Abstract
Nitric oxide (NO·) participates in the regulation of a wide array of biological processes and its deficit contributes to the severity of many diseases. Recently, a role of NO deficiency that occurs as a result of intravascular hemolysis and increases in levels of cell-free hemoglobin in the pathway of chronic anemic pathologies has been suggested. Experimental evidence for deoxyhemoglobin-catalyzed reduction of nitrite to NO· leads to the possibility of nitrite infusion-based therapies to correct NO· deficits. However, the presence of plasma hemoglobin also raises the possibility of deleterious free radical-mediated oxidative damage from the reaction between nitrite and oxyhemoglobin in the vasculature. We show that the conditions required for the reaction between nitrite and oxyhemoglobin to exhibit free radical-mediated autocatalytic kinetics are highly unlikely to occur in the plasma compartment, even during extensive hemolysis and with pharmacological nitrite doses. Although the presence of haptoglobin enhances the rate of the reaction between nitrite and oxyhemoglobin, common plasma antioxidants—ascorbate and urate, as well as catalase—prevent autocatalysis. Our findings suggest that pharmacological doses of nitrite are unlikely to cause free radical or ferrylhemoglobin formation in plasma originating from the reaction of nitrite with cell-free oxyhemoglobin in vivo.
Keywords: Oxyhemoglobin, Nitrite, Ferrylhemoglobin, Nitric oxide, Reactive nitrogen species, Ascorbic acid, Uric acid, Haptoglobin, Cell-free hemoglobin
Introduction
NO· is one of the most potent vasodilators and acts as a signaling molecule in a wide array of pathways in living systems. NO· is classically thought to be generated in vivo through the enzymatic activity of the nitric oxide synthases (NOS). NO· itself at physiological conditions is a short lived species. However, its role in signaling processes requires its immediate availability, which implies the existence of storage, or precursor species, with wide availability and lower reactivity, but easy convertibility into active NO·. The most well established precursor is the amino acid l-arginine which is converted to NO through the activity of NOS. More recently, nitrite ions have emerged as one of the possible precursors of NO· (for review see [1]). Nitrite is present in blood at close to micromolar concentrations (200–500 nM) [2], can be easily and quickly transported by the bloodstream within the body and is much less reactive than NO·. These facts, together with the discovery of nitrite-reductase activities of deoxyhemoglobin (deoxyHb) [1,3–7], nitric oxide synthase [8] and xanthine oxidoreductase [9] suggest that nitrite supplementation may be used to correct NO· deficiency in certain conditions such as stroke or ischemia-reperfusion injury [10–12].
However, significant increase of nitrite levels in plasma could also lead to undesirable oxidative damage within the vascular lumen by free radicals formed in the reaction of plasma oxyhemoglobin with nitrite. The presence of hemoglobin-derived oxidants in plasma had been previously associated with increased endothelial cell damage [13–15]. OxyHb levels in the plasma are in the range of 1 µM at normal conditions and can reach up to 30 µM in certain diseases [16]. It was reported that most of the cell-free Hb found in plasma is in ferrous state [17]. Reaction between nitrite and oxyHb may have two potential deleterious effects. First, it may significantly decrease the amount of nitrite available for reduction to NO· by proteins with nitrite-reductase activity, thereby limiting the usefulness of nitrite supplementation. Second, oxidation of oxyHb by nitrite has been shown to be capable of generating an autocatalytic free radical chain reaction that generates ferryl hemoglobin and nitrogen dioxide as intermediates. During the reaction of oxyHb with nitrite, H2O2 plays a pivotal role in the initiation phase, while the reaction propagation is maintained by the NO2 radical [18]. The end-products are metHb and nitrate. Ferryl species (ferryl-Hb(FeIV) and the ferrylHb radical) are important intermediates of the process [18–23]. Any extensive uncontrolled formation of free radical species in plasma and their consequent reactions with various biomolecules could result in tissue and organ damage [13–15]. However, most studies that have examined the kinetics and mechanism of this reaction have used a large excess of nitrite over Hb (10– to 100-fold), or oxyHb concentrations in the millimolar range. As the reaction mechanism is complex, the concentration dependencies examined at non-physiological reactant concentrations and ratios can not be extrapolated to the concentrations and ranges found in the physiological or pharmacological arena. It is therefore important to study this reaction under conditions expected to be found in the plasma in hemolytic disorders.
In case of hemolysis, cell-free hemoglobin and heme are rapidly cleared from bloodstream by highly specialized proteins, haptoglobin (Hp) and hemopexin, respectively [24,25]. Hp is a major Hb-binding protein in plasma, so we further focused on the effect of this protein. In this study, we used dimeric Hp 1-1 with two binding sites for Hb dimers, which is present in human blood at concentration ~20 µM. Hp 1-1 has a high affinity for Hb (Kd ~ 1012 M), and the resulting complex is very stable [24,26]. The physiological role of Hp–Hb complex formation seems to be mainly as aid in iron recycling from Hb by circulating leukocytes, and in preventing renal iron loading from Hb [27]. It has been reported that the Hb–Hp complex retains NO· scavenging activity [17,28,29] and its peroxidase activity is higher than the activity reported for Hb alone [30,31]. It is believed that Hp–Hb complex formation prevents oxidative damages caused by free Hb in vasculature [32,33].
In this paper, we used EPR to detect free radical species in plasma with added oxyHb and nitrite at equimolar Hb/nitrite ratios, and compared it to the reaction at a 10-fold excess of nitrite. In neither case were free radical species detected. When the same reaction was carried out in phosphate buffer, radical species formation was observed at the 10-fold excess of nitrite. Interestingly, metHb formed faster in plasma than in buffer at equimolar nitrite/oxyHb ratio and haptoglobin enhanced the reaction rate even at low nitrite regime. Systematic study of this reaction at a wide array of nitrite/oxyHb molar ratios, and the examination of the effect of various active compounds of plasma, such as antioxidants, haptoglobin and catalase, allowed us to determine conditions where the process exhibits full autocatalytic character and to clarify the importance of these species for free radical formation and suppression. A detailed understanding of possible redox reactions of cell-free hemoglobin in plasma upon nitrite addition is necessary for successful design of safe nitrite-based therapies for NO· deficiency.
Materials and methods
Materials
Fresh blood drawn from a healthy volunteer into test tubes containing heparin was immediately centrifuged for 20 min at 3000g to separate plasma from other blood components. Separated plasma was used immediately for EPR experiments.
OxyHb was prepared from fresh human blood according to Rossi-Fanelli et al. [34]. Hb aliquots were frozen in liquid nitrogen and stored at −80 °C. Sodium nitrite, ascorbic acid, uric acid, human haptoglobin phenotype 1-1 and catalase from bovine liver were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO).
Phosphate buffered saline (PBS, 10 mM phosphate buffer, 138 mM NaCl, 2.7 mM KCl, pH 7.4) contained 1 mM DTPA.
Electron paramagnetic resonance (EPR) spectroscopy
To determine if free radical formation from studied reaction occurs at physiological conditions, EPR experiments were carried out in plasma and PBS. For experiments done in plasma, oxyHb from stock solution (3 mM, PBS) was added into freshly separated plasma (final concentration of 30 µM). Samples with nitrite/oxyHb molar ratios of 1/1 and 10/1 were prepared in triplicates adding appropriate amounts of stock solution of NaNO2 (100 mM, in PBS). Plasma with added oxyHb and nitrite was incubated at 37 °C and aliquots for EPR experiments were withdrawn at the following time points: for nitrite/oxyHb equimolar ratio: 0, 1, 4, 6 and 11 h, and for nitrite/oxyHb molar ratio of 10/1 at 0, 15, 30, 45, 60, 90, 120 and 300 min after the beginning of incubation. The aliquots were immediately frozen in liquid nitrogen and stored at −80 °C until the analysis. Both, metHb and free radicals were measured.
For experiments done in PBS, samples with nitrite/oxyHb molar ratios of 2/1 and 10/1 were prepared and measured. Aliquots were taken at following time points: 2, 12 and 24 h for nitrite/oxyHb molar ratio of 2/1, and at 2, 3 and 6 h for nitrite/oxyHb molar ratio of 10/1.
Methemoglobin (metHb) was detected in g = 6 region at the temperature of 6 K using a Bruker Elexys EPR spectrometer (Bruker, MA), at microwave power of 2 mW, modulation amplitude 10 G, sweep time 84 s. Same samples were then scanned in g = 2 region at temperature of 90 K to detect free radical species using Bruker EMX EPR spectrometer (Bruker, MA) equipped with a loop-gap resonator, at microwave power 0.2 mW, modulation amplitude 2 G and sweep time 84 s.
The amount of metHb in samples in EPR measurements was calculated using freshly prepared metHb standard.
UV–visible spectroscopy
To follow and identify reaction products in reaction of oxyHb with nitrite in PBS at 37 °C, the concentration of oxyHb was kept constant at 30 µM. The appropriate amounts of stock solution of NaNO2 (100 mM, in PBS) were injected into oxyHb solution to obtain desired final nitrite concentrations. UV/vis spectra were automatically acquired in 450–700 nm range at every 15–360 s, depending on studied nitrite/Hb ratio. The following nitrite concentrations were used: 0, 3, 6, 15, 30, 60, 120, 300 and 450 µM, corresponding to nitrite/oxyHb molar ratios of 0, 1/10, 1/5, 1/2, 1/1, 2/1, 4/1, 10/1 and 15/1, respectively, and most reactions were carried out until completion. At conditions with completion times of more than 24 h at least one experiment was carried out to completion, otherwise experiments were stopped at 18–24 h marks for technical reasons. All spectra were recorded using UV/vis diode array spectrophotometer model HP 8453 (Agilent Technologies, Santa Clara, CA) equipped with 8-cell transporter and water bath temperature control. Samples were prepared and measured in triplicate for each nitrite/oxyHb ratio.
To examine the effect of ascorbic acid (AA) and uric acid (UA), we added freshly prepared solution of AA/UA into samples with 30 µM oxyHb to final concentrations of 5, 50, 100 and 500 µM for AA and 50, 100, 250 and 500 µM for UA. Immediately after the AA (UA) addition, nitrite was added to the final nitrite/oxyHb ratio of 10/1 and absorption spectra were acquired for 18 h with time intervals of 4 min.
To examine the role of H2O2 in radical chain formation, catalase from bovine liver was added at the beginning of experiment in appropriate amounts to achieve final activity of 50 kU/ml.
Human haptoglobin 1-1 (Hp) was added to a final concentration of 20 µM into solution of 14 µM or 7 µM oxyHb. Samples with nitrite/oxyHb molar ratios of 2/1 and 10/1 were prepared and kinetics of the reaction was followed using absorption spectroscopy. Control sample containing Hp/Hb without nitrite and samples of 14 µM or 7 µM Hb with added nitrite was included in each experimental set.
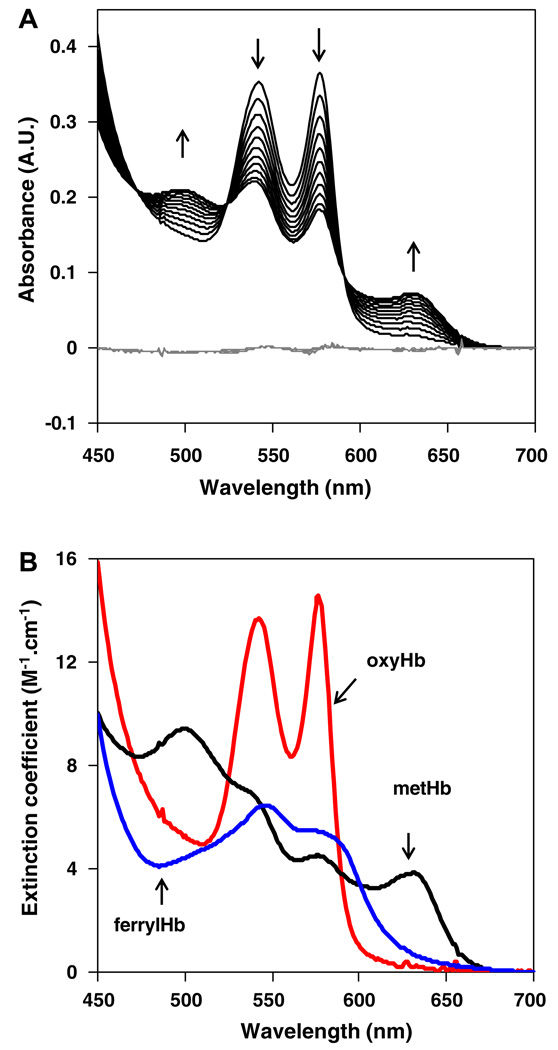
Multiple linear regression analysis (MLR) approach was used to determine contributions of each Hb species into the measured UV–vis spectra at each time point. A reference set of pure oxyHb, metHb and ferrylHb spectra were used (Fig. 1B) and the quality of fit was estimated using the plot of residuals. An illustrative spectral data set for nitrite/oxyHb molar ratio of 2/1 is shown in Fig. 1A, where arrows indicate temporal evolution of individual peaks and gray lines are plots of residuals after the MLR fit.
Fig. 1.
(A) Reaction of oxyHb with nitrite at nitrite/oxyHb molar ratio of 2/1 followed continuously for 20 h. OxyHb concentration 30 µM, nitrite 60 µM, PBS, 37 °C, pH 7.4, absorption spectra were recorded every 5 min, for clarity only spectra taken in 2 h intervals are plot. Directions of changes in absorption spectra of hemoglobin (black lines) in time are marked by arrows. The residuals between MLR analysis using set of absorption spectra from (B) are plot as gray lines. (B) Standard absorption spectra of pure oxyHb, metHb and ferrylHb used in MLR analysis.
Results
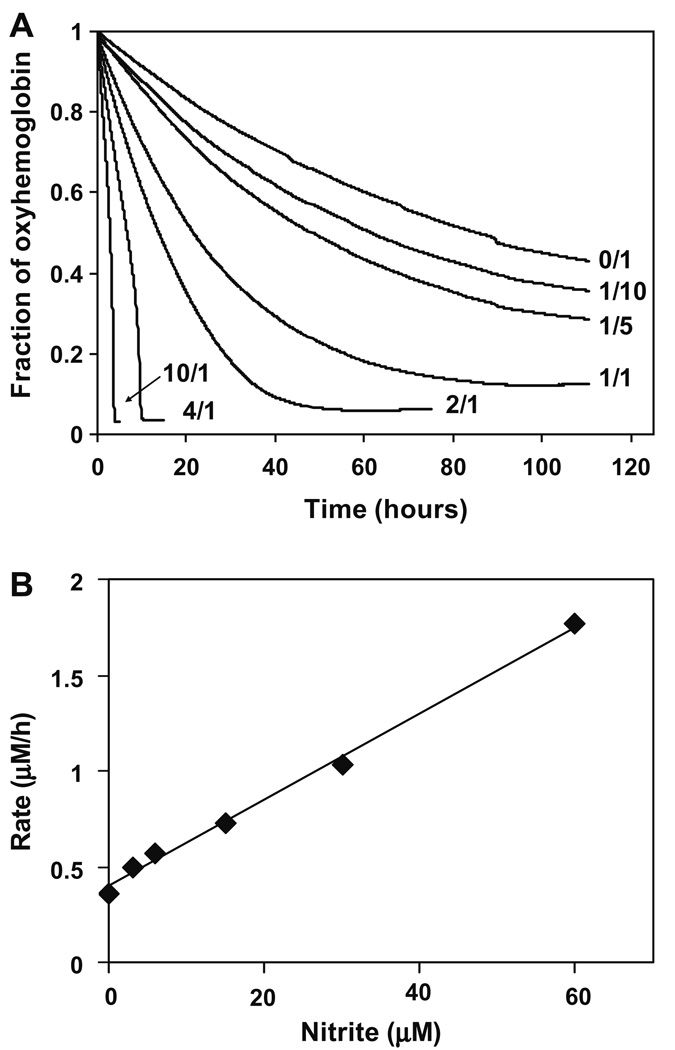
Absorption spectra were recorded during the reaction of oxyHb with nitrite in PBS, in a series of experiments with variable nitrite/oxyHb ratios at a constant oxyHb concentration of 30 µM. Contributions of individual Hb species were determined using MLR analysis using the basis spectra shown in Fig. 1. A complete plot of changes in the fraction of oxyHb as a function of time, for different nitrite/Hb molar ratio in PBS is in Fig. 2A. In the absence of nitrite slow autoxidation of oxyHb was observed. As expected, the reaction proceeds faster as nitrite concentrations increases, however, the kinetic profile was also dependent on nitrite concentration. At nitrite/oxyHb ratios below 4:1, oxyHb decay was monophasic. At ratios of 4:1 and greater, the reaction kinetics exhibited the autocatalytic profile that has been previously observed. Initial rate plotted as a function of nitrite concentration gave a bimolecular rate constant of 0.21 M−1 s−1 for the rate limiting step of the initial reaction (Fig. 2B).
Fig. 2.
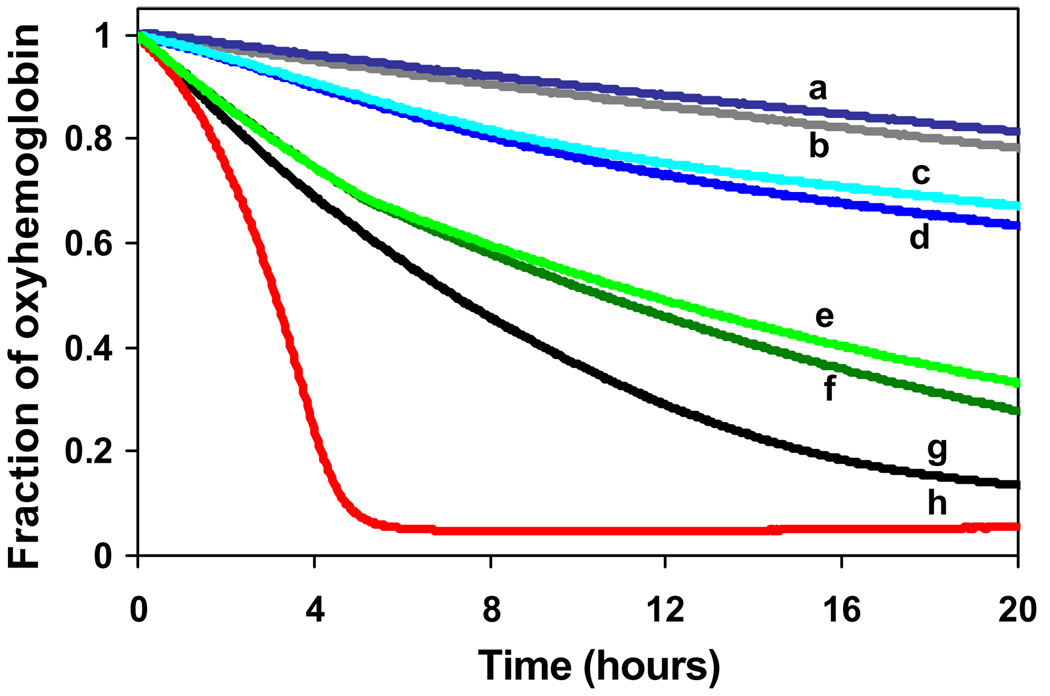
(A) OxyHb decay as function of time after addition of nitrite ions at different nitrite/oxyHb molar ratios in PBS at 37 °C and pH 7.4 and oxyHb concentration 30 µM. Two phase oxyHb decay was observed for nitrite/oxyHb molar ratios ≥ 4/1. Number on the right denotes the corresponding nitrite/oxyHb molar ratio. (B) Initial rates of oxyHb decay for all nitrite/Hb molar ratios studied. Hb concentration was kept constant at 30 µM in and all experiments were done in PBS at 37 °C and pH 7.4.
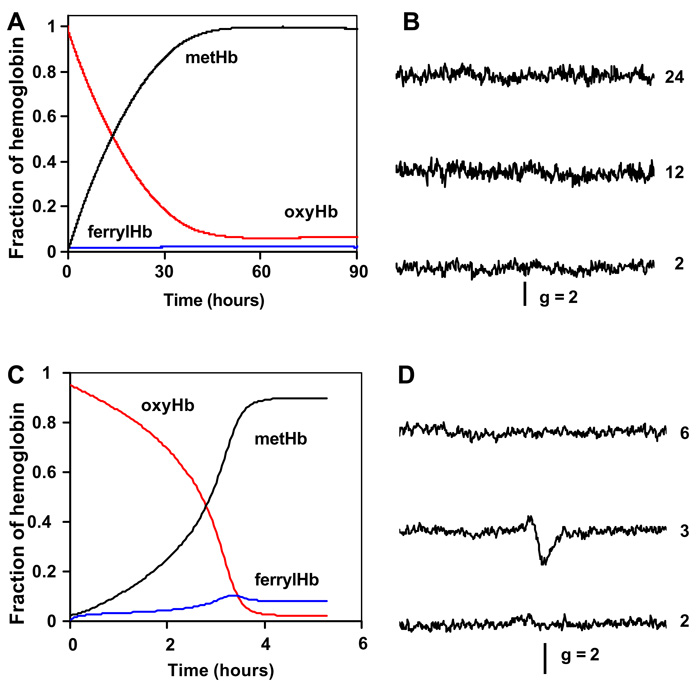
Full deconvolutions of the spectral changes observed during the reaction between nitrite and oxyHb are shown in Fig. 3. Each species is given in terms of its fractional contribution to the total amount of hemoglobin present. Under these experimental conditions oxyHb is oxidized only by a slow monotonic process, and the ferrylHb intermediate never achieves measurable levels. The removal of the ferryl species from the basis spectra did not alter the quality of fit (data not shown). EPR spectra in the g = 2 region did not reveal the formation of protein free radicals under these conditions (Fig. 3B). In the case of 10-fold excess of nitrite over oxyHb (Fig. 3C), all characteristics of an autocatalytic reaction were observed. The spectral deconvolution revealed a transient formation of ferryl hemoglobin species during the rapid phase of the reaction. In addition, a transient protein-based free radical coinciding with the fast phase of reaction was detected in g = 2 region (Fig. 3D).
Fig. 3.
(A) Multilinear regression analysis applied to the spectral set from Fig. 1A using standard oxyHb, ferrylHb and metHb spectra. (B) EPR scans in g = 2 region of aliquots taken from reaction mixture of nitrite/oxyHb 2/1 at different time points indicated by numbers on the right (time given in hours) (C) Multilinear regression analysis applied to the spectral set from experiment with nitrite/Hb molar ratio of 10/1 (300 µM nitrite and 30 µM oxyHb) using standard oxyHb, ferrylHb and metHb spectra. (D) EPR scans in g = 2 region of aliquots taken from reaction mixture of nitrite/oxyHb 10/1 at different time points indicated by numbers on the right (time given in hours).
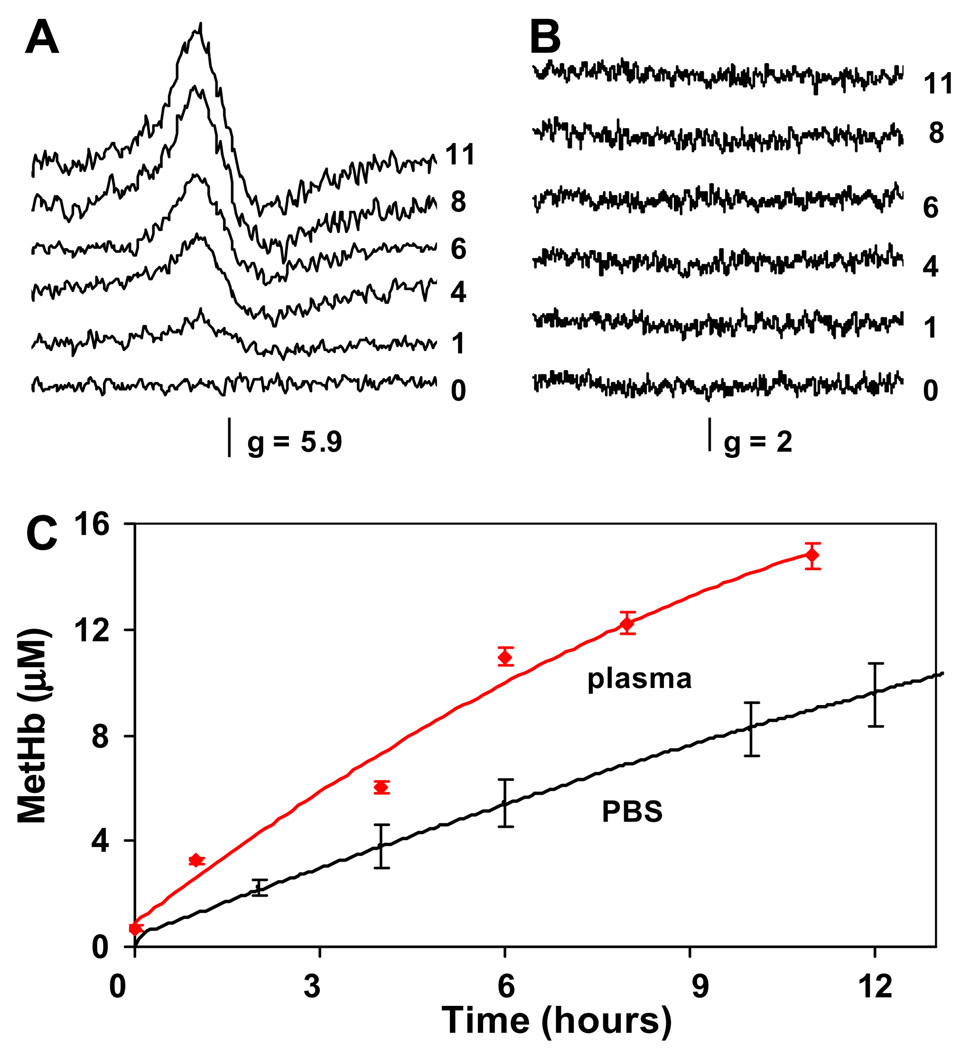
MetHb formation in plasma, detected by EPR spectroscopy, during the reaction of 30 µM oxyHb with nitrite at an equimolar nitrite/oxyHb ratio is shown in Fig. 4. EPR spectra for plasma samples in the metHb (g = 6) and free radical (g = 2) regions are shown in Fig. 4A and B, respectively. The development of the metHb signal with increased time of incubation is clearly seen in Fig. 4A as a function of time, however, no EPR signals were observed in the free radical region. In an attempt to assess nitrite concentration dependence of the reaction kinetics in plasma, a sample with an excess of nitrite at nitrite/oxyHb molar ratio of 10/1 was prepared and measured by EPR in g = 6 and g = 2 regions. The transient presence of metHb EPR signal during the course of the reaction was detected in the g = 6 region and no free radicals were detected in the g = 2 region (data not shown). The concentration of metHb in plasma was calculated using a metHb standard and is plotted in Fig. 4C as a function of the incubation time. For comparison, metHb concentration changes occurring during the reaction of oxyHb with nitrite in PBS under otherwise identical experimental conditions are also shown. Interestingly, the presence of plasma increased the rate of metHb formation by almost 2-fold.
Fig. 4.
(A) MetHb EPR spectra of aliquots taken from the reaction mixture of equimolar ratio of nitrite/oxyHb 1/1 in plasma at different time points indicated by numbers on the right (time given in hours). (B): EPR spectra of the same aliquots as in (B) scanned for the presence of free radical species in g = 2 region. (C) MetHb formation in plasma with added cell-free oxyhemoglobin after nitrite addition as determined using EPR spectroscopy (red line) compared with metHb formation in the same reaction carried on in PBS (pH 7.4) and followed using absorption spectroscopy (black line). Reaction conditions in both cases: oxyHb 30 µM, nitrite 30 µM, 37 °C. (For interpretation of color mentioned in this figure the reader is referred to the web version of the article.)
Fig. 5 summarizes the effect of presence of 20 µM haptoglobin (Hp) on the decay of oxyHb during its reaction with nitrite at equimolar nitrite/oxyHb ratio at an oxyHb concentration of 14 µM. The presence of Hp does not alter autooxidation of oxyHb in the absence of nitrite (lines a vs. b). In the absence of Hp, nitrite causes only monotonous oxyHb decay (line g). Interestingly, the presence of Hp in the reaction mixture of oxyHb resulted in biphasic kinetics (line h). Addition of 50 µM ascorbic acid significantly slows oxyHb decay in presence as well in the absence of Hp (lines c and d). A similar effect was found for 100 µM uric acid (data not shown). Addition of catalase (50 kU/ml) at the beginning of the experiment decreases the reaction rate regardless of Hp presence/absence (line e and f). Similar effects were observed during the reaction between 7 µM Hb, 7 µM nitrite, and 20 µM Hp (not shown). Addition of Hp into nitrite/oxyHb reaction mixture with 10-fold nitrite excess (a condition in which the reaction exhibits autocatalytic kinetics), causes dramatic shortening of the slow phase and acceleration of the development of rapid phase (data not shown).
Fig. 5.
Comparison of effects of ascorbic acid (AA) and catalase on the kinetics of oxyHb reaction with nitrite at equimolar ratio in presence/absence of Hp in PBS 37 °C. (a) oxyHb/Hp; (b) oxyHb; (c) oxyHb/Hp/nitrite/AA; (d) oxyHb/nitrite/AA, (e) oxyHb/nitrite/catalase; (f) oxyHb/Hp/nitrite/catalase; (g) oxyHb/nitrite; (h) oxyHb/Hp/nitrite. Following concentrations of reactants were used: oxyHb 14 µM, nitrite 14 µM, Hp 20 µM, AA 50 µM and catalase 50 kU/ml.
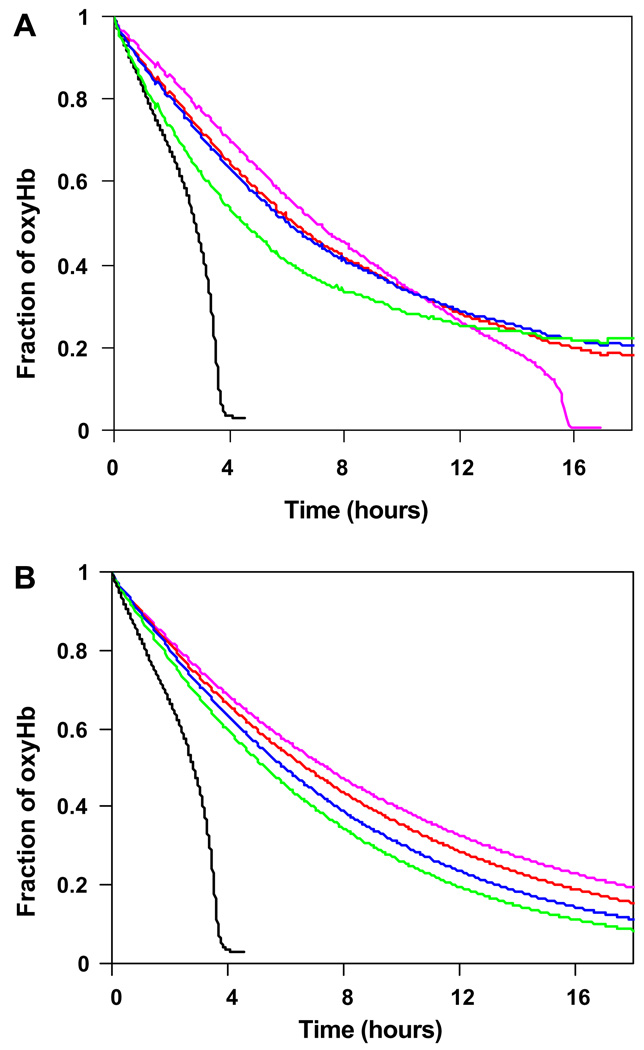
Fig. 6A and B show the potency of ascorbic and uric acids, respectively, to prevent the development of the rapid phase in reaction of oxyHb with nitrite at 10-fold excess of nitrite. Both antioxidants were tested at wide range of concentrations. As plotted in Fig. 6A, the presence of 5 µM ascorbic acid was enough to elongate the duration of slow “lag” phase from 3 h (without ascorbic acid) to 15 h before the reaction finally entered into the rapid phase. Further increases of ascorbic acid concentration to 50, 100 and 500 µM completely prevented the appearance of the rapid phase. Fig. 6B depicts the similar results observed when uric acid was used. All tested concentrations, 50, 100, 250 and 500 µM effectively prevented the development of the rapid phase of reaction.
Fig. 6.
(A) Effect of ascorbic acid on the reaction of nitrite with oxyHb. Experimental conditions: ascorbic acid 0 µM (black), 5 µM (purple), 50 µM (blue), 100 µM (red) and 500 µM (green), oxyHb 30 µM, nitrite/oxyHb 10/1, PBS, pH 7.4, 37 °C. (B) Effect of uric acid on the reaction of nitrite with oxyHb. Experimental conditions: uric acid 0 µM (black), 50 µM (purple), 100 µM (red), 250 µM (blue), 500 µM (green), oxyHb 30 µM, nitrite/oxyHb 10/1, PBS, pH 7.4, 37 °C. (For interpretation of color mentioned in this figure the reader is referred to the web version of the article.)
Discussion
The reaction of nitrite with oxyHb can involve multiple steps with complex concentration dependencies and many intermediate products. The reaction kinetics then are bimodal, with a slow phase (initiation), during which oxyHb is oxidized to metHb, that precedes a rapid propagation phase. The process is autocatalytic, as the propagation species is generated in multiple copies during one reaction cycle. Earlier attempts to explain the mechanism have been reviewed [35]. Our recent kinetic reexamination of this reaction suggests that the most crucial intermediates, H2O2 and NO2 radical act exclusively in the initiation and the propagation phases, respectively [18]. In this study we have attempted to examine this reaction in the context of nitrite supplementation in hemolytic disorders, and to ascertain if the oxidative intermediates generated during the progression of this reaction are likely to be of concern. For this reason we have focused on relevant concentration ranges of hemoglobin (up to 30 µM in plasma [16]) and on plasma components that may alter the reaction characteristics. As shown in this study, common plasma antioxidants (AA, UA) regulate the reaction by limiting the quantity of intermediate reactive products (as H2O2, NO2 radical, ferrylHb species).
Our EPR and spectrophotometric results for equimolar nitrite/oxyHb mixtures indicate that no free radicals are detected during the whole time course of the reaction. When 10-fold excess of nitrite over oxyHb was used, the situation in plasma and PBS vis a vis free radical formation differed dramatically; with an total absence of free radicals in plasma and clear transient presence of protein-based radical in PBS that coincided with the observed fast phase of the reaction (Fig. 3D). The observed monotonous kinetics of the reaction up to ~4-fold nitrite excess over oxyHb suggests that a burst of oxidizing reactive intermediates does not occur.
Natural levels of nitrite in plasma are in the nanomolar range of 200–500 nM [2]. Nitrite/oxyHb molar ratios of 1/1 and 10/1 in our study were chosen with two possible nitrite supplementation routes in mind. Supplementing nitrite by dietary sources with green leaf vegetables as a preferred source, 100 g of spinach would lead to total nitrite dose in range of 1 µmol/kg [1], or around 1 µM nitrite in blood. Therefore, concentrations of nitrite in plasma would unlikely to exceed its natural levels much more than 5-fold by dietary changes only. In this case, with extracellular oxyHb in plasma of ~1.4 µM, diet-modified nitrite/oxyHb molar ratio is ~1/1 without hemolysis and drops to ~1/30 during the hemolysis, so free radical formation in plasma is not expected. However, in case of acute NO· deficiency, more direct route to deliver nitrite is needed. In nitrite infusion-based therapies much higher levels of nitrite in plasma are possible, which could potentially lead to the possibility of free radical formation in the vasculature, such as ferrylHb species [13–15]. However, vasodilatory effects of nitrite occur even under a low pharmacologic concentration regime which is unlikely to generate a burst of oxidizing species. Infusion of nitrite into human forearm brachial artery to its final concentration in blood of 2 µM caused 30% increase of blood flow [4]. When 100-fold higher concentration of nitrite was used (200 µM), much higher increase of blood flow was observed (175%), but one could argue that achieving such a large increase in blood flow could be unnecessary or even dangerous in some cases. Also, intravenous infusion instead of intra-arterial could take advantage of the higher deoxyHb content of venous blood to make it possible to lower the necessary dose.
Reactive free radical species can be scavenged by antioxidants present in plasma. The two main antioxidants in plasma are ascorbic acid and uric acid with physiological concentration range of 20–60 µM and 300–400 µM, respectively [36–38]. The ability of both to destroy or at least significantly limit free radicals formed in case of 10-fold excess of nitrite over oxyHb in PBS suggests that the risk of free radical formation in nitrite-based therapies can be lowered by concurrent administration of antioxidants as previously shown [39]. FerrylHb and free radicals which are potential cause of oxidative stress in the vasculature, seem to be formed only at very non-physiological conditions of nitrite, such as may occur during nitrite poisoning [40] and should not play a major role in most processes involving cell-free plasma Hb in vivo.
Interestingly, at conditions of equimolar nitrite and oxyHb, the rate of metHb formation was significantly higher than in PBS (Fig. 4C). At normal conditions, cell-free hemoglobin and heme are rapidly bound to haptoglobin (Hp) and hemopexin, respectively [24,25] and cleared out of the blood. We hypothesized that the increased rate of metHb formation observed in plasma might be caused by the presence of Hp. Earlier it had been reported that heme environment in Hb in Hp–Hb complex is slightly altered when compared to free Hb [41,42]. Also, Hp binds primary to the Hb dimer.
It is known that the Hb–Hp complex retains NO· scavenging activity [17,28,29], but to our best knowledge no report had been made on how Hb-binding to Hp affects Hb reaction with nitrite ion. Several studies also report that Hp–Hb complex formation prevents oxidative damages caused by free Hb in vasculature [32,33].
Based on observed accelerated metHb formation from cell-free Hb in plasma (Fig. 4C) we further examine the effect of Hp presence on Hb-nitrite reaction kinetics. When the Hp–Hb complex was incubated with nitrite, we observed biphasic decay with a ferrylHb intermediate clearly present in MLR deconvolution (Fig. 5). This observation is in agreement with published data [43], where formation of ferrylHb from oxyHb as well as from metHb was not inhibited, but increased by Hp–Hb complex formation. However, according to the proposed reaction mechanism for oxyHb with nitrite [18], as well as the observed acceleration of metHb formation in plasma, we would expect to see some traces of protein-based free radicals in plasma, at least in the case of 10-fold nitrite excess, which we did not observe. The main difference between PBS and plasma in this regard is likely to be the presence of plasma antioxidants. To examine this hypothesis we used antioxidants added to the reaction mixture of nitrite/Hp–Hb in PBS at the beginning of the reaction. Indeed, after addition of 50 µM ascorbic acid and 100 µM uric acid only slow monotonic oxyHb decay, but not autocatalysis was observed.
Catalase added at the beginning of the reaction also prevented the autocatalytic burst both in presence and in absence of Hp, showing that Hp did not open an alternative reaction path leading to autocatalysis. We assume that formation of Hp–Hb complex with Hb dimer conformation in R state is responsible for enhancing the reaction kinetics.
In this study we provide insight into reaction of cell-free Hb with nitrite at low oxyHb and nitrite regimes as expected in plasma at physiological and pharmacological ranges and we also examine possible interactions with other plasma components. We found no autocatalytic burst of oxidant formation under these conditions, unlike what has been observed at high nitrite:Hb ratios and/or high Hb concentrations [19,44–48]. The presence of ferrylHb-based radicals was confirmed in case of 4-fold and higher excess of nitrite over oxyHb, and at low nitrite:oxyHb ratios when Hp–Hb complex was formed prior to the reaction. Addition of ascorbic and uric acids at concentrations normally found in plasma abolished radical formation, and added catalase scavenged H2O2. We conclude that effective pharmacological doses of nitrite, especially when complemented with antioxidant effects of plasma, are not likely to be limited vis a vis free radical or ferrylHb formation by this reaction in vivo during nitrite-based therapies in diseases accompanied by hemolysis or otherwise due to NO· deficiency [49,50]. Our study concentrated on cell-free hemoglobin, however, the major part of hemoglobin found in blood is of course encapsulated in red blood cells. One can ask about the consequences of nitrite infusion on this portion of hemoglobin. Based on the overwhelming ratio of hemoglobin over nitrite and the presence of antioxidants and metHb reductase system inside the cell we would predict that therapeutically desirable increases of nitrite concentration in plasma would have only a small effect on redox processes inside the red blood cell. Based on the results from our in vitro study, we believe that for the case of nitrite infusion therapy used for diseases accompanied by hemolysis, the determining factor vis a vis radical formation in the vasculature would be the cell-free hemoglobin. The risk of free radical formation in blood in direct nitrite infusion-based therapies can be further lowered by co-administration of sufficient doses of naturally occurring antioxidants, such as ascorbic acid (which itself could potentially participate in the reduction of nitrite to NO). However, this study concerns itself only with the reactions of nitrite in the plasma and therefore can not completely rule out any potentially detrimental effects of nitrite that may result from alternative routes of administration. Our results also suggest that pharmacological doses should be relatively low, below the nitrite/free Hb molar ratio of 4; however, to give recommendations for doses, a clinical trial is necessary.
Acknowledgments
Low temperature EPR measurements were carried out with the assistance of Dr. Brian Bennett from National Biomedical EPR Center, Department of Biophysics, Medical College of Wisconsin.
This work was supported by intramural NIH grant (A.N.S), NIGM grant GM55792 (N.H) and EB001980 from NIBIB that supports the National Biomedical Electron Paramagnetic Resonance Center at the Medical College of Wisconsin.
References
- 1.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO, III, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR, Jr, Feelisch M, Lundberg JO. The emerging biology of the nitrite anion. Nat. Chem. Biol. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 2.Dejam A, Hunter CJ, Pelletier MM, Hsu LL, Machado RF, Shiva S, Power GG, Kelm M, Gladwin MT, Schechter AN. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle MP, Pickerinq RA, DeWeert TM, Hoekstra JW, Pater D. Kinetics and the oxidation of human deoxyhemoglobin by nitrites. J. Biol. Chem. 1981;256:12393–12398. [PubMed] [Google Scholar]
- 4.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;12:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 5.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J. Biol. Chem. 2003;278:46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 6.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J. Clin. Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J. Biol. Chem. 2005;280:31126–31131. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 8.Gautier C, van Faassen E, Mikula I, Martasek P, Slama-Schwok A. Endothelial nitric oxide synthase reduces nitrite anions to NO under anoxia. Biochem. Biophys. Res. Commun. 2006;341:816–821. doi: 10.1016/j.bbrc.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalla A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. PNAS. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. PNAS. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duranski MR, Greer JJM, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet S-F, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripatara P, Patel NSA, Webb A, Rathod K, Lecomte FMJ, Mazzon E, Cuzzocrea S, Yaqoob MM, Ahluwalia A, Thiemermann C. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J. Am. Soc. Nephrol. 2007;18:570–580. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 13.Goldman DW, Breyer RJ, Yeh D, Brockner-Ryan BA, Alayash AI. Acellular hemoglobin-mediated oxidative stress toward endothelium: a role for ferryl ion. Am. J. Physiol. 1998;275:H1046–H1053. doi: 10.1152/ajpheart.1998.275.3.H1046. [DOI] [PubMed] [Google Scholar]
- 14.McLeod LL, Alayash AI. Detection of a ferrylhemoglobin intermediate in an endothelial cell model after hypoxia-reoxygenation. Am. J. Physiol. 1999;277:H92–H99. doi: 10.1152/ajpheart.1999.277.1.H92. [DOI] [PubMed] [Google Scholar]
- 15.D’Agnillo F, Wood F, Porras C, Macdonald VW, Alayash AI. Effects of hypoxia and glutathione depletion on hemoglobin- and myoglobin-mediated oxidative stress toward endothelium. Biochim. Biophys. Acta. 2000;1495:150–159. doi: 10.1016/s0167-4889(99)00163-9. [DOI] [PubMed] [Google Scholar]
- 16.Lezcano NE, Odo N, Kutlar A, Brambilla D, Adams RJ. Regular transfusion lower plasma free hemoglobin in children with sickle-cell disease at risk for stroke. Stroke. 2006;37:1424–1426. doi: 10.1161/01.STR.0000221173.97108.01. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Tanus-Santos JE, Reiter CD, Dejam A, Shiva S, Smith RD, Hogg N, Gladwin MT. Biological activity of nitric oxide in the plasmatic compartment. PNAS. 2004;101:11477–11482. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keszler A, Piknova B, Schechter AN, Hogg N. The reaction between nitrite and oxyhemoglobin: a mechanistic study. J. Biol. Chem. 2008;283:9615–9622. doi: 10.1074/jbc.M705630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosaka H, Imaizumi K, Tyuma I. Mechanism of autocatalytic oxidation of oxyhemoglobin by nitrite. An intermediate detected by electron spin resonance. Biochim. Biophys. Acta. 1982;702:237–241. doi: 10.1016/0167-4838(82)90508-8. [DOI] [PubMed] [Google Scholar]
- 20.Doyle MP, Pickering RA, Dykstra RL, Nelson CL, Boyer RF. Involvement of peroxide and superoxide in the oxidation of hemoglobin by nitrite. Biochem. Biophys. Res. Commun. 1982;105:127–132. doi: 10.1016/s0006-291x(82)80020-x. [DOI] [PubMed] [Google Scholar]
- 21.Herold S, Relunann FG. Kinetics of the reactions of nitrogen monoxide and nitrite with ferryl hemoglobin. Free Rad. Biol. Med. 2003;34:531–545. doi: 10.1016/s0891-5849(02)01355-2. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein S, Merenyi G, Samuni A. Kinetics and mechanism of •NO2 reacting with various oxidation states of myoglobin. J. Am. Chem. Soc. 2004;126:15694–15701. doi: 10.1021/ja046186+. [DOI] [PubMed] [Google Scholar]
- 23.Keszler A, Mason RP, Hogg N. Immuno-spin trapping of hemoglobin and myoglobin radicals derived from nitrite-mediated oxidation. Free Rad. Biol. Med. 2006;40:507–515. doi: 10.1016/j.freeradbiomed.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood. 1968;32:811–815. [PubMed] [Google Scholar]
- 25.Ascenzi P, Bocedi A, Visca P, Altruda F, Tolosano E, Berighelli T, Fasano M. Hemoglobin and heme scavenging. Life. 2005;57:749–759. doi: 10.1080/15216540500380871. [DOI] [PubMed] [Google Scholar]
- 26.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin. Chem. 1996;42:1589–1600. [PubMed] [Google Scholar]
- 27.Fagoonee S, Gburek J, Hirsch E, Marro S, Moestrup SK, Laurberg JM, Christensen EI, Silengo L, Altruda F, Tolosano E. Plasma protein haptoglobin modulates renal iron loading. Am. J. Pathol. 2005;166:973–983. doi: 10.1016/S0002-9440(10)62319-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards DH, Griffith TM, Ryley HC, Henderson AH. Haptoglobin-haemoglobin complex in human plasma inhibits endothelium dependent relaxation: evidence that endothelium derived relaxing factor acts as a local autocoid. Cardiovasc. Res. 1986;8:549–556. doi: 10.1093/cvr/20.8.549. [DOI] [PubMed] [Google Scholar]
- 29.Azarov I, He X, Jeffers A, Basu S, Ucer B, Hantgan RR, Levy A, Kim-Shapiro DB. Rate of nitric oxide scavenging by hemoglobin bound to haptoglobin. Nitric Oxide. 2008;18:296–302. doi: 10.1016/j.niox.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waks M, Yon J, Moretti J, Jayle MF. Comparative study of peroxidase activity of hemoglobin, methemoglobin and the hemoglobin-haptoglobin complex. Biochim. Biophys. Acta. 1963;67:417–424. doi: 10.1016/0006-3002(63)91847-x. [DOI] [PubMed] [Google Scholar]
- 31.Andreeva AP, Belostotskii VM, Rozenberg G.Ya. Comparative study of peroxidase activity of met- and oxyhemoglobin complexes with haptoglobin. Vopr. Med. Khim. 1973;4:378–381. [PubMed] [Google Scholar]
- 32.Melamed-Framk M, Lache O, Enav BI, Szafranek T, Levy NS, Ricklis RM, Levy AP. Structure-function analysis of the antioxidant properties of haptoglobin. Blood. 2001;98:3693–3698. doi: 10.1182/blood.v98.13.3693. [DOI] [PubMed] [Google Scholar]
- 33.Bamm VV, Tsemakhovich VA, Shaklai M, Shaklai N. Haptoglobin phenotypes differ in their ability to inhibit heme transfer from hemoglobin to LDL. Biochemistry. 2004;43:3899–3906. doi: 10.1021/bi0362626. [DOI] [PubMed] [Google Scholar]
- 34.Rossi-Fanelli A, Antonini E, Caputo A. Studies on the relation between molecular and functional properties of hemoglobin. I. The effect of salts on the molecular weight of human hemoglobin. J. Biol. Chem. 1961;236:391–396. [PubMed] [Google Scholar]
- 35.Kim-Shapiro DB, Gladwin MT, Patel RP, Hogg N. The reaction between nitrite and hemoglobin: the role of nitrite in hemoglobin-mediated hypoxic vasodilation. J. Inorg. Biochem. 2005;99:237–246. doi: 10.1016/j.jinorgbio.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 36.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. PNAS. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spranger T, Finckh B, Fingerhut R, Kohlshutter A, Beisiegel U, Kontush A. How different constituents of human plasma and low density lipoprotein determine plasma oxidizability by copper. Chem. Phys. Lipids. 1998;91:39–52. doi: 10.1016/s0009-3084(97)00092-3. [DOI] [PubMed] [Google Scholar]
- 38.Abuja PM. Ascorbate prevents prooxidant effects of urate in oxidation of human low density lipoprotein. FEBS Lett. 1999;446:305–308. doi: 10.1016/s0014-5793(99)00231-8. [DOI] [PubMed] [Google Scholar]
- 39.Dunne J, Caron A, Menu P, Alayash AI, Buehler PW, Wilson MT, Silaghi-Dumitrescu R, Faivre B, Cooper CE. Ascorbate removes the key precursors to oxidative damage by cell-free haemoglobin in vitro and in vivo. Biochem. J. 2006;399:513–524. doi: 10.1042/BJ20060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiese M, Weger N. Formation of ferrihaemoglobin with aminophenols in the human for the treatment of cyanide poisoning. Eur. J. Pharmacol. 1969;7:97–105. doi: 10.1016/0014-2999(69)90170-8. [DOI] [PubMed] [Google Scholar]
- 41.Hamaguchi H, Isomoto A, Miyake Y, Nakajima H. Some spectral properties of the human hemoglobin-haptoglobin complex. Biochemistry. 1971;10:1741–1745. doi: 10.1021/bi00786a001. [DOI] [PubMed] [Google Scholar]
- 42.Waks M, Kahn PC, Beychok S. Studies on structure of haptoglobin 1-1 and hemoglobin in the haptoglobin-hemoglobin complex. Biochem. Biophys. Res. Commun. 1971;45:1232–1239. doi: 10.1016/0006-291x(71)90150-1. [DOI] [PubMed] [Google Scholar]
- 43.Miller YI, Altamentova SM, Shaklai N. Oxidation of low-density lipoprotein by hemoglobin stems from heme-initiated globin radical: antioxidant role of haptoglobin. Biochemistry. 1997;36:12189–12198. doi: 10.1021/bi970258a. [DOI] [PubMed] [Google Scholar]
- 44.Rodkey F. A mechanism for the conversion of oxyhemoglobin to methemoglobin by nitrite. Clin. Chem. 1976;22:1986–1990. [PubMed] [Google Scholar]
- 45.Doyle M, Herman J, Dykstra L. Autocatalytic oxidation of hemoglobin induced by nitrite: activation and chemical inhibition. J. Free Rad. Biol. Med. 1985;1:145–153. doi: 10.1016/0748-5514(85)90019-4. [DOI] [PubMed] [Google Scholar]
- 46.Lissi E. Autocatalytic oxidation of hemoglobin by nitrite: a possible mechanism. J. Free Rad. Biol. Med. 1988;24:1535–1536. doi: 10.1016/s0891-5849(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 47.Kosaka H, Tyuma I. Mechanism of autocatalytic oxidation of oxyhemoglobin by nitrite. Environ. Health Perspect. 1987;73:147–151. doi: 10.1289/ehp.8773147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pietraforte D, Salzano AM, Scorza G, Minetti M. Scavenging of reactive nitrogen species by oxygenated hemoglobin: globin radicals and nitrotyrosines distinguish nitrite from nitric oxide reaction. Free Rad. Biol. Med. 2004;37:1244–1255. doi: 10.1016/j.freeradbiomed.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 49.Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, Tambouret R, Jassal DS, Raher MJ, Furutani E, Ichinose F, Gladwin MT, Rosenzweig A, Zapol WM, Picard MH, Bloch KD, Scherrer-Crosbie M. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H379–H384. doi: 10.1152/ajpheart.01172.2005. [DOI] [PubMed] [Google Scholar]
- 50.Kimura M, Ueda K, Goto C, Jitsuiki D, Nishioka K, Umemura T, Noma K, Yoshizumi M, Chayama K, Higashi Y. Repetition of ischemic preconditioning augments endothelium-dependent vasodilation in humans. Role of endothelium-derived nitric oxide and endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2007;27:1403–1410. doi: 10.1161/ATVBAHA.107.143578. [DOI] [PubMed] [Google Scholar]