Abstract
Objective
Corticosteroids are used in sepsis treatment to benefit outcome. However, discussion remains on which patients will benefit from treatment. Inter-individual variations in cortisol sensitivity, mediated through the glucocorticoid receptor, might play a role in the observed differences. Our aim was to study changes in mRNA levels of three glucocorticoid receptor splice variants in neutrophils of children with sepsis.
Patients and design
Twenty-three children admitted to the pediatric intensive care unit with sepsis or septic shock were included. Neutrophils were isolated at days 0, 3 and 7, and after recovery (>3 months). mRNA levels of the glucocorticoid receptor splice variants GR-α (determining most of the cortisol effect), GR-P (increasing GR-α effect) and GR-β (inhibitor of GR-α) were measured quantitatively.
Main results
Neutrophils from sepsis patients showed decreased levels of glucocorticoid receptor mRNA of the GR-α and GR-P splice variants on day 0 compared to after recovery. GR-α and GR-P mRNA levels showed a gradual recovery on days 3 and 7 and normalized after recovery. GR-β mRNA levels did not change significantly during sepsis. GR expression was negatively correlated to interleukin-6 (a measure of disease severity, r = −0.60, P = 0.009).
Conclusions
Children with sepsis or septic shock showed a transient depression of glucocorticoid receptor mRNA in their neutrophils. This feature may represent a tissue-specific adaptation during sepsis leading to increased cortisol resistance of neutrophils. Our study adds to understanding the mechanism of cortisol sensitivity in immune cells. Future treatment strategies, aiming at timing and tissue specific regulation of glucocorticoids, might benefit patients with sepsis or septic shock.
Keywords: Children, Corticosteroids, Cortisol sensitivity, Sepsis, Septic shock
Introduction
Sepsis is a systemic response to a severe infectious disease with a still high mortality and morbidity despite improving treatments. Cortisol has an important role in counter-balancing the immune activation to infection. An adequate cortisol stress response is essential for sepsis survival [1], but a continuing controversy exists concerning the potential benefit of adjunctive corticosteroids in severe sepsis; in this regard, it is extremely important to identify the population that might most benefit from such intervention and, in addition, identify the appropriate time course of such intervention [2, 3]. Inter-individual variations in the endogenous cortisol response to stress might play a role in the explanation of the observed differences [4, 5].
At a tissue level, the cortisol effects are determined by the glucocorticoid receptor [6–9]. Large interindividual variability of cortisol sensitivity has been found in a population-based study, with hypersensitive and resistant persons at the extremes [6]. We previously found that genetic variants of the glucocorticoid receptor are associated with altered cortisol sensitivity, an altered immune response, and altered inflammation [10–13]. These alterations might be mediated through changes in glucocorticoid receptor splice variant expression [14]. Three different 3′-splice variants of the glucocorticoid receptor have been reported: GR-α, the most abundant, binds ligand and is functionally active; GR-P is thought to enhance the function of GR-α [15]; and GR-β is a dominant negative inhibitor of GR-α action [9, 16]. Changes in levels of the different splice variants are thought to regulate glucocorticoid sensitivity (i.e., the response to cortisol) in a tissue-specific way [17, 18]. Previous animal studies show elevated glucocorticoid receptor m-RNA levels in septic rats correlate with protein levels of the receptor and with the number of hormone binding sites leading to increased glucocorticoid receptor hormone binding activity [19]. In humans, in vitro studies of human lymphocytes show that increased levels of GR-α and GR-β mRNA correlate with protein levels and with glucocorticoid sensitivity of the cells [20]. In lymphocytes of sepsis patients, increased cortisol sensitivity (measured by a thymidine incorporation assay in dexamethasone stimulated lymphocytes) has been found compared to controls [21]. Neutrophils play an important role in the defense against bacterial infections. Activation of neutrophils, through infection, leads to a pro-inflammatory state demonstrated by reduced apoptosis, increased adherence to the endothelium, extravasation, phagocytosis and production of pro-inflammatory cytokines during sepsis [22]. This leads to a better microbial clearance, which is beneficial for combating sepsis in the acute phase [22]. However, it also leads to tissue damage in prolonged sepsis [22], contributing to multiple organ failure. Increasing neutrophil activity by down-regulating cortisol sensitivity during sepsis could therefore enhance pathogen elimination but also augment the tissue injury. Neutrophils and their possible changes in cortisol sensitivity during sepsis have not been studied before at the level of the glucocorticoid receptor.
We hypothesized that neutrophils during sepsis would temporarily become more resistant to the anti-inflammatory effects of cortisol by transient down-regulation of their glucocorticoid receptor mRNA levels. Therefore, we aimed to study changes in glucocorticoid receptor mRNA levels in neutrophils of children with sepsis longitudinally.
Materials and methods
Patients
Patients diagnosed with sepsis were studied within 24 h after diagnosis. All children were admitted to the pediatric intensive care unit. Sepsis and septic shock were defined in accordance to the criteria set forth by the International Pediatric Sepsis Consensus Conference [23]. Shock was defined as persistent hypotension or evidence of poor end-organ perfusion [23]. Patients with neutropenia or immuno-suppressive therapies were excluded. The pediatric risk of mortality (PRISM) score was used to determine the severity of illness of individual patients [24]. A higher score means a higher risk of mortality. Blood samples were taken immediately after admission and informed consent, on day 0 (t = 0), and at 0900 hours on day 3 (t = 3), day 7 (t = 7), and after recovery (>3 months). Serum, stored at –20°C, was used for measurement of IL-6, using commercially available ELISA (IL-6, Quantikine HS).
To put the results in perspective and to analyze a possible age dependent effect, blood was obtained, for comparison, from 20 healthy adults of the laboratory staff, aged 21–58 years, who did not suffer from any acute or chronic illness.
The protocol was approved by the Medical Ethics Committee of the Erasmus University Medical Center Rotterdam and parents gave their written informed consent to the study.
Neutrophil isolation
All procedures for RNA isolation were performed immediately on fresh blood in all patients. Blood (5 ml) was drawn from an arterial catheter or venapuncture into heparinized tubes. Neutrophils were isolated by the Ficoll separation technique as described before [25]. Viability was tested by trypan blue and more than 95% of cells were viable. Purity of cell type subpopulation was analysed by cytospin and flow cytometric immuno-fluorescence analysis and was shown to be of high purity (>98%) [25].
RNA isolation, RT-reaction, and quantitative real time PCR
For RNA isolation, a cell suspension of 8 × 106 neutrophils in 200 μl saline was dissolved in 1 ml of trizol reagent (Invitrogen, Breda, The Netherlands) and incubated for 5 min at room temperature. After adding 0.2 ml chloroform, tubes were shaken vigorously for 15 s and incubated for 3 min at room temperature. After 10 min centrifugation at 4°C and 8,600g, the RNA, now in the aqueous phase, was transferred to a new tube, precipitated with 0.5 ml isopropanol, incubated for 10 min at room temperature and centrifuged for 10 min at 4°C and 8,600g. The supernatant was washed with 1 ml 70% ethanol, vortexed and centrifuged for 5 min at 4°C and 4,300g. The remaining RNA pellet was dissolved in 30 μl RNAse-free water and incubated for 10 min at 55°C. RNA concentrations were measured using a spectrophotometer (Nanodrop, Los Angeles U.S.A.). The RNA sample was stored at −80°C. cDNA was synthesized from 200 ng RNA in a total volume of 50 μl, using a reverse transcription (RT)-reaction as described previously [26].
Quantitative real time PCR was performed for GR-α, GR-P, and GR-β splice variants. Correction for assay variability was performed using the housekeeping gene hypoxanthine phosphoribosyltransferase (HPRT) of which expression levels are stable and not influenced by glucocorticoids (not shown). The primer sequences and reaction mix used have been previously described [26]. The reaction contained 2 μl cDNA template (corresponding to 8 ng total RNA in the RT-PCR), 2.5 μl reaction buffer, 2.5 μl MgCl2, 1 μl dNTP’s, 0.125 μl polymerase, 0.3 pmol/μl forward and reverse primer (0.5 pmol/μl for HPRT), and 0.2 pmol/μl probe, adding water to a total volume of 25 μl. The reactions were carried out in an ABI 7700 Sequence Detector System (Applied Biosystems, Nieuwerkerk aan den IJssel, The Netherlands). After initial heating at 95°C for 8 min, samples were subjected to 40 cycles of denaturation at 92°C for 15 s and annealing and synthesis for 1 min at 60°C. mRNA levels of the GR-α, GR-P, GR-β splice variants, and HPRT were calculated using the comparative CT Method, according to the manufacturer’s guidelines.
Statistical analysis
Data were analyzed using SPSS for windows, release 12.0.1 (SPSS, Chicago, IL, USA). For analysis of mean splice variant expression, a paired samples t test was used. The time course of splice variant mRNA levels at four different time points was analyzed using mixed model analysis, which allows the use of incomplete data in follow-up. We used logarithmically transformed values of age, CRP, IL-6, prism, cortisol, and ACTH to normalize the distribution for these variables. Correlations between splice variant mRNA levels and continuous variables were analyzed using Pearson’s correlation coefficient. For analysis of differences in splice variant levels between sexes, presence of shock, diagnosis or medication, the independent samples t-test was used. A P value <0.05 was considered significant.
Results
Twenty-three children with sepsis or septic shock were included in the study (Table 1), of whom 13 (57%) were male. Median age was 2.7 years (range 3 months to 14 years. The most frequent diagnosis was meningococcal sepsis in 15 (65%) patients. Fourteen (61%) were diagnosed with shock. Sixteen (70%) needed mechanical ventilation. Median admission duration on the intensive care unit was 2.0 days (range 1–16). Median PRISM score was 19 (range 1–38). Glucocorticoid treatment was used in 7 patients, etomidate in 5, midazolam in 13, and inotropic support in 15 patients. Median leukocyte count was 14 × 109/l (range 1.8–50). In the leukocyte differentiation, median percentage of granulocytes was 81% (range 26–94%), lymphocytes 14% (range 5–68%), monocytes 3% (range 0–9%) Median level of IL-6 was 54,722 pg/ml (range 44–467,433), C-reactive protein (CRP) at admission was 112 mg/l (range 25–408), cortisol 921 nmol/l (range 244–3,167), ACTH 4.8 pg/l (range 25–408) (Table 1). Of the 23 children who were included, 1 died within 24 h after diagnosis (patient 23, Table 1). All other children recovered and are alive to date.
Table 1.
Patient characteristics
| No | Sex | Age (years) | Diagnosis | Ventilated | Admission (days) | GC | PRISM score healthy = 0 | Cortisol (nmol/l) ref < 750 | ACTH (pmol/l) ref < 40 | CRP mg/l ref < 10 | IL-6 pg/ml ref < 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 1.2 | Meningococcal sepsis | No | 1 | No | 7 | 848 | 365 | ||
| 2 | F | 2.3 | Meningococcal sepsis | No | 1 | No | 13 | 1,349 | 3.5 | 84 | 10,494 |
| 3 | M | 2.7 | Meningococcal sepsis | No | 1 | No | 11 | 638 | 65 | ||
| 4 | F | 2.9 | Meningococcal sepsis | Yes | 1 | Yes | 17 | 1,645 | 156 | 334 | |
| 5 | F | 5.8 | Meningococcal sepsis | Yes | 2 | No | 11 | 1,006 | 4.8 | 229 | 135 |
| 6 | M | 2.4 | Meningococcal sepsis | No | 2 | Yes | 19 | 201 | 2,016 | ||
| 7 | M | 2.1 | Meningococcal sepsis | No | 2 | No | 5 | 619 | 2.0 | 89 | 112 |
| 8 | M | 3.0 | Meningococcal sepsis + shock | Yes | 15 | No | 15 | 696 | 238.0 | 85 | 30,481 |
| 9 | F | 4.0 | Meningococcal sepsis + shock | Yes | 7 | No | 37 | 782 | 141.0 | 25 | 362,333 |
| 10 | M | 0.3 | Meningococcal sepsis + shock | Yes | 13 | No | 14 | 995 | 39.3 | 150 | 13,892 |
| 11 | F | 1.7 | Meningococcal sepsis + shock | Yes | 2 | No | 23 | 601 | 5.2 | 104 | 15,866 |
| 12 | M | 5.1 | Meningococcal sepsis + shock | Yes | 4 | No | 19 | 1,047 | 13.6 | 51 | 49,526 |
| 13 | M | 1.4 | Meningococcal sepsis + shock | Yes | 5 | No | 31 | 244 | 179.0 | 78 | 467,433 |
| 14 | M | 1.8 | Meningococcal sepsis + shock | Yes | 1 | Yes | 14 | 394 | 2.4 | 176 | 688 |
| 15 | M | 2.1 | Meningococcal sepsis + shock | Yes | 2 | Yes | 30 | 2,901 | 1.9 | 408 | 547 |
| 16 | M | 2.8 | Sepsis + shock eci | Yes | 1 | Yes | 20 | 531 | 6.4 | 364 | 6,911 |
| 17 | M | 12.6 | Sepsis eci | No | 0 | No | 3 | 792 | 1.0 | 91 | 44 |
| 18 | M | 7.7 | Sepsis eci | No | 0 | Yes | 1 | 1,162 | 9.5 | 46 | 1,457 |
| 19 | F | 14.6 | Toxic shock syndrome | Yes | 3 | No | 21 | 3,167 | 1.0 | 112 | 50 |
| 20 | F | 3.3 | Toxic shock syndrome | Yes | 13 | No | 20 | 525 | 1.3 | 210 | |
| 21 | F | 0.7 | Pneumococcal sepsis + shock | Yes | 16 | Yes | 29 | 1,241 | 3.2 | 346 | 22,671 |
| 22 | F | 2.4 | Pneumococcal sepsis + shock | Yes | 16 | No | 38 | 2,344 | 141.0 | 53 | |
| 23 | M | 14.8 | Staphylococcal sepsis + shock | Yes | 1 | No | 29 | 1,948 | 4.1 | 323 |
F female, M male, GC glucocorticoid therapy: dexamethasone (no. 4, 6, 14, 16, 18, 21) or hydrocortisone (no. 15), ref healthy reference. One patient died (no. 23), all other children recovered and are alive to date
A blood sample was obtained from all 23 patients at day 0, from 11 patients at day 3, from 5 patients at day 7 and from 15 patients after recovery (>3 months after t = 0). The drop-outs on day 3 and 7 were due to prior discharge from the ICU. No recovery sample was obtained from 7 patients: 2 dropped out of the study because the parents refused venapuncture after recovery, 3 were missed during follow-up, and in 2 patients RNA isolation failed in the laboratory.
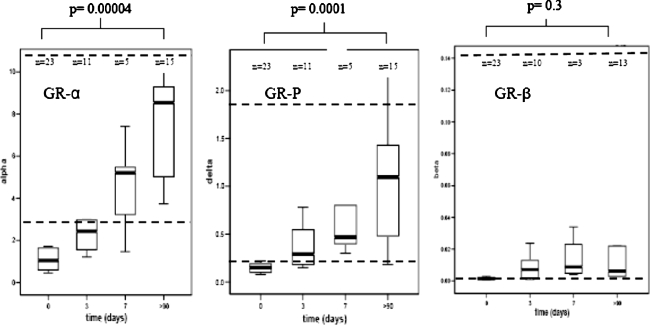
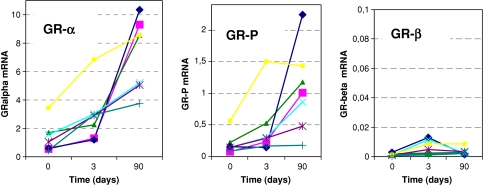
Glucocorticoid receptor mRNA levels are shown in Fig. 1. Sepsis children showed significantly lower levels of GR-α and GR-P in their neutrophils at t = 0 compared to after recovery (P = 0.00004 and 0.0001, respectively; Fig. 1). The GR-β levels did not change significantly (P 0.3; Fig. 1) [27]. Longitudinal analysis showed a linear increase for GR-α and GR-P mRNA (P = 0.000001 and 0.0001, respectively) from t = 0 to 3 and 7 days until recovery (Fig. 2). After recovery, mean GR-α, GR-P, and GR-β mRNA levels were not different in sepsis patients compared to the levels in 20 healthy adults (P value = 0.8, 0.9, and 1.0, respectively). The ±2 SD of the healthy adults is indicated by a dotted line in Fig. 1.
Fig. 1.
Levels of glucocorticoid receptor splice variants GR-α, GR-P, and GR-β mRNA in neutrophils of children with sepsis, measured at day 0, 3, 7 and after recovery (X-axis). Y-axis represents mRNA levels (copies) measured by real-time polymerase chain reaction. The dotted reference lines represent the ±2 SD of healthy adults
Fig. 2.
Longitudinal samples obtained from the seven sepsis patients with complete dataset for day 0, 3 and after recovery (X-axis). The Y-axis represents individual levels of glucocorticoid receptor splice variants GR-α, GR-P, and GR-β mRNA in neutrophils of these patients
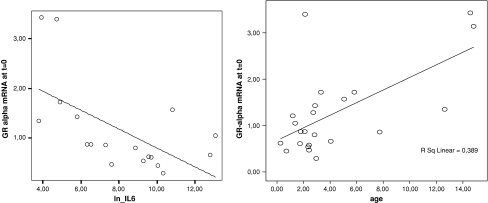
GR-α and GR-P mRNA at t = 0 were significantly correlated to IL-6 levels [correlation coefficient −0.60 (P = 0.009) and −0.49 (P = 0.04), respectively; Table 2, Fig. 3]. IL-6 was significantly correlated to PRISM score (correlation coefficient 0.60, P-value 0.008). GR-α at t = 0 was significantly correlated to age (Table 2 and Fig. 3). No correlation was found between age and IL-6 levels [correlation coefficient −0.164 (P = 0.52)]. After recovery, no correlation was found between age and GR mRNA levels. No correlation was found between GR splice variant levels at t = 0 and PRISM score, serum levels of CRP, cortisol, or ACTH (Table 2). No differences in glucocorticoid receptor mRNA levels were found in groups divided by gender, presence of shock, diagnosis, or length of stay in the intensive care unit (Table 3). No differences in glucocorticoid receptor mRNA levels were found in groups divided by medication, with the exception of significant lower mean GR-P levels in children who received glucocorticoid treatment compared to children without glucocorticoid treatment (Table 3).
Table 2.
Correlation of glucocorticoid receptor mRNA levels with patient characteristics
| CC | P value | CC | P value | CC | P value | |
|---|---|---|---|---|---|---|
| GR-α | GR-P | GR-β | ||||
| Age | 0.53 | 0.01 | 0.35 | 0.10 | −0.16 | 0.95 |
| PRISM | −0.16 | 0.47 | −0.14 | 0.52 | −0.17 | 0.94 |
| CRP | 0.25 | 0.91 | −0.79 | 0.72 | −0.54 | 0.82 |
| IL-6 | −0.60 | 0.009 | −0.49 | 0.04 | −0.17 | 0.51 |
| Cortisol | 0.30 | 0.18 | 0.15 | 0.52 | 0.15 | 0.52 |
| ACTH | 0.38 | 0.11 | −0.32 | 0.18 | 0.17 | 0.49 |
CC Correlation coefficient, GR glucocorticoid receptor, PRISM pediatric risk of mortality score, CRP C-reactive protein
Fig. 3.
Correlation between Interleukin-6 and GR-α mRNA levels at t = 0 and correlation between age and GR-α mRNA levels at t = 0
Table 3.
Mean glucocorticoid receptor mRNA expression at t = 0 grouped by sex, diagnosis or medication
| n | GR-α | P valuea | GR-P | P valuea | GR-β | P valuea | |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male | 13 | 1.27 | 0.92 | 0.18 | 0.89 | 0.0012 | 0.21 |
| Female | 10 | 1.23 | 0.19 | 0.0048 | |||
| Shock | |||||||
| Yes | 14 | 1.19 | 0.67 | 0.18 | 0.94 | 0.0014 | 0.19 |
| No | 9 | 1.36 | 0.19 | 0.0055 | |||
| Diagnosis: meningococcal | |||||||
| Yes | 15 | 1.11 | 0.29 | 0.15 | 0.23 | 0.0036 | 0.44 |
| No | 8 | 1.54 | 0.24 | 0.0013 | |||
| Length of ICU stay <7 days | |||||||
| Yes | 16 | 1.34 | 0.52 | 0.18 | 0.83 | 0.0032 | 0.66 |
| Nob | 7 | 1.07 | 0.20 | 0.0018 | |||
| Glucocorticoids | |||||||
| No | 16 | 1.45 | 0.14 | 0.23 | 0.04 | 0.0015 | 0.23 |
| Yes | 7 | 0.82 | 0.08 | 0.0052 | |||
| Ethomidate | |||||||
| No | 18 | 1.43 | 0.09 | 0.21 | 0.27 | 0.0031 | 0.70 |
| Yes | 5 | 0.65 | 0.11 | 0.0017 | |||
| Midazolam | |||||||
| No | 10 | 1.38 | 0.59 | 0.20 | 0.72 | 0.0013 | 0.44 |
| Yes | 13 | 1.17 | 0.17 | 0.0036 | |||
| Inotropic support | |||||||
| No | 8 | 1.48 | 0.71 | 0.2 | 0.73 | 0.0011 | 0.64 |
| Yes | 15 | 1.14 | 0.18 | 0.0036 | |||
aComparion of means by one-way ANOVA
bDeceased patient was analysed in the group of ICU stay >7 days
Discussion
This study shows that, in neutrophils of children with sepsis or septic shock, glucocorticoid receptor mRNA levels are suppressed (Figs. 1 and 2). During follow-up, the GR mRNA levels gradually increase at days 3 and 7 and have normalized after recovery (Figs. 1 and 2). Our study demonstrates a transient decline of glucocorticoid receptor mRNA levels in neutrophils during sepsis. This implicates enhanced cortisol resistance and thus augmented immune activation of neutrophils during sepsis. Activated neutrophils are important in fighting sepsis [28]. After bacterial invasion of the body, they produce an anti-infectious response by producing pro-inflammatory mediators like cytokines and nitric oxide [28, 29]. Exogenous glucocorticoids are known to generally suppress the neutrophil activation status. However, in sepsis patients with high endogenous cortisol levels, their numbers increase and the phagocytic and bactericidal capacity in circulating neutrophils of septic patients have been found to be higher than in controls [30]. This could be a useful endogenous adaptation in the acute phase of sepsis when fighting the bacterial infection has priority.
The decrease of GR mRNA levels in sepsis neutrophils that we found in our study could represent a tissue-specific effect. Other studies reported glucocorticoid receptor down-regulation as well as up-regulation in different tissues, measured by protein amounts (western blots) or by binding-assays. Reduced GR binding in liver, lung, and spleen was seen in endotoxin-treated rats [31, 32], while increased GR mRNA levels and binding activity were found in muscle [19, 33]. In humans, peripheral blood lymphocytes from sepsis patients showed an increased cortisol sensitivity which suggests increased amount of GR [21]. The currently available technologies provide the possibility of measuring mRNA levels in a more precise, quantitative way. To our knowledge, this is the first time that glucocorticoid receptor mRNA levels have been studied in neutrophils during sepsis. The decrease in GR mRNA levels in sepsis neutrophils found in our study differs from other tissues described in literature. This indicates a tissue-specific change in cortisol sensitivity during sepsis. Thus, while some tissues might benefit from high cortisol levels with increased cortisol sensitivity, other tissues may need to become cortisol-resistant to function optimally. More studies are needed aiming at unraveling the mechanism of tissue-specific transient changes of glucocorticoid sensitivity. An important barrier for studying tissues other than blood is the difficulty of obtaining these in humans. However, in vitro cell lines or postmortem material could be used. Development of tissue-specific corticosteroid therapy might be important for improving future treatment strategies.
In our study, glucocorticoid receptor mRNA levels were negatively correlated with IL-6 (Table 2, Fig. 3). IL-6 was also significantly correlated to the PRISM score (a measure of disease severity). This could imply that severity of disease plays a role in glucocorticoid receptor down-regulation. The gradual rise of glucocorticoid receptor mRNA levels on days 3 and 7 also coincided with the clinical improvement seen in the first week. Previously, IL-6 levels has been shown to be associated with outcome with sepsis nonsurvivors showing significantly higher mean IL-6 levels (1,195.5 × 103 pg/ml) compared to sepsis shock survivors (45.9 × 103 pg/ml) and sepsis survivors (0.4 × 103 pg/ml) [5]. No correlation was found between glucocorticoid receptor mRNA levels and PRISM score, which might be explained by the broader range of IL-6 (disentangling severity variation), compared to PRISM. On the other hand, the negative correlation of glucocorticoid receptor mRNA levels and IL-6 could represent a direct effect of pro-inflammatory cytokines like IL-6 on the down-regulation of glucocorticoid receptor mRNA expression. Previous in vitro studies showed that after stimulation of neutrophils with pro-inflammatory cytokines, a lower GR-α/GR-β ratio was found [9, 34, 35]. Another explanation could be that glucocorticoid receptor down-regulation is a cortisol-driven phenomenon as it coincides with elevation of cortisol levels. The significantly decreased levels of GR-P in children with glucocorticoid treatment may further support this hypothesis. However, the levels of GR-α in glucocorticoid-treated children are not significantly lower. GR-α mRNA levels were suppressed at all ages, but an age-dependent correlation was found at t = 0 in the direction of more GR down-regulation at a younger age (Table 2, Fig. 3). Thus, the ability of suppressing GR mRNA during sepsis might decrease with age. After recovery, no age-dependent variation was found in children. Also, no difference was found between GR mRNA levels in children after recovery and healthy adults (Fig. 1). We have no indication that the variation of GR mRNA levels at t = 0 was influenced by factors like gender, presence of shock, diagnosis, PRISM, plasma levels of cortisol, ACTH, CRP, length of stay in the intensive care unit, or use of medication, while no associations were found with any of these variables (Tables 2 and 3). However, our study results might be limited by sample size.
We aimed at studying children with the advantage of studying pure sepsis without interference of chronic disease or medication. However, the results might not apply to adults. In addition, the amount of blood that can be drawn from children is limited, so protein levels could not be measured. Measuring protein and mRNA levels simultaneously might be interesting to perform in adults. Although previous studies have shown correlations [19, 20], the measurement of mRNA levels may not necessarily correlate with protein levels in sepsis patients. We studied neutrophils only; studying other types of immune cells would be interesting, but in a previous study we found that it is hampered by difficulty of isolating pure cell subpopulations [25]. Ficoll-separated leukocytes of sepsis patients are not suitable for studying mononuclear cells because this fraction is highly contaminated with granulocytes. Interestingly, lymphocytes, which are prone to apoptosis, and neutrophils, which are prone to proliferation during sepsis, show opposite glucocorticoid receptor mRNA regulation. This apparent paradox deserves further study to identify tissue-specific regulators of GR expression and splice variants during sepsis.
In conclusion, children with sepsis showed a transient depression of the glucocorticoid receptor splice variants GR-α and GR-P mRNA in their neutrophils. This feature may represent a tissue-specific adaptation during sepsis leading to increased cortisol resistance of neutrophils. Understanding the mechanism of cortisol sensitivity in immune cells could lead to development of new treatment strategies. While some tissues benefit from glucocorticoid treatment, other tissues might not. Future treatment strategies, aimed at timing and tissue-specific regulation of glucocorticoids, might benefit patients with sepsis or other immune and inflammatory diseases.
Acknowledgment
This study was an investigator-driven study, supported by a grant from Pfizer Pharmaceuticals.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Webster JI, Sternberg EM (2004) Role of the hypothalamic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J Endocrinol 181:207–221 [DOI] [PubMed]
- 2.Jacobi J (2006) Corticosteroid replacement in critically ill patients. Crit Care Clin 22:245–253, vi [DOI] [PubMed]
- 3.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J (2008) Hydrocortisone therapy for patients with septic shock. N Engl J Med 358:111–124 [DOI] [PubMed]
- 4.Joosten KF, de Kleijn ED, Westerterp M, de Hoog M, Eijck FC, Hop WCJ, Voort EV, Hazelzet JA, Hokken-Koelega AC (2000) Endocrine and metabolic responses in children with meningoccocal sepsis: striking differences between survivors and nonsurvivors. J Clin Endocrinol Metab 85:3746–3753 [DOI] [PubMed]
- 5.den Brinker M, Joosten KF, Liem O, de Jong FH, Hop WC, Hazelzet JA, van Dijk M, Hokken-Koelega AC (2005) Adrenal insufficiency in meningococcal sepsis: bioavailable cortisol levels and impact of interleukin-6 levels and intubation with etomidate on adrenal function and mortality. J Clin Endocrinol Metab 90:5110–5117 [DOI] [PubMed]
- 6.Huizenga NA, Koper JW, de Lange P, Pols HA, Stolk RP, Grobbee DE, de Jong FH, Lamberts SW (1998) Interperson variability but intraperson stability of baseline plasma cortisol concentrations, and its relation to feedback sensitivity of the hypothalamo-pituitary-adrenal axis to a low dose of dexamethasone in elderly individuals. J Clin Endocrinol Metab 83:47–54 [DOI] [PubMed]
- 7.Miller AH, Spencer RL, Pearce BD, Pisell TL, Azrieli Y, Tanapat P, Moday H, Rhee R, McEwen BS (1998) Glucocorticoid receptors are differentially expressed in the cells and tissues of the immune system. Cell Immunol 186:45–54 [DOI] [PubMed]
- 8.Prigent H, Maxime V, Annane D (2004) Science review: mechanisms of impaired adrenal function in sepsis and molecular actions of glucocorticoids. Crit Care 8:243–252 [DOI] [PMC free article] [PubMed]
- 9.Zhou J, Cidlowski JA (2005) The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids 70:407–417 [DOI] [PubMed]
- 10.van den Akker EL, Koper JW, van Rossum EF, Dekker MJ, Russcher H, de Jong FH, Uitterlinden AG, Hofman A, Pols HA, Witteman JC, Lamberts SW (2008) Glucocorticoid receptor gene and risk of cardiovascular disease. Arch Intern Med 168:33–39 [DOI] [PubMed]
- 11.van den Akker EL, Nouwen JL, Melles DC, van Rossum EF, Koper JW, Uitterlinden AG, Hofman A, Verbrugh HA, Pols HA, Lamberts SW, van Belkum A (2006) Staphylococcus aureus nasal carriage is associated with glucocorticoid receptor gene polymorphisms. J Infect Dis 194:814–818 [DOI] [PubMed]
- 12.van den Akker EL, Russcher H, van Rossum EF, Brinkmann AO, de Jong FH, Hokken A, Pols HA, Koper JW, Lamberts SW (2006) Glucocorticoid receptor polymorphism affects transrepression but not transactivation. J Clin Endocrinol Metab 91:2800–2803 [DOI] [PubMed]
- 13.van Rossum EF, Lamberts SW (2004) Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Recent Prog Horm Res 59:333–357 [DOI] [PubMed]
- 14.Schaaf MJ, Cidlowski JA (2002) AUUUA motifs in the 3’UTR of human glucocorticoid receptor alpha and beta mRNA destabilize mRNA and decrease receptor protein expression. Steroids 67:627–636 [DOI] [PubMed]
- 15.de Lange P, Segeren CM, Koper JW, Wiemer E, Sonneveld P, Brinkmann AO, White A, Brogan IJ, de Jong FH, Lamberts SW (2001) Expression in hematological malignancies of a glucocorticoid receptor splice variant that augments glucocorticoid receptor-mediated effects in transfected cells. Cancer Res 61:3937–3941 [PubMed]
- 16.Bamberger CM, Bamberger AM, de Castro M, Chrousos GP (1995) Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest 95:2435–2441 [DOI] [PMC free article] [PubMed]
- 17.Pujols L, Xaubet A, Ramirez J, Mullol J, Roca-Ferrer J, Torrego A, Cidlowski JA, Picado C (2004) Expression of glucocorticoid receptors alpha and beta in steroid sensitive and steroid insensitive interstitial lung diseases. Thorax 59:687–693 [DOI] [PMC free article] [PubMed]
- 18.Lu NZ, Cidlowski JA (2004) The origin and functions of multiple human glucocorticoid receptor isoforms. Ann N Y Acad Sci 1024:102–123 [DOI] [PubMed]
- 19.Sun X, Fischer DR, Pritts TA, Wray CJ, Hasselgren PO (2002) Expression and binding activity of the glucocorticoid receptor are upregulated in septic muscle. Am J Physiol Regul Integr Comp Physiol 282:R509–R518 [DOI] [PubMed]
- 20.Goecke IA, Alvarez C, Henriquez J, Salas K, Molina ML, Ferreira A, Gatica H (2007) Methotrexate regulates the expression of glucocorticoid receptor alpha and beta isoforms in normal human peripheral mononuclear cells and human lymphocyte cell lines in vitro. Mol Immunol 44:2115–2123 [DOI] [PubMed]
- 21.Molijn GJ, Spek JJ, van Uffelen JC, de Jong FH, Brinkmann AO, Bruining HA, Lamberts SW, Koper JW (1995) Differential adaptation of glucocorticoid sensitivity of peripheral blood mononuclear leukocytes in patients with sepsis or septic shock. J Clin Endocrinol Metab 80:1799–1803 [DOI] [PubMed]
- 22.Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF (2006) Neutrophils in development of multiple organ failure in sepsis. Lancet 368:157–169 [DOI] [PubMed]
- 23.Goldstein B, Giroir B, Randolph A (2005) International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6:2–8 [DOI] [PubMed]
- 24.Pollack MM, Ruttimann UE, Getson PR (1988) Pediatric risk of mortality (PRISM) score. Crit Care Med 16:1110–1116 [DOI] [PubMed]
- 25.van den Akker EL, Baan CC, van den Berg B, Russcher H, Joosten K, Hokken-Koelega AC, Lamberts SW, Koper JW (2008) Ficoll-separated mononuclear cells from sepsis patients are contaminated with granulocytes. Intensive Care Med 34:912–916 [DOI] [PubMed]
- 26.Russcher H, Smit P, van den Akker EL, van Rossum EF, Brinkmann AO, de Jong FH, Lamberts SW, Koper JW (2005) Two polymorphisms in the glucocorticoid receptor gene directly affect glucocorticoid-regulated gene expression. J Clin Endocrinol Metab 90:5804–5810 [DOI] [PubMed]
- 27.van den Akker EL, Koper JW, Joosten K, De Jong FH, Hazelzet JA, Lamberts SW, Hokken-Koelega AC (2007) Glucocorticoid receptor expression is decreased in granulocytes of sepsis patients. Poster, ESPE Annual Meeting, Helsinki
- 28.Marshall JC (2005) Neutrophils in the pathogenesis of sepsis. Crit Care Med 33:S502–S505 [DOI] [PubMed]
- 29.Liberman AC, Druker J, Perone MJ, Arzt E (2007) Glucocorticoids in the regulation of transcription factors that control cytokine synthesis. Cytokine Growth Factor Rev 18:45–46 [DOI] [PubMed]
- 30.Ahmed NA, McGill S, Yee J, Hu F, Michel RP, Christou NV (1999) Mechanisms for the diminished neutrophil exudation to secondary inflammatory sites in infected patients with a systemic inflammatory response (sepsis). Crit Care Med 27:2459–2468 [DOI] [PubMed]
- 31.Duma D, Silva-Santos JE, Assreuy J (2004) Inhibition of glucocorticoid receptor binding by nitric oxide in endotoxemic rats. Crit Care Med 32:2304–2310 [DOI] [PubMed]
- 32.Stith RD, McCallum RE (1983) Down regulation of hepatic glucocorticoid receptors after endotoxin treatment. Infect Immun 40:613–621 [DOI] [PMC free article] [PubMed]
- 33.Sun X, Mammen JM, Tian X (2003) Sepsis induces the transcription of the glucocorticoid receptor in skeletal muscle cells. Clin Sci (Lond) 105:383–391 [DOI] [PubMed]
- 34.Strickland I, Kisich K, Hauk PJ, Vottero A, Chrousos GP, Klemm DJ, Leung DY (2001) High constitutive glucocorticoid receptor beta in human neutrophils enables them to reduce their spontaneous rate of cell death in response to corticosteroids. J Exp Med 193:585–593 [DOI] [PMC free article] [PubMed]
- 35.Xu Q, Leung DY, Kisich KO (2003) Serine-arginine-rich protein p30 directs alternative splicing of glucocorticoid receptor pre-mRNA to glucocorticoid receptor beta in neutrophils. J Biol Chem 278:27112–27118 [DOI] [PubMed]