Abstract
Background
Mutations and polymorphisms of OGG1, the major mammalian 8-oxoguanine repair activity, are associated with increased risk for several cancers. Decreased 8-oxoguanine repair capacity due to variant forms of the OGG1 gene is a common feature of numerous cancer cell lines. One such cell line, human KG-1 leukemia cells, has previously been demonstrated to be deficient in the excision of 8-oxoguanine from oxidatively damaged DNA. KG-1 cells have a homozygous R229Q amino acid substitution in OGG1 that has been presumed to alter the function of OGG1 and result in elevated levels of genomic 8-oxoG and hypersensitivity to 8-hydroxydeoxyguanosine nucleoside and ionizing radiation observed in KG-1 cells.
Methods
We characterized the enzymatic activity of R229Q OGG1 and the effect of the enzyme on cell survival following treatment with DNA damaging agents.
Results
R229Q OGG1 had activity similar to the wild-type enzyme, yet was easily heat inactivated at physiological temperature. R229Q OGG1 expressed in human cells had significantly lower activity than wild-type OGG1 and was also highly thermolabile. Expression of R229Q OGG1 sensitized KG-1 cells to killing by menadione and 8-hydroxydeoxyguanosine, but not ionizing radiation.
Conclusions
These results suggest that decreased 8-oxoguanine repair in KG-1 is due to thermolability of R229Q OGG1 and that the enzyme variant increases cellular susceptibility to killing resulting from oxidative DNA damage. The R229Q OGG1 variant is a validated polymorphism prevalent in world populations and not an isolated mutation in KG-1 cells, thus the R229Q OGG1 allele may be a novel marker for cancer susceptibility.
Keywords: R229Q, OGG1, 8-oxoguanine, KG-1, leukemia, polymorphism
Introduction
Reactive oxygen species (ROS) produce numerous forms of DNA damage, including 7,8-dihydro-8-oxoguanine (8-oxoG), a major mutagenic modification in DNA. 8-oxoG can mispair with adenine during DNA replication and, if left unrepaired, result in the fixation of G:C to T:A transversion mutations (1). Such transversions are common mutations at three major mutational hotspots in the p53 gene in lung and head and neck cancers (2). The primary mammalian enzyme for removing 8-oxoG from DNA is 8-oxoguanine-DNA glycosylase (OGG1) (3). In human tumor cells, the OGG1 locus is frequently missing or mutated and several cancer cell lines studied show decreased 8-oxoguanine excision activity, suggesting that in some cases, 8-oxoG repair capacity may be associated with cancer incidence. The importance of OGG1 as a tumor suppressor gene has been highlighted by numerous association studies linking single nucleotide polymorphisms (SNPs) in the OGG1 gene with elevated risk for lung, prostate, and orolaryngeal cancers (4). A single amino acid change in polymorphic S326C OGG1 has recently been shown to affect intracellular localization and alter repair activity, substrate specificity, molecular stoichiometry, and stimulation of the enzyme by AP-endonuclease 1 (5, 6). A decrease in 8-oxoG excision activity resulting from OGG1 deficiency or polymorphisms could be anticipated to lead to increased genomic 8-oxoG and promote the accumulation of ROS-induced mutations which may promote genomic instability and carcinogenesis.
An increase in 8-oxoG in DNA was recently reported for a model human leukemia cell line, KG-1, which was found to have a homozygous mutation in the OGG1 gene that results in the substitution of arginine 229 for glutamine (R229Q) (7) in the expressed enzyme. KG-1 cells have elevated levels of genomic 8-oxoG and are sensitive to killing via apoptosis when challenged with 8-hydroxydeoxyguanosine nucleoside (8-oxodG) or ionizing radiation exposure (7–9). Cellular extracts of KG-1 have markedly reduced 8-oxoG excision capacity when compared with leukemic cells expressing wild-type OGG1 (7), thus it was proposed that decreased repair of 8-oxoG and cellular sensitivity to ionizing radiation and 8-oxodG in KG-1 cells may be consequences of the R229Q mutation resulting in a dysfunctional OGG1 protein (7–9). We characterized the enzymatic activity of R229Q and determined the effect of R229Q expression on KG-1 survival following exposure to DNA damaging agents. Our results show that R229Q OGG1 is highly thermolabile and rapidly inactivated at physiological temperatures both in vitro and in vivo. Expression of both nuclear and mitochondrial R229Q OGG1 sensitized KG-1 cells to killing via an apoptotic pathway following exposure to menadione and 8-oxodG, thus R229Q promotes apoptosis following ROS and oxidized nucleoside exposure. Initially reported as a unique somatic mutation in KG-1 cells (7), we report that the R229Q allele is a documented polymorphism in human populations. With the significant incidence of the allele in the population, our observations of OGG1 structural destabilization and increased cell killing following induction of oxidative DNA damage resulting from the R229Q polymorphism suggest that the variant may be a potential marker for cancer susceptibility.
Materials and Methods
Cell culture
Cells were obtained from ATCC and maintained in the recommended growth medium (Gibco) in 5% CO2 at 37°C. KG-1 cells were transfected with Nucleofector R transfection reagents (Amaxa) according to the manufacturer’s instructions. HeLa cells were transfected using Fugene 6 transfection reagent (Roche) according to the manufacturer’s instructions. Nuclear extracts were prepared using NE-PER extraction reagents (Pierce). Mitochondria were prepared using a Mitochondria Isolation Kit for Mammalian Cells (Pierce). Mitochondrial extracts were prepared by lysing mitochondria from 20×106 cells in 25 μl of 1X SDS sample buffer at 95°C for 5 min.
Expression and purification of OGG1 enzymes
Wild-type and R229Q OGG1 were purified to homogeneity as described previously (6). Mammalian expression constructs were prepared by cloning the OGG1-1a coding sequence into pCMV-2B N-terminal FLAG-tag vector (Stratagene) for nuclear expression. The R229Q polymorphism was introduced into the OGG1 gene using a Quick Change XL mutagenesis kit (Stratagene). Mitochondrial vectors were prepared by cloning PCR-generated wild-type and R229Q OGG1 genes encoding a 13-amino acid C-terminal deletion into pCMV-4B C-terminal FLAG-tag vector (Stratagene). Plasmid sequences were confirmed by bi-directional DNA sequencing.
8-oxoguanine glycosylase activity assays
An HPLC-purified 31-mer oligonucleotide containing 8-oxoguanine (5′ GTG ACT ACG AGA CCT XAT GTG ACT GAG AGA G 3′, where X denotes 8-oxoG) was purchased from Midland Certified Reagent Company. A complementary oligo having C opposite 8-oxoG and an additional 5′ G was obtained from Invitrogen. Radiolabeled duplex DNA substrate having 8-oxoG paired with C was prepared by 3′-end labeling as described previously (6). OGG1 enzymes were reacted with duplex 8-oxoG:C substrate at 37°C in 20 mM Tris-HCl, pH 7.4, 100 mM NaCl, and 0.15 μg/μl BSA. Reactions were terminated by adding SDS and piperidine to 5% and 200mM, respectively, and heating to 95°C for 5 min. Reactions were electrophoresed on 20% acrylamide gels containing 7 M urea in TBE (Tris-borate-EDTA) buffer and radioactivity was quantified using a Storm Phosphorimager and Image Quant software (Molecular Dynamics).
Cell survival assays
Viability of KG-1 cells was measured following treatment with DNA damaging agents. Cells were transfected 24 hrs prior to treatments. Transfected cells (1×106) were plated in 100 mm dishes in 6 ml of growth medium. KG-1 cells were treated with menadione (Sigma) for 1 hr. Following menadione treatment, growth medium was replaced and cells were maintained for 7 days prior to analysis of cell survival. For 8-oxodG exposure, cells were maintained in medium supplemented with the indicated concentrations of 8-oxodG (8-hydroxy-2′-deoxy-guanosine, Sigma) for 7 days. Cells were exposed to ionizing radiation at a rate of 1 Gy/min in a Nordion Gammacell 40 Exactor Irradiator and analyzed for cell killing after 7 days. Cell viability was measured directly by flow cytometry. Cells were stained with Annexin V and PI (Roche) and 10,000 cells were analyzed for viability on a Becton Dickinson FACSCalibur (Becton Dickinson).
Results
R229Q OGG1 is thermolabile in vitro and in vivo
Since amino acid substitutions in proteins can often result in thermolability, we examined the effect of preincubating purified R229Q OGG1 and the wild-type enzyme (Fig. 1A) at 37°C prior to measuring excision activity. Surprisingly, purified R229Q OGG1 had 8-oxoG excision activity comparably to wild-type OGG1 (Fig. 2A). However, R229Q OGG1 was highly sensitive to heat inactivation and nearly completely inactivated by a 30 min preincubation at 37°C (Fig. 2B). Therefore, the R229Q substitution results in a thermolabile OGG1 protein that has near normal function at low temperature, but undergoes significant structural destabilization at physiological temperature. We then examined the effect of the R229Q polymorphism on OGG1 function with enzymes expressed in human cells. Wild-type and R229Q OGG1 enzymes were expressed at identical levels in KG-1 cells (Fig. 2C inset). Nuclear extracts from these transfectants were examined for 8-oxoG excision activity (Fig. 2C). Wild-type OGG1 expressed in nuclei produced high levels of 8-oxoG excision, while OGG1 activity in cells expressing R229Q OGG1 was roughly half that in cells expressing the wild-type enzyme (Fig. 2C). R229Q OGG1 expressed in vivo in KG-1 cells was easily inactivated by preincubating nuclear extracts prior to measuring enzymatic activity (Fig. 2C), in agreement with the observation of R229Q thermolability with purified enzyme (Fig. 2B). Purified OGG1 enzymes were expressed in E. coli at 16°C, whereas enzymes in nuclei of human cells were expressed at 37°C. The difference in temperature during enzyme expression explain why purified R229Q OGG1 had activity similar to the wild-type enzyme prior to preincubation, while R229Q expressed in human cells had significantly lower activity than the wild-type enzyme prior to preincubation. Similar thermolability of R229Q OGG1 was observed in nuclear extracts of HeLa cells expressing the polymorphic enzyme (data not shown). No change in the levels of wild-type or R229Q OGG1 in nuclear extracts was observed following preincubations (data not shown), thus R229Q OGG1 is heat inactivated and not selectively degraded in both KG-1 and HeLa cells.
Figure 1.
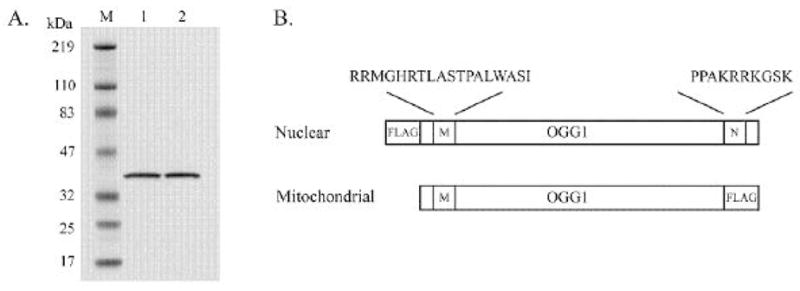
Purification of wild-type and R229Q OGG1 and mammalian expression constructs. (A) Lane 1, 0.5 μg wild-type OGG1, lane 2, 0.5 μg R229Q. (B) OGG1 coding sequences cloned into pCMV-2B (nuclear) or pCMV-4B (mitochondrial) mammalian FLAG-tag expression vectors (Stratagene). M and N denote intrinsic OGG1 mitochondrial and nuclear localization sequences, respectively. The nuclear vector encodes full-length OGG1 with an N-terminal FLAG-tag. The mitochondrial vector encodes OGG1 with the C-terminal 13 amino acids comprising the nuclear localization signal deleted and replaced with a FLAG-tag.
Figure 2.
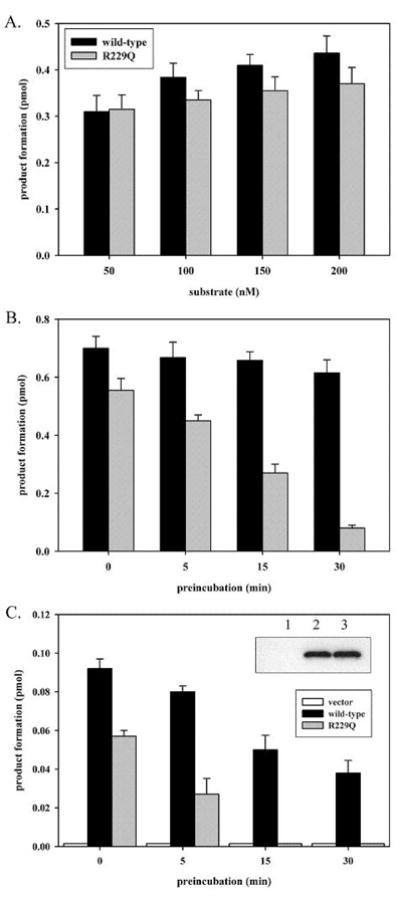
in vitro and in vivo thermolability of polymorphic R229Q OGG1. (A) Purified wild-type and R229Q OGG1 (12.5 nM) were reacted with 50–200 nM duplex 8-oxoG:C substrate for 5 min. Reactions were performed in 20 mM Tris-HCl, pH 7.4, 100 mM NaCl, and 0.15 μg/μl BSA. (B) Wild-type and R229Q OGG1 at a concentration of 5 ng/μl were preincubated at 37°C for 0, 5, 15 or 30 min. in 20 mM Tris-HCl, pH 7.4, 100 mM NaCl, 0.15 μg/μl BSA prior to being reacted at 12.5 nM with 250 nM 8-oxoG:C substrate for 10 min. (C) KG-1 cells were transfected with pCMV-2B vector or pCMV plasmids encoding N-terminally FLAG-tagged wild-type or R229Q OGG1. Nuclear extracts (2 μg) from KG-1 cells expressing wild-type or R229Q OGG1 at identical levels were assayed for 8-oxoG excision activity with 25 nM substrate for 5 min, with or without preincubating the extracts at 30°C for the indicated times prior to analysis. Panel C inset, anti-FLAG western blot of 2 μg of nuclear extract from KG-1 cells transfected with pCMV vector (lane 1), wild-type (lane 2), or R229Q (lane 3) expressing plasmids. Reactions were terminated and analyzed as described in Materials and Methods. All experiments were performed in triplicate and are shown with standard deviation.
Effects of wild-type and R229Q OGG1 expression on KG-1 survival
The possibility of a role for OGG1 and the R229Q polymorphism in cellular sensitivity to DNA damaging agents was examined by directly measuring the effect of expression of wild-type or R229Q OGG1 on the survival of KG-1 cells after treatment with menadione, 8-oxodG nucleoside, or ionizing radiation (Fig. 3). Since a previous report showed targeted expression of OGG1 to mitochondria increased cell survival following ROS treatment (10), effects of both nuclear and mitochondrially expressed OGG1s on KG-1 survival were investigated (Fig. 3). OGG1 enzymes expressed in KG-1 cells were highly active and resulted in a greater than 100-fold increase in intracellular 8-oxoG excision activity (Fig. 2C). Interestingly, expression of nuclear or mitochondrial wild-type OGG1 in KG-1 had no effect on cellular survival after menadione, 8-oxodG or radiation exposure, although all three treatments are known to introduce 8-oxoG into DNA. These results suggest that the susceptibility of KG-1 to killing following each treatment is not influenced by high levels of OGG1 activity (Fig. 3), therefore the decreased enzymatic activity of R229Q OGG1 may not underlie KG-1 sensitivity to DNA damaging agents. Expression of R229Q OGG1 in either the nucleus or mitochondria of KG-1 sensitized cells to killing via apoptosis following exposure to menadione and 8-oxodG, but not ionizing radiation (Fig. 3). Thereby, R229Q OGG1 expression is not generally cytotoxic but introduces selective sensitivity to menadione and 8-oxodG exposure.
Figure 3.
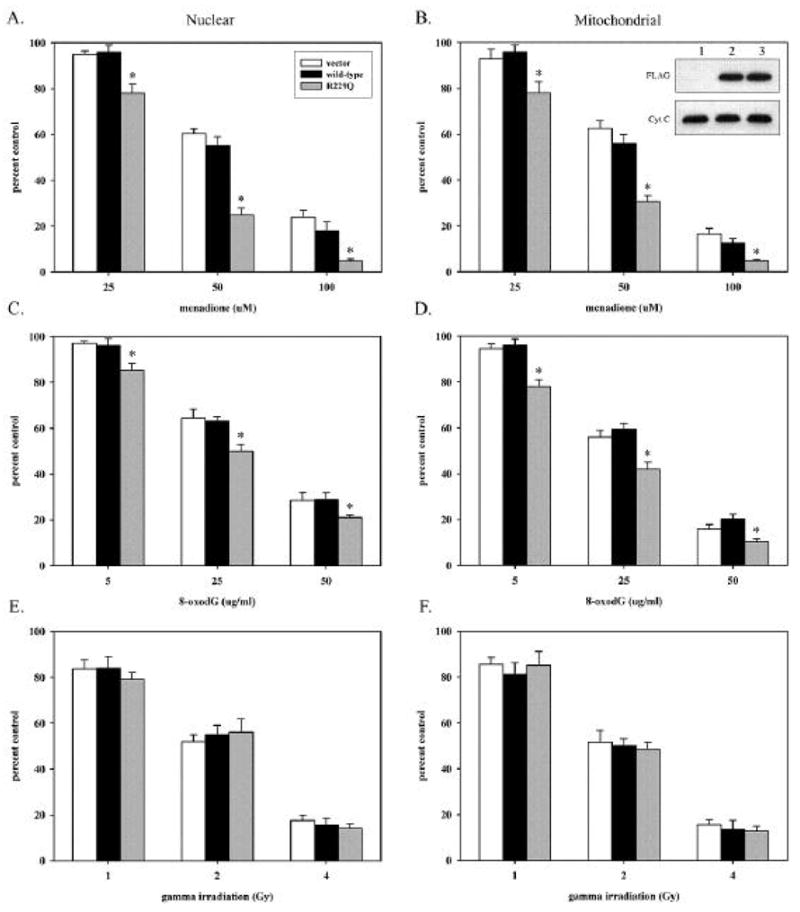
Effect of nuclear and mitochondrial wild-type and R229Q OGG1 expression on cell survival following menadione, 8-oxodG and ionizing radiation exposure in KG-1 cells. KG-1 cells were transfected with nuclear (left panels) or mitochondrial (right panels) pCMV vector, wild-type, or R229Q expressing plasmids and exposed to menadione (Panels A and B), 8-oxodG nucleoside (Panels C and D), or ionizing radiation (Panels E and F). Seven days after treatments, cells were assayed for viability by flow cytometry. For each treatment, 10,000 cells were analyzed. Each column represents the average of three independent experiments (30,000 cells total) with standard deviation. Statistically significant differences in survival (p<0.05) are indicated with an asterisk. Panel B inset, anti-FLAG and cytochrome C western blots of 2 μg of mitochondrial extract of KG-1 cells transfected with mitochondrial pCMV vector (lane 1), wild-type (lane 2), or R229Q (lane 3) expressing plasmids.
Discussion
Our observations of R229Q thermolability in vitro and in vivo explain previous reports of low 8-oxoguanine excision activity in KG-1 cells. Interestingly, thermolability of R229Q (Fig. 2B and C) is not the first reported instance of a thermolabile mammalian OGG1. SAMP1 mice, which exhibit premature senescence, shortened lifespan, and elevated levels of 8-oxoG in DNA, have a homozygous R304W mutation in OGG1, which results in a thermolabile OGG1 protein that is devoid of enzymatic activity (11). It was shown that knockout mice deficient in the repair of 8-oxoguanine have significant increases in both basal mutation frequency and late-onset lung tumor incidence compared to normal animals (12–14). Because of the destabilization of OGG1 resulting from the substitution of a large basic amino acid (arginine) for a small neutral amino acid (glutamine) at position 229 (Fig. 2), cells such as KG-1 harboring the R229Q variant can be anticipated to have a significantly elevated spontaneous mutation frequency due to increased 8-oxoguanine in DNA.
Though it is well established that OGG1 activity is required for repair of 8-oxoG and suppression of 8-oxoG related transversion mutations in vivo (15), the role of OGG1 in cell survival following DNA damage insults is less understood. OGG1 is believed to function as a tumor suppressor with a major role in protecting the genome from ROS-mediated genomic instability. However, since 8-oxoguanine in DNA is not cytotoxic, a clear role for OGG1 in short-term cell survival is not apparent. Because 8-oxoG does not pose a block to replicative DNA polymerases (16), its accumulation in nuclear DNA following ROS treatment could be expected to be largely mutagenic. If 8-oxoG is present at elevated levels in DNA, excessive levels of OGG1 activity may produce abundant abasic sites and overwhelm downstream repair machinery resulting in exposed cytotoxic abasic lesions, therefore lower OGG1 activity may favor survival in some instances. In contrast, the efficient removal of cytotoxic lesions formed by ROS exposure, which block DNA polymerases such as Tg (thymine glycol) and fapyA (4,6-diamino-5-formamidopyrimidine), is important for cell survival following DNA damage. In a report by Rosenquist, et al. (17), siRNA disruption of expression of NEIL1, an enzyme repairing Tg and fapyA, resulted in cellular sensitivity to ionizing radiation. Both menadione and ionizing radiation treatments produce increases in 8-oxoG in DNA via production of hydroxyl radical but also result in a myriad of cytotoxic lesions not repaired by OGG1, including Tg, numerous modified bases and single- and double-stranded breaks (18, 19). It was previously shown that exogenous 8-oxodG administered to KG-1 cells is not incorporated into DNA, but stimulates DNA synthesis via error-prone non-replicative polymerase β (POLB) which may result in increased incorporation of endogenous 8-oxodGTP into DNA (20). In addition to the incorporation of mutagenic 8-oxodG, activation of error-prone DNA synthesis by POLB may enhance the incorporation of modified or cytotoxic nucleotides that could contribute to the cell killing observed in KG-1 following 8-oxodG treatment. The introduction of cytotoxic lesions not repaired by OGG1 may explain the failure of the wild-type enzyme to enhance KG-1 survival following menadione, 8-oxodG and ionizing radiation exposure (Fig. 3). Thereby, OGG1 may have a minor role in KG-1 survival following DNA damaging agent exposure, as suggested by our results in survival experiments with cells expressing the wild-type OGG1 (Fig. 3). Our finding that expression of wild-type OGG1 in the nucleus and mitochondria of KG-1 cells does not significantly influence toxicity of DNA damaging agents suggests that DNA repair enzymes or pathways distinct from those dependent upon OGG1 may be causative in the DNA damaging agent sensitivity of KG-1 (Fig. 3). A recent study similarly showed that overexpression of OGG1 in the nucleus of SH-SY5Y neuroblastoma cells was found to reduce oxidative DNA damage in nuclear DNA but not significantly influence cell survival following ROS treatment (21). In contrast, in a recent study using TK6 lymphoblastoid cells, overexpression of OGG1 was found to decrease survival following gamma irradiation and increase survival following hydrogen peroxide exposure (22). In another study, OGG1 was conditionally targeted to mitochondria and was found to increase cell survival following ROS treatment (10). The effects of DNA glycosylases such as OGG1 on survival following DNA damage requires further investigation and may vary with cell type and be dependent on the type of DNA damage induced and intracellular levels and localization of the requisite repair enzymes.
Further sensitization of KG-1 cells to DNA damaging agents by overexpression of R229Q and the failure of the wild-type enzyme to enhance survival suggests that R229Q may sensitize cells to apoptosis via a mechanism not directly related to the repair of 8-oxoG (Fig. 3). Alternatively, the R229Q allele could function as a dominant negative, since expression of the wild-type OGG1 did not significantly increase survival of KG-1 cells endogenously expressing the R229Q variant (Fig. 3). It is unclear why overexpression of R229Q OGG1 in KG-1 significantly sensitized cells to menadione and 8-oxodG, but not ionizing radiation (Fig. 3). It is possible that differences in the spectrum of DNA and cellular damages introduced by agents used may underlie the lack of sensitization to ionizing radiation by R229Q OGG1. At any rate, cells other than KG-1 endogenously expressing the R229Q variant may have decreased survival ability following ROS exposure. This possibility is supported by the observation that HeLa cells expressing R229Q OGG1 were similarly sensitized to induced oxidative DNA damage (23).
In addition to R229Q OGG1 playing a significant role in the decreased cell survival of KG-1 following induction of DNA damage, the decreased stability and lower in vivo activity of the enzyme reported here may result in significant 8-oxoG accumulation in individuals carrying the R229Q allele. The observation that both OGG1 knockout mice tissue and KG-1 cells have dramatically elevated levels of 8-oxoG in genomic DNA compared to similar cells expressing wild-type OGG1 supports this possibility (3, 7). To our knowledge, KG-1 is the only documented R229Q OGG1 variant cancer cell line. We searched the NIH dbSNP database and found that R229Q is in fact a validated OGG1 polymorphism for which approximately 6% of the US population, or 18 million individuals, may be heterozygous (Table I). Such individuals may have decreased 8-oxoG repair capacity and increased cellular sensitivity to ROS-induced DNA damage that could promote susceptibility to carcinogenesis. Like the more prevalent cancer-associated S326C OGG1 polymorphism, the frequency of the R229Q OGG1 allele varies significantly with ethnicity, with particularly high incidence in a West African population (Table I). Individuals comprising the NIHPDR sample set are US residents who have ancestors from the major geographic regions of the world. Although ethnicity is not known for individuals in the NIHPDR, the exclusive presence of the R229Q allele in an African population, when looking at individual geographic regions, suggests individuals with the R229Q allele in the NIHPDR may be of African descent. On the basis of the sensitization to DNA damaging agents, thermolability and reduced 8-oxoguanine excision activity resulting from the R229Q polymorphism, in the context of the established in vivo mutagenicity of genomic 8-oxoguanine, the R229Q OGG1 allele may be an indicator for ROS-associated cancer susceptibility and warrants consideration in clinical cancer-genotype association studies.
Table 1.
Population distribution of the R229Q OGG1 polymorphism. The NIH Single Nucleotide Polymorphism database (dbSNP) (http://www.ncbi.nlm.nih.gov/projects/SNP/) was searched for validated OGG1 polymorphisms. Genotype frequencies and corresponding allele frequencies for individuals homozygous for wild-type OGG1 (G/G) and heterozygous for R229Q (A/G) are shown for 6 analyzed populations (refSNP ID: rs1805373). Sampled populations are NIHPDR, NIH polymorphism discovery resource; PDR90, 90-individual subset of NIHPDR; CEU, Utah residents with northern and western European ancestry; HCB, Han Chinese in Beijing, China; JPT, Japanese in Tokyo, Japan and YRI, Yoruba in Ibadan, Nigeria.
| Sample Collection | Genotypes | Alleles | ||||
|---|---|---|---|---|---|---|
| Population | Group | Individuals | G/G | A/G | G | A |
| NIHPDR | Global | 420 | 0.936 | 0.064 | 0.968 | 0.032 |
| PDR90 | Global | 78 | 0.936 | 0.064 | 0.968 | 0.032 |
| HapMap-CEU | European | 60 | 1.000 | - | 1.000 | - |
| HapMap-HCB | Asian | 45 | 1.000 | - | 1.000 | - |
| HapMap-JPT | Asian | 44 | 1.000 | - | 1.000 | - |
| HapMap-YRI | African | 60 | 0.800 | 0.200 | 0.900 | 0.100 |
|
| ||||||
| Total Samples | 707 | 0.938 | 0.062 | 0.969 | 0.031 | |
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Support: This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991;349:431–34. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- 2.Soussi T, Beroud C. Significance of TP53 mutations in human cancer: a critical analysis of mutations at CpG dinucleotides. Hum Mutat. 2003;21:192–200. doi: 10.1002/humu.10189. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura S. Involvement of mammalian OGG1(MMH) in excision of the 8-hydroxyguanine residue in DNA. Free Radic Biol Med. 2002;32:813–21. doi: 10.1016/s0891-5849(02)00778-5. [DOI] [PubMed] [Google Scholar]
- 4.Weiss JM, Goode EL, Ladiges WC, Ulrich CM. Polymorphic variation in hOGG1 and risk of cancer: a review of the functional and epidemiologic literature. Mol Carcinog. 2005;42:127–41. doi: 10.1002/mc.20067. [DOI] [PubMed] [Google Scholar]
- 5.Luna L, Rolseth V, Hildrestrand GA, Otterlei M, Dantzer F, Bjoras M, et al. Dynamic relocalization of hOGG1 during the cell cycle is disrupted in cells harbouring the hOGG1-Cys326 polymorphic variant. Nucleic Acids Res. 2005;33:1813–24. doi: 10.1093/nar/gki325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill JW, Evans MK. Dimerization and opposite base-dependent catalytic impairment of polymorphic S326C OGG1 glycosylase. Nucleic Acids Res. 2006;34:1620–32. doi: 10.1093/nar/gkl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyun JW, Choi JY, Zeng HH, Lee YS, Kim HS, Yoon SH, et al. Leukemic cell line, KG-1 has a functional loss of hOGG1 enzyme due to a point mutation and 8-hydroxydeoxyguanosine can kill KG-1. Oncogene. 2000;19:4476–79. doi: 10.1038/sj.onc.1203787. [DOI] [PubMed] [Google Scholar]
- 8.Hyun JW, Cheon GJ, Kim HS, Lee YS, Choi EY, Yoon BH, et al. Radiation sensitivity depends on OGG1 activity status in human leukemia cell lines. Free Radic Biol Med. 2002;32:212–20. doi: 10.1016/s0891-5849(01)00793-6. [DOI] [PubMed] [Google Scholar]
- 9.Hyun JW, Jung YC, Kim HS, Choi EY, Kim JE, Yoon BH, et al. 8-hydroxydeoxyguanosine causes death of human leukemia cells deficient in 8-oxoguanine glycosylase 1 activity by inducing apoptosis. Mol Cancer Res. 2003;1:290–99. [PubMed] [Google Scholar]
- 10.Rachek LI, Grishko VI, Musiyenko SI, Kelley MR, LeDoux SP, Wilson GL. Conditional targeting of the DNA repair enzyme hOGG1 into mitochondria. J Biol Chem. 2002;277:44932–37. doi: 10.1074/jbc.M208770200. [DOI] [PubMed] [Google Scholar]
- 11.Choi JY, Kim HS, Kang HK, Lee DW, Choi EM, Chung MH. Thermolabile 8-hydroxyguanine DNA glycosylase with low activity in senescence-accelerated mice due to a single-base mutation. Free Radic Biol Med. 1999;27:848–54. doi: 10.1016/s0891-5849(99)00141-0. [DOI] [PubMed] [Google Scholar]
- 12.Sakumi K, Tominaga Y, Furuichi M, Xu P, Tsuzuki T, Sekiguchi M, et al. Ogg1 knockout-associated lung tumorigenesis and its suppression by Mth1 gene disruption. Cancer Res. 2003;63:902–5. [PubMed] [Google Scholar]
- 13.Xie Y, Yang H, Cunanan C, Okamoto K, Shibata D, Pan J, et al. Deficiencies in mouse Myh and Ogg1 result in tumor predisposition and G to T mutations in codon 12 of the K-ras oncogene in lung tumors. Cancer Res. 2004;64:3096–3102. doi: 10.1158/0008-5472.can-03-3834. [DOI] [PubMed] [Google Scholar]
- 14.Russo MT, DeLuca G, Degan P, Parlanti E, Dogliotti E, Barnes DE, et al. Accumulation of the oxidative base lesion 8-hydroxyguanine in DNA of tumor-prone mice defective in both the Myh and Ogg1 DNA glycosylases. Cancer Res. 2004;64:4411–14. doi: 10.1158/0008-5472.CAN-04-0355. [DOI] [PubMed] [Google Scholar]
- 15.Sunaga N, Kohno T, Shinmura K, Saitoh T, Matsuda T, Saito R, et al. OGG1 protein suppresses G:C-->T:A mutation in a shuttle vector containing 8-hydroxyguanine in human cells. Carcinogenesis. 2001;22:1355–62. doi: 10.1093/carcin/22.9.1355. [DOI] [PubMed] [Google Scholar]
- 16.Avkin S, Livneh Z. Efficiency, specificity and DNA polymerase-dependence of translesion replication across the oxidative DNA lesion 8-oxoguanine in human cells. Mutat Res. 2002;510:81–90. doi: 10.1016/s0027-5107(02)00254-3. [DOI] [PubMed] [Google Scholar]
- 17.Rosenquist TA, Zaika E, Fernandes AS, Zharkov DO, Miller H, Grollman AP. The novel DNA glycosylase, NEIL1, protects mammalian cells from radiation-mediated cell death. DNA Repair (Amst) 2003;2:581–91. doi: 10.1016/s1568-7864(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 18.Morgan WA, Hartley JA, Cohen GM. Quinone-induced DNA single strand breaks in rat hepatocytes and human chronic myelogenous leukaemic K562 cells. Biochem Pharmacol. 1992;44:215–21. doi: 10.1016/0006-2952(92)90003-2. [DOI] [PubMed] [Google Scholar]
- 19.Piedade JA, Oliveira PS, Lopes MC, Oliveira-Brett AM. Voltammetric determination of γ radiation-induced DNA damage. Anal Biochem. 2006;355:39–49. doi: 10.1016/j.ab.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Kim JE, Hyun JW, Hayakawa H, Choi S, Choi J, Chung MH. Exogenous 8-oxo-dG is not utilized for nucleotide synthesis but enhances the accumulation of 8-oxo-Gua in DNA through error-prone DNA synthesis. Mutat Res. 2006;596:128–36. doi: 10.1016/j.mrfmmm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–91. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 22.Yang N, Chaudhry MA, Wallace SS. Base excision repair by hNTH1 and hOGG1: a two edged sword in the processing of DNA damage in gamma-irradiated human cells. DNA Repair (Amst) 2006;5:43–51. doi: 10.1016/j.dnarep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee A, Mambo E, Zhang Y, Deweese T, Sidransky D. Targeting of mutant hogg1 in mammalian mitochondria and nucleus: effect on cellular survival upon oxidative stress. BMC Cancer. 2006;6:235. doi: 10.1186/1471-2407-6-235. [DOI] [PMC free article] [PubMed] [Google Scholar]


