Abstract
Rationale
In order to assess participation in physical activities (PA) and disability in chronic obstructive pulmonary disease (COPD), we evaluated the use of an accelerometer and checklist to measure free-living PA.
Methods
17 males with stable COPD completed a daily activity checklist for 14 days. 10 subjects concurrently wore an accelerometer (FitSense, Southborough, MA) that records steps per day. Regression models assessed relationships between steps per day, number of daily checklist activities performed, and clinical measures of COPD status.
Results
The average steps per day ranged from 406 to 4,856. The median intra-subject coefficient of variation for steps per day was 0.52 (interquartile range [IQR] 0.41-0.58) and for number of daily checklist activities performed was 0.28 (IQR 0.22-0.32). A higher number of steps per day were associated with a greater distance walked on the 6-minute walk test and better health-related quality of life. A higher number of daily checklist activities performed was associated with a higher FEV1 % predicted and lower BODE index.
Conclusions
It is feasible to prospectively measure free-living PA in COPD using an unobtrusive accelerometer and simple activity checklist. There is low intra-subject variation in free-living PA, which is significantly associated with clinical measures of COPD status.
Keywords: accelerometer, ambulation, COPD, free-living physical activity, physical activity checklist, wearable sensor
INTRODUCTION
Improving exercise capacity and increasing physical activity is one of the main goals of treatment and research in chronic obstructive pulmonary disease (COPD) (1-3). Patients with severe COPD who have low exercise capacity have a higher probability of death compared to those with high exercise capacity (4;5). Reduced physical activity is associated with an increased risk of hospital admissions for COPD and respiratory mortality (6). Reduced physical activity in patients with COPD is also associated with higher levels of circulating markers of systemic inflammation, such as C-reactive protein and fibrinogen, that are associated with greater all-cause and cardiovascular mortality (7). Methods to measure disability in COPD by prospectively assessing participation in physical activities may lead to novel applications that allow maintenance of exercise benefits following a supervised pulmonary rehabilitation program, the early detection of COPD exacerbations, assessment of response to therapeutic interventions in COPD clinical trials, and recommendations for specific changes in physical activity to improve health outcomes in COPD.
While laboratory measurements of exercise capacity are well standardized (8-10), the methods to assess free-living physical activity, defined as “the level of activity that the patients, within their physical limitations, at their own pace, and in their own environment, typically perform,” have been limited (11-13). The pedometers and accelerometers used to date to measure physical activity in COPD have been limited by low accuracy at slow walking speeds (14), substantial intra-individual fluctuations in activity (15-17), high cost per device (11), need for users to record activity counts in a diary card (12), and need to position the device precisely on the waist and leg for accurate recordings (18). Physical activity questionnaires currently available assess recreational and occupational activities not typically performed by the relatively inactive, retired COPD population (11;19-22). Furthermore, health-related quality of life (HRQL) questionnaires assess the impact of COPD and symptoms on physical activity, rather than measure the amount or types of activities performed (12;23-25).
To overcome these limitations, we used the simple, inexpensive, and unobtrusive Actiped accelerometer (FitSense Technology, Inc., Southborough, MA) (26), which we have previously demonstrated to be accurate in a subgroup of subjects with COPD (27). In addition to steps per day, we developed a simple physical activity checklist to capture activities typically performed by patients with COPD. We used the Actiped accelerometer and physical activity checklist to (1) assess the feasibility of a simple program to monitor free-living physical activity over 2 weeks, (2) quantify the typical amount of free-living physical activity in which subjects with COPD participate, and (3) examine the relationships between steps per day, number of daily checklist activities performed, and various clinical measures of COPD status.
METHODS
Subjects
17 males with stable COPD were enrolled from a general pulmonary clinic between May and July, 2007. COPD was defined as having forced expiratory volume in 1 second (FEV1)/forced vital capacity <0.70 and a smoking history of > 10 pack-years or computed tomography evidence of emphysema. The protocol was approved by the VA Boston Healthcare System Committee on Human Research. Informed consent was obtained from each subject.
Accelerometer
Steps per day were measured with the Actiped accelerometer, an unobtrusive device that attaches to the shoe. It is relatively inexpensive at 150 USD per device, requires no maintenance by the user, and wirelessly transmits step counts to an internet-based database. In the clinic, all subjects performed 2 walks, one on a level course at usual walking speed and the other a 6-minute walk test (6MWT) at maximal walking speed while wearing an accelerometer. We confirmed that device accuracy [(device step counts/manual step counts)] × 100 was > 90% for both walks prior to sending the device home with each participant. Device accuracy was < 90% in 7 subjects with low usual walking speeds; therefore, 10 of the 17 subjects were given the accelerometer to wear at home for 14 days.
Participants were instructed to wear the accelerometer during all waking hours, except when bathing or showering. Subjects were contacted by phone at the end of each week to assess adherence and any device problems. The devices were returned to the study site by mail. When data were downloaded, it was discovered that in subjects who had high numbers of steps per day, the device did not have enough memory and overwrote stored data beginning with day 1. All subjects had data recorded for at least days 10 through 14. For the 10 subjects, there were a total of 86 person-days monitored. All accelerometer data that were available were analyzed.
Physical Activity Checklist (Appendix A)
We developed a physical activity checklist to complement the accelerometer data and assess upper extremity activities and lower extremity activities that may not be captured as ambulation. Physical activities selected for the activity checklist were related to self-care, home-management, movement, exercise, and recreation, and were based in part on the Pulmonary Functional Status & Dyspnea Questionnaire Activity Assessment (23). The checklist is self-administered and can be completed in approximately 10 minutes. Subjects indicated participation in an activity by circling yes or no and were asked to complete the one-page checklist every evening for 14 days.
Clinical Assessments
Exercise capacity was assessed with maximal distance walked on the 6MWT (8). Pulmonary function was assessed with FEV1 (28). We calculated the BODE (Body-Mass Index [BMI], Airflow Obstruction, Dyspnea, Exercise Capacity) Index, a multidimensional 10-point grading system, that assesses COPD severity (29).
Comorbidities of coronary artery disease (CAD), congestive heart failure (CHF), diabetes, and joint problems (osteoarthritis, hip or knee replacements, low back pain, or vertebral disc disease) were ascertained by interview and medical record review. Demographics and lifestyle activities that may affect level of physical activity such as educational status, current employment status, alcohol use, current smoking status, sleep quality, and participation in regular exercise were assessed by interview. We also assessed medication use, including prednisone use in the previous year, and use of supplemental oxygen.
Health-related quality of life (HRQL) was assessed by the Veterans RAND 36-Item Heath Survey (VR-36) (30) modified from the Medical Outcomes Study 36-item short form (SF-36) (25). We used Physical Component Summary (PCS) and Mental Component Summary (MCS) scores, with higher scores indicating better health status. The St. George's Respiratory Questionnaire (SGRQ) was used to assess respiratory-specific HRQL (24). Lower SGRQ total score indicates better health status. Dyspnea was quantified with the Medical Research Council (MRC) Dyspnea Scale (31) and the modified Borg rating scale (32). Depression was assessed with the Beck Depression Inventory (33).
Statistical Analysis
We examined summary statistics and plots of steps per day and number of daily checklist activities performed. To assess the degree of daily variation over 14 days in steps per day and number of daily checklist activities performed, we calculated the coefficient of variation (CV). We examined whether weekend days versus weekdays affected steps per day and number of daily checklist activities performed. Regression models accounting for repeated measures (PROC MIXED, SAS version 9.2; SAS Institute; Cary, NC) assessed the relationships between steps per day, number of daily checklist activities performed, and various measures of COPD status (34).
RESULTS
At the weekly telephone interviews, all subjects reported adherence to wearing the accelerometer and completing the activity checklist. No problems were reported with use of the accelerometer. All 238 (17 subjects × 14 days) activity checklists and 10 devices were returned by mail to the study site in a timely manner.
Subjects were white males with age 73 ± 8 years (mean ± SD), with moderate airways obstruction, mean FEV1 1.6 ± 0.68 L (57 ± 22 % predicted); most subjects were in GOLD stage II (1) (Table 1). Mean 6MW distance was 438 ± 99 m. Six subjects had concomitant CAD and 13 had joint problems. Two subjects had a history of CHF. The group was overweight and had mild depression, with mean Beck total score of 14 ± 12. Ten subjects reported participating in regular exercise, although only 4 had ever participated in a supervised pulmonary rehabilitation program. Thirteen subjects reported walking more than 10 minutes at a time on 3 or more days during a typical week. No subject experienced a COPD exacerbation during the study period. Characteristics for the group of 17 subjects as well as for the subgroups of 10 subjects who wore the accelerometer and the 7 who did not are presented in Table 1.
Table 1.
Subject Characteristics
| Variable | N=17 total | N=10 wore acc | N=7 did not wear acc |
|---|---|---|---|
| Age, yr, mean (SD) | 73(8) | 70(8) | 78(5) |
| Usual walking speed, mph, mean (SD) | 2.31 (0.41) | 2.49 (0.31) | 2.05 (0.42) |
| BMI, kg/m2, mean (SD) | 28(4) | 28(4) | 29(4) |
| Married, n (%) | 8(47) | 3(30) | 5(71) |
| Retired, n (%) | 12 (70) | 6(60) | 6(86) |
| Completed high school or more, n (%) | 14 (82) | 9(90) | 5(71) |
| Current smoker, n (%) | 3(18) | 3(30) | 0(0) |
| Regular drinkers, n (%) | 8(47) | 4(40) | 4(57) |
| Never get a good night's sleep, n (%) | 5(29) | 5(50) | 0(0) |
| Participate in regular exercise, n (%) | 10 (59) | 5(50) | 5(71) |
| FEV1 % predicted, mean (SD) | 57 (22) | 60 (25) | 53 (18) |
| GOLD Stage I Stage II Stage III Stage IV |
1(5) 10 (59) 2(12) 4(24) |
1(10) 6(60) 1(10) 2(20) |
0(0) 4(57) 1(14) 2(28) |
| Participated in pulmonary rehabilitation, n (%) | 4(24) | 4(40) | 0(0) |
| Current use of supplemental oxygen, n (%) | 5(29) | 3(30) | 2(28) |
| Current use of LAMA, n (%) | 10 (59) | 6(60) | 4(57) |
| Current use of LABA, n (%) | 12 (70) | 7(70) | 5(71) |
| Current use of inhaled corticosteroid, n (%) | 14 (82) | 8(80) | 6(86) |
| Used prednisone in past year, n (%) | 6(35) | 3(30) | 3(43) |
| Joint problems, n (%) | 13 (76) | 8(80) | 5(71) |
| Coronary artery disease, n (%) | 6(35) | 4(40) | 2(28) |
| Congestive heart failure, n (%) | 2(12) | 2(20) | 0(0) |
| Diabetes, n (%) | 5(29) | 3(30) | 2(28) |
| 6MW Test Distance, m, mean (SD) | 438 (99) | 467 (85) | 395 (108) |
| Dyspnea Borg Scale, mean (SD) | 1.7 (1.6) | 1.6(1.9) | 1.7 (1.4) |
| PCS, mean (SD) | 36 (12) | 36 (14) | 36(9) |
| MCS, mean (SD) | 50 (12) | 51 (10) | 49 (15) |
| SGRQ Total Score, mean (SD) | 44 (23) | 40 (22) | 49 (21) |
| Beck Depression Index, mean (SD) | 14 (12) | 13(9) | 15 (14) |
| BODE Index, mean (SD) | 4.0 (1.7) | 3.8 (1.5) | 4.3 (2.0) |
Definition of abbreviations: acc=accelerometer; BMI=body-mass index; LAMA=long-acting muscarinic antagonist; LABA=long-acting beta agonist; GOLD= Global Initiative for Chronic Obstructive Lung Disease; 6MW=6 minute walk; PCS=Physical Component Summary, MCS=Mental Component Summary, SGRQ=St. George's Respiratory Questionnaire, BODE=body-mass index, obstruction, dyspnea, exercise capacity.
The physical activity checklist included walking a dog and playing with grandchildren. Review of the responses completed by the 17 subjects showed that 13 subjects did not have a dog and 6 did not have grandchildren. These activities were excluded from the analyses, resulting in a maximum of 15 activities performed per day. The average number of daily checklist activities performed was 7±1 (range 0-13 per day). Table 2 shows frequency of participation in physical activities; there was no floor or ceiling effect. The most common activities performed (> 50% of days monitored) were preparing meals, climbing stairs, visiting friends or relatives, and traveling in a car, bus or train. Subjects were least likely to walk in a mall or store, go to a medical appointment, dig in the yard or garden, chop wood, or exercise at a gym or at home (< 29% of days). Walking in a grocery store, unloading groceries from car, performing an errand, picking up mail from mailbox, and washing and folding laundry, sweeping floors or making the bed were performed 39-48% of the days.
Table 2.
Number of times (%*) each activity was performed, n=17
| Physical Activity | YES | NO |
|---|---|---|
| Walk more than 5 minutes at a time | 181(76) | 37 (16) |
| Walk in a grocery store | 93 (39) | 142 (60) |
| Unload groceries from car and carry them into home | 102 (43) | 135 (57) |
| Walk in a mall or store like Home Depot | 59 (25) | 171 (72) |
| Perform an errand (go to the post office, bank, dry cleaner) | 102 (43) | 131 (55) |
| Go to a medical appointment | 34 (14) | 196(82) |
| Visit with relatives or friends | 138 (58) | 97 (41) |
| Prepare your own meal | 161 (68) | 76 (32) |
| Climb up stairs | 165 (69) | 73 (31) |
| Dig in yard or garden, chop wood, or shovel snow | 70 (29) | 162 (68) |
| Exercise at a gym (swim, bike, treadmill, weights) | 29 (12) | 209 (88) |
| Exercise at home (bike, treadmill, weights) | 51 (21) | 186 (78) |
| Pick up mail from the mailbox | 108 (45) | 129 (54) |
| Wash and fold laundry, sweep floors, or make the bed | 115(48) | 122 (51) |
| Travel in a car, bus, or train | 195 (82) | 42(18) |
May not add up to 100% because question was not answered or did not apply. Maximum number of times activity could be performed was 238 (17 subjects × 14 days).
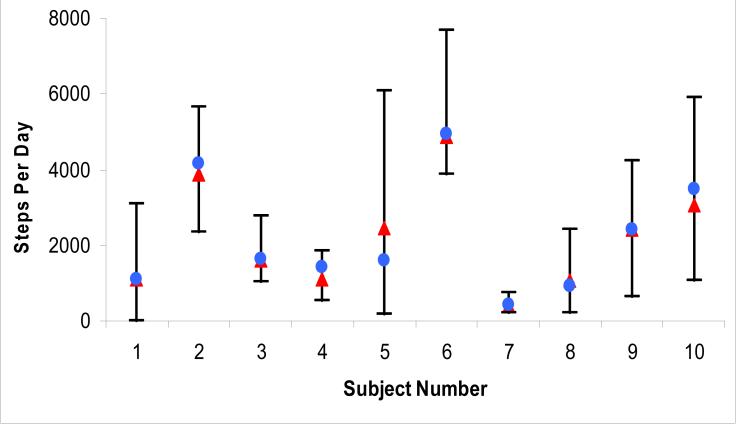
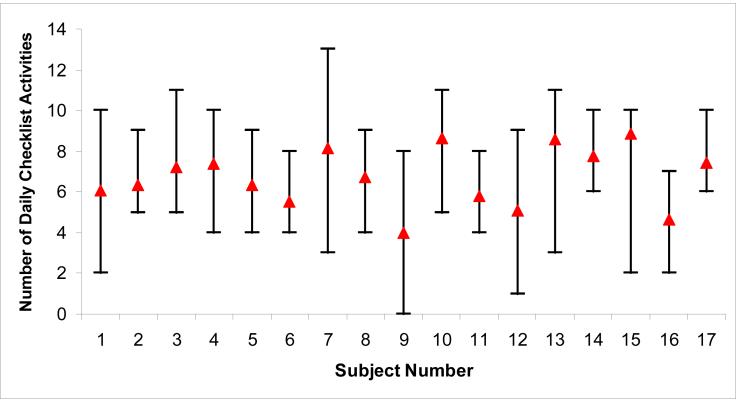
For the 10 subjects who wore the accelerometer at home, average steps per day for each subject ranged from 406 to 4,856, and the overall mean steps per day was 2,026 ± 1,783. Steps per day were significantly correlated with number of daily checklist activities. For each unit increase in checklist activities, there was an increase of 174 steps per day, p=0.04. Figures 1 and 2 shows the range of steps per day and number of daily checklist activities performed within and between subjects. The median intra-subject CV for steps per day was 0.52 (IQR 0.41-0.58). There was greater variation between subjects for steps per day, inter-subject CV of 0.64. Eight of the 10 subjects had an intra-subject CV (range 0.24-0.58) less than the inter-subject CV, indicating that the intra-subject variation in steps per day was relatively low. The intra-subject variation in number of daily checklist activities performed was lower (median CV 0.28 [IQR 0.22-0.32]) than the intra-subject variation in steps per day. The day of the week, categorized as either weekend or weekday, was not significantly associated with either steps per day or daily number of checklist activities performed.
Figure 1.
Range of steps per day within and between subjects, n=10.
Δ: mean daily step count for all days with data
Ο: mean daily step count for days 10 through 14
Figure 2.
Range of number of daily checklist activities within and between subjects, n=14. Subjects 1-10 are the same subjects as 1-10 in Figure 1.
Δ: mean number of daily checklist activities for 14 days
Steps per day were significantly associated with 6MWT distance, PCS and MCS, and the VR-36 Physical Function domain in regression models (Table 3). Number of daily checklist activities performed was significantly associated with FEV1 % predicted and BODE index (Table 4). Steps per day and number of daily checklist activities performed were significantly associated with comorbid joint problems. A history of joint problems was associated with fewer steps per day -2,742, 95% CI [-3,723, -1,761], p=0.0002 and fewer daily activities -1.1, 95% CI [-2.2, -0.083], p=0.04. Neither steps per day nor number of daily checklist activities was significantly associated with prior participation in pulmonary rehabilitation, report of regular exercise, prednisone use in the previous year, or use of supplemental oxygen use (p=0.12-0.93). Age, BMI, Beck depression score, and dyspnea assessed by modified Borg scale were not significantly associated with either steps per day or number of daily checklist activities performed (p=0.08-0.90).
Table 3.
Associations with Steps per Day, n=10
| Independent Variable | Unadjusted Coefficient [95 % CI] | P value |
|---|---|---|
| Checklist Activities | 174 [7,341] | 0.04 |
| 6MW Distance | 10 [4,17] | 0.01 |
| PCS | 67 [35,99] | 0.0003 |
| MCS | -72 [-125, -19] | 0.01 |
| VR-36 Physical Function | 29 [12,47] | 0.002 |
| Joint Problems | -2742 [-3723,-1761] | 0.0002 |
Each univariate model has steps per day as the dependent variable and one of the variables listed as the independent variable. Definition of abbreviations: 6MW=6 minute walk, PCS=Physical Component Summary, MCS=Mental Component Summary, VR-36=Veterans RAND 36-Item Health Survey.
Table 4.
Associations with Number of Daily Checklist Physical Activities Performed, n=17
| Independent Variable | Unadjusted Coefficient [95 % CI] | P value |
|---|---|---|
| FEV1 % predicted | 0.023 [0.005,0.041] | 0.01 |
| BODE Index | -0.34 [-0.58,-0.10] | 0.008 |
| Joint Problems | -1.1 [-2.2,-0.083] | 0.04 |
Each univariate model has number of daily checklist physical activities performed as the dependent variable and one of the variables listed as the independent variable. Definition of abbreviations: BODE=body-mass index, obstruction, dyspnea, exercise capacity.
DISCUSSION
We demonstrate that it is feasible for subjects with COPD to complete a simple daily activity checklist to monitor free-living PA for up to 2 weeks. In the subjects in whom we verified > 90% accuracy of the accelerometer to detect step counts, we also demonstrate that it is feasible for elderly subjects with COPD and other comorbidities to wear an unobtrusive, inexpensive, easy-to-use accelerometer. Using both the checklist and the accelerometer, we show that there is little day-to-day variability in physical activity in subjects with COPD and that free-living PA is significantly associated with clinical measures of COPD status. These types of methods may be used in future studies to prospectively assess participation in physical activities and measure disability in COPD.
Physical activity measured with our simple checklist was significantly associated with FEV1 % predicted and the BODE index demonstrating concurrent validity with different measures of COPD severity. Similarly, Pitta et al (35) found that BODE index increased significantly with each day of inactivity. We found no floor or ceiling effect for the 15 activities assessed, suggesting that the checklist can be sensitive to change if subjects engaged in fewer activities during a COPD exacerbation or engaged in more activities after a therapeutic intervention. We minimized the potential impact of seasonal variation on participation in physical activities by conducting the study over 3 months in the spring and summer. Our results show that the types of physical activities performed do not vary much from day-to-day within a subject, and that there is more day-to-day variation in steps walked per day. We acknowledge that it is possible that a greater variation in daily physical activities and step counts would have been observed in a study that included greater numbers of subjects across all GOLD stages, including women.
Currently, the devices most commonly used to measure physical activity in COPD are the Tritrac R3D (RT3 is newer version), the Dynaport Activity Monitor, and the SenseWear Pro Armband. Based on previous work using these devices, we currently know that, compared to healthy subjects, physical activity is reduced in patients with moderate to severe COPD (7;12;18). We also know that physical activity is only moderately correlated with the degree of airflow obstruction (12;18), indicating that physical activity captures a dimension of COPD disease status that is not assessed by current clinical measures. However, limitations of these devices have prohibited long-term monitoring and use in large scale clinical trials. The Tritrac R3D produces substantial intra-individual fluctuations in activity (16), the Dynaport Activity Monitor requires precise positioning on the waist and leg for accurate recordings (18), and the SenseWear Pro Armband is expensive at $1,000 USD per device. In the current study, we similarly show that patients with COPD walk considerably less than the 10,000 steps per day recommended for health promotion (36;37) and that steps per day are significantly associated with 6MWT distance and HRQL, but not with FEV1 % predicted. However, this is the first study to our knowledge that confirms previous findings with the use of an unobtrusive, simple accelerometer device.
It is difficult to identify a `gold standard' measure of actual (as opposed to reported) daily activity against which to test the accuracy of these devices. The devices used to date in COPD have different outcomes, with accuracy assessed in various ways. For example, the Tritrac R3D has the main outcome of vector magnitude units. Content and concurrent validity were demonstrated based on moderate to high Pearson correlation coefficients with 6MWT, FEV1, and dyspnea during monitoring in the home environment (15). The DynaPort Activity Monitor has its main outcome as time spent in different activities such as walking, standing, or lying. Accuracy has been assessed by comparisons with video recordings showing high agreement in protocols conducted in the clinic (38). The SenseWear Pro Armband has the main outcome of energy expenditure (7;39). This device has been validated against an exhaled breath metabolic system in the laboratory setting during slow to moderate pace walking (39).
We chose to use the Actihealth accelerometer since we were able to easily verify its accuracy against manual step counts for each subject in the clinic before home use. Although we were disappointed with its performance given its inaccuracy at low walking speeds observed in some persons with COPD, our results highlight the importance of verifying device accuracy in each subject being studied prior to use in the field. In keeping with our previous finding that usual walking speed is the most important predictor of device accuracy (27), the main difference between the 10 subjects who had >90 % accuracy and the 7 subjects who did not was usual walking speed, 2.49 mph versus 2.05 mph (Table 1). The issue of activity quantitation in COPD is still in the early stages of research and the technical issue of data accuracy in the COPD population needs to be addressed before the appropriate devices can be identified and used to assess free-living activity and promote physical activity in the COPD population. The devices will need to be inexpensive, easy to use, provide feedback to the subjects, and have capabilities to feedback data to health care providers either through the Internet or by telephone.
Furthermore, the Actihealth accelerometer had memory storage problems despite product labeling that it could store data for up to 30 days. Nevertheless, we found that average steps per day for the last 5 days of monitoring, for which all subjects had step count data, was nearly identical to average steps per day for all the days monitored for each subject suggesting that the data available were representative of the 14-day observation period (Figure 1). The device does not capture upper extremity activities or energy expenditure, but we coupled its use with an activity checklist that captured daily physical activities other than ambulation relevant to persons with COPD. It was not the purpose of this study to calculate the level of physical activity in absolute units such as energy expenditure. Our main objective was to test the feasibility of using an unobtrusive device and simple activity checklist under free-living conditions.
Given the number of observations obtained from repeated measurements over 2 weeks for each subject, there was enough statistical power to assess univariate correlations between steps per day, number of daily checklist activities, and other clinical variables. We found that steps per day were significantly associated with 6MWT distance, HRQL as measured by the PCS and MCS, and history of joint problems. Neither steps per day nor number of daily checklist activities performed was significantly associated with prior participation in pulmonary rehabilitation, report of regular exercise, prednisone use in the previous year, or supplemental oxygen use. Age, BMI, Beck depression score, and dyspnea assessed by the modified Borg scale were not significantly associated with either steps per day or number of daily checklist activities performed. Similarly, walking time has been correlated with 6MW distance, but not with use of oral corticosteroids, obesity, depression, arthritis, or back pain (18). FEV1 and BMI were not related to physical activity as assessed by a questionnaire, and dyspnea was not associated with physical activity in univariate models (22). These findings support the hypothesis that free-living physical activity captures a dimension of COPD disease status that is not assessed by current clinical measures. Sandland et al (40) showed that activity counts in subjects with COPD who received long term oxygen therapy were reduced by 79% compared to a healthy group. The difference in findings may be due to a much lower average FEV1 in their cohort of 9 subjects (0.66 L) compared to an average FEV1 of 1.6 L in our cohort. In order to assess the main cause of low steps per day in COPD, a larger study is needed to include variables that affect steps per day--such as lung function, HRQL, joint problems -in the same multivariate model.
CONCLUSION
In this report, we have demonstrated that free-living physical activity in subjects with COPD can be assessed with a simple activity checklist and, in the subgroup of subjects in whom the device is accurate, with the use of an unobtrusive, simple accelerometer. Using the accelerometer and the activity checklist, we show that there is low daily intra-subject variation in free-living PA which is significantly associated with clinical measures of COPD status. These types of methods may be used in future studies to prospectively assess participation in physical activities and measure disability in COPD.
ACKNOWLEDGEMENT
We thank Avron Spiro III, PhD for his thoughtful review of the manuscript.
The research reported here was supported by the Department of Veteran Affairs, Veterans Health Administration, Rehabilitation Research and Development Service through a VA Career Development Award to Dr. Moy.
Supported by NIH/NICHD RO1 HD42141 (Dr. Garshick).
ABBREVIATION LIST
- BODE
Body-Mass Index, Airflow Obstruction, Dyspnea, Exercise Capacity
- CAD
Coronary Artery Disease
- CHF
Congestive Heart Failure
- CI
Confidence Interval
- COPD
Chronic Obstructive Pulmonary Disease
- CV
Coefficient of Variation
- FEV1
Force Expiratory Volume in 1 second
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- HRQL
Health Related Quality of Life
- MCS
Mental Component Summary
- MPH
Miles per Hour
- PCS
Physical Component Summary
- SGRQ
St. George's Respiratory Questionnaire
- 6MWT
6-Minute Walk Test
Appendix A. Physical Activity Checklist
We are interested in the physical activities that you performed today. For each activity, circle Yes if you performed the activity today. Circle No if you did not
| DAY _ DATE | YES or NO |
|---|---|
| Walk more than 5 minutes at a time | YES NO |
| Walk dog □ I do not have a dog |
YES NO |
| Walk in a grocery store | YES NO |
| Unload groceries from car and carry them into home | YES NO |
| Walk in a mall or store like Home Depot | YES NO |
| Perform an errand (go to the post office, bank, dry cleaner) | YES NO |
| Go to a medical appointment | YES NO |
| Visit with relatives or friends | YES NO |
| Prepare your own meal | YES NO |
| Climb up stairs | YES NO |
| Dig in yard or garden, chop wood, or shovel snow | YES NO |
| Exercise at a gym (swim, bike, treadmill, weights) | YES NO |
| Exercise at home (bike, treadmill, weights) | YES NO |
| Pick up mail from the mailbox | YES NO |
| Play with grandchildren □ I do not have grandchildren |
YES NO |
| Wash and fold laundry, sweep floors, or make the bed | YES NO |
| Travel in a car, bus, or train | YES NO |
Footnotes
This work was performed at VA Boston Healthcare System, Boston, MA.
This study was initiated by the investigators who do not receive any financial support from FitSense, Inc. The results of the present study do not constitute endorsement of the product by the authors. None of the authors have any financial or other potential conflicts of interest to report.
Reference List
- (1).Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007 Sep 15;176(6):532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- (2).Croxton TL, Weinmann GG, Senior RM, Hoidal JR. Future research directions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002 Mar 15;165(6):838–44. doi: 10.1164/ajrccm.165.6.2108036. [DOI] [PubMed] [Google Scholar]
- (3).Croxton TL, Weinmann GG, Senior RM, Wise RA, Crapo JD, Buist AS. Clinical research in chronic obstructive pulmonary disease: needs and opportunities. Am J Respir Crit Care Med. 2003 Apr 15;167(8):1142–9. doi: 10.1164/rccm.200207-756WS. [DOI] [PubMed] [Google Scholar]
- (4).National Emphysema Treatment Trial Research Group A Randomized Trial Comparing Lung Volume Reduction Surgery with Medical Therapy for Severe Emphysema. N Engl J Med. 348:2059–2073. doi: 10.1056/NEJMoa030287. 5-22-2003. [DOI] [PubMed] [Google Scholar]
- (5).Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006 Jun 15;173(12):1326–34. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006 Sep;61(9):772–8. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Watz H, Waschki B, Boehme C, Claussen M, Meyer T, Magnussen H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional study. Am J Respir Crit Care Med. 2008 Apr 1;177(7):743–51. doi: 10.1164/rccm.200707-1011OC. [DOI] [PubMed] [Google Scholar]
- (8).ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002 Jul 1;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- (9).Sciurba F, Criner GJ, Lee SM, Mohsenifar Z, Shade D, Slivka W, et al. Six-minute walk distance in chronic obstructive pulmonary disease: reproducibility and effect of walking course layout and length. Am J Respir Crit Care Med. 2003 Jun 1;167(11):1522–7. doi: 10.1164/rccm.200203-166OC. [DOI] [PubMed] [Google Scholar]
- (10).ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003 Jan 15;167(2):211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- (11).Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur Respir J. 2006 May;27(5):1040–55. doi: 10.1183/09031936.06.00064105. [DOI] [PubMed] [Google Scholar]
- (12).Schonhofer B, Ardes P, Geibel M, Kohler D, Jones PW. Evaluation of a movement detector to measure daily activity in patients with chronic lung disease. Eur Respir J. 1997 Dec;10(12):2814–9. doi: 10.1183/09031936.97.10122814. [DOI] [PubMed] [Google Scholar]
- (13).Moy ML, Mentzer SJ, Reilly JJ. Ambulatory monitoring of cumulative free-living activity. IEEE Eng Med Biol Mag. 2003 May;22(3):89–95. doi: 10.1109/memb.2003.1213631. [DOI] [PubMed] [Google Scholar]
- (14).Storti KL, Pettee KK, Brach JS, Talkowski JB, Richardson CR, Kriska AM. Gait speed and step-count monitor accuracy in community-dwelling older adults. Med Sci Sports Exerc. 2008 Jan;40(1):59–64. doi: 10.1249/mss.0b013e318158b504. [DOI] [PubMed] [Google Scholar]
- (15).Steele BG, Holt L, Belza B, Ferris S, Lakshminaryan S, Buchner DM. Quantitating physical activity in COPD using a triaxial accelerometer. Chest. 2000 May;117(5):1359–67. doi: 10.1378/chest.117.5.1359. [DOI] [PubMed] [Google Scholar]
- (16).Nguyen HQ, Steele B, Benditt JO. Use of accelerometers to characterize physical activity patterns with COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2006;1(4):455–60. doi: 10.2147/copd.2006.1.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Steele BG, Belza B, Hunziker J, Holt L, Legro M, Coppersmith J, et al. Monitoring daily activity during pulmonary rehabilitation using a triaxial accelerometer. J Cardiopulm Rehabil. 2003 Mar;23(2):139–42. doi: 10.1097/00008483-200303000-00011. [DOI] [PubMed] [Google Scholar]
- (18).Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005 May 1;171(9):972–7. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- (19).Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001 Jul;33(7):1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- (20).Dubbert PM, Vander Weg MW, Kirchner KA, Shaw B. Evaluation of the 7-day physical activity recall in urban and rural men. Med Sci Sports Exerc. 2004 Sep;36(9):1646–54. doi: 10.1249/01.mss.0000139893.65189.f2. [DOI] [PubMed] [Google Scholar]
- (21).Kriska AM. A Collection of Physical Activity Questionnaires for Health-Related Research. Med Sci Sports Exerc. 1997;29(6) Supplement. [PubMed] [Google Scholar]
- (22).Garcia-Aymerich J, Felez MA, Escarrabill J, Marrades RM, Morera J, Elosua R, et al. Physical activity and its determinants in severe chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2004 Oct;36(10):1667–73. doi: 10.1249/01.mss.0000142378.98039.58. [DOI] [PubMed] [Google Scholar]
- (23).Lareau SC, Carrieri-Kohlman V, Janson-Bjerklie S, Roos PJ. Development and testing of the Pulmonary Functional Status and Dyspnea Questionnaire (PFSDQ) Heart Lung. 1994 May;23(3):242–50. [PubMed] [Google Scholar]
- (24).Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis. 1992 Jun;145(6):1321–7. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- (25).Ware JE. The SF-36 health survey. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2nd ed. Lippincott-Raven; Philadelphia, PA: 1996. [Google Scholar]
- (26).Weyand PG, Kelly M, Blackadar T, Darley JC, Oliver SR, Ohlenbusch NE, et al. Ambulatory estimates of maximal aerobic power from foot -ground contact times and heart rates in running humans. J Appl Physiol. 2001 Jul;91(1):451–8. doi: 10.1152/jappl.2001.91.1.451. [DOI] [PubMed] [Google Scholar]
- (27).Moy ML, Garshick E, Matthess K, Lew R, Reilly J. Accuracy of a uniaxial accelerometer in chronic obstructive pulmonary disease. JRRD. 2008;45:611–617. doi: 10.1682/jrrd.2007.09.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).American Thoracic Society statement. Lung function testing: selection of reference values and interpretative strategies. Am J Respir Crit Care Med. 1991;144:1202–18. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- (29).Celli BR, Cote CG, Marin JM, Casanova C, Montes de OM, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004 Mar 4;350(10):1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- (30).Kazis LE, Miller DR, Clark JA, Skinner KM, Lee A, Ren XS, et al. Improving the response choices on the veterans SF-36 health survey role functioning scales: results from the Veterans Health Study. J Ambul Care Manage. 2004 Jul;27(3):263–80. doi: 10.1097/00004479-200407000-00010. [DOI] [PubMed] [Google Scholar]
- (31).Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988 Mar;93(3):580–6. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- (32).Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81. [PubMed] [Google Scholar]
- (33).Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961 Jun;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- (34).Kleinbaum D, Kupper L, Muller K, Nizam A. 3rd ed. Duxbury Press; Boston: 1998. Applied regression analysis and other multivarible methods. [Google Scholar]
- (35).Pitta F, Troosters T, Probst VS, Lucas S, Decramer M, Gosselink R. Potential consequences for stable chronic obstructive pulmonary disease patients who do not get the recommended minimum daily amount of physical activity. J Bras Pneumol. 2006 Jul;32(4):301–8. doi: 10.1590/s1806-37132006001100008. [DOI] [PubMed] [Google Scholar]
- (36).Tudor-Locke C, Bassett DR., Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- (37).Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007 Nov 21;298(19):2296–304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- (38).Pitta F, Troosters T, Spruit MA, Decramer M, Gosselink R. Activity monitoring for assessment of physical activities in daily life in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2005 Oct;86(10):1979–85. doi: 10.1016/j.apmr.2005.04.016. [DOI] [PubMed] [Google Scholar]
- (39).Patel SA, Benzo RP, Slivka WA, Sciurba FC. Activity monitoring and energy expenditure in COPD patients: a validation study. COPD. 2007 Jun;4(2):107–12. doi: 10.1080/15412550701246658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Sandland CJ, Singh SJ, Curcio A, Jones PM, Morgan MD. A profile of daily activity in chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2005 May;25(3):181–3. doi: 10.1097/00008483-200505000-00011. [DOI] [PubMed] [Google Scholar]