Abstract
Homeostatic signaling systems have been implicated in the modulation of presynaptic neurotransmitter release, but the underlying mechanisms remain unknown. In a screen that was performed blind to gene identity we isolated mutations in Drosophila ephexin (Rho-type guanine nucleotide exchange factor) that disrupt the homeostatic enhancement of presynaptic release following impairment of postsynaptic glutamate receptor function at the Drosophila NMJ. We show that Ephexin is sufficient presynaptically for synaptic homeostasis and localizes in puncta throughout the nerve terminal. However, ephexin mutations do not alter other aspects of neuromuscular development including morphology or active zone number. We then show that, during synaptic homeostasis, Ephexin functions primarily with Cdc42 in a signaling system that converges upon the presynaptic CaV2.1 calcium channel. Finally, we show that Ephexin binds the Drosophila Eph receptor (Eph) and Eph mutants disrupt synaptic homeostasis. Based on these data we propose that Ephexin/Cdc42 couples synaptic Eph signaling to the modulation of presynaptic CaV2.1 channels during the homeostatic enhancement of presynaptic release.
INTRODUCTION
Homeostatic signaling systems, operating at the level of individual nerve and muscle cells, are believed to interface with the mechanisms of neural plasticity to ensure that neural function remains stable over time (Burrone and Murthy, 2003; Davis, 2006; Davis and Bezprozvanny, 2001; Frank et al., 2006; Marder and Goaillard, 2006; Marder and Prinz, 2002; Perez-Otano and Ehlers, 2005; Turrigiano and Nelson, 2004). There are two general mechanisms by which homeostatic regulatory systems have been proposed to control neuronal excitability. In one paradigm, chronic perturbations of neuronal activity have been observed to cause compensatory changes to ion channel or neurotransmitter receptor abundance (Davis, 2006; Marder and Prinz, 2002; Turrigiano and Nelson, 2004). In a second paradigm, the homeostatic modulation of synaptic transmission can be expressed as a change in presynaptic vesicle release, observed at both central (Murthy et al., 2001; Thiagarajan et al., 2005) and peripheral synapses (Davis, 2006). The most well characterized example of this type of homeostatic signaling is observed at the neuromuscular junction (Davis, 2006; Davis et al., 1998; Frank et al., 2006; Petersen et al., 1997). For example, impaired postsynaptic neurotransmitter receptor function at the Drosophila NMJ initiates a rapid, compensatory increase in presynaptic release that offsets impaired receptor function and restores muscle excitation (Davis, 2006; Frank et al., 2006). This homeostatic signaling system can be initiated by genetic mutation of the glutamate receptor subunit GluRIIA (Petersen et al., 1997), genetic alteration of glutamate receptor function (Davis et al., 1998), acute pharmacological inhibition of glutamate receptor function (Frank et al., 2006) and perturbation of muscle membrane excitability (Frank et al., 2006; Paradis et al., 2001).
Work at the Drosophila NMJ has begun to uncover genes that are required for homeostatic compensation. It was recently demonstrated that homeostatic modulation of presynaptic release (synaptic homeostasis) is blocked by mutations that disrupt the function of a presynaptic CaV2.1 calcium channel subunit encoded by the gene cacophony (cac) (Frank et al., 2006). The observation that synaptic homeostasis, following inhibition of postsynaptic glutamate receptors, is blocked by mutations in a presynaptic calcium channel has been taken as further molecular evidence for the existence of a retrograde signal at this NMJ (Davis, 2006; Frank et al., 2006). The nature of this homeostatic, retrograde signal has been of great interest. Recent data have provided evidence that Bone Morphogenic Protein (BMP) signaling can function as a retrograde signal at the Drosophila NMJ, coupling the developmental growth of the muscle to the growth of the presynaptic nerve terminal (Aberle et al., 2002; McCabe et al., 2003). Although this same BMP signaling system is required for synaptic homeostasis at the Drosophila NMJ, the BMPs do not function as the retrograde signal that directly modulates presynaptic neurotransmitter release (Goold and Davis, 2007). Rather, the BMPs confer competence upon the motoneurons such that they are able to express homeostatic plasticity (Goold and Davis, 2007). Thus, the nature of the retrograde signal that directly mediates the homeostatic modulation of presynaptic release remains unknown. Likewise, nothing is known regarding the signaling systems that reside within the presynaptic nerve terminal that couple retrograde signaling to the modulation of CaV2.1 calcium channels that appears necessary for a homeostatic change in synaptic vesicle release.
Here we identify and characterize core components of a presynaptic signaling system that is necessary for the homeostatic modulation of presynaptic release at the Drosophila NMJ. We provide evidence that Ephexin (Exn), a Rho-type guanine nucleotide exchange factor (Rho-GEF), is a presynaptic protein that is required for the homeostatic modulation of vesicle release. We then provide evidence that, during synaptic homeostasis, Exn acts primarily via Cdc42 within a signaling system that converges upon the CaV2.1 calcium channel. Finally, we show that Drosophila Ephexin, like its vertebrate homologue, binds to the Eph receptor. We then show that Eph mutants disrupt the homeostatic modulation of presynaptic release at the Drosophila NMJ. Therefore, we hypothesize that the Drosophila Eph receptor may receive a retrograde signal at the presynaptic nerve terminal. Exn and Cdc42 could then couple Eph activation to the modulation of CaV2.1 calcium channels necessary for the homeostatic modulation of presynaptic release. These data not only significantly advance our understanding of the presynaptic signaling systems that mediate a homeostatic modulation of presynaptic release, but also define a function for Rho-GTPase signaling in the modulation of synaptic transmission at a fast, glutamatergic synapse.
RESULTS
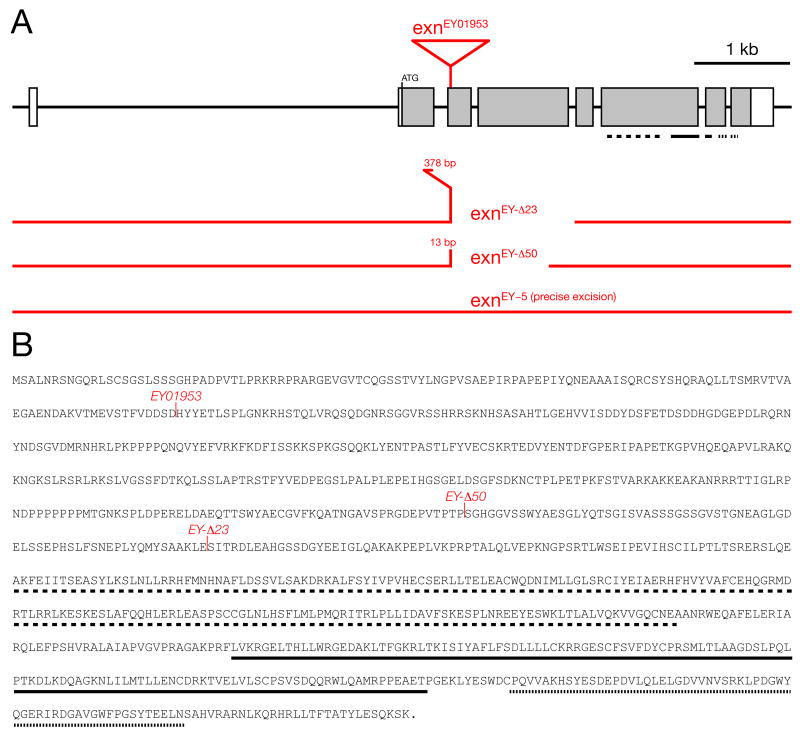
As part of an ongoing, large-scale screen, we have identified mutations in the gene CG3799 that disrupt the homeostatic modulation of presynaptic neurotransmitter release at the Drosophila NMJ. The gene CG3799 is annotated as the Drosophila homologue of the vertebrate Rho-type guanine nucleotide exchange factor (Rho-GEF) termed exn. The N-terminal half of CG3799 is not conserved. However, the C-terminal half is highly homologous to vertebrate exn, including the presence of a Dbl Homology-Plextrin Homology (DH-PH) domain (Rho-GEF domain) and a SH3 domain (Shamah et al., 2001; Sahin et al., 2005). These homologies strongly predict that CG3799 functions as a Rho-GEF. Based upon sequence homology, domain organization and the current annotation of this gene in FlyBase, we refer to this gene as Drosophila exn (Figure 1).
Figure 1. Organization and mutation of the Drosophila exn locus.
A) Genomic overview of the exn gene. The insertion site of the P-element exnEY01953 and the extent of the deletions in the excision alleles exnEY-Δ23 and exnEY-Δ50 are indicated. The allele exnEY-5 is a precise excision of the exnEY1953 P-element insertion. B) Primary amino acid sequence of the Exn protein. exn encodes a 1051 amino acid long protein that contains a RhoGEF domain (dashed line), a PH domain (solid line) and an SH3 domain (dotted line). Domain location is also indicated in (A). The insertion of exnEY01953 and the distal break points of the exnEY-Δ23 and exnEY-Δ50 deletions are indicated.
To complete a genetic analysis of Drosophila exn we took advantage of a P-element insertion mutation in the exn gene (exnEY01953; BDGP gene disruption project) (Bellen et al., 2004). This transposon insertion resides within the coding sequence of exon 2 (Figure 1A) and, as such, is predicted to cause a severe loss of gene function. In order to perform a genetic analysis of Drosophila exn we generated additional alleles. We first excised the P(EY01953) transposon and screened for both imprecise and precise P-element excisions. We isolated two imprecise excisions of the P(EY01953) transposon termed exnEY-Δ50 and exnEY-Δ23. These new mutations are deletions that remove large portions of the exn gene and are predicted to be null mutations (Figure 1). We also isolated exnEY-5, which is a precise excision of the P(EY01953) transposon (Figure 1A). We used these genetic tools to further test the requirement of exn for the homeostatic modulation of presynaptic release at the Drosophila NMJ.
exn mutations disrupt synaptic homeostasis
Mutations in the GluRIIA subunit of the muscle-specific glutamate receptor (GluRIIASP16) cause a significant decrease in the average spontaneous miniature release event (mepsp) amplitude (Petersen et al., 1997). The decrease in mepsp amplitude is offset by a significant increase in presynaptic transmitter release as assessed by calculation of quantal content (see Methods). This increase in quantal content has been confirmed to represent a change in presynaptic transmitter release by analysis of failures (Petersen et al., 1997) and analysis of short-term synaptic plasticity (Frank et al., 2006). This effect has been interpreted as evidence for a homeostatic modulation of presynaptic transmitter release, a conclusion supported by additional electrophysiological experiments using pharmacological agents to impair postsynaptic glutamate receptor function (Frank et al., 2006).
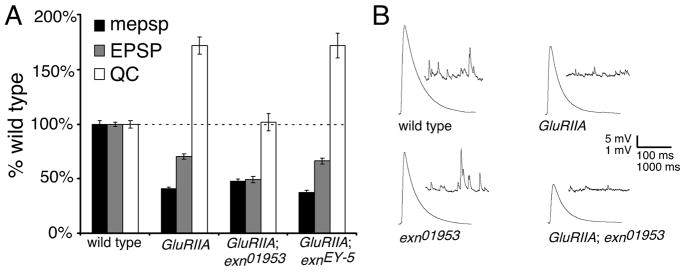
To analyze the requirements of exn for synaptic homeostasis we generated double mutants of all exn alleles with the glutamate receptor mutation, GluRIIASP16. We first demonstrate that spontaneous miniature release event amplitude is significantly decreased in the GluRIIASP16; exnEY01953 double mutant, an effect that is similar to that in the GluRIIASP16 mutant alone (Figure 2A). However, the homeostatic increase in presynaptic transmitter release is severely impaired in these double mutant animals (Figure 2; p=0.01). These data suggest that disruption of the exn gene prevents the normal expression of synaptic homeostasis at the Drosophila NMJ. To confirm the specificity of this effect, we examined animals in which we place the precise P-element excision allele (exnEY-5) in the background of the GluRIIASP16 mutation. In these animals, the significant decrease in mepsp amplitude is offset by an increase in presynaptic release, restoring EPSP amplitudes toward control values, thereby demonstrating normal homeostatic modulation of presynaptic transmitter release (Figure 2A).
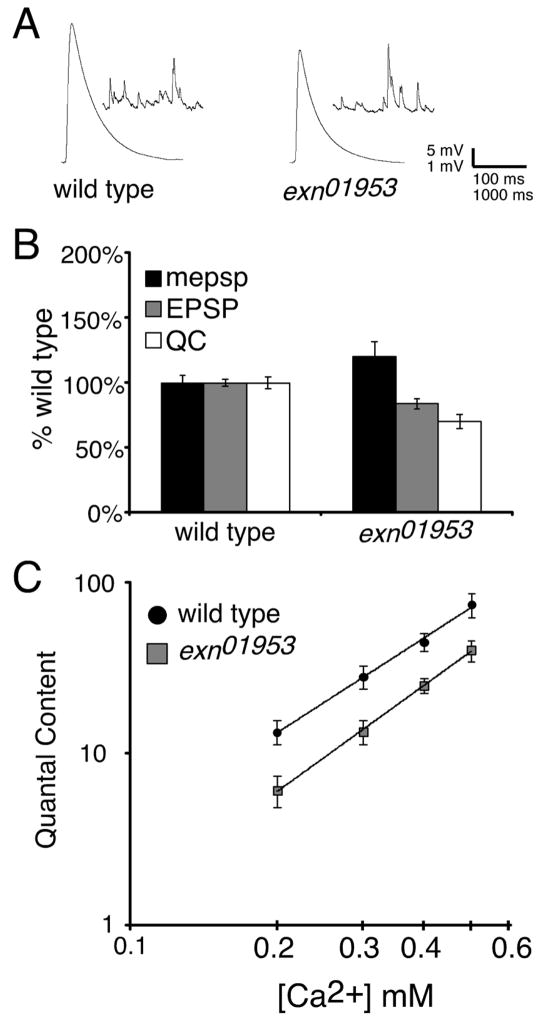
Figure 2. Impaired synaptic homeostasis at the exn mutant NMJ.
A) Average values for mepsp (black), EPSP (gray), and quantal content (white) relative to wild-type control. The GluRIIASP16 mutation causes a decrease in quantal size (mepsp). Decreased mepsp amplitude is partially offset by a homeostatic increase in presynaptic release (QC) (p<0.001; Student’s T-test). The GluRIIASP16; exnEY01953 double mutant NMJs show a decrease in mepsp amplitude without a corresponding increase in QC (p>0.2 compared to wild type). A genotype composed of the precise excision allele (EY-5) and GluRIIASP16 shows a robust homeostatic increase in QC (p<0.001) similar to GluRIIASP16 alone (p>0.9 compared to GluRIIASP16 alone). We also note a significant increase in mepsp amplitude comparing GluRIIASP16; exnEY01953 to the revertant GluRIIASP16; exnEY-5 (p<0.05). B) Representative electrophysiological traces as indicated. Statistically significant differences are calculated according to an unpaired, two-tailed Student’s T-test. Data are presented as average values (± SEM). Data relevant to this figure are also presented in supplemental table 1.
Presynaptic Ephexin is sufficient to rescue synaptic homeostasis
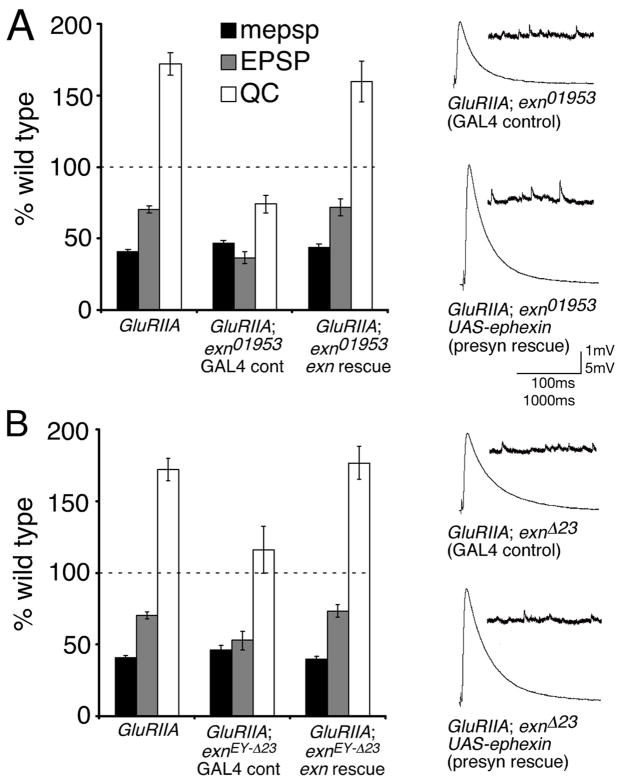
The exn gene appears to be ubiquitously expressed throughout most tissues in the Drosophila embryo including the central and peripheral nervous systems, as assessed by RNA in situ hybridization (data not shown). Therefore, we performed genetic rescue experiments to determine if exn functions in the presynaptic neuron during the homeostatic modulation of presynaptic release. To do so, we generated transgenic animals that allow the expression of a UAS-YFP-exn transgene (YFP is placed at the extreme N-terminus of the exn cDNA, see methods). We then expressed UAS-YFP-exn using a pan-neuronal GAL4 driver (elav-GAL4) in the GluRIIA; exn double mutant background. We performed the rescue experiments using both the exn P-element insertion mutation exnEY10953 and the putative null allele exnEY-Δ23 in the GluRIIA mutant background. We find that presynaptic expression of UAS-YFP-exn is sufficient to fully rescue the expression of synaptic homeostasis in both the GluRIIA; exnEY10953 and the GluRIIA; exnEY-Δ23 double mutant backgrounds (Figure 3). These experiments demonstrate that presynaptic expression of exn is sufficient to restore synaptic homeostasis in an exn mutant background.
Figure 3. Exn is sufficient presynaptically for synaptic homeostasis.
A) Average values for mepsp (black), EPSP (gray), and quantal content (QC; white) relative to wild-type control. Values are presented for GluRIIASP16 alone, GluRIIASP16; exnEY01953 double mutants bearing a UAS-YFP-exn construct driven by the presynaptic elav-GAL4 driver (exn rescue), or for sibling-matched controls with no driver (GAL4 control). Expression of UAS-YFP-exn significantly restores the homeostatic increase in release (p<0.001 compared to sibling matched GAL4 control). Representative traces are shown at right. B) Values presented as in (A). Experiments are performed as in (A) except that the exnEY-Δ23 mutation is used. Representative traces are shown at right. Data are presented as average values (± SEM). Data relevant to this figure are presented in supplemental table 1.
To visualize Exn localization within the presynaptic nerve terminal, we imaged YFP-Exn in animals that express UAS-YFP-exn using the elaV-GAL4 driver. Since presynaptic expression of YFP-Exn is sufficient to rescue defective synaptic homeostasis in the exn mutant, the distribution of ectopically expressed YFP-Exn should reflect, at least in part, the endogenous localization of Exn during the process of synaptic homeostasis. In support of this approach, numerous synaptic proteins have been shown to have appropriate sub-synaptic localization when overexpressed epitope-tagged proteins are visualized (Heerssen et al., 2008; Marek and Davis, 2002; Sweeney and Davis, 2002).
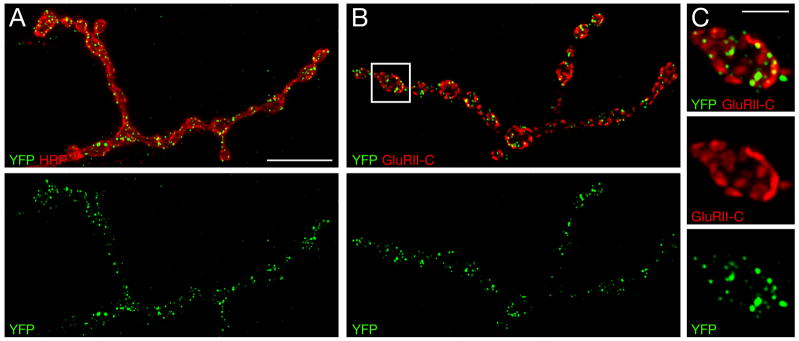
We find that YFP-Exn is efficiently trafficked to the presynaptic nerve terminal at the NMJ (Figure 4). YFP-Exn localizes to small puncta that are distributed throughout the presynaptic nerve terminal. These YFP-Exn puncta are found in close proximity to active zones, defined by co-staining with antibodies that recognize postsynaptic glutamate receptor clusters (Figure 4B, C). However, our image analysis indicates that YFP-Exn is not strictly an active zone associated protein since it is not found in direct opposition to all glutamate receptor clusters, whereas the active zone associated protein Bruchpilot (Brp) is found opposed to each postsynaptic glutamate receptor cluster (Pielage et al., 2006; Wagh et al., 2006). From these data, we conclude that Exn is trafficked to the presynaptic nerve terminal and resides in a position where it could reasonably participate in the modulation of presynaptic vesicle release.
Figure 4. YFP-Exn distribution within the presynaptic nerve terminal.
A–C) Overexpression of YFP-exn in motoneurons. A) NMJ co-stained for YFP-Exn (green) and presynaptic membranes (HRP, red). YFP-Exn is present in discrete puncta that are distributed throughout the presynaptic nerve terminal. B and C) The NMJ co-stained for YFP (green) and postsynaptic glutamate receptors (GluRII-C, red). C) Higher magnification of the boxed area in (B) shows accumulation of YFP-Exn opposite postsynaptic GluRII-C clusters. Scale bar in (A)–(B) 10 μm. Scale bar in (C) 2.5 μm.
Calcium cooperativity of neurotransmitter release is normal in exn mutants
We next examined baseline synaptic transmission in the exn mutant background. We observe a small, but statistically significant, defect in synaptic vesicle release in the exn mutants compared to wild type controls (Figure 5A, B). We also observe a trend toward an increase in mepsp amplitude (p>0.10). We assayed presynaptic release over a range of external calcium concentrations (0.2 to 0.5mM). We find a statistically significant decrease in presynaptic release in exn compared to wild type at each calcium concentration tested (Figure 5C). Nevertheless, our data indicate that the calcium cooperativity of vesicle release remains normal in the exn mutant background. These data highlight a requirement for exn within the presynaptic nerve terminal, either during NMJ development, which could have a basal requirement for synaptic homeostasis, or in the regulation of synaptic vesicle release. Since synaptic homeostasis is normal in mutant backgrounds that disrupt baseline synaptic transmission to a far greater extent than exn (including mutations in syx1A and cysteine string protein; Goold and Davis, 2007), we speculate that the change in baseline synaptic transmission is not the primary cause of impaired synaptic homeostasis, though it could contribute. We also find that the percent decrease in quantal content in exn compared to wild type is significantly less than the percent decrease in quantal content in GluRIIA; exn double mutant animals compared to GluRIIA mutants (Figure 2; p<0.05). Again, these data suggest that Exn has an important function during synaptic homeostasis.
Figure 5. Impaired presynaptic release without a change in release cooperativity in exnEY01953 mutants.
A) Representative electrophysiological traces of wild-type and exnEY01953 NMJs. B) Average values for mepsp (black), EPSP (gray), and quantal content (white) relative to wild-type control. exnEY01953 mutant NMJs display a small, yet significant deficit in neurotransmission (p<0.05). C) Quantal content was quantified for wild type (black) and exnEY01953 (gray) over the range of extracellular calcium concentrations indicated. Statistically significant differences are indicated according to an unpaired, two-tailed Student’s T-test. Data are presented as average values (± SEM).
Normal number of active zones in exn mutants
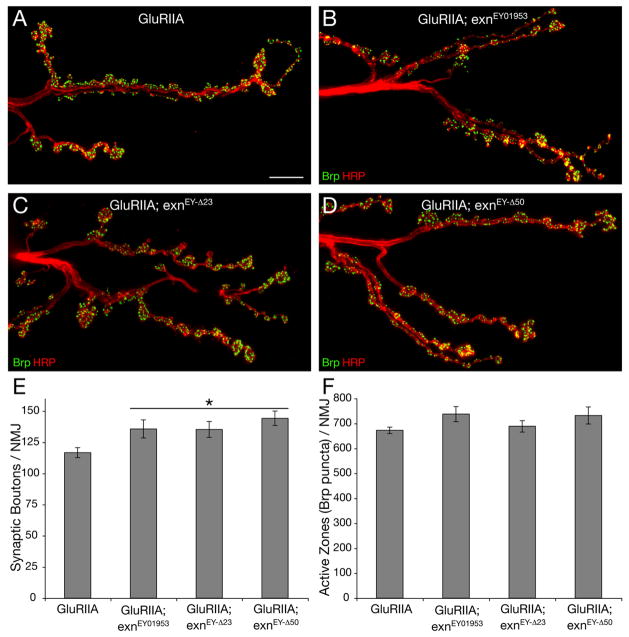
We next determined whether exn mutations alter anatomical NMJ development or active zone number. It has been established that synaptic growth is normal in the GluRIIASP16 mutant background (Petersen et al., 1997), and that the rapid induction of synaptic homeostasis is protein synthesis independent and occurs without a change in NMJ morphology or active zone number (Frank et al., 2006). However, it remains possible that a defect in NMJ development could disrupt the NMJ such that it could not express homeostatic plasticity. Therefore, we examined NMJ morphology and active zone number in GluRIIASP16 animals compared to the GluRIIASP16; exn double mutant animals. We stained NMJs with anti-HRP and anti-Bruchpilot (Brp) antibodies, which mark the presynaptic membrane and presynaptic active zone, respectively (Pielage et al., 2006; Wagh et al., 2006). We find that the GluRIIASP16; exn double mutants have a slight, though statistically significant, increase in bouton number (Figure 6). Thus, decreased NMJ growth cannot account for impaired synaptic homeostasis in these animals. We also quantified the average number of active zones per NMJ using anti-Brp to define active zones as done previously (Pielage et al., 2006). Active zones were visualized and quantified in three-dimensions, allowing us to quantify total active zone number per NMJ. We find no difference in average active zone number per NMJ in GluRIIA mutants versus GluRIIASP16; exn double mutants (Figure 6F). Thus, the exn-dependent block of synaptic homeostasis is not a secondary consequence of defective morphological development or a deficit in presynaptic active zone number. It is formally possible that the small increase in bouton number reflects a response of the NMJ to impaired synaptic homeostasis. However, this seems unlikely because the change is small and there is no change in total active zone number.
Figure 6. Normal active zone number at exn mutant NMJ.
A–D) Partial view of muscle 6/7 NMJs in segment A2 co-stained for the presynaptic active zone protein Brp (green) and the presynaptic membrane marker HRP (red) for the genotypes indicated. Gross NMJ morphology is normal in each mutant. E) Quantification of the number of synaptic boutons at muscles 6/7 in segment A2. All exn mutations lead to a slight but significant (p<0.05) increase in bouton number compared to GluRIIA mutations alone (n ≥ 8). F) Quantification of the number of presynaptic active zones marked by Brp staining of muscle 6/7 NMJs in segment A2 (see text). There is no significant difference in the total number of Brp-positive active zones in exn mutants compared to the GluRIIA control background (n ≥ 9). Scale bar in (A)–(D) 10 μm. Statistically significant differences are indicated according to an unpaired, two-tailed Student’s T-test. Data are presented as average values (± SEM).
Exn-dependent signaling converges upon presynaptic CaV2.1 calcium channels to achieve homeostatic modulation of presynaptic release
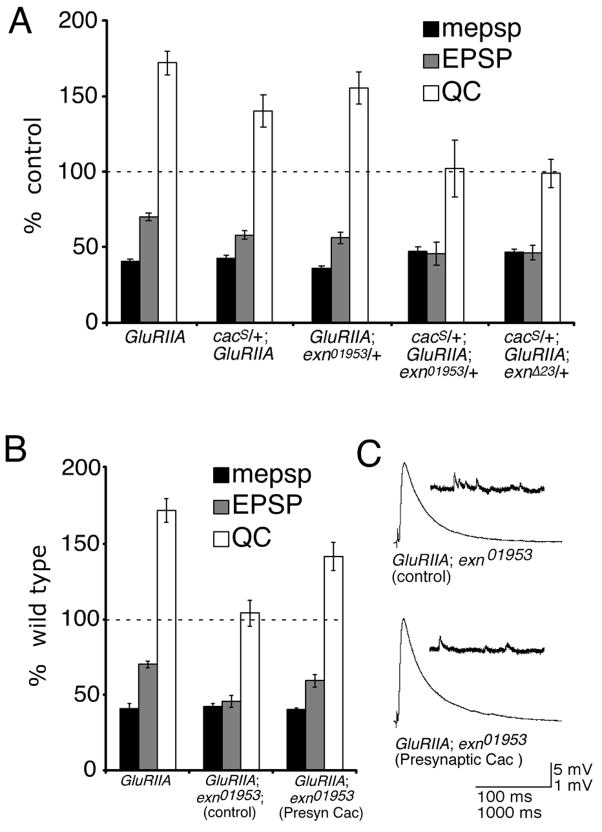
In a previous study, we demonstrated that cacophony (cac) mutations, which impair the pore-forming subunit of the CaV2.1 presynaptic calcium channel, completely block synaptic homeostasis at the Drosophila NMJ (Frank et al., 2006). To test for a genetic interaction between exn and cac, we generated double heterozygous mutant combinations (cac/+; exn/+) within a GluRIIA mutant background. The exn/+ heterozygous mutations (exnEY01953/+ and exnEY-Δ23/+) alone had no significant effect on synaptic homeostasis when placed in the GluRIIA mutant background (Figure 7A; p>0.2; and data not shown), while the presence of the cacS/+ mutation (a point mutation) in the GluRIIA mutant background showed a mild suppression (24% decrease; p<0.05) of homeostatic compensation (Figure 7A). However, the presence of both heterozygous mutations in the GluRIIA mutant (cacS/+;GluRIIA; exn/+) caused a complete block of homeostatic compensation (Figure 7A). A quantitatively similar genetic interaction is observed between a cac null mutation (cacHC129) and exn/+. In these experiments the presence of the heterozygous cacHC129/+ mutation had little effect on quantal content in the GluRIIA mutant background (p>0.05), but blocked synaptic homeostasis when combined with the heterozygous exn/+ mutation in the GluRIIA mutant (p<0.01 when compared with either GluRIIA; exn/+ or cacHC129/+; GluRIIA; data not shown). A strong genetic interaction between heterozygous mutations, such as observed here, can be taken as evidence that the two genes function within the same biological process, acting either within the same signaling system or in parallel signaling pathways.
Figure 7. Genetic interaction between exn and presynaptic calcium channels encoded by cac (CaV2.1).
A) mepsp (black), EPSP (gray), and quantal content (QC; white) are plotted relative to genetic controls lacking the GluRIIA mutation. cacS/+; GluRIIASP16 and exnEY01953/+; GluRIIASP16 NMJs display robust homeostatic compensation that is slightly suppressed relative to control (p<0.05 for cacS/+; GluRIIASP16). By contrast, when a double heterozygous mutant combination is assayed in the GluRIIA mutant background (cacS/+; GluRIIASP16; exnEY01953/+) or (cacS/+; GluRIIASP16; exnEY-Δ23/+) the NMJs show no homeostatic increase in quantal content compared to control. B, C) Presynaptic expression of GFP-cac restores homeostatic compensation to the GluRIIA; exn double mutant. B) Average values as in (A). For the rescue experiment, values are presented for GluRIIASP16; exnEY01953 double mutants bearing a UAS-GFP-cac construct driven by the presynaptic elav-GAL4 driver (presynaptic Cac), or for sibling-matched controls with no driver (control). In the absence of elav-GAL4, no rescue is observed (control). In the presence of elav-GAL4, a significant homeostatic increase in QC is observed (p<0.01 compared to sibling matched controls). The level of homeostatic compensation does not reach GluRIIA alone (p<0.05). C) Representative electrophysiological traces as indicated. Statistically significant differences are indicated according to an unpaired, two-tailed Student’s T-test. Data are presented as average values (± SEM). Data for (A) are presented in Supplemental Table 3. Data for (B) are presented in Supplemental Table 1.
To further examine the relationship between Exn and Cac in this homeostatic signaling system, we asked whether expression of cac (UAS-GFP-cac) in the GluRIIA; exn double mutant animals could rescue the defect in synaptic homeostasis. The UAS-GFP-cac channel is expressed, localizes to presynaptic active zones (Wagh et al., 2006) and is sufficient to rescue viability in the cac null mutation demonstrating that this channel incorporates into presynaptic active zones. As a control, we first verified that expression of the UAS-GFP-cac cDNA alone has no effect on baseline synaptic transmission (data not shown), confirming previously published data (Kawasaki et al., 2002). Next we demonstrate that synaptic homeostasis is significantly restored when UAS-GFP-cac is expressed in the exn; GluRIIA double mutant background (Figure 7B, C; p<0.01). Since expression of UAS-GFP-cac alone has no effect on baseline transmission, the partial rescue of synaptic homeostasis cannot be attributed to a non-specific potentiation of release. Rather, we conclude that Cac over-expression compensates for a specific defect caused by the exn mutation that is responsible for the block of synaptic homeostasis in a GluRIIA mutant background. These genetic data are consistent with a model in which the Cac channel resides downstream of Exn in a presynaptic, homeostatic signaling system at the Drosophila NMJ.
Evidence for altered presynaptic calcium channel activity in the GluRIIA mutant background
To this point our genetic data support the conclusion that exn is required upstream of the cac channel during synaptic homeostasis, but the data do not address whether modulation of the presynaptic calcium channel normally occurs in a GluRIIA mutant background. Therefore, we turned to the use of Cadmium (Cd2+) and Nickel (Ni2+), which are described as indiscriminant calcium channel antagonists (Mintz et al., 1995). In cultured Drosophila neurons, both divalents block the CaV2.1 calcium current (Peng and Wu, 2007). Here we demonstrate that presynaptic release (quantal content) in the GluRIIA mutant background is significantly more sensitive to extracellular application of Cd2+ (3μM) than is the wild type NMJ (Supplemental Figure 2A). There is an even greater disparity between wild type and GluRIIA when using Ni2+ (0.5mM) instead of Cd2+ (Supplemental Figure 2A). Although both Cd2+ and Ni2+ reduce mepsp amplitude (Supplemental Figure 2B), the percent change in mepsp amplitude cannot account for increased sensitivity of presynaptic release to both Cd2+ and Ni2+ observed in the GluRIIA mutant. Finally, there is no effect of these divalents on resting membrane potential (Supplemental Figure 2C). The differential effects of Cd2+ and Ni2+ are consistent with the conclusion that synaptic homeostasis in the GluRIIA mutant is achieved, at least in part, through an effect on presynaptic calcium channels that could include a change in calcium channel number, activity or localization. This conclusion is also consistent with our previously published observation that CaV2.1 channel mutations block synaptic homeostasis in the GluRIIA mutant (Frank et al., 2006). When taken together with our genetic analyses described above, these data support a model in which Exn-dependent signaling converges upon the presynaptic CaV2.1 calcium channel to achieve a homeostatic modulation of presynaptic release.
Ephexin signaling during the rapid induction of synaptic homeostasis
We recently demonstrated that application of a sub-blocking concentration of the glutamate receptor antagonist philanthatoxin (PhTx) can induce a compensatory, homeostatic increase in presynaptic release in approximately 10 minutes (Frank et al., 2006). Thus, application of PhTx can be used to probe the mechanisms responsible for the rapid induction of homeostatic signaling, in contrast to assaying homeostatic compensation in a GluRIIA mutant animal, which reveals the effects of persistent homeostatic signaling over a period of 3–4 days. Several mutations, including alleles of cac block both PhTx-dependent and GluRIIA-dependent synaptic homeostasis, indicating that the rapid induction and sustained expression of synaptic homeostasis share molecular mechanisms (Frank et al., 2006; Goold and Davis, 2007). Therefore, we applied PhTx (4μM) to the exn mutant as done previously (Frank et al., 2006; Goold and Davis, 2007). To our surprise we find that the PhTx-dependent, rapid induction of synaptic homeostasis is normal in the exn mutant (Supplemental Figure 1). These data suggest that exn may be dispensable for the rapid induction of synaptic homeostasis. To further examine the role of Exn-dependent signaling during the rapid induction of synaptic homeostasis, we tested the cac/+; exn/+ double heterozygous mutant animals that we show disrupt synaptic homeostasis in the GluRIIA mutant background (see above). Each heterozygous mutant alone has no effect on the rapid induction of homeostatic signaling following PhTx application. However, the double heterozygous mutant animals show a severe disruption of synaptic homeostasis (Supplemental Figure 1). Quantitatively identical results were obtained when using two independent cac alleles in this experiment. Thus, although exn mutations alone do not block the rapid induction of synaptic homeostasis, we provide evidence that the larger signaling system that includes both exn and cac is required for both the rapid induction and sustained expression of synaptic homeostasis. Why, then, does exn have a greater effect on synaptic homeostasis in the GluRIIA mutant background? The GluRIIA mutation is a persistent homeostatic stress and might place an increased burden on the full functionality of the homeostatic signaling system. Alternatively, Exn signaling may have a more pronounced role during the sustained expression of synaptic homeostasis. To date, the molecular mechanisms that convert the rapid induction of synaptic homeostasis into the persistent expression of synaptic homeostasis remain unknown.
Evidence that Exn acts primarily via Cdc42 during synaptic homeostasis
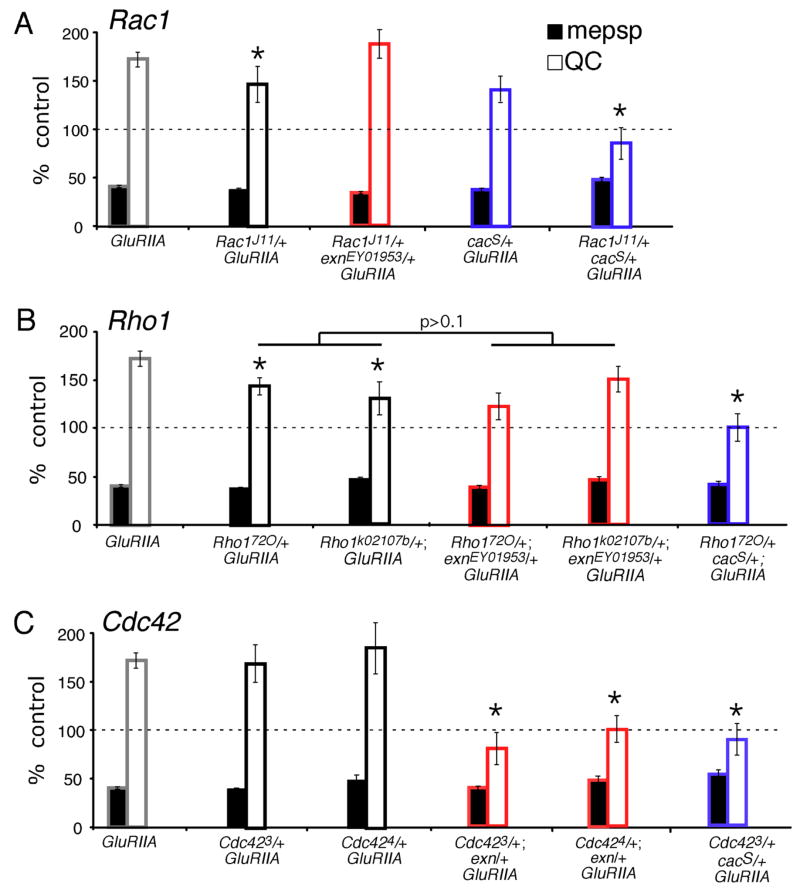
The Exn RhoGEF domain is conserved in Drosophila. Therefore, we sought to determine which Rho-type GTPase(s) might function with Exn during the mechanisms of synaptic homeostasis. The Drosophila homolog of RhoA is called Rho1. There exist several published loss-of-function Rho1 alleles, including Rho172O, which is a P-element transposon excision allele that removes the translational start site of Rho1 (Strutt et al., 1997). A second allele that we used is Rho1k02107b, which is an intronic P-element transposon insertion (Magie et al., 1999). Both homozygous mutations are embryonic lethal (Magie et al., 1999; Strutt et al., 1997). To test for defects in synaptic homeostasis, we generated animals with a Rho1/+ heterozygous mutation in a GluRIIA mutant background. We find that the Rho172O/+ heterozygotes and the Rho1k02107b/+ heterozygotes both cause a partial, but statistically significant (p≤0.05), suppression of synaptic homeostasis (Figure 8B). We next tested whether Rho1 and exn interact genetically by generating transheterozygous Rho1/+, exn/+ animals in a GluRIIA mutant background. However, we did not observe any enhancement of the Rho1/+ mutant phenotype in these animals. These data suggest that loss of Rho1 influences the expression of synaptic homeostasis, but Rho1 is not the primary GTPase involved in Exn signaling during synaptic homeostasis.
Figure 8. Rho-GTPases participate in the mechanism of synaptic homeostasis.
Quantification for mepsp (black) and quantal content (QC; white) are plotted relative to control values in the absence of the GluRIIA mutation (100%). A) A heterozygous Rac1J11/+ mutant (black) has a slight, but significant (p<0.05) decrease in synaptic homeostasis when compared to GluRIIA alone (black). This effect is not enhanced by a heterozygous exn mutation (Rac/+ with exn/+ in the GluRIIA mutant background; red). The heterozygous cacS mutation does not abolish homeostatic increase in QC in the GluRIIA mutant (blue). However, the double heterozygous cacS/+ Rac1J11/+ animals show a block of homeostatic compensation in the GluRIIA mutant background (blue, right). B) Heterozygous Rho1/+ mutations (black) display a partial reduction in synaptic homeostasis (*; p<0.05). This suppression of synaptic homeostasis is not significantly enhanced by removing one copy of exn (red; p>0.1). Synaptic homeostasis is completely blocked in double heterozygous cacS/+; Rho1/+ animals in the GluRIIA mutant background (blue; p<0.001 compared to GluRIIA alone). C) Heterozygous Cdc42/+ animals display a normal homeostatic increase in QC (black; p>0.9 compared to GluRIIA). Homeostatic compensation is eliminated when the double heterozygous mutant combination of Cdc42 and exn (red) are placed in the GluRIIA mutant background (p<0.001 compared to GluRIIA) and when the double heterozygous combination of Cdc42/+ with cacS/+ is placed in the GluRIIA mutant background (blue; p<0.001 compared to GluRIIA). Statistically significant differences are indicated according to an unpaired, two-tailed Student’s T-test. Data are presented as average values (± SEM). Data relevant to this figure are presented in supplemental table 2.
We conducted similar experiments with Rac1 and Cdc42 mutations. For Rac1, we used the Rac1J11 allele, a null missense mutation (G60E) that alters a glycine that is ubiquitous in the GTPase superfamily (Hakeda-Suzuki et al., 2002; Ng et al., 2002). As with Rho1/+ heterozygotes, the Rac1J11/+ heterozygous mutation causes a mild suppression of synaptic homeostasis when placed in the GluRIIA mutant background (Figure 8A). Again, the Rac1/+ mutant showed no further effect on synaptic homeostasis when combined with a heterozygous exn/+ mutation (Figure 8A). Indeed, this double heterozygous mutant combination in the GluRIIA background shows normal homeostatic compensation, underscoring that Rac1 is unlikely to be the GTPase that functions with Exn during synaptic homeostasis.
Next, we tested a role for Cdc42. We examined three independent loss-of-function alleles, Cdc422, Cdc423 and Cdc424 (Fehon et al., 1997; Genova et al., 2000). Cdc423 is an embryonic lethal missense mutation, G114D; Cdc424 is an embryonic lethal splice acceptor alteration; and Cdc422 alters a splice donor sequence (Fehon et al., 1997; Genova et al., 2000). Synaptic homeostasis in the GluRIIA mutant was unaffected by the presence of a Cdc423/+ or Cdc424/+ heterozygous mutation (Figure 8C). However, in this case, the Cdc42 alleles showed a strong genetic interaction with exn. Synaptic homeostatic signaling was completely blocked in animals that are double heterozygous for Cdc42/+; exn/+ in the GluRIIA mutant background (Figure 8C). We extended this analysis to the third Cdc42 allele (Cdc422) with quantitatively similar results (data not shown). Finally, having implicated Cdc42 in the mechanisms of synaptic homeostasis we controlled for effects on synapse morphology. There is no deficit in synaptic bouton number in Cdc423/+; GluRIIA or Cdc424/+; GluRIIA or Cdc423/+; GluRIIA; exn/+ compared to wild type or GluRIIA controls (data not shown; p>0.1, N>10 for all genotypes). Together, these data indicate that Cdc42 is the primary small GTPase that functions with Exn during the homeostatic modulation of presynaptic release.
Finally, since exn shows a strong genetic interaction with mutations in the CaV2.1 channel (cac/+), we asked whether mutations in Rac1, Rho1 and Cdc42 might show a genetic interaction with mutations in cac as well. To our surprise, although Cdc42 is the only GTPase to show a strong genetic interaction with exn, heterozygous mutations in all three GTPases block synaptic homeostasis when combined with a heterozygous cac/+ mutation in the GluRIIA mutant background (Figure 8). From these data, we conclude that loss of a single copy of cac has rendered the presynaptic nerve terminal highly sensitive to mutations in Rho1, Rac1 and Cdc42 during synaptic homeostasis. It is possible that the heterozygous cac mutation has rendered the presynaptic terminal sensitive to any mutation that impairs, even modestly, synaptic homeostasis. Consistent with such a possibility, the Rho1/+ and Rac1/+ heterozygous mutations cause a mild suppression of synaptic homeostasis and show a strong genetic interaction with cac (Figure 8).
The Ephrin receptor binds Exn and is required for synaptic homeostasis
Exn was originally identified as an Eph-receptor interacting protein that can convey signaling from the EphA receptor to the actin cytoskeleton through activation of the small GTPases RhoA, Rac and Cdc42 (Shamah et al., 2001; Sahin et al., 2005). In contrast to vertebrates, the Drosophila genome encodes only a single Eph receptor that shows equal similarity to both the A and B classes of Eph receptors (Dearborn et al., 2002; Scully et al., 1999). During development, the Eph receptor is highly expressed throughout the nervous system and is targeted to axons (Boyle et al., 2006; Scully et al., 1999). Therefore, the Eph receptor is present within developing neurons and is a prime candidate to receive a trans-synaptic signal involved in synaptic homeostasis.
First, we tested whether the interaction between Drosophila Exn and the Eph receptor is conserved. Using a yeast-2-hybrid approach we demonstrate that the kinase-domain of the Eph receptor binds to the C-terminal Rho-GEF domain of Exn (Figure 9). We also observe an interaction between the Eph kinase domain and the SH3 domain of Exn (Figure 9). These data are similar to the interaction observed in vertebrates, though an interaction mediated by the SH3 domain of Exn was not previously observed in vertebrate homologues (Shamah et al., 2001; Sahin et al., 2005).
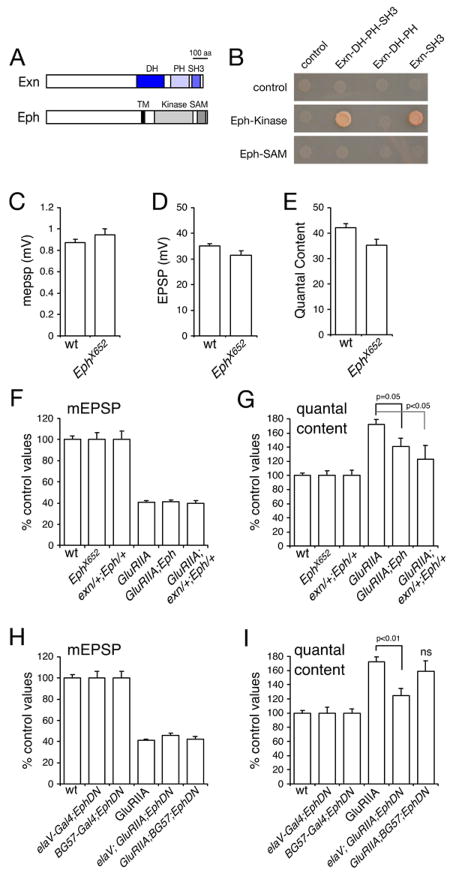
Figure 9. An Eph receptor mutation disrupts synaptic homeostasis.
A) Diagrams of Drosophila Exn and Eph are shown at left. B) Data from a yeast two-hybrid system demonstrating an interaction between the Eph receptor kinase domain with constructs encoding either the DH-PH and SH3 domains of Ephexin or just the two single domains. C–E) Quantification of mepsp amplitude (C), EPSP amplitude (D) and quantal content (E) comparing wild type and the Ephx652 mutant. A significant difference (p<0.05) is found comparing EPSP amplitudes and comparing quantal contents. F) Quantification of mEPSP amplitudes for the indicated genotypes. Values are presented as normalized to values for each control genotype. Control genotypes (wt, EphX652, exn/+; Eph/+) are normalized to themselves (100%). Experimental genotypes are normalized to the appropriate control (GluRIIA is normalized to wt. GluRIIA; Eph is normalized to Eph. GluRIIA; exn/+; Eph/+ is normalized to exn/+; Eph/+). This normalization procedure takes into account any baseline transmission defect in the control genotype. All genotypes use EphX652 though this notation is shortened in some columns for display purposes. G) Quantification of quantal content normalized to control genotypes as in (F). Statistically significant differences are indicated. H) Quantification of mEPSP amplitudes for the indicated genotypes. Data are normalized as in (F). For display purposes elaV-GAL4 is shortened to elaV in column 4 and BG57-GAL4 is shortened to BG57 in column 5. (I) Quantification of quantal content for the indicated genotypes (notation as in H). Data are normalized as in (F). Statistically significant differences are indicated according to an unpaired, two-tailed Student’s T-test. Data are presented as average values (± SEM). Data relevant to this figure are presented in supplemental table 1.
These data suggest that Exn could function downstream of the Eph receptor at the presynaptic nerve terminal of the Drosophila NMJ. To test a potential requirement of the Drosophila Eph receptor in synaptic homeostasis, we analyzed a previously published, molecularly characterized Eph receptor deletion mutation, Ephx652 (Boyle et al., 2006) by electrophysiology. First, we demonstrate that Ephx652 mutants have a slight, but significant (p<0.05), defect in quantal content and EPSP amplitude (Figure 9D,E). However, there is no significant difference in average mepsp amplitude (Figure 9C). The calculation of quantal content (average EPSP/average mepsp) could be influenced by the trend toward larger mepsp amplitudes (p>0.1; Figure 9C) combined with the slight decrease in EPSP amplitudes (p=0.05; Figure 9D). As such, we conclude that the Ephx652 mutation has only a minor effect on baseline synaptic transmission. We also assessed whether the Ephx652 mutation disrupts synaptic growth. We find that bouton numbers in the GluRIIA;; Ephx652 double mutant are not significantly different from the GluRIIA mutant alone (p>0.2, data not shown; N>10). Thus, the Eph receptor does not appear to have a role in synapse specification in the peripheral neuromuscular system, or in the developmental growth of the NMJ.
To assess the function of the Eph receptor during synaptic homeostasis we recorded from the NMJs of homozygous GluRIIASP16;; Ephx652 mutant animals. In these double mutants, mepsp amplitudes are significantly decreased, as observed in GluRIIASP16 mutants alone (Figure 9F). However, the average homeostatic increase in presynaptic release observed in the GluRIIA mutants is significantly suppressed in the GluRIIA;; Ephx652 double mutant (Figure 9G; p=0.05). In this analysis we normalize each experimental genotype to the appropriate genetic control. Control genotypes (wt, EphX652, exn/+; Eph/+) are normalized to themselves (100%) whereas experimental genotypes are normalized to the appropriate control (GluRIIA is normalized to wt, while GluRIIA; Eph is normalized to Eph and the GluRIIA; exn/+; Eph/+ genotype is normalized to exn/+; Eph/+). This method of normalization takes into account the slight defect in baseline synaptic transmission. Thus, we can conclude that homeostatic compensation is significantly disrupted in the Eph mutant over and above the slight disruption of baseline synaptic transmission (see also supplemental Table 1). However, homeostatic compensation is not completely blocked.
Next, we sought additional evidence to support a role for the Eph signaling in synaptic homeostasis. We took advantage of a previously published dominant negative Eph receptor (UAS-EphDN) that includes the extracellular domain but lacks the cytoplasmic signaling domains (Dearborn et al., 2002). Neuronal expression of UAS-EphDN significantly disrupts synaptic homeostasis (Figure 9H, I). By contrast, muscle-specific expression of UAS-EphDN does not significantly impair synaptic homeostasis. It should be noted, however that muscle specific expression of UAS-EphDN does impair baseline synaptic transmission (see supplemental Table 1). These data are consistent with the conclusion that Eph could signal upstream of presynaptic ephexin during synaptic homeostasis. Finally, we took advantage of our previous observation that synaptic homeostasis is sensitive to partial losses of gene function (Figure 8). Here we show that a double-heterozygous combination of the exn/+ and Eph/+ mutants significantly disrupts synaptic homeostasis, while neither heterozygous mutation alone disrupts synaptic homeostasis (Figure 9F, G). These data provide further support for the conclusion that Eph receptors could signal with Ephexin during the process of homeostatic compensation.
DISCUSSION
Considerable evidence has accumulated to support the existence of a homeostatic signaling system at the Drosophila NMJ that originates in muscle and feeds back to modulate neurotransmitter release from the presynaptic nerve terminal (Davis, 2006). Here, we significantly advance our molecular understanding of this fundamental form of synaptic plasticity. We provide evidence that the Drosophila Eph receptor, Exn and Cdc42 are core components of a homeostatic signaling system at the Drosophila NMJ. We also provide evidence that this signaling system ultimately converges upon CaV2.1 calcium channels, representing a plausible mechanism for the modulation of presynaptic vesicle release.
Recent evidence demonstrates that the Drosophila Eph receptor is neuronally expressed (Boyle et al., 2006). Additional evidence indicates that the Drosophila Ephrin homologue is expressed in Drosophila muscle (Tsuda et al., 2008). Based upon our data and these additional studies, we present a model for homeostatic signaling at the Drosophila NMJ. We hypothesize that the Eph receptor functions as a presynaptic receptor for a homeostatic, retrograde signal derived from muscle. The activated Eph receptor signals via Exn and Cdc42 to eventually achieve increased calcium influx via the CaV2.1 calcium channel and, thereby, alter presynaptic release.
It is somewhat surprising that mutations in the Eph receptor only partially disrupt synaptic homeostasis. There are several possible explanations. One possibility is that the EphX652 deletion mutation we have used is not a molecular null. This possibility has recently been suggested by an RT-PCR analysis (Tsuda et al., 2008). Another possibility is that the Eph receptor is an important component of homeostatic signaling, but is not the only presynaptic receptor that participates in homeostatic retrograde signaling at the NMJ. This would be consistent with previously proposed models of synaptic homeostasis based upon the use of multiple feedback signaling systems functioning in parallel (Davis, 2006). In either case, our data implicate the Eph receptor and downstream signaling as important components of the homeostatic signaling systems that control presynaptic neurotransmitter release.
If the Drosophila Eph receptor functions to detect a retrograde signal at the NMJ during synaptic homeostasis, then there exist two prominent possibilities for the identity of the homeostatic retrograde signal. One candidate is Drosophila Ephrin, recently shown to be expressed in Drosophila muscle (Tsuda et al., 2008). Another candidate suggested by recent work in C. elegans and Drosophila is Vap33. The Vap33 protein can be cleaved and secreted and can function as an Eph receptor ligand during C. elegans fertilization. Vap33 has been placed at the Drosophila NMJ both pre- and postsynaptically, making it a candidate for a muscle-derived retrograde signal (Pennetta et al., 2002). However, unlike mutations in Drosophila Eph and exn, Vap33 mutations cause a severe perturbation of NMJ growth and morphology (Pennetta et al., 2002). As such, Vap33 may be involved in a separate signaling system, or may have diverse functions in addition to those involved in synaptic homeostasis and in addition to those mediated by the putative synaptic Eph receptor.
Molecular mechanisms underlying the rapid induction versus sustained expression of synaptic homeostasis
It is interesting to note that exn mutations have a greater effect on synaptic homeostasis when placed in the GluRIIA mutant background compared to application of sub-blocking concentrations of PhTx, which cause a similar decrease in postsynaptic mepsp amplitude. PhTx-dependent signaling induces a homeostatic increase in presynaptic release in a time frame of seconds to minutes (Frank et al., 2006). By contrast, the GluRIIA mutant represents a persistent stress that requires a continuous increase in presynaptic release for several days. One possibility is that the GluRIIA mutant alters NMJ development in some fundamental manner to permanently compensate for decreased postsynaptic excitability, and this is the process most directly affected by the exn mutation. Several lines of evidence suggest that this is not the case. GluRIIA mutants have normal bouton numbers, normal muscle size (Petersen et al., 1997) and normal active zone number, estimated by quantification of the T-bar associated protein Bruchpilot as well as ultrastructurally (GWD and Richard Fetter, unpublished data). Thus, there is no evidence for a compensatory change in the anatomy or architecture of the GluRIIA NMJ. The homeostatic increase in vesicle release probability also seems very similar in PhTx treated animals and GluRIIA mutants. Both conditions have increased quantal content (estimated by average EPSP/average mepsp and by the method of failures; Petersen et al., 1997; Frank et al., 2006). In addition, both conditions show pronounced synaptic depression during prolonged stimulation (Frank et al., 2006), arguing that PhTx and the GluRIIA mutant both induce a similar change in presynaptic release probability and there has been no further developmental compensation in the GluRIIA mutant background. However, by analogy with the molecularly distinct, sequential phases of long-term potentiation (LTP) it remains possible that there will be molecular distinctions between the rapid induction and sustained expression of homeostatic signaling at the Drosophila NMJ. Eph-Exn signaling may represent one such molecular distinction.
Thus, we consider two scenarios. One possibility is that the Exn is not the only GEF or signaling molecule downstream of the Eph receptor involved in synaptic homeostasis. Consistent with this possibility, we find that both Rho1/+ and Rac1/+ heterozygous mutations disrupt synaptic homeostasis in the GluRIIA mutant background but do not interact genetically with exn during synaptic homeostasis. Thus, there may be sufficient signaling capacity in this GTPase system to induce a rapid homeostatic response following PhTx application. However, a perturbation that persists for several days such as the GluRIIA mutation, may place a burden on the homeostatic signaling system and reveal a requirement for Eph-Exn signaling. This would be possible if homeostatic signaling did not cause a persistent change to the presynaptic terminal, but had to be continually updated during a persistent stress such as the GluRIIA mutation. Alternatively, it is possible that Eph-Exn signaling is not the retrograde signal that is required for the rapid induction of synaptic homeostasis. Rather, this represents a retrograde signaling system that is primarily invoked to consolidate or sustain homeostatic signaling over time. In this scenario, Eph-Exn signaling could be considered a form of feedback signaling that ensures an appropriate level of homeostatic signaling is sustained over time. By analogy with homeostatic signaling systems in other tissues and other organisms, it has been proposed that multiple types of trans-synaptic feedback regulation will be necessary to execute and sustain an appropriate homeostatic signaling response (Davis, 2006).
Rho-GTPase signaling and the modulation of presynaptic transmitter release
Our data define a role for Rho-type GTPases in the modulation of vesicle release at a fast glutamatergic synapse. Rho-GTPases play a prominent role in many biological processes including regulation of the actin cytoskeleton and protein trafficking (Ridley, 2006). However, relatively little is known about the function of Rho-type GTPases during the regulation of calcium channel function. Rac1 and Cdc42 have been implicated in modulation of calcium channel function downstream of the Bradykinin receptor (Wilk-Blaszczak et al., 1997), but little else is known. By contrast, there is considerable evidence that the RGK family of small GTP binding proteins including Rad, Gem, Rem and Rem2 function to control calcium channel trafficking (Correll et al., 2008; Jarvis and Zamponi, 2007). The RGK family members all contain a Ras-like GTPase core and, like Rho-GTPases, are members of the Ras-GTPase superfamily. The RGK family members have been implicated in regulation of L-type calcium channel surface expression through the modulation of channel trafficking from the ER (Correll et al., 2008; Jarvis and Zamponi, 2007). It is interesting to speculate that Rho-GTPase signaling downstream of the Eph receptor could be involved in similar trafficking of CaV2.1 type calcium channels.
There is also evidence for Rho-GTPase signaling in the regulation of vesicle release. For example, Rho-GTPases including Cdc42 have also been implicated in the mechanisms of secretory granule fusion (Malacombe et al., 2006). In these studies, it is often hypothesized that the Rho-GTPases act primarily on the cytoskeleton and indirectly affect vesicle release. However, recent studies in C. elegans provide a different view. In these studies, Rho-GTPase signaling appears to modulate synaptic transmission via two independent pathways. In one pathway, RHO-1 inhibits DGK-1 (diacylglycerol kinase), leading to increased formation of DAG. Increased DAG is believed to recruit UNC-13 to the synapse and, thereby, potentiate synaptic transmission (Hiley et al., 2006; McMullan et al., 2006). There is also evidence for RHO-1-dependent modulation of presynaptic release that is independent of DAG and UNC-13, though this signaling pathway remains to be defined (Hiley et al., 2006; McMullan et al., 2006).
Here we provide evidence that Exn and Cdc42 are involved in the homeostatic modulation of presynaptic release. There is an important distinction to be made with respect to prior studies cited above. We have not eliminated the function of any Rho-GTPase in our assays. Rather, we demonstrate that the mechanisms of synaptic homeostasis are highly sensitive to changes in the level of Rho-GTPase signaling. Consistent with this fact, we do not find a dramatic alteration in the development of the NMJ and there is no change in active zone number in the exn mutant. By contrast, loss of other putative actin-regulatory proteins present at the Drosophila NMJ such as NSF (Stewart et al., 2002; Stewart et al., 2005), Nervous-wreck (Coyle et al., 2004), Dap160/Intersectin (Marie et al., 2004) and Profilin (unpublished data) cause a dramatic change in synapse morphology including an increase in the formation of small, highly branched synaptic boutons termed satellite boutons. Since we do not find any substantial or consistent correlation between a change in NMJ anatomy and impaired synaptic homeostasis, we do not think that the exn, Cdc42/+ (or Rho1/+ or Rac1/+) mutations used in our study drastically affect the presynaptic actin cytoskeleton during NMJ development (see Rodal et al., 2008). Thus, we do not favor the hypothesis that Eph-Exn signaling acts primarily on the cytoskeleton with secondary effects on synaptic homeostasis.
At present, our data do not allow us to conclude whether changes in CaV2.1 channel conductance, abundance or organization are the primary target of Exn signaling and synaptic homeostasis. Although not definitive, we do present some evidence to suggest that modulation of CaV2.1 channel abundance may be involved in synaptic homeostasis. Specifically, we show that presynaptic expression of UAS-Cac-GFP partially restores synaptic homeostasis in an exn mutant. The simplest interpretation, though certainly not the only interpretation, is that we have restored channel abundance and that loss of channel abundance is the cause of the exn-mediated defect in synaptic homeostasis. This would be consistent with a mild defect in synaptic transmission without a change in release cooperativity in the exn mutant animals. According to this idea, exn mutations may limit the available CaV2.1 protein and prevent any subsequent homeostatic increase in channel abundance. Exn-Cdc42 signaling could alter the trafficking of CaV2.1 channels as suggested by the work on RGK GTPases (see above). Alternatively, Exn-Cdc42 signaling could regulate the availability of presynaptic sites for channel incorporation at the active zone, a possibility suggested by the “slot” model for calcium channel scaffolding at vertebrate central synapses (Cao et al., 2004).
MATERIALS AND METHODS
Electrophysiology
Recordings were taken from muscle 6 in abdominal segment 2 or 3 of third instar larvae as previously described (Davis et al., 1998). Recordings were made in HL3 saline with the following components (and concentrations): NaCl (70 mM), KCl (5 mM), MgCl2 (10 mM), NaHCO3 (10 mM), Sucrose (115 mM = 3.9%), Trehalose (4.2 mM = 0.16%), HEPES (5.0 mM = 0.12%), and CaCl2 (0.5 mM unless specified). Quantal content was estimated for each recording by calculating the average EPSP/average mepsp. Quantal contents were calculated for each recording and then averaged across all NMJs for a given genotype. To assess calcium cooperativity, quantal contents were corrected for nonlinear summation according to established methods (Martin, 1955; Davis et al., 1996). For acute pharmacological homeostatic challenge, Philanthotoxin-433 (Sigma Aldrich) was used, as previously described (Frank et al., 2006). For divalent cation blockade of Ca2+ channels, CdCl2 or NiCl2 were used at specified concentrations.
Synapse Morphology and Anatomical Analyses by Immunocytochemistry
Third instar larval preparations were fixed for 2 min in Bouin’s fixative (Sigma Aldrich) and incubated overnight at 4° C with primary antibodies. Secondary antibodies were applied for 2 hours at room temperature. The following antibodies were used: mouse anti-Brp 1:100 (Wagh et al., 2006); mouse anti-Synapsin 1:50 (Developmental Studies Hybridoma Bank, Iowa); mouse anti-GFP 1:500 (Molecular Probes) and rabbit anti-GluRIIC 1:1000 (Marrus et al., 2004). Alexa conjugated secondary antibodies and Cy3 conjugated goat anti-HRP were used at 1:200–1:800 (Jackson Immunoresearch Laboratories, Molecular Probes). Larval preparations were mounted in Vectashield (Vector) and imaged at room temperature using an Axiovert 200 (Zeiss) inverted microscope, a 100x Plan Apochromat objective (aperture 1.4), and a cooled CCD camera (Coolsnap HQ, Roper). Intelligent Imaging Innovations (3I) software was used to capture, process, and analyze images.
Generation of UAS-YFP-exn
The UAS-YFP-exn construct was generated by amplifying the full length exn open reading frame using the GH03693 cDNA template (Drosophila Genomics Resource Center, Indiana) and then direct cloning into the UAS-N-terminal Venus (EYFP) vector (T. Murphy, Drosophila Genomics Resource Center, Indiana) using the Gateway™ cloning system (Invitrogen). The following primers were used to amplify the exn open reading frame: 5′-CACCATGTCGGCGTTGAATCGCAGCAA and 5′-TCACTTGCTCTTTTGCGACTCCAGGTAGG. The construct was confirmed by sequencing. Transgenic flies were generated by standard injection methods. The yeast two-hybrid binding assay between Exn and Eph was conducted using standard techniques and reagents (Golemis et al., 2008; Terman et al., 2002). The protein domains tested for interaction are as indicated (Figure 9). PCR amplification of DNA encoding the protein domains was done using the exn cDNA GH03693 as a template (Drosophila Genomics Resource Center, Indiana).
Genetics and Drosophila Husbandry
Ephexin was identified as a gene involved in synaptic homeostasis using an assay to screen for genes that when mutated or knocked down cause impaired motility or development of the GluRIIA mutant animals. Genes were screened blind to identity and rescreened before selection and identification. Subsequently, genes identified in the first round of screening were examined for electrophysiological defects in synaptic homeostasis in the GluRIIA mutant background.
Drosophila melanogaster stocks with the following mutations, UAS transgenes, or GAL4 drivers were used in the course of this study. Chromosome X: w1118 (Hazelrigg et al., 1984), cacS (Smith et al., 1998), Cdc422 (Fehon et al., 1997; Genova et al., 2000), Cdc423 (Fehon et al., 1997; Genova et al., 2000), Cdc424 (Fehon et al., 1997; Genova et al., 2000), C155 (elaV)-GAL4 (Lin and Goodman, 1994), cacHC129 (Kawasaki et al., 2002). Chromosome II: GluRIIASP16 (Petersen et al., 1997), Rho172O (Strutt et al., 1997), Rho1k02107b (Magie et al., 1999), UAS-YFP-exnFL10a (this study), UAS-YFP-exnFL4 (this study). Chromosome III: exnEY01953 (BDGP gene disruption project) (Bellen et al., 2004), exnEY-Δ23 (this study), exnEY-Δ50 (this study), exnEY-5 (this study), Rac1J11 (Hakeda-Suzuki et al., 2002; Ng et al., 2002), BG57-GAL4 (Budnik et al., 1996), UAS-YFP-exnFL2 (this study), UAS-EphDN (Dearborn et al., 2002). Chromosome IV: EphX652 (Boyle et al., 2006). Precise and imprecise excisions were generated using standard protocols and Δ2–3 as a transposase source. Animals were kept in chambers with controlled temperature and humidity (Forma Scientific). Experimental and control animals were reared in parallel and treated identically in all experiments. Homeostatic challenge was provided by the GluRIIASP16 null mutation (Petersen et al., 1997). The w1118 strain (Hazelrigg et al., 1984) was used as a wild-type control genotype.
Supplementary Material
Acknowledgments
We would like to thank Bruno Marie and Benjamin Eaton for their help in the screening effort that identified exn. We thank Mario Lioubin and Peter Clyne for contributing to the generation of additional exn alleles and to the generation yeast-two-hybrid data. These studies were supported by NIH Grant number NS39313 to GWD. CAF was supported by an NIH NRSA (NS049694) and by an NIH K99 Career Development Award Grant NS062738, JP was supported by a fellowship of the Deutsche Forschungsgemeinschaft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M, Nighorn A, Thomas JB. Drosophila Eph receptor guides specific axon branches of mushroom body neurons. Development. 2006;133:1845–1854. doi: 10.1242/dev.02353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Koh YH, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, Murthy VN. Synaptic gain control and homeostasis. Curr Opin Neurobiol. 2003;13:560–567. doi: 10.1016/j.conb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Cao YQ, Piedras-Renteria ES, Smith GB, Chen G, Harata NC, Tsien RW. Presynaptic Ca2+ channels compete for channel type-preferring slots in altered neurotransmission arising from Ca2+ channelopathy. Neuron. 2004;43:387–400. doi: 10.1016/j.neuron.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Correll RN, Pang C, Niedowicz DM, Finlin BS, Andres DA. The RGK family of GTP-binding proteins: regulators of voltage-dependent calcium channels and cytoskeleton remodeling. Cell Signal. 2008;20:292–300. doi: 10.1016/j.cellsig.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle IP, Koh YH, Lee WC, Slind J, Fergestad T, Littleton JT, Ganetzky B. Nervous wreck, an SH3 adaptor protein that interacts with Wsp, regulates synaptic growth in Drosophila. Neuron. 2004;41:521–534. doi: 10.1016/s0896-6273(04)00016-9. [DOI] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Davis GW, Bezprozvanny I. Maintaining the stability of neural function: a homeostatic hypothesis. Annu Rev Physiol. 2001;63:847–869. doi: 10.1146/annurev.physiol.63.1.847. [DOI] [PubMed] [Google Scholar]
- Davis GW, DiAntonio A, Petersen SA, Goodman CS. Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron. 1998;20:305–315. doi: 10.1016/s0896-6273(00)80458-4. [DOI] [PubMed] [Google Scholar]
- Davis GW, Goodman CS. Genetic analysis of synaptic development and plasticity: homeostatic regulation of synaptic efficacy. Curr Opin Neurobiol. 1998a;8:149–156. doi: 10.1016/s0959-4388(98)80018-4. [DOI] [PubMed] [Google Scholar]
- Dearborn R, Jr, He Q, Kunes S, Dai Y. Eph receptor tyrosine kinase-mediated formation of a topographic map in the Drosophila visual system. J Neurosci. 2002;22:1338–1349. doi: 10.1523/JNEUROSCI.22-04-01338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon RG, Oren T, LaJeunesse DR, Melby TE, McCartney BM. Isolation of mutations in the Drosophila homologues of the human Neurofibromatosis 2 and yeast CDC42 genes using a simple and efficient reverse-genetic method. Genetics. 1997;146:245–252. doi: 10.1093/genetics/146.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova JL, Jong S, Camp JT, Fehon RG. Functional analysis of Cdc42 in actin filament assembly, epithelial morphogenesis, and cell signaling during Drosophila development. Dev Biol. 2000;221:181–194. doi: 10.1006/dbio.2000.9671. [DOI] [PubMed] [Google Scholar]
- Golemis EA, Serebriiskii I, Finley RL, Jr, Kolonin MG, Gyuris J, Brent R. Interaction trap/two-hybrid system to identify interacting proteins. Curr Protoc Mol Biol. 2008;Chapter 20 doi: 10.1002/0471142727.mb2001s46. Unit 20 21. [DOI] [PubMed] [Google Scholar]
- Goold CP, Davis GW. The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control. Neuron. 2007;56:109–123. doi: 10.1016/j.neuron.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, Harms M, Nardine T, Luo L, Dickson BJ. Rac function and regulation during Drosophila development. Nature. 2002;416:438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- Hazelrigg T, Levis R, Rubin GM. Transformation of white locus DNA in drosophila: dosage compensation, zeste interaction, and position effects. Cell. 1984;36:469–481. doi: 10.1016/0092-8674(84)90240-x. [DOI] [PubMed] [Google Scholar]
- Heerssen H, Fetter RD, Davis GW. Clathrin dependence of synaptic-vesicle formation at the Drosophila neuromuscular junction. Curr Biol. 2008;18:401–409. doi: 10.1016/j.cub.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley E, McMullan R, Nurrish SJ. The Galpha12-RGS RhoGEF-RhoA signalling pathway regulates neurotransmitter release in C. elegans. Embo J. 2006;25:5884–5895. doi: 10.1038/sj.emboj.7601458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis SE, Zamponi GW. Trafficking and regulation of neuronal voltage-gated calcium channels. Curr Opin Cell Biol. 2007;19:474–482. doi: 10.1016/j.ceb.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Kawasaki F, Collins SC, Ordway RW. Synaptic calcium-channel function in Drosophila: analysis and transformation rescue of temperature-sensitive paralytic and lethal mutations of cacophony. J Neurosci. 2002;22:5856–5864. doi: 10.1523/JNEUROSCI.22-14-05856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DM, Goodman CS. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron. 1994;13:507–523. doi: 10.1016/0896-6273(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Magie CR, Meyer MR, Gorsuch MS, Parkhurst SM. Mutations in the Rho1 small GTPase disrupt morphogenesis and segmentation during early Drosophila development. Development. 1999;126:5353–5364. doi: 10.1242/dev.126.23.5353. [DOI] [PubMed] [Google Scholar]
- Malacombe M, Ceridono M, Calco V, Chasserot-Golaz S, McPherson PS, Bader MF, Gasman S. Intersectin-1L nucleotide exchange factor regulates secretory granule exocytosis by activating Cdc42. Embo J. 2006;25:3494–3503. doi: 10.1038/sj.emboj.7601247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Marder E, Prinz AA. Modeling stability in neuron and network function: the role of activity in homeostasis. Bioessays. 2002;24:1145–1154. doi: 10.1002/bies.10185. [DOI] [PubMed] [Google Scholar]
- Marek KW, Davis GW. Transgenically encoded protein photoinactivation (FlAsH-FALI): acute inactivation of synaptotagmin I. Neuron. 2002;36:805–813. doi: 10.1016/s0896-6273(02)01068-1. [DOI] [PubMed] [Google Scholar]
- Marie B, Sweeney ST, Poskanzer KE, Roos J, Kelly RB, Davis GW. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43:207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Marrus SB, Portman SL, Allen MJ, Moffat KG, DiAntonio A. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J Neurosci. 2004;24:1406–1415. doi: 10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR. A further study of the statistical composition on the end-plate potential. J Physiol. 1955;130:114–22. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O’Connor MB. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- McMullan R, Hiley E, Morrison P, Nurrish SJ. Rho is a presynaptic activator of neurotransmitter release at pre-existing synapses in C. elegans. Genes Dev. 2006;20:65–76. doi: 10.1101/gad.359706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416:442–447. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Peng IF, Wu CF. Drosophila cacophony channels: a major mediator of neuronal Ca2+ currents and a trigger for K+ channel homeostatic regulation. J Neurosci. 2007;27:1072–1081. doi: 10.1523/JNEUROSCI.4746-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennetta G, Hiesinger PR, Fabian-Fine R, Meinertzhagen IA, Bellen HJ. Drosophila VAP-33A directs bouton formation at neuromuscular junctions in a dosage-dependent manner. Neuron. 2002;35:291–306. doi: 10.1016/s0896-6273(02)00769-9. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Pielage J, Fetter RD, Davis GW. A postsynaptic spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J Cell Biol. 2006;175:491–503. doi: 10.1083/jcb.200607036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Rodal AA, Motola-Barnes RN, Littleton JT. Nervous wreck and Cdc42 cooperate to regulate endocytic actin assembly during synaptic growth. J Neurosci. 2008;28:8316–25. doi: 10.1523/JNEUROSCI.2304-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, Schmidt S, Wright TM, Shamah SM, O’Connell S, Cowan CW, et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Sandrock AW, Jr, Dryer SE, Rosen KM, Gozani SN, Kramer R, Theill LE, Fischbach GD. Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science. 1997;276:599–603. doi: 10.1126/science.276.5312.599. [DOI] [PubMed] [Google Scholar]
- Scully AL, McKeown M, Thomas JB. Isolation and characterization of Dek, a Drosophila eph receptor protein tyrosine kinase. Mol Cell Neurosci. 1999;13:337–347. doi: 10.1006/mcne.1999.0752. [DOI] [PubMed] [Google Scholar]
- Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, Greenberg ME. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- Smith LA, Peixoto AA, Kramer EM, Villella A, Hall JC. Courtship and visual defects of cacophony mutants reveal functional complexity of a calcium-channel alpha1 subunit in Drosophila. Genetics. 1998;149:1407–1426. doi: 10.1093/genetics/149.3.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol [A] 1994;175:179–91. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Mohtashami M, Rivlin P, Deitcher DL, Trimble WS, Boulianne GL. Dominant-negative NSF2 disrupts the structure and function of Drosophila neuromuscular synapses. J Neurobiol. 2002;51:261–271. doi: 10.1002/neu.10059. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Pearce J, Bajec M, Khorana R. Disruption of synaptic development and ultrastructure by Drosophila NSF2 alleles. J Comp Neurol. 2005;488:101–111. doi: 10.1002/cne.20603. [DOI] [PubMed] [Google Scholar]
- Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Davis GW. Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron. 2002;36:403–416. doi: 10.1016/s0896-6273(02)01014-0. [DOI] [PubMed] [Google Scholar]
- Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell. 2002;109:887–900. doi: 10.1016/s0092-8674(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Han SM, Yang Y, Tong C, Lin YQ, Mohan K, Haueter C, Zoghbi A, Harati Y, Kwan J, et al. The amyotrophic lateral sclerosis 8 protein VAPB is cleaved, secreted, and acts as a ligand for Eph receptors. Cell. 2008;133:963–977. doi: 10.1016/j.cell.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Wilk-Blaszczak MA, Singer WD, Quill T, Miller B, Frost JA, Sternweis PC, Belardetti F. The monomeric G-proteins Rac1 and/or Cdc42 are required for the inhibition of voltage-dependent calcium current by bradykinin. J Neurosci. 1997;17:4094–4100. doi: 10.1523/JNEUROSCI.17-11-04094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.