (Maurer et al., 2000; de la Torre et al., 2004). Because HHMA and HHA are both catechols, they can undergo further autooxidation to the corresponding ortho-quinones, which are readily conjugated with glutathione (GSH) to form glutathionyl adducts (Hiramatsu et al., 1990). The GSH adducts of HHMA and HMA appear to be transported into the brain via blood-brain barrier GSH transporters, where they are subsequently metabolized to the corresponding N-acetylcysteine (NAC) adducts (Bai et al., 2001). Direct injection of the GSH and N-acetylcysteine conjugates of HHMA or HMA into rat brain produces not only the acute neurobehavioral effects of the parent drug but also its selective serotonergic neurotoxicity (Miller et al., 1996; Bai et al., 1999). Moreover, because multidose administration of MDMA is typical of drug intake during rave parties, the effects of multiple doses of MDMA on the concentration of catechol-thioether metabolites in rat brain were determined. The data revealed that thioether metabolites, especially the NAC conjugates, accumulate in rat brain after multidose administration of MDMA (Erives et al., 2008). The ability of these metabolites to generate reactive oxygen species and to arylate proteins, in combination with their ability to modulate the activity of proteins involved in the regulation of neurotransmitter transport (Jones et al., 2004), suggests that they play an important role in the development of MDMA-mediated serotonergic neurotoxicity.
Fig. 1.
Pathways involved in the metabolic disposition of MDMA in humans (information obtained in part from Maurer et al., 2000). Major pathways are outlined with thicker lines.
Although catechol-thioether metabolites of MDMA have been identified in rat brain (Jones et al., 2005), there is no evidence for their formation in humans after MDMA exposure. GSH adducts are metabolized by γ-glutamyl transpeptidase and subsequently by aminopeptidase M to the corresponding cysteine conjugates, which are ultimately N-acetylated to form the N-acetylcysteine derivative (also referred to as mercapturic acid). In humans, a noninvasive approach to demonstrate the formation of GSH adducts of HHMA and HHA after MDMA exposure would be the detection of the mercapturate derivatives in urine. In the present study, an analytical methodology for the detection of MDMA-derived mercapturates in human urine has been developed and applied to samples obtained from recreational users of MDMA (1.5 mg/kg, 75–100 mg dose) in a controlled setting. Finally, because enzymes regulating the formation (CYP2D6) and the inactivation (COMT) of catechol metabolites of MDMA in humans are highly polymorphic, the contribution of genetic variability to interindividual differences in the urinary concentration of mercapturates has also been examined.
Materials and Methods
Chemicals and Reagents. Ultrapure water was obtained using a Milli-Q purification system (Millipore, Molsheim, France). HPLC-grade acetonitrile, methanol, hydrochloric acid, sodium acetate, potassium hydrogen phosphate, potassium dihydrogen phosphate, sodium hydroxide, trifluoroacetic acid, metabisulfite, dithiothreitol (DTT), formic acid, ammonia, ammonium formate, and ammonium chloride were obtained from Merck (Darmstadt, Germany). EDTA, N-acetyl-l-cysteine, and mushroom tyrosinase (2033 U/mg) were obtained from Sigma-Aldrich (St. Louis, MO). N-Acetyl-l-cysteine methyl ester was obtained from Fluka Biochemika (Riedel-de Haën, Seelze, Germany). Bond Elut PBA [phenylboronic acid (PBA), 500 mg of sorbent] columns were purchased from Varian, Inc. (Harbor City, CA) and mounted on a Vac Elut vacuum manifold (Supelco, Bellefonte, PA).
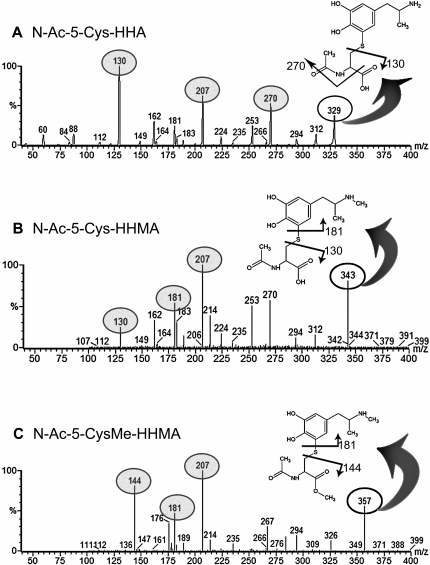
Synthesis of N-Acetylcysteine and N-Acetylcysteine-Methyl Ester Adducts of HHMA and HHA. 5-(N-Acetylcystein-S-yl)-3,4-dihydroxymethamphetamine-HHMA (N-Ac-5-Cys-HHMA), 5-(N-acetylcystein-S-yl)-3,4-dihydroxyamphetamine-HHA (N-Ac-5-Cys-HHA), and 5-(N-acetylcystein-S-yl)-methylesther-3,4-dihydroxymethamphetamine [N-Ac-5-Cys-O-Me-HHMA, as internal standard (IS)] were synthesized following an experimental procedure similar to that described previously (Jones et al., 2005). In brief, 3 mM HHMA or HHA, 8 mM N-acetyl-l-cysteine or N-acetyl-l-cysteine methyl ester, and 1016 IU of tyrosinase from mushroom (2033 U/mg) in 100 ml of phosphate buffer (50 mM), pH 7.4, previously degassed with argon were incubated for 30 min at room temperature. The reaction was quenched with 2 ml of 88% formic acid. The reaction mixture was concentrated by lyophilization, and adducts were isolated by semipreparative reverse-phase HPLC-UV (conditions described under Analytical HPLC-UV and Semipreparative HPLC-UV). Collected fractions were lyophilized, and the structure and purity of the compound were determined by NMR and high-performance liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS). HPLC-MS/MS revealed a single compound with a molecular ion corresponding to each product synthesized: N-Ac-5-Cys-HHA (m/z 329), N-Ac-5-Cys-HHMA (m/z 343), and N-Ac-5-Cys-O-Me-HHA (m/z 357). The molecular ion, once further fragmented, gives rise to several daughter ions, including those of N-acetylcysteine (m/z 161.9), N-acetylcysteine methyl ester (m/z 175.9), HHMA (m/z 182.9), and HHA (m/z 168.9).
Working Standards. Solutions of 1 mg/ml N-Ac-5-Cys-HHMA, N-Ac-5-Cys-HHA, and N-Ac-5-Cys-O-Me-HHMA (IS) were prepared in methanol. Working solutions of 0.1, 1, 10, and 100 μg/ml of each compound were prepared by dilution of the corresponding 1 mg/ml stock solution.
Preparation of Calibration and Quality Control Samples. Calibration curves and QC samples were prepared by adding appropriate volumes of working solutions to test tubes, each containing 5 ml of drug-free urine. QC samples were prepared with solutions different from those used for the preparation of calibration curves. Final concentrations in the calibration curves were 4, 7, 15, 20, and 30 ng/ml N-Ac-5-Cys-HHMA and N-Ac-5-Cys-HHA. Control urine samples containing appropriate analytes at different concentrations were prepared in drug-free samples in 5-ml aliquots. The concentrations of QC samples were as follows: 4, 12, and 26 ng/ml of both metabolites.
1H NMR spectra were obtained in deuterated methanol solutions on Varian ANOVA 500 and Unity 300 spectrometers. Chemical shifts are reported in parts per million relative to the multiplet at 3.39 ppm of deuterated methanol.
Analytical HPLC-UV. A LaChrom Pump L-7100 (Hitachi and Merck) with a manual injector coupled to a UV LC-75 spectrophotometric detector (PerkinElmer Life and Analytical Sciences, Waltham, MA) was used, with a Lichrosphere RP-18 5 μm (4 × 250 mm) column. The HPLC solvent system was a gradient and consisted of two phases: A, 0.1% TFA in water; and B, 20% water-80% acetonitrile with 0.095% TFA. The step linear gradient for elution was from 0% B to 5% B in 1 min and from 5% B to 60% B in 30 min with a flow rate 1 ml/min, and the eluate was monitored at 215 nm.
Semipreparative HPLC-UV. A Waters LC 4000 HPLC system (Waters, Milford, MA) coupled to an UV 4000 Series Spectrophotometric Detector (Hitachi and Merck) was used with a X-Terra MS C18 column (10 μm, 19 × 250 mm). The same mobile phases and gradient as those described for the analytical HPLC-UV system were used for semipreparative HPLC but with a flow rate of 12 ml/min.
HPLC-MS/MS. Extracted urine samples were analyzed in a Micromass Quattro micro API triple quadrupole mass spectrometer (Waters, Etten-Leur, The Netherlands) equipped with an electrospray ionization (ESI) probe coupled to an Alliance HPLC system (Waters). An Atlantis T3 3 μm (2.1 × 20 mm) column (Waters) was used. The mobile phase flow rate was 0.25 ml/min. A binary mobile phase was used: A, 100% 0.1% formic acid in water; and B, 100% acetonitrile. The linear gradient for elution was 0 to 10% B in 15 min. The ESI probe was operated in positive mode, and the ESI settings were as follows: capillary voltage, 3000 V; source temperature, 120°C; desolvation temperature, 420°C; cone voltage, 20 V; and cone gas and desolvation gas flow rates, 34 and 609 l/h, respectively. Initial screening of the samples was performed in positive mode in the first quadrupole based on full MS measurements between 50 and 500 m/z only (Q1 MS mode). After the Q1 MS scan, selected reactions monitoring was performed. The fragmentation channels monitored for [M + H]+ to product ions were for N-Ac-5-Cys-HHMA m/z 343 → 130, m/z 343 → 181, m/z 343 → 207, and m/z 343 → 130; for N-Ac-5-Cys-HHA m/z 329 → 130, m/z 329 → and 207, and m/z 329 → 270; and for N-Ac-5-Cys-O-Me-HHMA (IS) m/z 357 → 144, m/z 357 → 181, and m/z 357 → 207. The maximum ionization time for each product ion scan was 2 s with an isolation width of 0.9 m/z. Quantitative analysis was based on peak area ratios of the 343 and 329 atomic mass unit ions relative to the internal standard ion at 357 atomic mass units. On each day of analysis, a 5-point calibration curve ranging from 3 to 30 ng/ml was performed in duplicate.
Genotyping. Samples of 1 ml of whole blood were taken for DNA extraction and CYP2D6 genotyping (DrugMEt; Jurilab Ltd., Kuopio, Finland). The following allelic variants were determined: *1, *2, *4, *5, *9, *10, *35, and *41. In addition, deletions (*3) and gene duplications (*1xn and *2xn) were determined.
The COMT Val/Met (rs4680) and P2 promoter (rs2097603) allelic variants were determined using the 5′ exonuclease TaqMan assay performed using an ABI 7900HT Sequence Detection System (real-time polymerase chain reaction) supplied by Applied Biosystems (Foster City, CA). Primers and fluorescent probes for the Val/Met assay were obtained from Applied Biosystems by submission of the polymorphic sequence of the COMT gene to Assay-by-Design Service (p/n 4332728, COMT_V158MS1AG). According to assay design the primers have the following sequences: forward 5′-CCCAGCGGATGGTGGAT-3′ and reverse 5′-CAGGCATGCACACCTTGTC-3′. The reporter probes for the real-time polymerase chain reaction have the following sequences: FAM-TCGCTGGCGTGAAG-3′ and VICTTCGCTGGCATGAAG-3′. For the P2 promoter assay, primers have the following sequences according to those published previously (Chen et al., 2004): forward 5′-GCCGTGTCTGGACTGTGAGT-3′ and reverse 5′-GGGTTCAGAATCACGGATGTG-3′. The reporter probes have the following sequences: 6FAMAACAGACAGAAAAGTTTCCCCTTCCCA-3′ and VICCAGACAGAAAAGCTTCCCCTTCCCATA-3′. Reaction conditions were those described in the ABI PRISM 7900HT User's Guide. Endpoint fluorescent signals were detected on the ABI 7900 system, and the data were analyzed using the Sequence Detector System software, version 2.1.
Subjects and Dosing. The study was conducted in accordance with the Declaration of Helsinki (2000), approved by the local institutional review board (Clinical Research Ethical Committee of the Municipal Institute of Health Care), and authorized by the Spanish Medicines Agency of the Spanish Ministry of Health. All subjects gave their written informed consent before inclusion in the study and were compensated for their participation.
Each subject was interviewed by a physician to exclude concomitant medical conditions and underwent a general physical examination, routine laboratory tests, urinalysis, and 12-lead electrocardiogram. Subjects were interviewed by a psychiatrist (Psychiatric Research Interview for Substance and Mental Disorders for DSM-IV) to exclude those with a history of or actual major psychiatric disorders (schizophrenia, psychosis, and major affective disorder). Fifteen individuals (11 males and 4 females) fulfilled the inclusion criteria and had a mean age of 26 years (range 19–33 years), mean body weight of 69.6 kg (range 54.2–91.2 kg), and mean height of 181 cm (range 171–196 cm). Subjects reported an average of 26 previous experiences (range 6–100 previous experiences) with MDMA. All but four were current smokers. None met the criteria of abuse or drug dependence (except for nicotine dependence). All had previous experience with other psychostimulants, cannabis, or hallucinogens. None had a history of adverse medical or psychiatric reactions after MDMA consumption. The subjects were phenotyped with dextromethorphan for CYP2D6 enzyme activity, and all were categorized as extensive metabolizers (Schmid et al., 1985). All subjects received a single 1.5 mg/kg dose of MDMA. MDMA was obtained from the Spanish Ministry of Health, and MDMA soft gelatin capsules were prepared and supplied by the Department of Pharmacy of Hospital del Mar (Barcelona, Spain).
Sample Collection. Plasma samples were collected at 0, 0.3, 0.6, 1, 1.5, 2, and 4 h after MDMA administration. Urine samples were collected before and after drug administration at the 0 and 0 to 4 h time period. Urine was collected in refrigerated plastic containers covered with aluminum foil to prevent light exposure. Samples were immediately acidified with 0.5 ml of 6 M HCl, distributed in aliquots, and stored at –20°C until further analysis. Concentrations of MDMA and metabolites in plasma and urine were determined by gas chromatography coupled to mass spectrometry after an analytical procedure described previously (Pizarro et al., 2002).
Preparation of Urine Sample for the Analysis of N-Acetyl-Cysteinyl Adducts. For the determination of N-Ac-5-Cys-HHMA and N-Ac-5-Cys-HHA, 5 ml of urine and 25 μl of the appropriate IS solution were transferred into 15-ml screw-capped glass tubes. Urine pH was adjusted to 9.5 with ammonium chloride buffer (pH 9.5). After vortex mixing, the sample was filtered through a 0.22-μm centrifuge filter at 3500 rpm for 5 min. Bond Elut PBA (500-mg) columns were conditioned by washing with 5 ml of ammonium chloride buffer (pH 9.5). Urine samples were applied to the column and forced to pass through. After application of the sample, the column was washed twice with 3 ml of H2O/MeOH (95:5%). Analytes were then eluted with 2 ml of TFA-1 M/MeOH (30:70%) and 20 mM DTT. The eluate was evaporated for 20 min under a stream of nitrogen in a water bath at 39°C to evaporate the methanol and then frozen and lyophilized. The dried extract was reconstituted in 100 μl of the mobile phase with 9 mM Na2S2O5 and 5 mM EDTA as preservatives, transferred to a 0.22-μm centrifuge tube and centrifuged at 15,000 rpm for 5 min. The supernatant was then transferred into 200-μl injection vials, and a 40-μl aliquot was injected into the LC-ESI-MS/MS system.
Validation Procedure. Validation of the method was performed according to a 4-day protocol. Linearity was determined by checking different calibration curves (n = 10 in 4 consecutive days) at five different concentrations of N-Ac-5-Cys-HHMA (4, 7, 15, 20, and 30 ng/ml) and N-Ac-5-Cys-HHA (4, 7, 15, 20, and 30 ng/ml). Peak area ratios between N-Ac-5-Cys-HHMA or N-Ac-5-Cys-HHA and the internal standard (N-Ac-5-Cys-O-Me-HHMA) were used for calculations. A weighted (1/concentration) least-squares regression analysis was used (SPSS computer software package, version 12.0 for Windows; SPSS Inc., Chicago, IL). By quantifying a quadruplicate of the lower concentration of the calibration curves, we estimated the limits of detection and quantification as 3 and 10 standard deviations of the calculated concentrations, respectively. Intermediate precision was calculated with the relative S.D. of concentrations calculated for quality control samples (5, 12, and 26 ng/ml N-Ac-5-Cys-HHMA or N-Ac-5-Cys-HHA), and the interassay accuracy was the relative error of the calculated concentrations. Five replicates at three different concentrations of N-Ac-5-Cys-HHMA or N-Ac-5-Cys-HHA (4, 7, 15, 20, and 30 ng/ml) spiked in blank urine were used for the determination of intra-assay precision (expressed as coefficient of variation for specific added target concentrations) and accuracy (expressed as percentage error of concentration found as compared with target added concentrations). Interday precision and accuracy were determined on 3 different experimental days. Analytical recoveries were calculated by comparison between peak areas of the calibration samples analyzed with the normal procedure and those obtained after the same amounts of reference substances and IS were added to blank urine after extraction. Recoveries were analyzed at three different concentrations (4, 15, and 30 ng/ml) using four replicates for each concentration evaluated. The stability of N-Ac-5-Cys-HHMA and N-Ac-5-Cys-HHA in urine was evaluated in two freeze/thaw cycles. The test involved a comparison of replicate stability samples, which had been frozen and thawed two times with a fresh urine sample that had not been frozen.
Results
Sample Preparation. Because the catecholamine-like properties of HHMA and HHA conjugates makes them very unstable and sensitive to oxidation and decomposition, the whole process from sample collection to HPLC-MS/MS determination was performed as quickly as possible, keeping samples cold and protected from light during the entire procedure. Moreover, addition of preservatives was necessary to prevent oxidation. More precisely, EDTA prevents catalytic oxidation by metal ions, whereas sodium metabisulfite acts as a reducing agent. Because these two preservatives interfere with our solid-phase extraction process, we used 20 mM DTT as a preservative during the extraction and added EDTA and sodium metabisulfite to the samples after the extraction to further protect the analytes from oxidation during the remainder of the analysis.
PBA has proven to be especially effective in the isolation of catecholamines from biological fluids because boronate groups have a high specificity for cis-diol-containing compounds such as catechols. We chose Varian Bond Elut PBA columns for solid-phase extraction; these columns are packed with phenylboronic acid covalently linked to a silica gel surface. The specificity of this sample preparation method is based on the difference in affinity for PBA between the catecholamines and potentially interfering compounds present in the sample matrix.
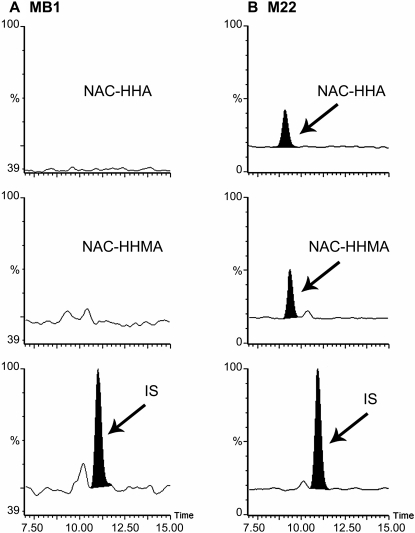
LC-MS/MS Analysis. The analytes were separated on an Atlantis T3 column that produces good separation of small polar compounds with good retention and selectivity. Quantitation by ESI-MS was performed in multiple reaction monitoring (MRM) mode to enhance sensitivity and precision; three fragment ions of high abundance were used for the detection of each compound. The full-scan MS/MS spectra as well as the proposed fragmentation patterns for N-Ac-5-Cys-HHMA, N-Ac-5-Cys-HHA, and N-Ac-5-Cys-O-Me-HHMA are shown in Fig. 2. A representative MRM chromatogram of the second lowest calibrator sample (7 ng/ml N-Ac-5-Cys-HHMA and N-Ac-5-Cys-HHA and 5 ng/ml IS) is shown in Fig. 3. Quantitation was performed by comparison of peak area ratio (analyte versus IS) with calibration curves obtained with spiked calibrators. We used the methyl ester analog of N-Ac-5-Cys-HHMA (N-Ac-5-Cys-O-Me-HHMA) as the IS for both the HHMA and HHA adducts because these two compounds are structurally very similar and adding the methyl ester analog of N-Ac-5-Cys-HHA as an IS would only add more complexity to the separation of these structurally similar compounds, without improving quantitation.
Fig. 2.
Full-scan MS/MS spectra and the proposed patterns of fragmentation for N-Ac-5-Cys-HHMA (A), N-Ac-5-Cys-O-Me-HHMA (B) (IS), and N-Ac-5-Cys-HHA (C). Fragment ions used for detection and quantification of each compound are highlighted in gray.
Fig. 3.
LC-MS/MS chromatograms of blank human urine (A) and human urine spiked with N-Ac-5-Cys-HHMA and N-Ac-5-Cys-HHA (B), 7 ng/ml each, and 5 ng/ml of IS.
Method Validation. Standard curve plots for the analytes were linear in the range of tested concentrations (4–30 ng/ml). Intra- and interassay accuracy and precision results satisfactorily met current acceptance criteria in the validation of bioanalytical methods (see Supplemental Data). Analytical recoveries (80.5% for N-Ac-5-Cys-HMA and 90.5% for N-Ac-5-Cys-HHMA) and calculated limits of detection and quantification (1.5 and 4.3 ng/ml N-Ac-5-Cys-HHA and 4.3 and 12.9 ng/ml N-Ac-5-Cys-HHMA) were considered adequate for the purpose of the study. The freeze/thaw stability test showed that neither N-Ac-5-Cys-HHMA nor N-Ac-5-Cys-HHA was stable in urine during freeze/thaw cycles (data not shown).
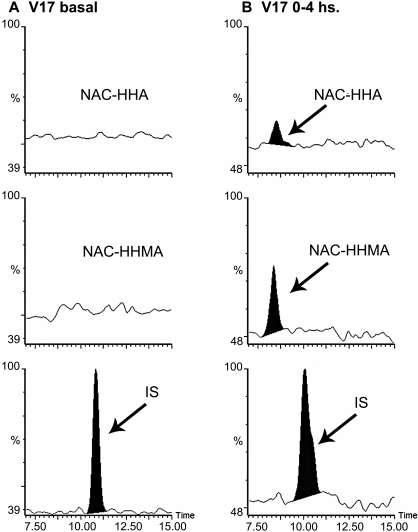
Analysis of N-Ac-5-Cys-HHMA and N-Ac-5-Cys-HHA in Human Urine After MDMA Consumption. The optimized LC-MS/MS analysis was applied to urine samples from healthy recreational users of MDMA, who were given a single 1.5 mg/kg oral dose of MDMA. Urine samples were collected before and after drug administration at the 0 and 0 to 4 h time period. Figure 4 shows MRM chromatograms of urine from a volunteer before (t = 0) and 0 to 4 h after MDMA intake. Estimated adduct concentrations for this volunteer were 2.1 and 7.2 ng/ml for N-Ac-5-Cys-HHA and N-Ac-5-Cys-HHMA, respectively.
Fig. 4.
LC-MS/MS chromatograms of urine samples from a volunteer before MDMA intake (t = 0) (A), and a 0 to 4 h sample from a volunteer ingesting 80 mg of (R,S)-MDMA (B).
MDMA, MDA, HMMA, and HMA were also determined in urine. Tables 1 and 2 and Fig. 5 summarize urinary excretion of MDMA and its metabolites, including the thioether adducts of HHMA and HHA.
TABLE 1.
Urinary excretion of MDMA and its metabolites (males)
|
Volunteer Code
|
CYP2D6 Genotype
|
MDMA
|
Dose
|
Excreted 0 to 4 h (Males)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MDMA | MDA | HMMA | HMA | N-Ac-5-Cys-HHMA | N-Ac-5-Cys-HHA | Total | ||||
| mg | mg/kg | μmol | ||||||||
| 11 | *1/*4 | 90 | 1.4 | 33.7 | 0.94 | 9.9 | 1.13 | 0.0016 | 0.0002 | 45.64 |
| 12 | *1/*10 | 80 | 1.5 | 12.8 | 0.65 | 12.3 | 1.11 | 0.0041 | ND | 26.88 |
| 13 | *1/*2 | 100 | 1.4 | 9.4 | 0.75 | 26.3 | 1.50 | 0.0062 | ND | 37.88 |
| 14 | *1/*9 | 100 | 1.1 | 4.5 | 0.37 | 6.2 | 0.77 | 0.0163 | 0.0040 | 11.84 |
| 15 | *2/*41 | 90 | 1.5 | 9.7 | 0.76 | 7.1 | 1.44 | ND | 0.0012 | 19.08 |
| 16 | *1/*2 | 100 | 1.4 | 3.3 | 0.58 | 19.9 | 1.30 | 0.0102 | 0.0025 | 25.16 |
| 18 | *9/*10 | 100 | 1.2 | 2.7 | 0.30 | 2.9 | 0.52 | 0.0022 | 0.0017 | 6.43 |
| 20 | *2/*4 | 100 | 1.4 | 25.1 | 0.67 | 11.5 | 0.51 | 0.0010 | 0.0004 | 37.85 |
| 21 | *1/*2 | 100 | 1.5 | 10.9 | 0.50 | 16.5 | 0.95 | 0.0160 | 0.0036 | 28.85 |
| 22 | *1/*2 | 100 | 1.5 | 45.2 | 2.25 | 9.8 | 1.70 | 0.0012 | 0.0028 | 58.91 |
| 23 | *1/*2 | 80 | 1.4 | 68.3 | 3.04 | 16.8 | 3.52 | 0.0031 | 0.0055 | 91.67 |
| Mean | 20.5 | 0.98 | 12.7 | 1.31 | 0.0062 | 0.0024 | 35.47 | |||
| S.D. | 6.3 | 0.25 | 2.0 | 0.24 | 0.0018 | 0.0005 | 7.21 | |||
| Recovery as % of dose | 4.0 | 0.19 | 2.5 | 0.25 | 0.0012 | 0.0005 | 6.94 | |||
ND, not detected.
TABLE 2.
Urinary excretion of MDMA and its metabolites (females)
|
Volunteer Code
|
CYP2D6 Genotype
|
MDMA
|
Dose
|
Excreted 0-4 h (Females)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MDMA | MDA | HMMA | HMA | N-Ac-5-Cys-HHMA | N-Ac-5-Cys-HHA | Total | ||||
| mg | mg/kg | μmol | ||||||||
| 17 | *2/*10 | 80 | 1.3 | 46.4 | 1.64 | 16.34 | 0.9 | 0.0042 | 0.0013 | 65.36 |
| 19 | *1/*2 | 75 | 1.5 | 19.7 | 0.96 | 32.93 | 1.8 | 0.0088 | 0.0018 | 55.41 |
| 26 | *1/*10 | 75 | 1.5 | 15.5 | 1.35 | 38.92 | 2.2 | 0.0049 | 0.0015 | 58.03 |
| 27 | *1/*35 | 75 | 1.4 | 7.9 | 0.49 | 16.86 | 1.1 | 0.0091 | 0.0019 | 26.39 |
| Mean | 22.4 | 1.11 | 26.26 | 1.5 | 0.0070 | 0.0020 | 51.30 | |||
| S.D. | 8.4 | 0.25 | 5.71 | 0.3 | 0.0013 | 0.0001 | 8.57 | |||
| Recovery as % of dose | 4.3 | 0.21 | 5.07 | 0.3 | 0.0013 | 0.0003 | 9.88 | |||
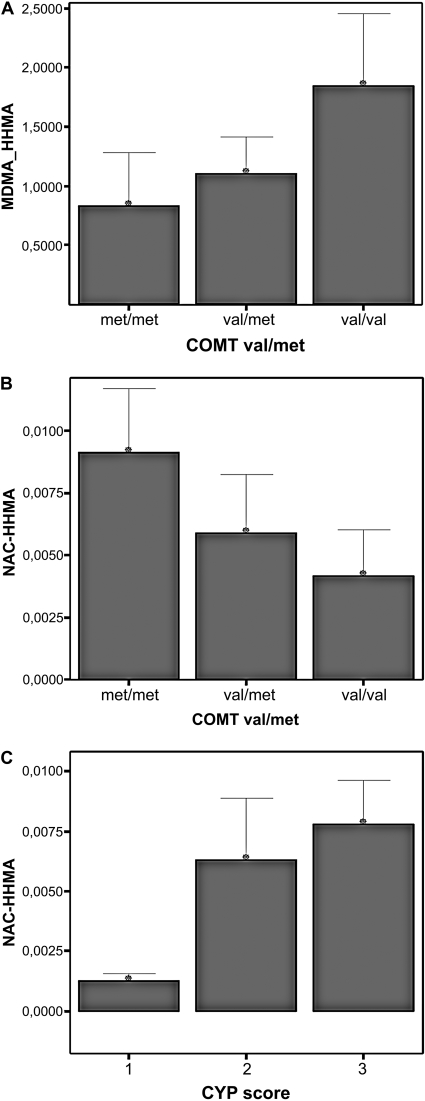
Fig. 5.
The relationship of COMT genotypes with the ratio MDMA vs. HMMA (A) and of COMT genotypes (B) and CYP2D6 scores (C) with urinary N-Ac-5-Cys-HHMA excretion. Cytochrome P450 (CYP) score 1, subjects heterozygous for a wild-type allele (*1 or *2) and a nonfunctional allele (*4); CYP score 2, subjects bearing alleles partially functional (*9, *10, or *41); CYP score 3, homozygous wild type (*1, *2, or *35). Bars show means. Error bars show mean ± 1.0 S.E.
There is a significant correlation between N-Ac-5-Cys-HHMA recovered in urine and the composite parameters MDMA-HMMA (ratio of urinary recoveries, 0–4 h, r =–0727, p < 0.003, n = 14) (Fig. 5A) and MDA-HMA (ratio of urinary recoveries, 0–4 h, r =–0569, p < 0.034, n = 14) but not with MDMA or HMMA taken alone. The recovery of N-Ac-5-Cys-HHMA was related marginally to the CYP2D6 score (p < 0.1) (Fig. 5C) and to the COMT genotype (p < 0.1) (Fig. 5B) of subjects. The recovery of N-Ac-5-Cys-HHMA was 2-fold higher among met/met subjects compared with the value for the val/val subjects (0.091 ± 0.005 versus 0.041 ± 0.003 μmol/4 h, n = 4 for each genotype). A similar trend was observed for MDMA-HMMA and COMT genotype.
Discussion
We have, for the first time in humans, identified and quantified catechol-thioether metabolites of MDMA (N-Ac-5-Cys-HHMA and N-Ac-5-Cys-HHA). The identification of MDMA mercapturates lends credence to the hypothesis that the metabolic bioactivation of MDMA has the potential to contribute to MDMA neurotoxicity in humans. Preliminary data on polymorphisms of genes involved in the metabolism of MDMA reveal that specific genotypic profiles may constitute risk factors for the development of neurotoxicity.
The fraction of the MDMA dose recovered as thioether adducts excreted in urine 4 h after MDMA ingestion is ∼0.002%. Approximately 1.6% of a dose of MDA (23 μmol s.c.) is excreted in bile as 5-(glutathion-S-yl)-α-methyldopamine within 5 h. This translates to ∼50% of the dose of MDA that causes both neurobehavioral and neurochemical changes (Miller et al., 1996). Moreover, because polyphenolic-GSH conjugates and the corresponding cysteine and NAC conjugates are biologically reactive, quantitation of their biliary and urinary excretion probably represents a minimum estimate of in vivo formation. Indeed, after administration of 2-hydroxy-1-(glutathion-S-yl)-17β-estradiol to rats, only 15% of the dose was recovered in urine and 5% in feces, several days after administration (Elce, 1972), and up to 96% of an infused dose of polyphenolic-GSH conjugates is retained in the animal in the in situ perfused rat kidney model (Hill et al., 1994; Rivera et al., 1994). These findings emphasize that quantitation of N-Ac-5-Cys-HHMA and N-Ac-5-Cys-HHA in urine probably underestimates the contribution of this metabolic pathway to MDMA disposition. It is also relevant to note that these metabolites are extremely potent serotonergic toxicants (Miller et al., 1997; Bai et al., 1999; Jones et al., 2005). As little as 7 nmol of N-Ac-5-Cys-HHMA is sufficient to produce decreases in striatal and cortical 5-HT concentrations when injected directly into the brain (Jones et al., 2005). Finally, multiple doses of MDMA result in the accumulation of N-Ac-5-Cys-HHMA and N-Ac-5-Cys-HHA in rat brain (Erives et al., 2008). Thus, the fraction of MDMA excreted in urine needs to be considered in the context of the fraction of MDMA converted to N-Ac-5-Cys-HHMA and N-Ac-5-Cys-HHA in vivo, in combination with the neurotoxic potency of these metabolites.
The 0 to 4 h time period for the collection of urine samples was selected on the premise that CYP2D6 would remain active during this time period and before complete inactivation. The low urinary recovery during the first 4 h after ingestion could also be explained by the accumulation of these metabolites in the brain, as shown in rats (Erives et al., 2008), and by the fact that the kinetics of mercapturate formation and excretion is unknown, with larger recoveries possible over extended urine collection periods.
The fraction of the catechol metabolites converted to neurotoxic metabolites varies greatly between individuals exposed to similar doses of MDMA (Tables 1 and 2). This finding is somewhat expected, because the enzymes that participate in the formation (CYP2D6) and inactivation (COMT) of HHMA and HMA are highly polymorphic in the human population (de la Torre and Farré, 2004). There is a significant correlation between N-Ac-5-Cys-HHMA recovered in urine and the ratio of MDMA/HMMA but not with MDMA or HHMA taken alone. Again, this is not unexpected because the formation of the N-Ac-5-Cys adduct depends on the availability of the intermediary catechol metabolite, which in turn is to some extent a balance between its generation via O-demethylenation of MDMA (CYP2D6) and its removal via COMT-mediated O-methylation to HMMA. In concordance with this view, the recovery of N-Ac-5-Cys-HHMA is also moderately correlated to the CYP2D6 score and the COMT genotype of subjects (p < 0.1). All of the subjects exhibited the same CYP2D6 phenotype (extensive metabolizers). The extensive metabolizer phenotype is explained by the combination of several allelic variants displaying different degrees of functionality (Tables 1 and 2; Fig. 5C). In the present study the relevance of the CYP2D6 genetic polymorphism on N-Ac-5-Cys adduct formation can only be partially delineated on the basis of different activity rates within each particular genotype. It would be necessary to include more extreme phenotypes (poor metabolizers and ultra-rapid metabolizers) to fully assess the contribution of the CYP2D6 polymorphism to N-Ac-5-Cys adduct formation. However, the effect of genetic polymorphisms in CYP2D6 on thioether catechol adduct formation and subsequent neurotoxicity is likely to be moderate and possibly only relevant during the first hours after MDMA ingestion because the majority of hepatic CYP2D6 is inactivated within 1 h after a recreational dose of MDMA (Yang et al., 2006; O'Mathúna et al., 2008) and because when CYP2D6 is inhibited before MDMA intake with paroxetine, a potent mechanism-based inactivator of this enzyme (Bertelsen et al., 2003), only a 30% increase of the area under the curve of MDMA in plasma is observed (Segura et al., 2005). Prolonging the urine collection after MDMA intake would assist in assessing the correlation (or lack thereof) between CYP2D6 polymorphism and N-Ac-5-Cys adduct formation.
Considering that the pattern of MDMA consumption often involves repeated doses and that CYP2D6 MDMA-induced inactivation gives rise to a phenomenon of phenocopying, which converts subjects toward a poorer metabolizer phenotype, independent of their original genotype (O'Mathúna et al., 2008), we would expect genetic polymorphisms and activity of COMT to be a more important determinant of NAC adduct formation and of susceptibility to MDMA-mediated neurotoxicity. Consistent with this view, administration of the COMT inhibitor entacapone 30 min before MDMA dosing to rats exacerbates the 5-HT depletion produced by MDMA, an effect not related either to changes on core body temperature, because the MDMA-induced hyperthermic response was not significantly altered, or to the serum l-tyrosine levels, which where higher in the MDMA-treated rats than inn the MDMA/entacapone-treated rats, suggesting that toxic MDMA catechol metabolites are responsible for the MDMA toxicity in the entacapone-treated rats (Goñi-Allo et al., 2008a). Moreover, although the HMMA and HMA plasma concentrations were significantly lower in the entacapone-treated animals, plasma concentrations of HHMA and HHA were unchanged, suggesting that these compounds are cleared by alternative pathways (Goñi-Allo et al., 2008b), for example, thioether adduct formation. Taken together, these results support the idea that the COMT activity level may be relevant in terms of susceptibility to MDMA neurotoxicity.
The observed gender differences in the urinary excretion of MDMA and its metabolites can be partly explained by the more rapid renal process in males, including clearance of drugs metabolized by CYP2D6 (Schwartz, 2003). More studies with female subjects are needed to draw any further conclusions on gender differences in MDMA metabolism. The urinary excretion of the catechols HHMA and HHA needs to be estimated to determine whether there are gender differences in the fraction of these metabolites that are converted to thioether adducts.
In summary, we report the first published data on the urinary excretion of catechol-thioether metabolites of MDMA. The neurotoxicity of these metabolites is well established in rats. Because the metabolic pathways of MDMA in humans are similar to those in rats, with differences only in the relative kinetics of metabolism (de la Torre and Farré, 2004), it is likely that these metabolites are also present in human brain with the potential to achieve neurotoxic concentrations. The dose administrated in this study (1.5 mg/kg, 75–100 mg) represents a typical recreational single dose (Parrott, 2002). Although it is not well established whether a single recreational dose is likely to produce long-term serotonergic deficits in humans, it can be expected that multiple or regular use of MDMA in humans (the typical pattern of MDMA consumption) may lead to long-term serotonergic damage similar to that seen in animal studies. Thus, catechol-thioether metabolites of MDMA may contribute to MDMA neurotoxicity in humans. Factors that influence interindividual differences in the formation of these adducts will be major determinants of the susceptibility of humans to MDMA neurotoxicity. In this respect, although much attention has been focused on the CYP2D6 polymorphism with respect to the generation of thioether adducts and neurotoxicity, polymorphisms in COMT seem to be more relevant. A more detailed examination of the influence of COMT polymorphism on NAC adduct formation is warranted. An assessment of thioether adducts in human plasma would assist in understanding their pharmacokinetics, which would also be of relevance to the possible prediction of a neurotoxic response, especially in view of the fact that a critical threshold concentration of neurotoxic metabolites seems to be necessary to produce a permanent neurotoxic effect (Jones et al., 2005). The extent of metabolic bioactivation, modulated by environmental and genetic factors, may be a major contributing factor to susceptibility to MDMA neurotoxicity.
Supplementary Material
Acknowledgments
We thank George Tsaprailis and Yelena Feinstein (Southwest Environmental Health Sciences Center Proteomics Facility Core) for the HPLC-MS and HPLC-MS/MS analyses.
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse [Grant 1R0-1DA017987-10A2]; a postdoctoral grant by the Secretaria de Estado de Universidades e Investigación del Ministerio de Educación y Ciencia, Spain (to N.P.); a de Gestió d'Ajuts Universitaris i de Recerca predoctoral fellowship Generalitat de Catalunya, Spain (to X.P.); Ministerio de Educación y Ciencia (Spain) [Grant SAF2005-0189]; and Generalitat de Catalunya (Spain) [Grant 2005SGR00032]. We acknowledge assistance from the National Institute of Environmental Health Sciences-supported Southwest Environmental Health Sciences Center [Grant P30-ES06694] at the University of Arizona.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.026393.
ABBREVIATIONS: MDMA, 3,4-methylenedioxymethamphetamine; 5-HT, serotonin, 5-hydroxytryptamine; MDA, 3,4-methylenedioxyamphetamine; HHMA, 3,4-dihydroxymethamphetamine or N-methyl-α-methyldopamine; HHA, 3,4-dihydroxyamphetamine or α-methyldopamine; GSH, glutathione; NAC, N-acetylcysteine; HMA, 4-hydroxy-3-methoxy-amphetamine; COMT, catechol-O-methyltransferase; HPLC, high-performance liquid chromatography; DTT, dithiothreitol; PBA, phenylboronic acid; N-Ac-5-Cys-HHMA, 5-(N-acetylcystein-S-yl)-3,4-dihydroxymethamphetamine; N-Ac-5-Cys-HHA, 5-(N-acetylcystein-S-yl)-3,4-dihydroxyamphetamine; N-Ac-5-Cys-O-Me-HHMA, 5-(N-acetylcystein-S-yl)-methylesther-3,4-dihydroxymethamphetamine; IS, internal standard; MS/MS, tandem mass spectrometry; QC, quality control; TFA, trifluoroacetic acid; LC, liquid chromatography; MS, mass spectrometry; MRM, multiple reaction monitoring; HMMA, 4-hydroxy-3-methoxymethamphetamine.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
References
- Aguirre N, Galbete JL, Lasheras B, and Del Río J (1995) Methylendioxymethamphetamine induces opposite changes in central pre- and postsynaptic 5-HT1A receptors in rats. Eur J Pharmacol 281 101–105. [DOI] [PubMed] [Google Scholar]
- Bai F, Lau SS, and Monks TJ (1999) Glutathione and N-acetylcysteine conjugates of α-methyldopamine produce serotonergic neurotoxicity: possible role in methylenedioxyamphetamine-mediated neurotoxicity. Chem Res Toxicol 12 1150–1157. [DOI] [PubMed] [Google Scholar]
- Bai F, Jones DC, Lau SS, and Monks TJ (2001) Serotonergic neurotoxicity of 3,4-(±)methylenedioxyamphetamine and 3,4-(±)-methylendioxymethamphetamine (Ecstasy) is potentiated by inhibition of γ-glutamyl transpeptidase. Chem Res Toxicol 14 863–870. [DOI] [PubMed] [Google Scholar]
- Barrionuevo M, Aguirre N, Del Río JD, and Lasheras B (2000) Serotonergic deficits and impaired passive-avoidance learning in rats by MDEA: a comparison with MDMA. Pharmacol Biochem Behav 65 233–240. [DOI] [PubMed] [Google Scholar]
- Bertelsen KM, Venkatakrishnan K, Von Moltke LL, Obach RS, and Greenblatt DJ (2003) Apparent mechanism-based inhibition of human CYP2D6 in vitro by paroxetine: comparison with fluoxetine and quinidine. Drug Metab Dispos 31 289–293. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, and Lyles J (2007) Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res 11 183–202. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, et al. (2004) Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in post-mortem human brain. Am J Hum Genet 75 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Roset PN, Pizarro N, Abanades S, Segura M, Segura J, and Camí J (2004) Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit 26 137–144. [DOI] [PubMed] [Google Scholar]
- de la Torre R and Farré M (2004) Neurotoxicity of MDMA (Ecstasy): the limitations of scaling from animals to humans. Trends Pharmacol Sci 25 505–508. [DOI] [PubMed] [Google Scholar]
- Elce JS (1972) Metabolism of a glutathione conjugate of 2-hydroxyoestradiol-17 in the adult male rat. Biochem J 126 1067–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erives GV, Lau SS, and Monks TJ (2008) Accumulation of neurotoxic thioether metabolites of 3,4-(±)-methylenedioxymethamphetamine in rat brain. J Pharmacol Exp Ther 324 284–291. [DOI] [PubMed] [Google Scholar]
- Goñi-Allo B, Puerta E, Mathúna BO, Hervias I, Lasheras B, de la Torre R, and Aguirre N (2008a) On the role of tyrosine and peripheral metabolism in the 3,4-methyelendioxymethamphetamine-induced serotonin neurotoxicity in rats. Neuropharmacology 54 885–900. [DOI] [PubMed] [Google Scholar]
- Goñi-Allo B, O Mathúna B, Segura M, Puerta E, Lasheras B, de la Torre R, and Aguirre N (2008b) The relationship between core body temperature and 3,4-methyelendioxymethamphetamine metabolism in rats: implications for neurotoxicity. Psychopharmacology 197 263–278. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, and Colado MI (2003) The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”). Pharmacol Rev 55 463–508. [DOI] [PubMed] [Google Scholar]
- Hill BA, Davison KL, Dulik DM, Monks TJ, and Lau SS (1994) Metabolism of 2-(glutathion-S-yl)hydroquinone and 2,3,5-(triglutathion-S-yl)hydroquinone in the in situ perfused rat kidney: relationship to nephrotoxicity. Toxicol Appl Pharmacol 129 121–132. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Kumagai Y, Unger SE, and Cho AK (1990) Metabolism of methylenedioxymethamphetamine: formation of dihydroxymethamphetamine and a quinone identified as its glutathione adduct. J Pharmacol Exp Ther 254 521–527. [PubMed] [Google Scholar]
- Jones DC, Duvauchelle C, Ikegami A, Olsen CM, Lau SS, de la Torre R, and Monks TJ (2005) Serotonergic neurotoxic metabolites of ecstasy identified in rat brain. J Pharmacol Exp Ther 313 422–431. [DOI] [PubMed] [Google Scholar]
- Jones DC, Lau SS, and Monks TJ (2004) Thioether metabolites of 3,4-(±)-methylenedioxyamphetamine and 3,4-(±)-methylenedioxymethamphetamine inhibit hSERT function and simultaneously stimulate dopamine uptake into hSERT expressing SK-N-MC cells. J Pharmacol Exp Ther 311 298–306. [DOI] [PubMed] [Google Scholar]
- Maurer HH, Bickeboeller-Friedrich J, Kraemer T, and Peters FT (2000) Toxicokinetics and analytical toxicology of amphetamine-derived designer drugs (`Ecstasy'). Toxicol Lett 112–113 133–142. [DOI] [PubMed] [Google Scholar]
- Miller RT, Lau SS, and Monks TJ (1996) Effects of intra cerebroventricular administration of 5-(glutathion-S-yl)-α-methyldopamine on brain dopamine, serotonin, and norepinephrine concentrations in male Sprague-Dawley rats. Chem Res Toxicol 9 457–465. [DOI] [PubMed] [Google Scholar]
- Miller RT, Lau SS, and Monks TJ (1997) 2,5-Bis-(glutathion-S-yl)-α-methyldopamine, a putative metabolite of (±)-3,4-methylenedioxyamphetamine, decreases brain serotonin concentrations. Eur J Pharmacol 323 173–180. [DOI] [PubMed] [Google Scholar]
- Monks TJ, Jones DC, Bai F, and Lau SS (2004) The role of metabolism in 3,4-(±)-methylenedioxyamphetamine and 3,4-(±)-methylenedioxymethamphetamine (Ecstasy) toxicity. Ther Drug Monit 26 132–136. [DOI] [PubMed] [Google Scholar]
- Nichols DE (1986) Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens: identification of a new therapeutic class: entactogens. J Psychoactive Drugs 18 305–313. [DOI] [PubMed] [Google Scholar]
- O'Mathúna B, Farré M, Rostami-Hodjegan A, Yang J, Cuyàs E, Torrens M, Pardo R, Abanades S, Maluf S, Tucker GT, et al. (2008) The consequences of 3,4-methylenedioxymethamphetamine induced CYP2D6 inhibition in humans. J Clin Psychopharmacol 28 523–529. [DOI] [PubMed] [Google Scholar]
- Parrott AC (2002) Recreational Ecstasy/MDMA, the serotonin syndrome and serotonergic neurotoxicity. Pharmacol Biochem Behav 71 837–844. [DOI] [PubMed] [Google Scholar]
- Pizarro N, Ortuño J, Farré M, Hernández-López C, Pujadas M, Llebaria A, Joglar J, Roset PN, Mas M, Segura J, et al. (2002) Determination of MDMA and its metabolites in blood and urine by gas chromatography-mass spectrometry and analysis of enantiomers by capillary electrophoresis. J Anal Toxicol 26 157–165. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Yuan J, and McCann UD (2000) (±)3,4-Methylenedioxymethamphetamine (“Ecstasy”)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology 42 5–10. [DOI] [PubMed] [Google Scholar]
- Rivera MI, Hinojosa LM, Hill BA, Lau SS, and Monks TJ (1994) Metabolism and toxicity of 2-bromo-(diglutathion-S-yl)-hydroquinone and 2-bromo-3-(glutathion-S-yl)hydroquinone in the in situ perfused rat kidney. Drug Metab Dispos 22 503–510. [PubMed] [Google Scholar]
- Schmid B, Bircher J, Preisig R, and Küpfer A (1985) Polymorphic dextromethorphan metabolism: co-segregation of oxidative O-demethylation with debrisoquin hydroxylation. Clin Pharmacol Ther 38 618–624. [DOI] [PubMed] [Google Scholar]
- Schwartz JB (2003) The influence of sex on pharmacokinetics. Clin Pharmacokinet 42 107–121. [DOI] [PubMed] [Google Scholar]
- Segura M, Farré M, Pichini S, Peiró AM, Roset PN, Ramírez A, Ortuño J, Pacifici R, Zuccaro P, Segura J, et al. (2005) Contribution of cytochrome P450 2D6 to 3,4-methylenedioxymethamphetamine disposition in humans: use of paroxetine as a metabolic inhibitor probe. Clin Pharmacokinet 44 649–660. [DOI] [PubMed] [Google Scholar]
- Stone DM, Johnson M, Hanson GR, and Gibb JW (1988) Role of endogenous dopamine in the central serotonergic deficits induces by methylenedioxymethamphetamine. J Pharmacol Exp Ther 247 79–87. [PubMed] [Google Scholar]
- Yang J, Jamei M, Heydari A, Yeo KR, de la Torre R, Farré M, Tucker GT, and Rostami-Hodjegan A (2006) Implications of mechanism-based inhibition of CYP2D6 for the pharmacokinetics and toxicity of MDMA. J Psychopharmacol 20 842–849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.