Abstract
Studying the effects of saturated and unsaturated fatty acids on biological and model (liposomes) membranes could provide insight into the contribution of biophysical effects on the cytotoxicity observed with saturated fatty acids. In vitro experiments suggest that unsaturated fatty acids, such as oleate and linoleate, are less toxic, and have less of an impact on the membrane fluidity. To understand and assess the biophysical changes in the presence of the different fatty acids, we performed computational analyses of model liposomes with palmitate, oleate, and linoleate. The computational results indicate that the unsaturated fatty acid chain serves as a membrane stabilizer by preventing changes to the membrane fluidity. Based on a Voronoi tessellation analysis, unsaturated fatty acids have structural properties that can reduce the lipid ordering within the model membranes. In addition, hydrogen bond analysis indicates a more uniform level of membrane hydration in the presence of oleate and linoleate as compared to palmitate. Altogether, these observations from the computational studies provide a possible mechanism by which unsaturated fatty acids minimize biophysical changes and protect the cellular membrane and structure. To corroborate our findings, we also performed a liposomal leakage study to assess how the different fatty acids alter the membrane integrity of liposomes. This showed that palmitate, a saturated fatty acid, caused greater destabilization of liposomes (more “leaky”) than oleate, an unsaturated fatty acid.
Keywords: saturated, unsaturated, fatty acid, membrane fluidity, cytotoxicity, liposomes
1. Introduction
Free fatty acids (FFAs), derived from dietary triglycerides (TGs) and phospholipids, are aliphatic monocarboxylic acids, which are among the most important energy sources for cells and tissues [1, 2]. Typically containing a lipid chain between 4 to 28 carbons, FFAs are classified according to the degree of unsaturation: saturated, monounsaturated, and polyunsaturated [3, 4, 5, 6]. The FFA concentration in the plasma is regulated by plasma protein albumin, that leaves about 10 nmol/L of FFA unbounded, which is believe to induce most of the toxic effects [7, 8]. As shown by Fig. 1, which represents our current results, and other recent in vitro studies, saturated FFAs significantly induce toxic effects on various cells types, however, unsaturated FFAs are shown to reduce and/or prevent toxic effects [9, 10, 11, 12, 13, 14]. This effect of saturated FFAs (i.e., palmitate) is dose dependent, inducing significant cytotoxicity and cell death at much lower FFA concentrations of 0.4 mM [15].
Figure 1.
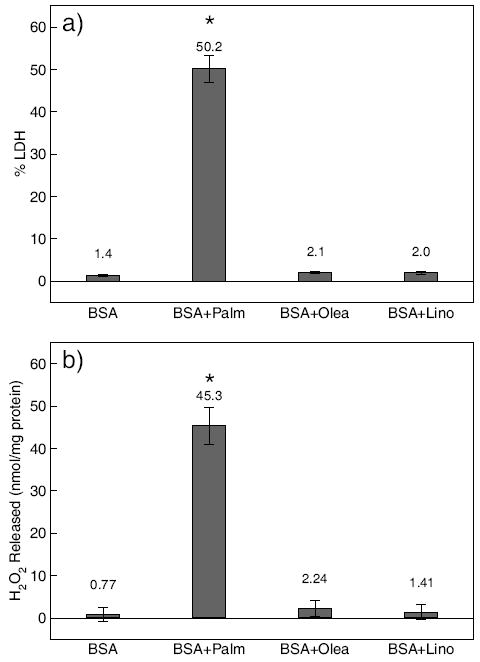
a) HepG2 cells cytotoxicity in response to FFAs. Confluent HepG2 cells in BSA medium were exposed to 0.7 mM palmitate (Palm), oleate (Olea), or linoleate (Lino). LDH released was measured after 48 hrs. b) Effects of FFAs on H2O2 release. Confluent HepG2 cells in BSA medium were treated with 0.7 mM palmitate (Palm), oleate (Olea), or linoleate (Lino) for 48 hrs. The H2O2 released into the medium was measured and normalized to total cellular protein. Error bars are standard deviation of three independent experiments. “*” indicates statistical difference from control, respectively (p < 0.05).
Until recently, studies on the mechanism of FFA-induced cell death focused on the production of potential toxic intermediates, such as, reactive oxygen species (ROS), ceramides [16], reduced mitochondrial potential [17] and mitochondrial Bcl-2/Bax ratio [11]. Given the hydrophobic nature of saturated FFAs, the induced toxicity may be attributed to their hydrophobic effects on the cellular membrane, since the cytotoxicity was not completely prevented upon treatment with mitochondrial complex inhibitors or free radical scavengers [12]. In our previous study, we found that saturated fatty acids (FA), such as palmitate, embedded within the phospholipid bilayers significantly decreased membrane fluidity [13]. These observations were confirmed by molecular dynamics (MD) simulations [13]. Currently, there is very limited knowledge on whether biophysical effects are involved in preventing or reducing the toxicity of unsaturated FFAs on cells.
Numerous studies examined the effects of unsaturated FAs on liposomes, often used as model membranes. For example, Lee et al. exposed liposomes containing different amounts of oleic, linoleic, and arachidonic acid to oxidizing medium and found that the fragments of FA peroxidation significantly increased the amount of monocyte chemotaxis and monocyte adhesion, suggesting that the oxidation products of linoleic and arachidonic acids can trigger cellular immune response [18]. Furthermore, Watabe et al. examined the decomposition rate of unsaturated FAs in DPPC liposomes, containing photoporphyrin IX (PpIX), from light irradiations and determined the oxidation rate, from fast to slow: arachidonic acid < oleic acid < α-linoleic acid < linoleic acid [19]. Although oleic acid contains fewer double-bonds than linoleic derivatives, it has a greater oxidation rate because the location of its double-bond is in close proximity to the PpIX molecules embedded within the bilayer. In summary, these experimental studies were based on the resulting products of lipid oxidation, and have not addressed the direct interactions between the lipid constituents and the FAs themselves in this process.
Recently, Wong-Ekkabut et al. investigated the structural properties of 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphatidylcholine (PLPC) bilayers in the presence of varying concentrations of oxidized phospholipids and fatty acid derivatives [20]. The fatty acids considered were 9-trans, cis-hydroperoxide linoleic acid, 13-trans, cis-hydroperoxide linoleic acid, 9-oxo-nonanoic acid, and 12-oxo-9-dodecenoic acid. Their results showed an inverse correlation between the degree of oxidation and membrane thickness. By increasing the fatty acid content in the bilayers, they found an increase in the bilayer surface area while the bilayer thickness was significantly reduced. Their permeation analysis showed that water molecules were able to penetrate more easily through the bilayer, thus destabilizing the bilayer structure. They attributed the toxicity effect of the oxidized phospholipids to an increase in the membrane permeability.
In our previous studies, we found that trehalose, a non-reducing disaccharide, widely used as a stabilizer and preservant, had a protective role in palmitate-induced toxicity [13]. Unlike trehalose, which is impermeable to membranes [21], both saturated and unsaturated FFAs can be transported through the membrane into cells through passive and active transport. Once in the membrane, FAs can modify the membrane properties by altering the membrane fluidity and affecting cellular function [22, 23]. Based on this fact, we proposed that, unlike saturated FAs, the presence of unsaturated FAs stabilizes the membrane and maintain its fluidity.
A seleted number of studies using molecular dynamics (MD) simulations have been reported investigating, separately, the biological functions of fatty acid molecules and their interactions with phospholipid bilayers [24, 25, 20]. Of closest relevance is the study by Hyvönen et al., which considered the membrane phospholipid component of lyso-PC molecules (a PC headgroup with glycerol backbone and palmitic acid chain) and linoleate/linoleic acid and find that the bilayers became unstable, as a result of penetration of the water into the core region of the bilayer [24].
To compare the effect of saturated and unsaturated FAs, we conducted cell membrane characterization experiments on HepG2 cells and liposomes exposed to palmitate, oleate, and linoleate. MD simulations were used to confirm the experimental results and determine the role of palmitate, oleate, and linoleate on model DOPC bilayers. Although the interactions between phospholipid liposome and FAs have been extensively studied in the past [26, 27], the phase transition and leakage studies of DOPC liposomes containing saturated and unsaturated FAs were necessary to relate the experimental and bilayer computational results. These studies helped to interpret changes to the membrane fluidity and phase transition temperature caused by FAs embedded within the phospholipid bilayers, and to identify the role of unsaturated FAs in preventing these changes during the early stage of FAs adsorption. Insight into these mechanism will add to our understanding of the biophysical processes that are induced by FFAs that eventually may be linked to cytotoxicity.
2. Experimental Materials and Methods
2.1. Cell Culture
Human hepatocellular carcinoma cell line, HepG2 (American Type Culture Collection, Manassas, VA), was cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS, American Type Culture Collection) and 2% penicillin-streptomycin (Invitrogen). They were seeded in 6-well plates and incubated at 37 °C in humidified atmosphere containing 10% CO2. After the cells reached confluence, the media were replaced with 2 mL of control (4% fatty-acid free BSA - bovine serum albumin) or FFA (0.7 mM palmitate, oleate, or linoleate with 4% BSA) media and changed every 24 hrs. The BSA level used was close to physiological levels [28]. 0.7 mM FFAs was employed in this study because the plasma FFA levels in obese and type 2 diabetic patients have been reported to be approximately this level [29, 30, 31, 32]. Experiments were conducted after 48 hrs of treatment.
2.2. Cytotoxicity Assay and Membrane Fluidity
Experimental protocols for cytotoxicity assay and cell membrane characterization (membrane fluidity measurements) are described in detail in our previous study [13]. In short, cell viability was assessed by lactate dehydrogenase (LDH) leakage through the membrane into the medium and the changes in membrane fluidity were measured using two different stearic acid derivatives, 5-n-doxylstearic acid (5-n-SASL) and 16-n-doxylstearic acid (16-n-SASL) (Invitrogen). The 5-n-SASL probe monitors the portion of the membrane closest to the phospholipid headgroups, while the 16-n-SASL reflects changes in the middle/end of the phospholipid hydrocarbon chains.
2.3. Liposome Preparation and DSC Measurement
To correlate the fluidity measurements to the computational studies, a simpler model cell membrane was used. Liposomes (multi-lamellar vesicles or MLVs) made of DOPC (Avanti Polar Lipids, Alabaster, AL) and palmitic acid (palmitate), oleic acid (oleate), or linoleic acid (linoleate) (Sigma-Aldrich, St. Louis, MO) were prepared by the thin film method according to the protocol from Avanti Polar Lipids (see our previous publication for details [13]). Differential scanning calorimetry (DSC) analysis was performed on the liposome samples at a scan rate of 1°C/min to determine the phase transition temperature.
2.4. Liposomal Leakage Study
Large unilamellar vesicles loaded with Suforhodamine B (SRB, Invitrogen) were prepared following the procedure described in ref. [33]. 5 mg of DOPC was hydrated in 0.5 mL of buffer containing 10 mM Tris-HCl (pH 8.0), 50 μM EGTA and 50 mM SRB. After 3 hrs, the solution underwent five freeze/thaw cycles at -10/+35°C. The multilamellar liposomes were loaded in an 1 mL syringe and then pressed through a 0.1-μm polycarbone membrane, eleven times, and Avanti-microextruder (Avanti Polar Lipids, Inc). After the extrusions, the lipid solution was purified using a HiTrap™ desalting column (GE Healthcare) that was equilibrated with a buffer containing 10 mM Tris-HCl (pH 8.0), 50 μM EGTA, and 40 mM KCl to remove unloaded SRB molecules. After removing excess SRB molecules from the SRB-loaded LUV, the fluorescence intensity of the SRB-loaded LUV was monitored with a fluorescence microplate reader (excitation wavelength, 565 nm; emission wavelength, 586 nm) with and without a non-ionic detergent (NP-40, USB corporation). In the presence of detergent, only the SRB-loaded LUV fractions changed fluorescence due to dissociation of the SRB excimers. SRB-loaded LUV fraction was diluted at a ratio 1 to 25 in buffer (10 mM Tris-HCl (pH 8.0), 50 μM EGTA , and 40 mM KCl). In the presence of 1 mM CaCl2, palmitate or oleate was added to SRB-loaded LUV, and the fluorescence intensity was measured. In addition, 4% NP-40 was added to the same concentration of SRB-loaded LUV samples to determine the maximum fluorescence intensity of the total SRB molecules loaded in the liposomes.
3. Simulation Details
MD simulations were performed to investigate the role of unsaturated FAs (oleate and linoleate) in the structure and integrity of phospholipid bilayers in comparison to pure bilayer and bilayers containing saturated FAs (palmitate). The phospholipid bilayers used in this study were composed of DOPC with a total of 200 lipid molecules (100 per leaflet). This model bilayer was chosen for these studies because it gives an approximate representation of the phospholipid constituents in HepG2 cells [34], and matches the liposomal model used in this study. Several concentrations of palmitate, oleate, and linoleate were introduced into the phospholipid bilayer systems (see Table 1 for the actual compositions). The structure of molecules considered in this study are shown in Fig. S1 in the Supporting Information.
Table 1.
Compositions of fatty acids in the phospholipid bilayers. All bilayers contain 200 DOPC (100 per leaflet) at a hydration of 40 water per lipid. Each bilayer was constructed to have equal number of fatty acids per leaflet. Number of fatty acids per system: e.g., 10 palmitate in System 2, 10 oleate in System 3, etc. FA concentrations (mole%) are shown in the last column.
| System | Palmitate | Oleate | Linoleate | Concentration |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 |
| 2-4 | 10 | 10 | 10 | 5 |
| 5-7 | 22 | 22 | 22 | 11 |
| 8-10 | 36 | 36 | 36 | 18 |
| 11-13 | 50 | 50 | 50 | 25 |
To avoid aggregation of FFAs in the aqueous phase, the bilayer structures were initially constructed to have various concentrations of FAs embedded into the bilayer prior to equilibration (Systems 2-13 in Table 1). Simulations of these systems were used to address the overall changes in the membrane structure in the presence of FAs at the early stage of FA adsorption to the bilayer.
To investigate the effect of Ca2+ ions in the leakage study of liposomes containing FAs, another set of simulations were performed containing 5 and 10 Ca2+ ions with the corresponding number of Cl− ions to counterbalance the charges. All systems listed in Table 1 were performed with and without Ca2+. The ions were inserted in the aqueous phase by randomly selecting water molecules and converting them into Ca2+ or Cl−. The corresponding concentration of the ions were selected to represent the 1 mM CaCl2 solution used in the leakage experiment. Although extensive computational studies of the lipid bilayers in the presence of ions have been described elsewhere [35, 36, 37, 38, 39, 40, 41], our bilayer simulations aimed at investigating the localization of FAs in the vicinity of membrane-bounded Ca2+. As lengthy simulations are required for typical systems with ions, the simulations presented in this study are insufficient to observed the localization of FAs; however, attractive movement of the FAs toward the membrane-bounded Ca2+ may provide important evidence suggesting FAs aggregation within the membrane matrix.
The force-field for DOPC and water are consistent with those employed in previous studies, which includes intramolecular parameters for bonds, angles, proper dihedral, and improper dihedral [42, 43]. The Ryckaert-Bellemans potential was used for the torsion potential of the lipid hydrocarbon chains [44] with modification of lipid tail force-field for the double-bonds [45, 46]. Non-bonded interactions were described by the parameters from Berger et al. [47, 48, 49] and partial atomic charges were obtained from Chiu et al. [50]. All FAs were modeled in the protonated state and described using parameters derived from the lipid force-field. The carboxylic acid group was based on the parameters of glutamic acid, which were available from the Gromos force-field [51]. The single point charge (SPC) model was adopted for water [52]. Parameters from the OPLS-AA force-field were used for Ca2+ and Cl− ions [48]. The united-atom representation was used for the methyl/methylene groups in the acyl chains of DOPC, palmitate, oleate, and linoleate.
All simulations were performed in the constant pressure and constant temperature (NPT) ensemble, at a pressure of 1 bar and at a temperature of 310 K (see our previous study for more details on the parameters used in the simulations [13]). A time-step of 3 fs was used for all simulations with the total simulation times of 105 ns for bilayers without CaCl2 and 90 ns for bilayers with CaCl2. Coulombic and van der Waals cutoff interactions were at 1.0 nm. Long-range electrostatic interactions were corrected with the particle-mesh Ewald method (PME) [53, 54]. Periodic boundary conditions were applied in all directions. Trajectories were collected every 3 ps. All simulations were performed with the GROMACS 3.3.3 software package [55, 56, 57] (single-precision mode) in parallel.
4. Experimental Results
Saturated FAs are more cytotoxic, as determined by peroxide and LDH measurements, and alter the membrane fluidity, as measured by electron paramagnetic resonance (EPR) spectrometry, more significantly than unsaturated FAs. Cell membranes are very complex to model, thus to more directly compare the experimental results with the computational analyses, we performed phase transition and leakage studies of DOPC liposomes with FAs. Although liposomes are model bilayers representing a reduced number of constituents contained in cell membranes, results obtained from these studies can still provided some indication on potential biophysical effects that may be at play in cellular membranes.
4.1. Cytotoxicity and Peroxide Measurements
Previously we found that the level of cytotoxicity in HepG2 cells induced by saturated FFA (i.e., palmitate), as measured by the relative amount of LDH released, increased in a dose-dependent manner [14, 15]. Here we determined whether unsaturated FFAs (i.e., oleate and linoleate) would induce significant levels of cytotoxicity, even at the highest concentration of palmitate that was used previously [14, 15]. LDH release provides some measure of the membrane integrity. Control consisted of HepG2 cells exposed to DMEM with 4% BSA. From Fig. 1a, our measurements indicate that, unlike palmitate, oleate and linoleate did not induce cytotoxicity in HepG2 cells. Correspondingly, we found a correlation in the LDH and H2O2 released into the medium. The amount of H2O2 released after exposure of HepG2 cells to the various FFAs are shown in Fig. 1b. Palmitate induced the highest level of ROS production as determined by H2O2.
4.2. Membrane Fluidity Measurements
FFAs are typically hydrophobic in nature, therefore, we investigated the non-specific cytotoxic effects due to their hydrophobicity (see previous publication for more details [13]). From our previous study, we found palmitate altered the membrane fluidity significantly [13]. Here we wanted to determine whether unsaturated FFAs similarly impacted the membrane fluidity. The membrane fluidity of HepG2 cells were measured after 48 hrs of exposure to saturated and unsaturated FAs using EPR. The control was HepG2 cells exposed to DMEM with 4% BSA. Using 5-n-SASL and 16-n-SASL as probes to monitor the ordering of the lipid tails near the phospholipid head-groups and the center of the bilayer core, respectively, an increase in the S order parameter and peak height ratio for HepG2 cells was observed for the palmitate cultures, as shown in Figs. 2a and b, respectively. No significant changes were observed for HepG2 cells exposed to either oleate or linoleate, in comparison to the control. The results suggest a greater reduction of the local membrane fluidity due to the greater hydrophobic effect of saturated than unsaturated FAs.
Figure 2.
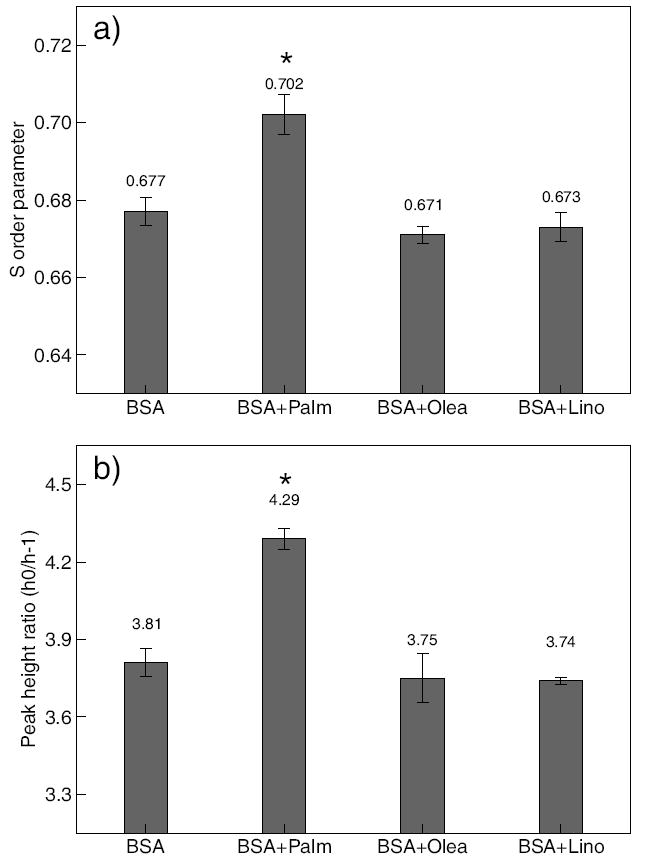
Effect of FFAs exposure on cellular membrane fluidity. Cells were treated with 0.7 mM palmitate (Palm), oleate (Olea), or linoleate (Lino) for 48 hrs. Cellular membrane fluidity was measured using EPR. a) Values are order parameter for 5-n-SASL labeled HepG2 cells. b) Values are peak height ratio for 16-n-SASL labeled HepG2 cells. Error bars are standard deviation of three independent experiments. “*” indicates statistical difference from control, respectively (p < 0.05).
4.3. DSC Measurements and Liposomal Leakage Study
Cell membranes are very complex, thus to more directly compare the experimental results with the subsequent computational analyses, we performed phase transition and leakage studies of DOPC liposomes with FAs. We measured the phase transition temperature of DOPC liposomes by DSC. The phase transition temperature obtained from the DSC thermographs for DOPC liposomes with increasing mole fractions of palmitate, oleate, or linoleate are shown in Fig. 3. The results demonstrate a significant increase in the phase transition temperature of DOPC liposomes with increasing concentration of palmitate, however, slight changes are observed for DOPC liposomes containing oleate and linoleate. This suggests that palmitate increases the ordering of the phospholipids in the liposomes, which correlates well with the decrease in the local membrane fluidity as measured by EPR of the HepG2 cells (lower fluidity, higher phase transition temperature).
Figure 3.
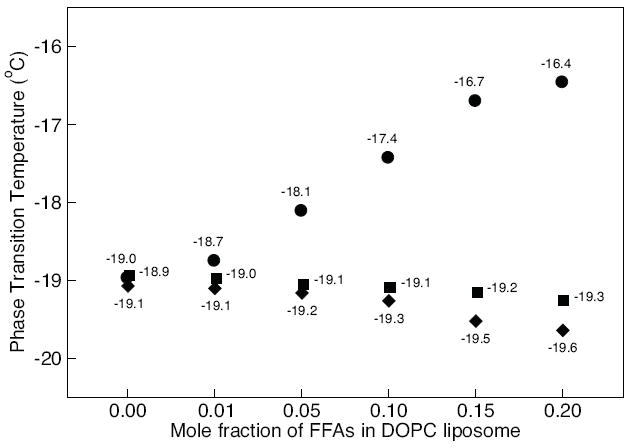
Phase transition temperature of DOPC liposomes with increasing concentrations of palmitate (circles), oleate (diamonds), and linoleate (squares). The mole fractions of FFAs in DOPC liposome were varied from 0.00 to 0.20. Phase transition was measured with DSC. Error bars (standard deviation of three independent experiments) are smaller than the symbols size.
To investigate whether palmitate can more readily induce liposome membrane fluidization than the unsaturated FFAs, which could have implications on membrane integrity, we performed a leakage study using DOPC liposome loaded with SRB. A previous study had shown that linoleate did not induce membrane permeabilization, therefore we evaluated only oleate, as the unsaturated FFA in the current study [58, 33]. Fig. 4 shows that the release of SRB molecules from the liposome increased with increasing palmitate concentrations, as indicated by the increase in fluorescence intensity. On the other hand, the fluorescence intensity for liposomes exposed to oleate remained relatively unchanged, indicating that SRB remained encapsulated in the liposome. The maximum SRB release was 3,348±200. Although this result does not correlate with the LDH release from HepG2 or explain how the membrane integrity is compromised due to palmitate, it does suggest that palmitate can alter the liposome bilayer and compromise its integrity. Liposomes are model bilayers representing a reduced number of constituents contained in cell membranes, thus results obtained from this study could provided some insights into potential biophysical effects that may be at play and perhaps relevant to cellular membranes.
Figure 4.
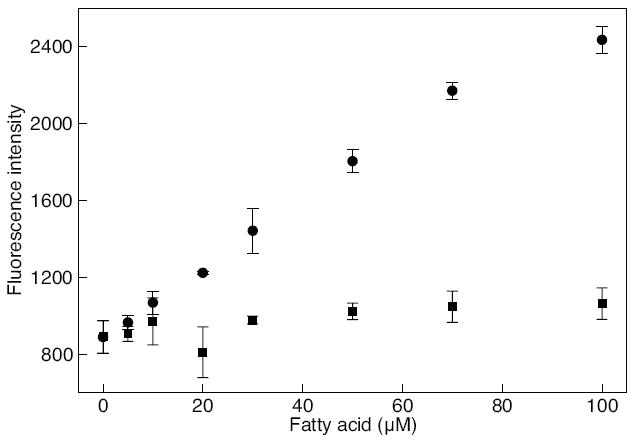
At a constant concentration of SRB-loaded LUV, 1 mM CaCl2 was added to the sample, followed by the addition of fatty acids, palmitate (circle) or oleate (square), at varying concentrations. The mole fractions of FFAs in SRB-loaded LUV were varied from 0.00 to 0.20. The fluorescence intensity was measured at 565/586 nm using a fluorescence microplate reader. All experiments were performed in triplicates and the error bars represent standard deviations.
5. Computational Results
The computational studies performed allowed us to obtain a molecular level understanding on how unsaturated FAs interact with the phospholipid bilayers in comparison to the effects induced by saturated FAs. Therefore, a number of quantities were analyzed to characterize the effect of unsaturated FAs (oleate and linoleate) on the properties of DOPC bilayers, including: bilayer surface area, bilayer thickness, lipid tail order parameters, area per phospholipid/FA, and hydrogen bonding. All results were calculated from the ensemble average over the last 60 ns of the simulations. Results for the bilayer systems without FA are also shown for comparison. Interactions of other types of phospholipid bilayers containing POPC and palmitate have been reported in our previous study [13] and, where appropriate, are mentioned here for comparison. The actual FA content in the bilayers is shown in Table 1. Note that the bilayers were constructed by having equal number of fatty acids on each leaflet, a condition necessary to obtain a stable system.
5.1. Bilayer Surface Area
The first property we analyzed is the bilayer surface area (cross-sectional area of the simulation box). This demonstrates the global effect of FAs embedded within the bilayers (see previous publication for more details [13]). As shown in Fig. 5 (closed symbols), the bilayer surface area increases with increasing FA concentration. This is seen because each FA occupies an additional volume within the bilayer and, due to its alignment with the phospholipid tails, the bilayer expands in the lateral dimensions. A similar behavior was observed in our previous study using POPC embedded with palmitate [13]. Furthermore, our results indicate that oleate or linoleate induce a larger increase in the bilayer surface area than palmitate. This is seen because the kinked lipid tail of unsaturated FAs occupy a larger lateral surface area when packed within the bilayers compared to a straight-chain lipid tail of saturated FAs. Based on this analysis, unsaturated FAs induce greater lateral expansion between phospholipids, possibly decreasing the packing between phospholipids and increasing membrane fluidity.
Figure 5.
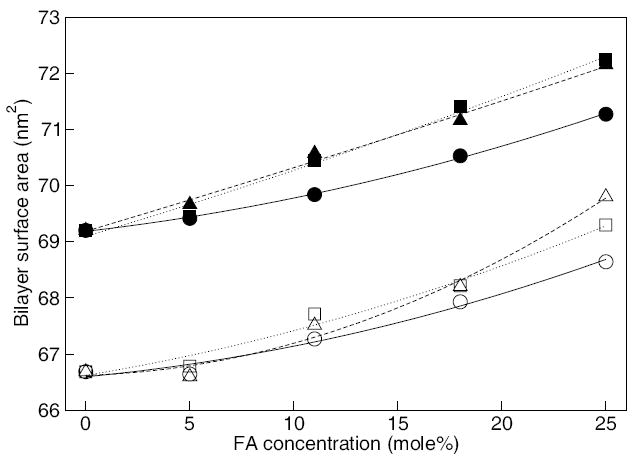
Surface area of the bilayers containing palmitate (circles), oleate (squares), and linoleate (triangles). Open and closed symbols are for the identical bilayer systems with and without Ca2+ ions, respectively. Lines are drawn as a guide. Statistical error estimates are less than the size of the symbols for all the simulations (see Table 2) and are omitted for clarity.
5.2. Area per phospholipid and FA
To further investigate the effect of FA inside the membrane, a 2-D Voronoi tessellation analysis [59] was conducted on the equilibrium bilayer structures with and without FAs (see our previous publication for more details about this analysis [13]). For the analysis considered, the Voronoi plane was defined by the position of carbonyl carbon atoms of the lipid and carboxylic carbon atoms of the FAs, as these were located at about the same depth in the bilayer. The result of a Voronoi tessellation analysis is a plot representing the spatial distribution of the molecules in the bilayer. Note that only a single snapshot at the end of the simulation was used as a representative distribution of the molecules in the bilayer. Using this method, the area occupied by the phospholipid and FA molecules (calculated from the area of the Voronoi polygons) were determined and compared, as shown in Fig. 6. In Fig. 6a, the area per phospholipid decreases with increasing palmitate, oleate, or linoleate concentrations. For palmitate systems, the area per phospholipid decreases almost linearly with the concentration. This behavior was observed in our previous study using POPC bilayers embedded with various palmitate concentrations [13]. Unlike palmitate, the area per phospholipid for the oleate and linoleate system appears to decrease non-linearly and reach values greater than the palmitate system. This indicates that the packing between phospholipids are more complex in the presence of unsaturated FA.
Figure 6.
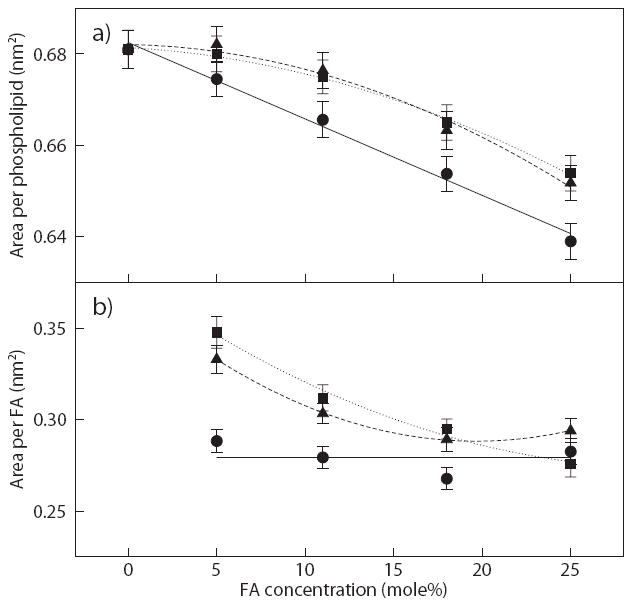
Average area per a) phospholipid and b) FA obtained from Voronoi tessellation analysis for the corresponding bilayers with palmitate (circles), oleate (squares), and linoleate (triangles). Solid, dotted, and dash lines are drawn as guide, respectively. Error bars are estimated standard error of the mean area of Voronoi polygons.
Using the same analysis, the area per FA was calculated, as shown in Fig. 6b. From the figure, the area per palmitate remains constant (within statistical uncertainty), whereas the area per oleate and linoleate, originally starts high at 5 mole% and then significantly decreases until reaching the value obtained for palmitate. As reported in our previous study [13], we observed the same behavior for palmitate systems and concluded that the area per palmitate remains unchanged mainly because palmitate, a fatty acid molecule with a long and straight hydrocarbon tail, is laterally incompressible within the bilayer. In the case of the oleate and linoleate systems, the double-bond in the lipid generally produces a large surface area as shown in the bilayer surface analysis in Fig. 5. Therefore, oleate and linoleate have the ability to absorb the lipid ordering by reducing its area per FA. Note that at the highest oleate concentration (25 mole%), the area per FA are about the same, which means that oleate and linoleate may have reached a maximum lipid ordering by straightening the unsaturated FA chain. Although the three different FAs reach approximately the same area per FA at high concentration, the area per phospholipid remains largely different among bilayer systems. This is partially related to the kinked lipid tail of unsaturated FAs that disrupt the ordering of the phospholipid chains and induce a slight increase in the area per phospholipid. In support of these findings, Koenig et al. utilized a combination of NMR and X-ray diffraction techniques to compare membrane compressibility between saturated and unsaturated phospholipids and found that saturated chains are significantly less compressible than polyunsaturated chains [60]. From this analysis, we are able to determine, in part, one aspect of the role of saturated and unsaturated FAs embedded within the bilayer structure.
5.3. Hydrogen Bonding
Hydrogen bond analysis of water with the phospholipids was performed to investigate the effect of the hydration with increasing FA concentrations. First, radial distribution functions (RDF) between phospholipid oxygen atoms and water were calculated to determine the hydration radius for each phospholipid oxygen (four oxygens in the phosphate group and four oxygens in the ester group), which corresponded to the distance to the first minimum in the RDFs. Fig. S4 in the Supporting Information shows the RDFs for DOPC bilayers containing 11 mole% FAs (RDFs for the other FA compositions are not shown for the sake of brevity, but they are all similar to those in Fig. S4). The values of the hydration shell for all bilayer systems are reported in Table S1, also available in the Supporting Information. Using the hydrogen bond analysis previously described by Brady and Schmidt [61] with the first hydration shell from the RDFs, Fig. 7 shows the average number of hydrogen bonds per phospholipid between lipid oxygen atoms and water for all systems considered. As seen in the figure, the average number of hydrogen bonds significantly reduces with increasing palmitate concentration. This is caused by the increased packing of the phospholipids (reduced area per FA – see Fig. 6a), thus resulting in the removal of potential binding sites for water. On the other hand, the number of hydrogen bonds for oleate and linoleate systems remains relatively constant regardless of their concentrations. This is related to the fact that oleate and linoleate help to maintain the spacing between phospholipids, thus resulting in a suitable area per phospholipid and level of hydration. This analysis demonstrates the contrasting role of saturated and unsaturated fatty acids; even though unsaturated FAs can be as easily incorporated into the phospholipids bilayer as palmitate, their presence in the bilayer is actually beneficial, as they cause little perturbation to the bilayer structure and help to maintain the hydration level.
Figure 7.
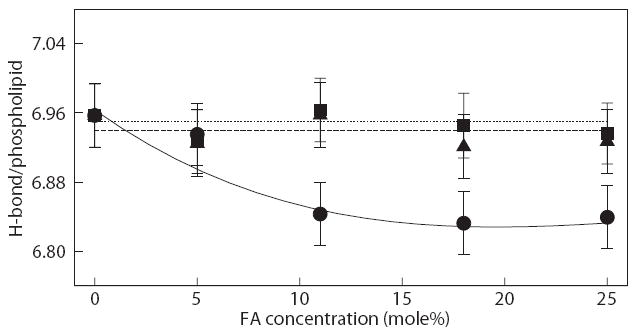
Average number of hydrogen bonds per phospholipid for bilayers with palmitate (circles), oleate (square) and linoleate (triangle); water as H-donors. Solid, dotted, and dash lines are drawn as guide, respectively. Error bars represent standard deviations.
5.4. Effect of Ca2+ Ions on phospholipids Bilayers with FAs
It was observed in the leakage studies that DOPC liposomes containing specific FAs become unstable when Ca2+ ions were present, resulting in an intensified release with increasing FA concentrations. To address the role of Ca2+ ions, simulations were also performed for the FAs systems in the lipid bilayer with Ca2+ (and Cl− as counter ions). It was observed from the simulations that all of the Ca2+ ions quickly deposited onto the bilayer surface. This is consistent with previous experimental results investigating the distribution and penetration depth of mono-, di- and trivalent ions [62, 63]. The initial hypothesis was that the presence of highly positive charged ions may further induce the aggregation of FAs in the bilayer, resulting in the formation of a phospholipid-Ca2+ complex that may destabilize the liposome membrane [64].
To probe this hypothesis, we first determined the overall structural properties of the phospholipid bilayers containing Ca2+. The properties analyzed included the bilayer surface area and the lipid tail order parameter. As shown in Fig. 5, the average bilayer surface area with Ca2+ is slightly lower than those for the bilayers without Ca2+ (the difference in the bilayer surface area translates to less than 0.2 nm in the actual dimensions of the simulation box). This behavior is consistent with other studies considering ions [35, 36, 40]. Also seen in Fig. 5 is that the systems with and without Ca2+ exhibit the same trend. Analysis of the lipid tail order parameters also gave evidence that the Ca2+ ions had no significant impact to the bilayer properties (see Fig. S5 in the Supporting Information).
To determine the impact of Ca2+ ions in the aggregation of FAs in the bilayer, we measured the average distance between Ca2+ ions and the carboxylic oxygen in the FAs. The carboxylic oxygen was chosen because its position in the bilayer is about the same depth as the bound Ca2+ ions on the surface. Fig. S6 shows the normalized Ca2+-FA distribution distance for the bilayers containing 11 mole% FA calculated in the interval between 20-30 ns and 80-90 ns. These intervals were chosen because they represent an early and final state of the systems. From the plots in Fig. S6, there is no significant change in the distribution of the FAs in the bilayer relative to the Ca2+ after 50 ns. In other word, the aggregation of FA within the bilayer near the Ca2+ ions was not observed from the simulations, as the average distribution did not shift to a lower distance. The same observation is seen for the other systems with different FA concentrations (see Fig. S6 in the Supporting Information).
6. Discussion
Unlike most FFAs, unbound palmitate has been shown to be toxic to HepG2 cells at 0.4 mM and higher [15]. On the other hand, unsaturated FFAs have both positive and negative effects among the different types of cells. We found in this study, from LDH release and peroxide measurements, that oleate and linoleate are less harmful to HepG2 cells than palmitate at the same concentration of 0.7 mM. The EPR measurements indicate that there is a significant change in membrane fluidity in the presence of palmitate, compared to oleate and linoleate. This change is mainly associated with the greater hydrophobic effect of saturated FAs, which resulted in reduced membrane fluidity. To compare the biochemical and biophysical processes associate with the change in membrane fluidity in the presence of palmitate, oleate, or linoleate at a molecular level, we studied the effect of these FFAs on model cell membranes (DOPC lipid bilayers) using MD simulations. Due to a relatively short simulation time considered (~100 ns), only the effect of FAs at the early stage of FAs adsorption is considered. To investigate the long-term effect of FAs on biological membranes, the liposomal leakage studies is used to monitor the stability of biological membrane by varying FFA concentrations.
A number of structural properties were analyzed from a series of phospholipid bilayer simulations containing FAs. From the average size of the bilayer surface area, we found that oleate and linoleate significantly expand the DOPC bilayer surface compared to palmitate. This was expected because of the presence of kinked lipid tails (oleate and linoleate) slightly increased the molecular volume for each FA. As a result, the bilayer surface area increased when unsaturated FAs were packed inside the bilayer. Using the component density profiles, the average bilayer thickness was estimated but the results did not show significant differences between bilayers containing the same FA concentrations. This implied that FAs expanded the spacing between phospholipids but had little effect on the packing between each phospholipid. To verify this, the lipid tail order parameters were calculated. The results showed an increase in the order parameters with increasing FA concentrations, however, no significant differences among FAs were observed. These results indicated a packing competition between FA and DOPC, which resulted in an increase in the bilayer surface area and lipid tail order parameters. Based on these findings, a more discrete analysis was necessary to differentiate the properties of the various FAs.
The local effect of FAs was calculated using a Voronoi tessellation analysis. Using this method, the results indicated that the unsaturated FAs have the ability to help reduce the ordering between phospholipids. With an increase in FA concentrations, we found that the area per phospholipid decreased linearly for palmitate but non-linearly with either oleate or linoleate. To explain this, the average area per FA was calculated. The results showed that the area per oleate or linoleate were significantly larger than palmitate at low concentration but decreased non-linearly to the value for palmitate at high concentration. This reduction indicated a straightening of the unsaturated FA chain with increasing FA concentrations. This was not observed for palmitate because the lipid chain is largely linear due to chain saturation. From this analysis, it is evident that unsaturated FAs partially help maintain membrane fluidity by reducing the packing between phospholipids, whereas saturated FAs only increase the packing between phospholipids thus reducing membrane fluidity.
Since the packing between phospholipids was partially preserved for the oleate and linoleate systems, it was hypothesized that the level of hydration of the bilayer is maintained. Extensive hydrogen bond analysis was performed for each bilayer systems and the results indicated that the number of hydrogen bond per phospholipid is relatively the same with increasing oleate or linoleate concentrations. Significant reduction was observed for palmitate. These results indicated that the presence of saturated FAs increased the packing between phospholipids which expelled the water in the vicinity of the bilayer surface, evidence of reduced membrane fluidity. On the other hand, unsaturated FAs helped reduce the packing between phospholipids and preserve the level of hydration, thus maintaining membrane fluidity.
Based on the computational analysis, it is observed that the adsorption of palmitate induces larger physical changes to the phospholipid component in the bilayer than oleate or linoleate. One possibility is that saturated FAs are more likely to phase separate in DOPC bilayer than unsaturated FAs. From a biological membrane perspective, the possible outcome of these changes may compromise membrane integrity and lead to cell death. The liposomal leakage experiments presented here also raised challenging questions on the effect of FAs on biological membranes. First, there was an increase in the leakage of encapsulated SRB molecules with increasing palmitate concentrations. Since the leakage increased almost linearly with palmitate concentrations, it suggests that palmitate did not completely solubilize the liposome membranes like non-ionic detergents, but rather facilitated the release of SRB. Second, since the experiments are conducted in minutes to hours, it is possible that palmitate aggregates within the membranes due to the hydrophobicity and mobility of palmitate, which may increase the membrane permeability and eventually the formation of pores [25]. Lastly, there was little to no leakage of SRB for bilayers exposed to oleate concentrations. This suggested that oleate is less likely to penetrate and in turn aggregate within the membranes, which may be attributed to its lower mobility in the membrane. It should be noted that, as model membranes, cellular response is absent in liposomal systems, and as such, the leakage study isolated the biophysical effects of FAs. Additional systematic studies among FAs will be required to translate the effects of FAs in liposomes to cells.
One aspect of the experiments that may also contribute to the leakage of the bilayers is the requirement for a buffer solution, which in this case contained Ca2+. Simulations of the phospholipid bilayers and FAs with Ca2+ showed that the ions had little impact in the overall properties of the phospholipid bilayer and that, within the time considered (~100 ns), the aggregation of FAs was unaltered. The results from these simulations were insufficient to determine the role of Ca2+ ions in the leakage of liposomes containing palmitate. There are a number of variables that must be better understood from both the experiments and simulations to further our knowledge of the possible interactions that interplay between phospholipids, FAs, and ions. One uncertainty in both the experiments and simulations is the protonation state of the FAs. In the simulations, all FAs were modeled in the protonated state (zero net charge). Unprotonated FAs (−1 net charge) can significantly impact their interactions with Ca2+ ions, as these would be more pronounced and possibly enhance the lateral diffusion of FAs in the bilayer, which would lead to their aggregation. Another factor is the time required for aggregation. The simulations performed with and without Ca2+ were about 100 ns long, which may be insufficient to visibly observe the formation of FA aggregates in the bilayer. Either significantly longer simulations or the application of coarse-grained models at the expense of less accurate molecular interactions would be required. In this context, there are currently very few computational studies that tested the limits of atomistic and coarse-grained membrane simulations and found conclusive evidence of lipid ordered domain formation (lipid rafts) [65, 66]. Although the liposome results do not correlate directly with the LDH release seen in the HepG2 cells, they both suggest that the bilayer membrane integrity is compromised when exposed to palmitate.
7. Conclusions
In this study, we performed a series of experimental and computational measurements to gain insight into how unsaturated FAs, oleate and linoleate, interact with HepG2 cells in comparison to a saturated FA, palmitate. Experimentally, we found that healthy HepG2 cells exposed to oleate and linoleate did not induce LDH and H2O2 release into the medium, compared to palmitate. Furthermore, it is observed from EPR measurements that the local membrane fluidity is increased slightly for HepG2 cells exposed to oleate and linoleate. The leading hypothesis for the observed result is that the presence of saturated FA potentially increases the chain packing between cellular lipids, however, the kinked lipid chains of the unsaturated FA disrupted their normal packing, thus increasing membrane fluidity.
The computational component of this study aimed at interpreting and understanding the experimental results, providing knowledge at the molecular level into the role of unsaturated FAs in maintaining the membrane fluidity and integrity. As illustrated by the results, the average structural properties, such as bilayer surface area, thickness, and order parameter were insufficient in differentiating the effects of saturated and unsaturated FAs on the bilayers. Both saturated and unsaturated FAs increased the bilayer surface area, thickness, and lipid tail order parameter with increasing FA concentrations. As a result, it was necessary to consider the local effect of FAs embedded in the bilayers. Using Voronoi tessellation analysis, the results demonstrated the ability of unsaturated FAs to reduce the order packing between phospholipids and FAs within the bilayer. Furthermore, water-phospholipid hydrogen bond analysis indicated that the average number of hydrogen bonds remained relatively the same regardless of oleate or linoleate concentrations, however, significantly reduced for palmitate. From this analysis, we confirmed that the role of unsaturated FA is very different in that unsaturated FAs induced fewer changes to the phospholipid component of the bilayer and helped maintain the level of hydration. Simulations with Ca2+ were performed to obtain insight into the impact of ions on the bilayer properties and aggregation of FAs as a means to understand the leakage of the liposomes. The results from these simulations did not show significant changes to the phospholipid bilayer nor evidence for enhanced FA aggregation in the presence of Ca2+, however, the protonation state of the FAs and simulation time are factors that need to be better understood and addressed in future experiments and simulations.
Table 2.
Average bilayer surface area and thickness averaged over the last 60 ns of the simulations. Surface area is estimated from the x- and y-dimensions of the simulation box. Thicknesses are estimated from the DOPC density profiles shown in Fig. S2 in the Supporting Information.
| Bilayer surface area (nm2) | Bilayer thickness (nm) | |||||
|---|---|---|---|---|---|---|
| FA (mol%) | palmitate | oleate | linoleate | palmitate | oleate | linoleate |
| 0 | 69.20±0.03 | 69.20±0.03 | 69.20±0.03 | 2.85±0.04 | 2.85±0.04 | 2.85±0.04 |
| 5 | 69.42±0.04 | 69.46±0.04 | 69.20±0.03 | 2.97±0.04 | 2.91±0.04 | 2.91±0.04 |
| 11 | 69.84±0.04 | 70.45±0.04 | 70.57±0.04 | 3.04±0.04 | 3.06±0.04 | 2.99±0.04 |
| 18 | 70.53±0.04 | 71.41±0.03 | 71.16±0.04 | 3.11±0.04 | 3.12±0.04 | 3.08±0.04 |
| 25 | 71.27±0.04 | 72.24±0.04 | 72.15±0.04 | 3.17±0.04 | 3.16±0.04 | 3.18±0.04 |
Acknowledgments
This work was funded in part by the MSU Foundation, the National Science Foundation (BES 0425821), and the National Institutes of Health (1R01GM079688-01). EPR measurements were graciously provided by Dr. John L. McCracken in the Department of Chemistry, respectively, at Michigan State University. AKS acknowledges the support from DuPont as a DuPont Young Professor. Computational resources were provided by Virginia Tech Advanced Research Computing (System X).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
References
- 1.Galli C, Marangoni F. Recent advances in the biology of n-6 fatty acids. Nutrition. 1997;13(1112):978–985. doi: 10.1016/s0899-9007(97)00341-9. [DOI] [PubMed] [Google Scholar]
- 2.Yamashita A, Sugiura T, Waku K. Acyltransferases and transacylases involved in fatty acid remodeling of phospholipids and metabolism of bioactive lipids in mammalian cells. J Biochem -Tokyo. 1997;122(1):1–16. doi: 10.1093/oxfordjournals.jbchem.a021715. [DOI] [PubMed] [Google Scholar]
- 3.Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. Journal of Lipid Research. 1985;26(2):194–202. [PubMed] [Google Scholar]
- 4.Rudel LL, Haines JL, Sawyer JK. Effects on plasma lipoproteins of monounsaturated, saturated, and polyunsaturated fatty acids in the diet of African green monkeys. Journal of Lipid Research. 1990;31(10):1873–82. [PubMed] [Google Scholar]
- 5.Nydahl MC, Gustafsson IB, Vessby B. Lipid-lowering diets enriched with monounsaturated or polyunsaturated fatty acids but low in saturated fatty acids have similar effects on serum lipid concentrations in hyperlipidemic patients. American Journal of Clinical Nutrition. 1994;59(1):115–22. doi: 10.1093/ajcn/59.1.115. [DOI] [PubMed] [Google Scholar]
- 6.Gardner CD, Kraemer HC. Monounsaturated versus polyunsaturated dietary fat and serum lipids. a meta-analysis. Arteriosclerosis Thrombosis and Vascular Biology. 1995;15(11):1917–27. doi: 10.1161/01.atv.15.11.1917. [DOI] [PubMed] [Google Scholar]
- 7.Saifer A, Goldman L. The free fatty acids bound to human serum albumin. J Lipid Res. 1961;2(3):268–270. [Google Scholar]
- 8.Spector AA. Fatty acid binding to plasma albumin. J Lipid Res. 1975;16(3):165–79. [PubMed] [Google Scholar]
- 9.El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M. Saturated fatty acids synergize with elevated glucose to cause pancreatic β-cell death. Endocrinology. 2003;144(9):4154–63. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- 10.Andrade LN, de Lima TM, Curi R, Castrucci AM. Toxicity of fatty acids on murine and human melanoma cell lines. Toxicol In vitro. 2005;19(4):553–60. doi: 10.1016/j.tiv.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Ji J, Zhang L, Wang P, Mu YM, Zhu XY, Wu YY, Yu H, Zhang B, Chen SM, Sun XZ. Saturated free fatty acid, palmitic acid, induces apoptosis in fetal hepatocytes in culture. Exp Toxicol Pathol. 2005;56(6):369–76. doi: 10.1016/j.etp.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava S, Chan C. Hydrogen peroxide and hydroxyl radicals mediate palmitate-induced cytotoxicity to hepatoma cells: Relation to mitochondrial permeability transition. Free Radic Res. 2007;41(1):38–49. doi: 10.1080/10715760600943900. [DOI] [PubMed] [Google Scholar]
- 13.Leekumjorn S, Wu YF, Sum AK, Chan C. Experimental and computational studies investigating trehalose protection of HepG2 cells from palmitate-induced toxicity. Biophys J. 2008;94(7):2869–2883. doi: 10.1529/biophysj.107.120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava S, Chan C. Application of metabolic flux analysis to identify the mechanisms of free fatty acid toxicity to human hepatoma cell line. Biotechnology and Bioengineering. 2008;99(2):399. doi: 10.1002/bit.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Chan C. Repression of pkr mediates palmitate-induced apoptosis in HepG2 cells through Bcl-2. Cell Research. doi: 10.1038/cr.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Z-H, Mu Y-M, Wang B-A, Li X-L, Lu J-M, Li J-Y, Pan C-Y, Yanase T, Nawata H. Saturated free fatty acids, palmitic acid and stearic acid, induce apoptosis by stimulation of ceramide generation in rat testicular Leydig cell. Biochem Biophys Res Commun. 2003;303(4):1002–1007. doi: 10.1016/s0006-291x(03)00449-2. [DOI] [PubMed] [Google Scholar]
- 17.Kong JY, Rabkin SW. Palmitate-induced apoptosis in cardiomyocytes is mediated through alterations in mitochondria: Prevention by cyclosporin a. Biochim Biophys Acta-Mol Cell Biol L. 2000;1485(1):45–55. doi: 10.1016/s1388-1981(00)00028-7. [DOI] [PubMed] [Google Scholar]
- 18.Lee C, Barnett J, Reaven PD. Liposomes enriched in oleic acid are less susceptible to oxidation and have less proinflammatory activity when exposed to oxidizing conditions. J Lipid Res. 1998;39(6):1239–1247. [PubMed] [Google Scholar]
- 19.Watabe N, Ishida Y, Ochiai A, Tokuoka Y, Kawashima N. Oxidation decomposition of unsaturated fatty acids by singlet oxygen in phospholipid bilayer membranes. J Oleo Sci. 2007;56(2):73–80. doi: 10.5650/jos.56.73. [DOI] [PubMed] [Google Scholar]
- 20.Wong-Ekkabut J, Xu ZT, Triampo W, Tang IM, Tieleman DP, Monticelli L. Effect of lipid peroxidation on the properties of lipid bilayers: A molecular dynamics study. Biophys J. 2007;93(12):4225–4236. doi: 10.1529/biophysj.107.112565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen T, Acker JP, Eroglu A, Cheley S, Bayley H, Fowler A, Toner M. Beneficial effect of intracellular trehalose on the membrane integrity of dried mammalian cells. Cryobiology. 2001;43(2):168–81. doi: 10.1006/cryo.2001.2360. [DOI] [PubMed] [Google Scholar]
- 22.Awad AB, Spector AA. Modification of the fatty acid composition of Ehrlich ascites tumor cell plasma membranes. Biochim Biophys Acta-Biomembranes. 1976;426(4):723–31. doi: 10.1016/0005-2736(76)90137-1. [DOI] [PubMed] [Google Scholar]
- 23.Burns CP, Luttenegger DG, Dudley DT, Buettner GR, Spector AA. Effect of modification of plasma membrane fatty acid composition on fluidity and methotrexate transport in L1210 murine leukemia cells. Cancer Res. 1979;39(5):1726–32. [PubMed] [Google Scholar]
- 24.Hyvönen MT, Oorni K, Kovanen PT, Ala-Korpela M. Changes in a phospholipid bilayer induced by the hydrolysis of a phospholipase A(2) enzyme: A molecular dynamics simulation study. Biophy J. 2001;80(2):565–578. doi: 10.1016/S0006-3495(01)76038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knecht V, Mark AE, Marrink SJ. Phase behavior of a phospholipid/fatty acid/water mixture studied in atomic detail. J Am Chem Soc. 2006;128(6):2030–4. doi: 10.1021/ja056619o. [DOI] [PubMed] [Google Scholar]
- 26.Langner M, Hui S. Effect of free fatty acids on the permeability of 1,2-dimyristoyl-sn-glycero-3-phosphocholine bilayer at the main phase transition. BBA-Biomembranes. 2000;1463(2):439–47. doi: 10.1016/s0005-2736(99)00236-9. [DOI] [PubMed] [Google Scholar]
- 27.Rogerson ML, Robinson BH, Bucak S, Walde P. Kinetic studies of the interaction of fatty acids with phosphatidylcholine vesicles (liposomes) Colloids Surf B Biointerfaces. 2006;48(1):24–34. doi: 10.1016/j.colsurfb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Cirelli N, Lebrun P, Gueuning C, Delogne-Desnoeck J, Van-bellinghen A-M, Graff G, Meuris S. Physiological concentrations of albumin stimulate chorionic gonadotrophin and placental lactogen release from human term placental explants. Hum Reprod. 2001:441–448. doi: 10.1093/humrep/16.3.441. [DOI] [PubMed] [Google Scholar]
- 29.Boden G, Chen X, Capulong E, Mozzoli M. Effects of free fatty acids on gluconeogenesis and autoregulation of glucose production in type 2 diabetes. Diabetes. 2001;50:810–816. doi: 10.2337/diabetes.50.4.810. [DOI] [PubMed] [Google Scholar]
- 30.Mensink M, Blaak EE, van Baak MA, Wagenmakers AJ, Saris WH. Plasma free fatty acid uptake and oxidation are already diminished in subjects at high risk for developing type 2 diabetes. Diabetes. 2001;50:2548–2554. doi: 10.2337/diabetes.50.11.2548. [DOI] [PubMed] [Google Scholar]
- 31.Skowronski R, Hollenbeck CB, Varasteh BB, Chen YD, Reaven GM. Regulation of non-esterified fatty acid and glycerol concentration by insulin in normal individuals and patients with type 2 diabetes. Diabetic Med. 1991;8:330–333. doi: 10.1111/j.1464-5491.1991.tb01605.x. [DOI] [PubMed] [Google Scholar]
- 32.Woerle HJ, Popa E, Dostou J, Welle S, Gerich J, Meyer C. Exogenous insulin replacement in type 2 diabetes reverses excessive hepatic glucose release, but not excessive renal glucose release and impaired free fatty acid clearance. Metabolism. 2002;51:1494–1500. doi: 10.1053/meta.2002.35203. [DOI] [PubMed] [Google Scholar]
- 33.Agafonov A, Gritsenko E, Belosludtsev K, Kovalev A, Gateau-Roesch O, Saris NEL, Mironova GD. A permeability transition in liposomes induced by the formation of ca2+/palmitic acid complexes. BBA-Biomembranes. 2003;1609(2):153–160. doi: 10.1016/s0005-2736(02)00666-1. [DOI] [PubMed] [Google Scholar]
- 34.Kuo P, Weinfeld M, Loscalzo J. Effect of membrane fatty acyl composition on LDL metabolism in HepG2 hepatocytes. Biochemistry. 1990;29(28):6626–32. doi: 10.1021/bi00480a011. [DOI] [PubMed] [Google Scholar]
- 35.Pandit SA, Berkowitz ML. Molecular dynamics simulation of dipalmitoylphosphatidylserine bilayer with Na+ counterions. Biophys J. 2002;82(4):1818–1827. doi: 10.1016/S0006-3495(02)75532-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Böckmann RA, Hac A, Heimburg T, Grubmüller H. Effect of sodium chloride on a lipid bilayer. Biophys J. 2003;85(3):1647–1655. doi: 10.1016/S0006-3495(03)74594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Böckmann RA, Grubmüller Multistep binding of divalent cations to phospholipid bilayers: A molecular dynamics study. Angew Chem Int Ed. 2004;43(8):1021–1024. doi: 10.1002/anie.200352784. [DOI] [PubMed] [Google Scholar]
- 38.Mukhopadhyay P, Monticelli L, Tieleman DP. Molecular dynamics simulation of a palmitoyl-oleoyl phosphatidylserine bilayer with na+ counterions and nacl. Biophys J. 2004;86(3):1601–1609. doi: 10.1016/S0006-3495(04)74227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurtovenko AA. Asymmetry of lipid bilayers induced by monovalent salt: Atomistic molecular-dynamics study. J Chem Phys. 2005;122(24):244902. doi: 10.1063/1.1942489. journal of Chemical Physics. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen UR, Leidy C, Westh P, Peters GH. The effect of calcium on the properties of charged phospholipid bilayers. BBA-Biomembranes. 2006;1758(5):573–582. doi: 10.1016/j.bbamem.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 41.Shinoda K, Shinoda W, Mikami M. Molecular dynamics simulation of an archaeal lipid bilayer with sodium chloride. Phys Chem Chem Phys. 2007;9(5):643–650. doi: 10.1039/b611543h. [DOI] [PubMed] [Google Scholar]
- 42.Marrink SJ, Berger O, Tieleman P, Jähnig F. Adhesion forces of lipids in a phospholipid membrane studied by molecular dynamics simulations. Biophys J. 1998;74(2):931–943. doi: 10.1016/S0006-3495(98)74016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tieleman DP, Berendsen HJC. A molecular dynamics study of the pores formed by Escherichia coli OmpF porin in a fully hydrated palmitoyloleoylphosphatidylcholine bilayer. Biophys J. 1998;74(6):2786–2801. doi: 10.1016/S0006-3495(98)77986-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryckaert JP, Bellemans A. Molecular dynamics of liquid normal-butane near its boiling-point. Chem Phys Lett. 1975;30(1):123–125. [Google Scholar]
- 45.Bachar M, Brunelle P, Tieleman DP, Rauk A. Molecular dynamics simulation of a polyunsaturated lipid bilayer susceptible to lipid peroxidation. J Phys Chem B. 2004;108(22):7170–7179. [Google Scholar]
- 46.Martinez-Seara H, Róg T, Pasenkiewicz-Gierula M, Vattulainen I, Karttunen M, Reigada R. Effect of double bond position on lipid bilayer properties: Insight through atomistic simulations. J Phys Chem B. 2007;111(38):11162–11168. doi: 10.1021/jp071894d. [DOI] [PubMed] [Google Scholar]
- 47.Berger O, Edholm O, Jähnig F. Molecular dynamics simulations of a fluid bilayer of dipalmitoylphosphatidylcholine at full hydration, constant pressure, and constant temperature. Biophys J. 1997;72(5):2002–2013. doi: 10.1016/S0006-3495(97)78845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jorgensen WL, Tirado-Rives J. The OPLS potential function for proteins. Energy minimizations for crystals of cyclic peptides and crambin. J Am Chem Soc. 1988;110:1657–1666. doi: 10.1021/ja00214a001. [DOI] [PubMed] [Google Scholar]
- 49.Essex JW, Hann MM, Richards WG. Molecular dynamics simulation of a hydrated phospholipid-bilayer. Philos T Roy Soc B. 1994;344(1309):239–260. doi: 10.1098/rstb.1994.0064. [DOI] [PubMed] [Google Scholar]
- 50.Chiu SW, Clark M, Balaji V, Subramaniam S, Scott HL, Jakobsson E. Incorporation of surface tension into molecular dynamics simulation of an interface: A fluid phase lipid bilayer membrane. Biophys J. 1995;69(4):1230–1245. doi: 10.1016/S0006-3495(95)80005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Gunsteren WF, Berendsen HJC. Gromos-87 manual, Biomos BV, Nijenborgh 4, 9747. AG Groningen; The Netherlands: 1987. [Google Scholar]
- 52.Berendsen HJC, Postma JPM, van Gunsteren WF, Hermans J, Pullman B. Intermolecular Forces. Reidel, Dordrecht; The Netherlands: 1981. [Google Scholar]
- 53.Darden T, York D, Pedersen L. Particle mesh Ewald: an N · log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- 54.Essman U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG. A smooth particle mesh Ewald method. J Chem Phys. 1995;103:8577–8593. [Google Scholar]
- 55.Berendsen HJC, van der Spoel D, van Drunen R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput Phys Commun. 1995;91(13):43–56. [Google Scholar]
- 56.Lindahl E, Hess B, van der Spoel D. GROMACS 3.0: A package for molecular simulation and trajectory analysis. J Mol Model. 2001;7(8):306–317. [Google Scholar]
- 57.Van der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJC. Gromacs: fast, flexible, and free. J Comput Chem. 2005;26(16):1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 58.Mironova GD, Gateau-Roesch O, Levrat C, Gritsenko E, Pavlov E, Lazareva AV, Limarenko E, Rey C, Louisot P, Saris NE. Palmitic and stearic acids bind ca2+ with high affinity and form nonspecific channels in black-lipid membranes. possible relation to ca2+-activated mitochondrial pores. J Bioenerg Biomembr. 2001;33(4):319–31. doi: 10.1023/a:1010659323937. [DOI] [PubMed] [Google Scholar]
- 59.Shinoda W, Okazaki S. A Voronoi analysis of lipid area fluctuation in a bilayer. J Chem Phys. 1998;109(4):1517–1521. [Google Scholar]
- 60.Koenig BW, Strey HH, Gawrisch K. Membrane lateral compressibility determined by NMR and X-ray diffraction: Effect of acyl chain polyunsaturation. Biophys J. 1997;73(4):1954–1966. doi: 10.1016/S0006-3495(97)78226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brady JW, Schmidt RK. The role of hydrogen bonding in carbohydrates: Molecular dynamics simulations of maltose in aqueous solution. J Phys Chem. 1993;97:958–966. [Google Scholar]
- 62.Akutsu H, Seelig J. Interaction of metal ions with phosphatidylcholine bilayer membranes. Biochemistry. 1981;20(26):7366–73. doi: 10.1021/bi00529a007. [DOI] [PubMed] [Google Scholar]
- 63.Roux M, Bloom M. Ca2+, Mg2+, Li+, Na+, and K+ distributions in the headgroup region of binary membranes of phosphatidylcholine and phosphatidylserine as seen by deuterium NMR. Biochemistry. 1990;29(30):7077–89. doi: 10.1021/bi00482a019. [DOI] [PubMed] [Google Scholar]
- 64.Altenbach C, Seelig J. Ca2+ binding to phosphatidylcholine bilayers as studied by deuterium magnetic resonance. Evidence for the formation of a Ca2+ complex with two phospholipid molecules. Biochemistry. 1984;23(17):3913–20. doi: 10.1021/bi00312a019. [DOI] [PubMed] [Google Scholar]
- 65.Niemela PS, Ollila S, Hyvonen MT, Karttunen M, Vattulainen I. Assessing the nature of lipid raft membranes. PLoS Comput Biol. 2007;3(2):304–312. doi: 10.1371/journal.pcbi.0030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Risselada HJ, Marrink SJ. The molecular face of lipid rafts in model membranes. Proc Natl Acad Sci USA. 2008;105(45):17367–17372. doi: 10.1073/pnas.0807527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


