Abstract
Smooth pursuit eye movement (SPEM) abnormalities in schizophrenia, although well described, are poorly understood. SPEMs are initiated by motion of an object image on the retina. During initiation, the eyes accelerate until they approximate target velocity and a state of minimal retinal motion is achieved. Pursuit is maintained through predictive eye movements based on extraretinal signals and corrections based on deviations from the fovea. Here, initiation and predictive pursuit responses were used to estimate the contributions of retinal and extraretinal signals to pursuit maintenance in schizophrenia patients’ relatives. Relatives exhibited normal initiation, but had lower predictive pursuit gain compared with controls. Relatives had normal gain during pursuit maintenance, presumably by greater reliance on retinal error. This was confirmed by group differences in regression coefficients for retinal and extraretinal measures, and suggests that schizophrenia SPEM deficits involve reduced ability to maintain or integrate extraretinal signals, and that retinal error may be used to compensate.
Descriptors: Smooth pursuit eye movements, Schizophrenia, Predictive pursuit, Extra-retinal processing, Smooth pursuit initiation
A wealth of evidence, including a recent preliminary report citing linkage of pursuit abnormality to chromosome 6p21 in relatives of schizophrenic patients, suggests that the smooth pursuit deficit marks the genetic liability to schizophrenia (Arolt et al., 1996; Clementz & Sweeney, 1990; Levy, Holzman, Matthysse, & Mendell, 1994). Although many aspects of the smooth pursuit deficit associated with the schizophrenia phenotype(s) have been well described, only recently have investigators focused on understanding the underlying neuronal mechanisms of these eye movement abnormalities (Clementz & McDowell, 1994; Ross et al., 1995; Sweeney et al., 1998). The smooth pursuit function is a highly developed and complex system served by a widely distributed neuronal network (Leigh & Zee, 1991). In recent years, many of the normal functions of the component processes of the smooth pursuit system in humans, and the underlying neurophysiological substrates in monkeys, have become known (Assad & Maunsell, 1995; Barnes, Barnes, & Chakraborti, 2000; Barton et al., 1996; Komatsu & Wurtz, 1989; Krauzlis, 2001; Lisberger, Morris, & Tychsen, 1987; MacAvoy, Gottlib, & Bruce, 1991; Newsome, Wurtz, Dersteler, & Mikami, 1985; Newsome, Wurtz, & Komatsu, 1988; Schmid, Rees, Frith, & Barnes, 2001; Suh, Leung, & Kettner, 2000). Examination of smooth pursuit in schizophrenia within a neurophysiology-based framework has an advantage over traditional methods, in that it is likely to identify specific brain regions that are associated with observed deficits. In addition, the identification of a functionally specific deficit is likely to be more sensitive and specific in identifying the phenotype compared with traditional global measures.
Normally, the pursuit system needs motion information to generate smooth pursuit eye movements. During initiation of smooth pursuit, the slippage of the image of the target on the retina, referred to hereafter as retinal motion, stimulates smooth pursuit (Lisberger et al., 1987; Newsome et al., 1985). The processing of retinal motion occurs in the mediotemporal (MT) region (Lisberger & Movshon, 1999; Newsome et al., 1985, 1988). Anticipation of image motion, at times, contributes to the initiation of smooth pursuit, and is thought to be mediated by the frontal eye fields (FEF; Braun, Boman, & Hotson, 1996; MacAvoy et al., 1991; Newsome et al., 1988). Once eye velocity approximates target velocity, retinal motion is minimal. Although highly trained monkeys are able to follow a moving target based exclusively on retinal motion processing for about a second, data suggest that predictive pursuit based on extraretinal motion signals plays an important role in maintaining pursuit performance in humans (Barnes & Asselman, 1992; Braun et al., 1996; van den Berg, 1988). There are at least two sources of extraretinal motion signals. One is a memory trace of the previous retinal motion mediated by the posterior parietal region (Assad & Maunsell, 1995). The second is a copy of the smooth pursuit motor command, the so-called efference copy, which is thought to be mediated by the medial superior temporal region (MST; Barton et al., 1996; Komatsu & Wurtz, 1989). Normally, potent extraretinal input drives the system during pursuit maintenance, with minor corrections based on retinal velocity error information (Barnes & Asselman, 1991; Lisberger et al., 1987; van den Berg 1988). The FEFs are thought to integrate this extraretinal motion information into a smooth pursuit response (MacAvoy et al., 1991).
Earlier studies have shown that patients with chronic schizophrenia and unmedicated or recently medicated first-break patients exhibit poor smooth pursuit performance in response to extraretinal motion information in the absence of retinal motion signals (Thaker, Ross, Buchanan, & Adami, 1996; Thaker et al., 1999). Subsequent studies in nonpsychotic, first-degree relatives of patients with schizophrenia have found a similar deficit— suggesting that this abnormality is marking the genetic liability (Thaker et al., 1998).
In the current paper, responses to retinal motion signals were examined in the relatives of patients with schizophrenia in order (a) to determine whether the smooth pursuit abnormality that is observed in relatives is specific to the extraretinal component, and (b) to test a model that explains the differences in smooth pursuit maintenance in relatives compared to controls. Non-psychotic, first-degree relatives of patients with schizophrenia were recruited for this study. This sample was enriched by disproportionately recruiting relatives with schizophrenia spectrum personality (SSP) symptoms. The comparison group also consisted of a subgroup with SSP but no family history of psychoses, thus controlling for SSP symptoms. In this context, note that cases of SSP when encountered in the community are likely to be heterogeneous in their origins and not necessarily schizophrenia related (Chapman, Chapman, Kwapil, Eckblad, & Zinser, 1994; Kendler et al., 1993; Lyons, Toomey, Faraone, & Tsuang, 1994; Reiss, Hagerman, Vinogradov, Abrams, & King, 1988; Squires-Wheeler, Skodol, Friedman, & Erlenmeyer-Kimling, 1988; Stanley, Turner, & Borden, 1990).
One might expect a modest schizophrenia-related impairment in pursuit initiation to the extent that anticipatory eye movements during initiation involve the predictive mechanism (Barnes & Asselman, 1991; Braun et al., 1996; Croft, Lee, Bertolot, & Gruzelier, 2001). Our hypothesis, however, predicts a larger difference between relatives and controls in predictive pursuit measures based on extraretinal motion information in the absence of retinal motion information (Thaker et al., 1996). Thus, first-degree biological relatives of patients with schizophrenia are likely to depend more on retinal motion information to maintain pursuit. Relative contributions of retinal and extraretinal motion signals to pursuit maintenance were examined using regression analyses.
Methods
Participants
Participants were recruited and assessed using methods described in detail in earlier publications (Kunkel, Adami, Zetlmeisl, Ross, & Thaker, 1998; Thaker et al., 1998). Briefly, participants were recruited from two sources: (a) first degree, biological relatives of schizophrenic patients (defined according to DSM-III-R criteria; American Psychiatric Association, 1987), and (b) the community through newspaper advertisements. To recruit community subjects with schizophrenia spectrum personality traits, the newspaper advertisements specifically listed SSP symptoms; some of the advertisements focused only on negative symptoms. All participants gave informed written consent in accordance with University of Maryland Institutional Review Board guidelines. Participants were paid $10/hour for participating. Participants underwent clinical evaluations, which included the Structured Clinical Interview for DSM-III-R(SCID) and Structured Interview for DSM-III-R Personality Disorders (SIDP-R; Pfohl, Blum, Zimmerman, & Stangl, 1989). The latter interview was expanded to include additional questions probing magical thinking, perceptual distortions, and negative symptoms (Kendler, Lieberman, & Walsh, 1989; Kirkpatrick, Buchanan, McKenney, Alphs, & Carpenter, 1989). Community subjects underwent interviews for Family History Research Diagnostic Criteria (FH-RDC; Andreasen, Rice, Endicott, Reich, & Coryell, 1986). The interrater reliabilities among the clinical interviewers were above 0.81 (kappa) on these instruments. All available information was reviewed in a diagnostic meeting to reach DSM-III-R, Axis I diagnoses. Personality traits were also evaluated and participants were assigned to SSP groups according to modified DSM-III-R criteria (three or more paranoid traits, three or more schizoid traits, or four or more schizotypal traits). Individuals without SSP traits but with other personality disorders were excluded.
Participants were divided into four subgroups based on the subject source and the presence of schizotypal, schizoid, or paranoid personalities. The four groups were: community participants (a) with (n=29) and (b) without SSP (n=41) and relatives (c) with (n=23) and (d) without SSP (n=42). The community participants did not have a family history of psychoses according to FH-RDC. The mean (±SD) ages of the four groups were 32.4±8.7, 34.4± 8.7, 36.6±7.6, and 34.7±8.0 years, respectively. These values were not statistically different. The mean (±SD) two-factor Hollingshead socioeconomic status (SES) scores in the four subject groups were 3.41±0.87, 2.73±0.94, 3.43±0.95, and 2.98±0.92, respectively (Hollingshead & Redlich, 1988). Analysis of variance revealed that the SSP groups exhibited significantly lower SES compared with non-SSP community and family volunteers, F(1,131)= 10.31, p<.001.
Laboratory Procedures
Ramp mask ramp task
A target (a cross in a 0.25° by 0.25° box) was presented on a 15-in. flat monitor placed 27 in. away from a chin support frame (approximately 28 in. from the eyes). A trial started with a fovea-petal step-ramp with unpredictable onset. Three target speeds were presented, 9.4°, 14.0°, and 18.7°/s across different trials. The initial step size was varied across trials depending on the target speed used in that trial, so that the target crossed the center point after 133 ms of motion. Initiation data were collected from a total of 25 trials, presented in two blocks of 12 and 13 trials. After the initial step-ramp, the target continued moving in a horizontal plane back and forth at a constant velocity. One sweep across the monitor from one extreme point to the other constituted a half-cycle, which had an amplitude of 24° of visual angle. After four to six half-cycles, the target was unpredictably masked (made invisible) for 500 ms. Of the 25 trials presented, 15 had the mask appearing variably sometime during a half-cycle. In this condition, five trials were presented at each target speed. In the remaining trials, the mask occurred at the end of a half-cycle. In this condition, only two target speeds, 14.0° and 18.7°/s, were presented. At the end of each trial, the target was stationary for a period of 1.5 s at a 12° point to the left or right of center, at which point the target returned to center. Another calibration step of 12° was inserted in the opposite direction to the previous 12° fixation. Participants were instructed to follow the moving target even when it became briefly invisible.
Ocular motor data acquisition and analysis
Eye movement data were collected using an infrared technique (Applied Science Laboratory 210 model; sampling rate 333 Hz with a time constant of 4 ms), a 16-bit analogue-to-digital converter, and data acquisition and analysis software (Aqcknowledge, Goleta, CA and Igor, Wavemetrics, Lake Oswego, OR). The analysis was conducted blind to the participant’s identity. The methods for the analysis of smooth pursuit eye movements have been published previously and are only briefly described here (Ross et al., 1996; Thaker et al., 1998; van den Berg 1988). The digital data were filtered off-line using a low pass filter at a cutoff frequency of 75 Hz. Saccades were identified based on velocity (>35°/s) and acceleration (>600°/s2) criteria. Saccades were replaced in the eye velocity record by linear interpolation between the two points just after and before the saccade. The record was then low-pass filtered at 20 Hz. Interpolated data, which originally were part of the saccade, were identified as missing data points so as not to be included in subsequent calculations.
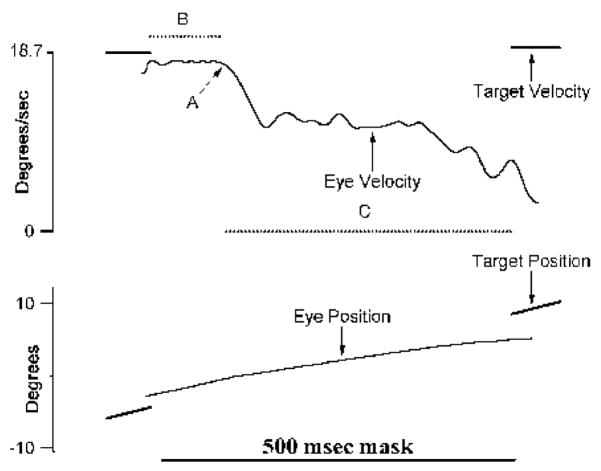
Mean pursuit initiation gain and acceleration from the first 100 ms of pursuit onset were obtained. Closed-loop gain that provides a measure of pursuit maintenance was measured from half-cycles that preceded the half-cycle with the mask. This was calculated using the standard method of averaging eye velocity during pursuit maintenance and then dividing by the target velocity. For this analysis, eye velocity from 133 ms before and after the change in direction of the target was not used. Residual predictive pursuit gain, obtained from masks occurring during a half-cycle and after the eye velocity was stabilized (Becker & Fuchs, 1985; Thaker et al., 1998) was used as a proxy measure of the response to only extraretinal motion (see Figure 1 for an operational definition of residual predictive pursuit gain). The stabilized SPEM response in the absence of target motion information is not likely to be contaminated by retinal motion information, and has been shown to differentiate patients with schizophrenia from normal controls (van den Berg, 1988).
Figure 1.
The top panel shows eye and target velocity data, and the bottom panel corresponding position data from a 500-msmask occurring during a ramp. Eye velocity data were filtered at 20 Hz. As one can see, the eye velocity remained unchanged for about 95 ms after the target was extinguished (B), presumably still influenced by the prior closed-loop response. After this initial period, the eye velocity stabilized to a lower level (58% of the closed-loop response (C), arguably the response based on extraretinal motion signals. Residual predictive gain was calculated by dividing average eye velocity during C by expected target velocity. The transition point from closed-loop to extraretinal response, which is marked by A, was identified by an algorithm. The program searches for the time point within the mask when the eye velocity first decreases by 50% of the premask value. From this point backwards, the algorithm searches for the local minimum or maximum value (depending on target direction) by analyzing the smoothed first and second derivatives. This is identified as the transition point.
Smooth pursuit measures from the mask period when the mask occurred at the beginning of a half-cycle were not considered here because data were only available for 14.0° and 18.7°/s target presentations. These data are presented elsewhere (Thaker et al., 1998).
Data Analysis
For each subject, the data were collapsed across trials to obtain mean values, which were averaged to get the group means. Subjects were included if they finished 60% or more of the trials in a given condition. Previous analyses showed no significant effects of target direction in the half-cycle in which the mask occurred, the number of half-cycles before the occurrence of the mask, or their interactions with group membership. Thus, the data were collapsed across these factors. Smooth pursuit performance during initiation and target masking were examined using mixed design repeated measures analyses of variance (ANOVA) with two within-subject factors (pursuit phase and target velocity), and two between-subject factors (subject source and SSP status). In addition, mixed design repeated measures analyses of variance were used to assess group differences in pursuit latency and mean eye acceleration at the three target velocities.
Repeated measures were run using the SPSS MANOVA procedure in order to avoid inflation of type I error rates associated with violations of the assumption of sphericity (Vasey & Thayer 1987). In no instance were multivariate results different from those that would be obtained assuming sphericity and employing Greenhouse–Geisser corrections. Significant interactions were probed using simple effects procedures described by Levine (1991). The relationships between the closed-loop gain and the gain of responses to retinal and extraretinal motion signals were modeled by separate regression analyses in relatives and community subjects. Data from all three target velocities were included in these analyses.
Results
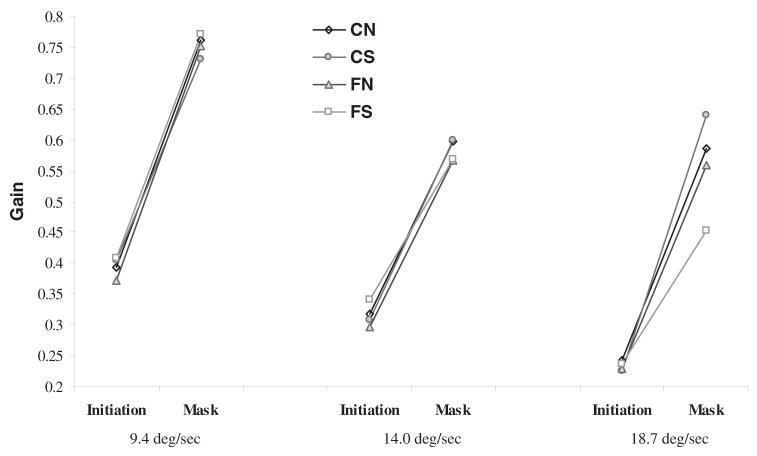
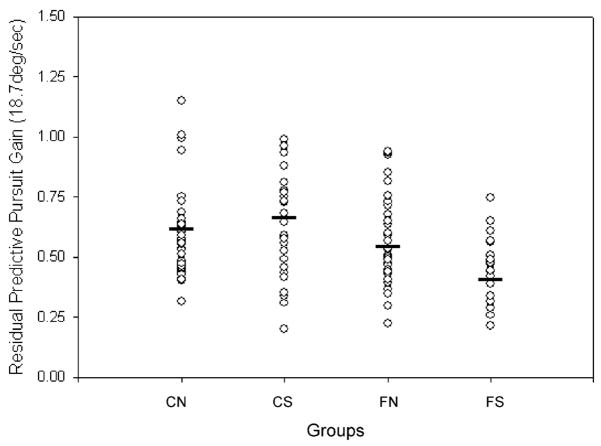
Smooth pursuit gain during maintenance was not significantly different between relatives and community subjects. Pursuit gain in response to retinal motion (i.e., during initiation) and to extraretinal motion were examined. A repeated measures ANOVA revealed a four-way interaction involving Subject Source × SSP Status × Target Velocity × Pursuit Phase (i.e., initiation vs. mask), F(2,262)=3.12, p<.05 (see Figure 2). Separate repeated measures employing the MANOVA procedure with simple effects were conducted for initiation and mask pursuit gain. No significant main effects or interactions were found for pursuit gain during initiation. However, the analysis of pursuit gain during the mask (i.e., residual predictive pursuit gain) revealed a significant Subject Source × SSP Status × Velocity interaction, F(2,262)=4.84, p<.01. Lower order effects included a main effect of velocity, F(2,262)=71.51, p<.001. A simple effects analysis of the three-way interaction (examining subject source, SSP status, and subject source by SSP status within each level of velocity) revealed a significant Subject Source × SSP Status interaction at a target velocity of 18.7°/s, F(1,131)=6.49, p=.01. A lower order effect of subject source (i.e., family status) was also observed, F(1,131)=11.74, p<.005. Post hoc analyses of the interaction showed that the SSP relatives exhibited significantly lower residual predictive pursuit gain compared with the two community subject groups (see Table 1 and Figure 3); performance in the non-SSP relatives was not statistically different from any of the other three groups.
Figure 2.
Smooth pursuit gain during initiation and during the mask. CN: community normals, CS: community subjects with spectrum personality symptoms, FN: normal relatives, and FS: relatives with spectrum personality symptoms.
Table 1.
Measures of Pursuit under Open- and Closed-Loop Conditions
| Community subjectsa |
First-degreee relativesa |
|||
|---|---|---|---|---|
| Target velocity (°/s) | Non-SSP (n = 41) | SSP (n = 29) | Non-SSP (n = 42) | SSP (n = 23) |
| Closed-loop condition | ||||
| Pursuit gain | ||||
| 9.4 | 0.88 (0.10) | 0.88 (0.11) | 0.87 (0.14) | 0.86 (0.14) |
| 14 | 0.86 (0.13) | 0.85 (0.16) | 0.85 (0.16) | 0.80 (0.22) |
| 18.7 | 0.82 (0.15) | 0.82 (0.15) | 0.75 (0.18) | 0.76 (0.17) |
| Mask condition | ||||
| Residual | ||||
| 9.4 | 0.76 (0.18) | 0.73 (0.20) | 0.75 (0.21) | 0.77 (0.15) |
| 14 | 0.60 (0.12) | 0.60 (0.14) | 0.57 (0.15) | 0.57 (0.21) |
| 18.7 | 0.59 (0.17) | 0.64 (0.21) | 0.56 (0.18) | 0.45 (0.13)b |
| Open-loop condition | ||||
| Pursuit latency (in ms) | ||||
| 9.4 | 212 (41) | 201 (47) | 204 (33) | 205 (40) |
| 14 | 195 (33) | 182 (42) | 187 (35) | 189 (39) |
| 18.7 | 200 (38) | 195 (36) | 195 (36) | 196 (33) |
| Open-loop acceleration (in °/s/s) | ||||
| 9.4 | 68 (39) | 65 (23) | 60 (22) | 59 (25) |
| 14 | 77 (49) | 74 (31) | 71 (30) | 76 (18) |
| 18.7 | 88 (44) | 79 (37) | 76 (40) | 75 (26) |
Mean (±SD) values are shown.
SSP relatives exhibited significantly lower residual predictive pursuit gain compared with community groups at 18.7°/s target velocity. Confirmed by a significant Subject Source × SSP Status × Velocity interaction, F(2,262)=4.84, p<.01, and post hoc comparisons (see Results).
Figure 3.
Individual data for residual predictive pursuit gain (18.7°/s) in the four groups. CN: community normals, CS: community subjects with spectrum personality symptoms, FN: normal relatives, and FS: relatives with spectrum personality symptoms. Solid bars indicate the means for the four groups.
The smooth pursuit latencies at initiation were similar in relatives and community subjects (193±34 and 192±29 ms, respectively). Eye acceleration during the first 100 ms of initiation was not statistically different across groups (Table 1).
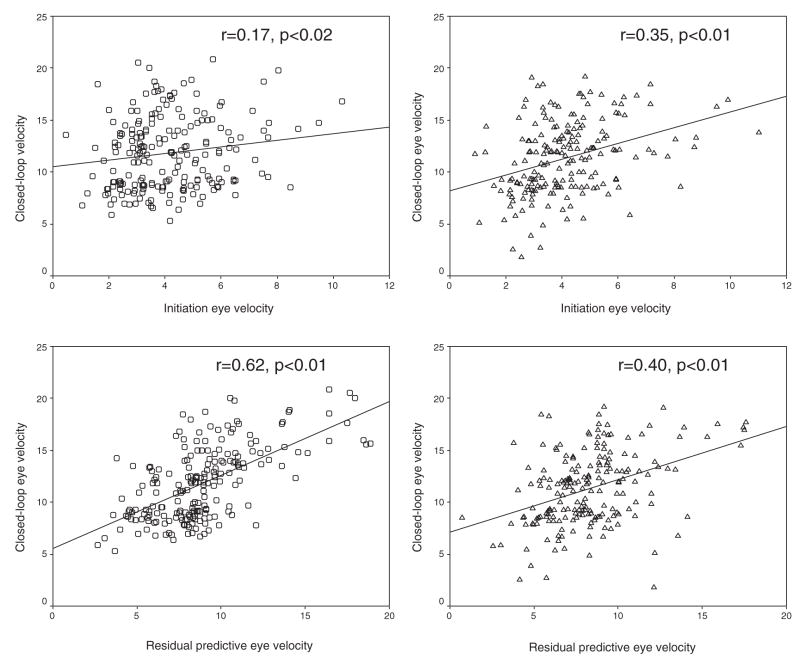
Regression analyses were used to separately model how smooth pursuit is maintained in relatives and community subjects by examining the relationships between eye velocity during pursuit maintenance and its two components: responses to retinal and extraretinal motion signals. The results are summarized in Table 2. In both groups, retinal and extraretinal components significantly predicted the eye velocity during pursuit maintenance (p<.01). Bivariate correlations between the dependent and the two independent variables are shown in Figure 4. Standardized regression coefficients (Beta values) associated with the two independent variables were significantly different in the two samples. The standardized regression coefficient associated with retinal velocity was significantly smaller in community subjects (β=.127, SE=0.054) compared with the relatives (β=0.329, SE=0.062; z= −2.46, p≤.02 (Cohen & Cohen, 1983). In contrast, the coefficient associated with extraretinal velocity was significantly greater in the community subjects (β=.612, SE=0.054) compared with the relatives (β=.377, SE=0.062; z=2.86, p<.002). Separate regression analyses in subgroups of relatives showed similar findings to those obtained for the entire family cohort: Standardized regression coefficients (SE) values associated with the initiation eye velocity were .37 (0.07) and .27 (0.13) in the nonSSP and SSP relatives, respectively; and, were .47 (0.07) and .17 (0.12) for eye velocity during the mask.
Table 2.
Regression Analyses in Community Subjects and Relatives. Model: Pursuit Maintenance Eye Velocity=Betar × Retinal Eye Velocity + Betaer × Extraretinal Eye Velocitya
| Group | Multiple R | Eye velocity (closed-loop)a | Betar | SEr | Eye velocity (retinal)a | Betaer | SEer | Eye velocity (extraretinal)a |
|---|---|---|---|---|---|---|---|---|
| Community subjects | 0.63b | 11.8±3.5 | .13 | 0.05 | 4.2±1.9 | .61 | 0.05 | 8.9±3.1 |
| Relatives | 0.50b | 11.3±3.5 | .30 | 0.06 | 4.1±1.6 | .38 | 0.06 | 8.2±2.8 |
| p valuesc | .02 | .01 |
Mean (±SD) values for eye velocities were obtained from (a) the maintenance phase (i.e., closed-loop condition), (b) the initiation phase (response to only retinal signals), and (c) the mask period (response to only extraretinal signals).
Regression model was significant in the community subjects, F(2,209)=7.04, p<.001, as well as in relatives, F(2,196)=32.8, p<.001.
Results of statistical analyses comparing regression coefficients (betas) in the community subjects and relatives (Cohen & Cohen, 1983).
Figure 4.
The four scatter graphs illustrate bivariate correlations between eye velocity under closed-loop condition (y-axes) and during initiation (top two graphs) and during the mask (bottom two graphs). The two graphs on the left are from the community subjects and on the right from the relatives.
Discussion
The current study examined components of smooth pursuit eye movements in relatives of patients with schizophrenia and a well-matched comparison group recruited from the community. Smooth pursuit responses to retinal motion signals were examined and the contributions of retinal and extraretinal components to the closed-loop pursuit response were modeled in each group. Results show that smooth pursuit is maintained primarily by extraretinal information in community control subjects, consistent with the hypothesis that pursuit maintenance is predominantly driven by extraretinal information (Barnes et al., 1991; Lisberger et al., 1987; van den Berg, 1988). Relatives of patients with schizophrenia exhibit normal pursuit initiation, which suggests that they are able to process target image motion on the retina and produce a normal oculomotor response based on this information. However, relatives showed a specific deficit in their pursuit response involving extraretinal motion signals. Both retinal and extraretinal components significantly contributed to the prediction of eye velocity during pursuit maintenance in each group, but the relationships between the dependent and independent variables in the regression models were significantly different between the groups. These analyses suggest that pursuit maintenance among relatives is more heavily dependent on retinal motion information processing compared with community subjects, who are able to maintain pursuit based on extraretinal information processing. This is consistent with experiments suggesting that retinal and extraretinal signals interact to produce a motion percept (Turano & Massof, 2001).
The finding of normal pursuit initiation in relatives of patients with schizophrenia was inconsistent with our hypothesis (that, to the extent that prediction plays a role in the initiation response, relatives would show a modest deficit). It is likely that by varying the duration of the fixation period prior to the onset of target motion, we were able to make the target onset unpredictable. This would then minimize the role of prediction in pursuit initiation. Furthermore, the findings are in apparent disagreement with a study in our laboratory in patients with schizophrenia and other published studies in patients and their relatives (Clementz & McDowell, 1994; Clementz, Reid, McDowell, & Cadenhead, 1995; Ross et al., 1996). Several factors including differences in the tasks (differences in the extent to which the target onset was predictable) and subject groups studied can explain these inconsistencies. In our previous study in patients with schizophrenia, the target onset was less variable and the affected group consisted of subjects with overt psychosis, most on antipsychotic and other medications, and some with the deficit syndrome. The latter is known to be associated with early visual processing difficulties (Buchanan, Strauss, Breier, Kirkpatrick, & Carpenter, 1997). Furthermore, recent data suggests that impairment in initiation is specific to patients with the deficit syndrome and their relatives (Hong, Avila, Adami, Elliot, & Thaker, in press). Both the relatives and control subjects in the study by Clementz et al. were older than participants in the current study (Clementz et al., 1995). The statistical correction procedures used to adjust for age may have been biased by the fact that the effects of age on eye movement measures are often nonlinear, with severe deterioration in function after the age of 50 (Ross et al., 1999).
Findings of abnormal pursuit response involving extraretinal information processing and normal response to retinal motion information implicate several neuroanatomic regions that subserve the smooth pursuit eye movement system. Several regions in the brain are thought to be involved in the maintenance of an internal representation of previous target and/or eye velocity information during brief target extinction periods. MST and its projection areas in the dorsolateral pontine nucleus and cerebellar structures, particularly the flocculus (Komatsu & Wurtz, 1989; Lisberger et al., 1987) are involved in maintaining an efference copy. Neurons in the posterior parietal cortex are thought to encode previous retinal slip velocity information (Ferrera, Rudooph, & Maunsell, 1994; Trestman et al., 1995). Such extraretinal motion information is thought to be integrated into a predictive smooth pursuit response by frontal eye fields (MacAvoy et al., 1991). Consistent with the above postulations, a study in our laboratory noted that abnormal smooth pursuit eye movements in schizophrenic patients were associated with changes in cerebral glucose metabolism in frontal and posterior parietal cortical regions (Ross et al., 1995).
In conclusion, data are presented that suggest that individuals with the genetic liability to schizophrenia have a specific deficit in processing and/or utilizing extraretinal motion signals during smooth pursuit eye movements. Identification of a functionally specific abnormality in smooth pursuit is informative regarding the underlying abnormality in neuronal circuitry associated with genetic risk for schizophrenia. In addition, a functionally specific measure is likely to improve the accuracy of identifying disease-related phenotypes. Indeed, a recent report using Receiver Operating Characteristic Curve analysis has shown that the predictive accuracy of predictive pursuit measures in identifying schizophrenia risk is significantly higher compared with root mean square error and closed-loop (Avila, McMahon, Elliott, & Thaker, 2002).
Acknowledgments
The work presented in the paper was supported by NIH grant MH49826 and MH40279.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: Author; 1987. [Google Scholar]
- Andreasen NC, Rice J, Endicott J, Reich T, Coryell W. The family history approach to diagnosis. How useful is it? Archives of General Psychiatry. 1986;43:421–429. doi: 10.1001/archpsyc.1986.01800050019002. [DOI] [PubMed] [Google Scholar]
- Arolt V, Lencer R, Nolte A, Muller-Myhsok B, Purmann S, Schurmann M, Leutelt J, Pinnow M, Schwinger E. Eye tracking dysfunction is a putative phenotypic susceptibility marker of schizophrenia and maps to a locus on chromosome 6p in families with multiple occurrence of the disease. American Journal of Medical Genetics. 1996;67:564–579. doi: 10.1002/(SICI)1096-8628(19961122)67:6<564::AID-AJMG10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Assad JA, Maunsell JH. Neuronal correlates of inferred motion in primate posterior parietal cortex. Nature. 1995;373:518–521. doi: 10.1038/373518a0. [DOI] [PubMed] [Google Scholar]
- Avila M, McMahon RP, Elliott AR, Thaker GK. Neurophysiological markers of genetic liability to schizophrena: Sensitivity and specificity to specific quantitative eye movement measures. Journal of Abnormal Psychology. 2002;111:259–267. [PubMed] [Google Scholar]
- Barnes GR, Asselman PT. The mechanism of prediction in human smooth pursuit eye movements. Journal of Physiology. 1991;439:439–461. doi: 10.1113/jphysiol.1991.sp018675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GR, Asselman PT. Pursuit of intermittently illuminated moving targets in the human. Journal of Physiology. 1992;445:617–637. doi: 10.1113/jphysiol.1992.sp018943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GR, Barnes DM, Chakraborti SR. Ocular pursuit responses to repeated, single-cycle sinusoids reveal behavior compatible with predictive pursuit. Journal of Neurophysiology. 2000;84:2340–2355. doi: 10.1152/jn.2000.84.5.2340. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Simpson T, Kiriakopoulos E, Stewart C, Crawley A, Guthrie B, Wood M, Mikulis D. Functional MRI of lateral occipitotemporal cortex during pursuit and motion perception. Annals of Neurology. 1996;40:387–398. doi: 10.1002/ana.410400308. [DOI] [PubMed] [Google Scholar]
- Becker W, Fuchs AF. Prediction in the oculomotor system: Smooth pursuit during transient disappearance of a visual target. Experimental Brain Research. 1985;57:562–575. doi: 10.1007/BF00237843. [DOI] [PubMed] [Google Scholar]
- Braun DI, Boman DK, Hotson JR. Anticipatory smooth eye movements and predictive pursuit after unilateral lesions in human brain. Experimental Brain Research. 1996;110:111–116. doi: 10.1007/BF00241380. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Strauss ME, Breier A, Kirkpatrick B, Carpenter WTJ. Attentional impairments in deficit and nondeficit forms of schizophrenia. American Journal of Psychiatry. 1997;154:363–370. doi: 10.1176/ajp.154.3.363. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. Journal of Abnormal Psychology. 1994;103:171–183. doi: 10.1037//0021-843x.103.2.171. [DOI] [PubMed] [Google Scholar]
- Clementz BA, McDowell JE. Smooth pursuit in schizophrenia: Abnormalities of open- and closed-loop responses. Psychophysiology. 1994;31:79–86. doi: 10.1111/j.1469-8986.1994.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Reid SA, McDowell JE, Cadenhead KS. Abnormality of smooth pursuit eye movement initiation: Specificity to the schizophrenia spectrum? Psychophysiology. 1995;32:130–134. doi: 10.1111/j.1469-8986.1995.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA. Is eye movement dysfunction a biological marker for schizophrenia? A methodological review. Psychological Bulletin. 1990;108:77–92. doi: 10.1037/0033-2909.108.1.77. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Cohen R. Applied multiple regression/correlation analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983. [Google Scholar]
- Croft RJ, Lee A, Bertolot J, Gruzelier JH. Associations of P50 suppression and desensitization with perceptual and cognitive features of “unreality” in schizotypy. Biological Psychiatry. 2001;50:441–446. doi: 10.1016/s0006-3223(01)01082-4. [DOI] [PubMed] [Google Scholar]
- Ferrera VP, Rudolph KK, Maunsell JH. Responses of neurons in the parietal and temporal visual pathways during amotion task. Journal of Neuroscience. 1994;14:6171–6186. doi: 10.1523/JNEUROSCI.14-10-06171.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A, Redlich S. Social class and mental illness: A community study. New York: John Willey; 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Avila M, Adami H, Elliot A, Thaker GK. Components of the smooth pursuit functions in deficit and nondeficit schizophrenia. Schizophrenia Research. doi: 10.1016/s0920-9964(02)00388-2. (in press) [DOI] [PubMed] [Google Scholar]
- Kendler KS, Lieberman JA, Walsh D. The Structured Interview for Schizotypy (SIS): A preliminary report. Schizophrenia Bulletin. 1989;15:559–571. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D. The Roscommon Family Study III. Schizophrenia-related personality disorders in relatives. Archives of General Psychiatry. 1993;50:781–788. doi: 10.1001/archpsyc.1993.01820220033004. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT. The schedule for the deficit syndrome: An instrument for research in schizophrenia. Psychiatry Research. 1989;30:119–123. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Modulation of pursuit eye movements by stimulation of cortical areas MT and MST. Journal of Neurophysiology. 1989;62:31–47. doi: 10.1152/jn.1989.62.1.31. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Extraretinal inputs to neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. Journal of Neurophysiology. 2001;86:2629–2633. doi: 10.1152/jn.2001.86.5.2629. [DOI] [PubMed] [Google Scholar]
- Kunkel R, Adami H, Zetlmeisl M, Ross DE, Thaker GK. Recruitment of non-patient volunteers with schizophrenia spectrum personality symptoms. Schizophrenia Research. 1998;34:181–186. doi: 10.1016/s0920-9964(98)00102-9. [DOI] [PubMed] [Google Scholar]
- Leigh JR, Zee DS. The neurology of eye movements. Philadelphia, PA: F. A. Davis; 1991. [Google Scholar]
- Levine G. A guide to SPSS for analysis of variance. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. [Google Scholar]
- Levy DL, Holzman PS, Matthysse S, Mendell NR. Eye tracking and schizophrenia: A selective review. Schizophrenia Bulletin. 1994;20:47–62. doi: 10.1093/schbul/20.1.47. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annual Review of Neuroscience. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annual Review of Neuroscience. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Movshon JA. Visual motion analysis for pursuit eye movements in area MT of macaque monkeys. Journal of Neuroscience. 1999;19:2224–2246. doi: 10.1523/JNEUROSCI.19-06-02224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MJ, Toomey R, Faraone SV, Tsuang MT. Comparison of schizotypal relatives of schizophrenic versus affective probands. American Journal of Medical Genetics. 1994;54:279–285. doi: 10.1002/ajmg.1320540318. [DOI] [PubMed] [Google Scholar]
- MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth-pursuit eye movement representation in the primate frontal eye field. Cerebral Cortex. 1991;1:95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Dursteler MR, Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. Journal of Neuroscience. 1985;5:825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements II. Differentiation of retinal from extraretinal inputs. Journal of Neurophysiology. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M, Stangl D. Structured Interview for DSM-III–R Personality (SIDP–R) Ames, IA: Department of Psychiatry, University of Iowa; 1989. [Google Scholar]
- Reiss AL, Hagerman RJ, Vinogradov S, Abrams M, King RJ. Psychiatric disability in female carriers of the fragile X chromosome. Archives of General Psychiatry. 1988;45:25–30. doi: 10.1001/archpsyc.1988.01800250029005. [DOI] [PubMed] [Google Scholar]
- Ross DE, Thaker GK, Buchanan RW, Lahti AC, Medoff D, Bartko JJ, Moran M, Hartley J. Association of abnormal smooth pursuit eye movements with the deficit syndrome in schizophrenic patients. American Journal of Psychiatry. 1996;153:1158–1165. doi: 10.1176/ajp.153.9.1158. [DOI] [PubMed] [Google Scholar]
- Ross DE, Thaker GK, Holcomb HH, Cascella NG, Medoff DR, Tamminga CA. Abnormal smooth pursuit eye movements in schizophrenic patients are associated with cerebral glucose metabolism in oculomotor regions. Psychiatry Research. 1995;58:53–67. doi: 10.1016/0165-1781(95)02724-b. [DOI] [PubMed] [Google Scholar]
- Ross RG, Olincy A, Harris JG, Radant A, Adler LE, Compagnon N, Freedman R. The effects of age on a smooth pursuit tracking task in adults with schizophrenia and normal subjects. Biological Psychiatry. 1999;46:383–391. doi: 10.1016/s0006-3223(98)00369-2. [DOI] [PubMed] [Google Scholar]
- Schmid A, Rees G, Frith C, Barnes G. An fMRI study of anticipation and learning of smooth pursuit eye movements in humans. Neuro Report. 2001;12:1409–1414. doi: 10.1097/00001756-200105250-00023. [DOI] [PubMed] [Google Scholar]
- Squires-Wheeler E, Skodol AE, Friedman D, Erlenmeyer-Kimling L. The specificity of DSM-III schizotypal personality traits. Psychological Medicine. 1988;18:757–765. doi: 10.1017/s0033291700008461. [DOI] [PubMed] [Google Scholar]
- Stanley MA, Turner SM, Borden JW. Schizotypal features in obsessive-compulsive disorder. Comprehensive Psychiatry. 1990;31:511–518. doi: 10.1016/0010-440x(90)90065-z. [DOI] [PubMed] [Google Scholar]
- Suh M, Leung HC, Kettner RE. Cerebellar flocculus and ventral paraflocculus Purkinje cell activity during predictive and visually driven pursuit in monkey. Journal of Neurophysiology. 2000;84:1835–1850. doi: 10.1152/jn.2000.84.4.1835. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Srinivasagam NM, Keshavan MS, Schooler NR, Haas GL, Carl JR. Eye tracking abnormalities in schizophrenia: Evidence for dysfunction in the frontal eye fields. Biological Psychiatry. 1998;44:698–708. doi: 10.1016/s0006-3223(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Buchanan RW, Adami HM, Medoff DR. Smooth pursuit eye movements to extraretinal motion signals: Deficits in patients with schizophrenia. Psychiatry Research. 1999;88:209–219. doi: 10.1016/s0165-1781(99)00084-0. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Buchanan RW, Moran MJ, Lahti A, Kim CE. Does pursuit abnormality in schizophrenia represent a deficit in the predictive mechanism? Psychiatry Research. 1996;59:221–237. doi: 10.1016/0165-1781(95)02759-9. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Cassady SL, Adami HM, LaPorte D, Medoff DR, Lahti A. Smooth pursuit eye movements to extraretinal motion signals: Deficits in relatives of patients with schizophrenia. Archives of General Psychiatry. 1998;55:830–836. doi: 10.1001/archpsyc.55.9.830. [DOI] [PubMed] [Google Scholar]
- Trestman RL, Keefe RS, Mitropoulou V, Harvey PD, deVegvar ML, Lees-Roitman S. Cognitive function and biological correlates of cognitive performance in schizotypal personality disorder. Psychiatry Research. 1995;59:127–136. doi: 10.1016/0165-1781(95)02709-2. [DOI] [PubMed] [Google Scholar]
- Turano KA, Massof RW. Nonlinear contribution of eye velocity to motion perception. Vision Research. 2001;41:385–395. doi: 10.1016/s0042-6989(00)00255-8. [DOI] [PubMed] [Google Scholar]
- van den Berg AV. Human smooth pursuit during transient perturbations of predictable and unpredictable target movement. Experimental Brain Research. 1988;72:95–108. doi: 10.1007/BF00248504. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: A multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]