Abstract
Purpose
To estimate the effect and toxicity of bimonthly low-dose leucovorin (LV) and fluorouracil (5-FU) bolus plus continuous infusion(LV5FU2) with docetaxel combination chemotheraphy in patients with inoperable or postoperative relapsed gastric cancer.
Materials and Methods
Total 27 patients are enrolled in this study. LV 20 mg/m2 (bolus), 5FU 400 mg/m2 (bolus), 5-FU 600 mg/m2 (24-hour continuous infusion) on day 1, 2, 15, and 16, docetaxel 60 mg/m2 (1-hour infusion) on day 15 every 4 weeks.
Results
Total of 141 cycles were administered and response rate were 36.8% with 2 complete response (10.5%) and 5 partial response (26.3%) in 19 evaluable patients. The median response duration is 8.1 months (95% CI, 4.0~12.1). The median progression-free survival time is 6.7 months (95% CI, 5.0~8.5) and the median overall survival time is 11.9 months (95% CI, 4.8~19.1). The grade 3-4 toxcity of neutropenia (24.8%) and anemia (11.3%), neutropenic fever (2.8%) is observed. The grade 1 toxcity of injection site reaction is observed all patients and the grade 1-2 toxcity of alopecia is observed 60%.
Conclusions
LV5FU2 with docetaxel combination chemotheraphy is effective and tolerable in patients with inoperable or postoperative relapsed gastric cancer.
Keywords: Docetaxel, Fluorouracil, Stomach neoplasms
INTRODUCTION
Gastric cancer is an important malignant tumor with the first incidence and the second cause of mortality in Korea. The incidence was decreased in West. Nonetheless, it still shows a high incidence in Japan and Korea (1). In gastric cancer, the only therapeutic mode that allows complete cure is to detect early and perform radical resection. At the time of diagnosis, radical resection could be performed only in approximately 1/3 patients, and it recurs in 50~80% patients after surgery (2). For inoperable or recurrent gastric patients, palliative chemotherapy rather than best supportive care prolongs survival period, however the standardized palliative chemotherapy has not been established (3).
5-Flurouracil (5-FU) is an antagonist of pyrimidine that the major component of nucleic acid. It was developed in the 1950s, and is a useful drug for gastric cancer and colon cancer. But, the optimal administration method is controversial. The action mechanism, side effects, resistance mechanism of 5-FU administered as bolus or continuous infusion is different from each other. Administered as bolus, the main mechanism of action is to suppress RNA synthesis, and neutropenia, mucositis, and diarrhea are major side effects. As continuous infusion, the main mechanism of action is to suppress thymidilate synthase, and stomatitis and dermatitis are its major side effects (4). Leucovorin (LV) is biochemical modulator that amplifies the action of 5-FU. The optimal administration mode is controversial, and in comparison with high dose combination therapies, the response rate of low dose combination therapies is not inferior (5).
The synergic effect of 5FU bolus and continuous infusion has been proven in vitro. LV5FU2 (Gramont regimen) that bolus and continuous infusion 5-FU together with LV at 2 weeks interval, is more effectiveness and less side effects in comparison with only bolus injection in metastatic colon cancer. It has been widely used in Europe, and its synergic effect in combination with platinum, irinotecan, and other drugs was shown. It becomes the basis of FOLFOX4 (oxaliplatin, 5-FU, LV) that has been widely used for colon cancer (6,7).
We modified a high dose of LV contained in conventional LV5FU2 to a low dose, and administered to inoperable or recurrent gastric adenocarcinoma patients. In administered patients, symptoms were improved and acceptable side effects were shown, nonetheless, none of patients showed response in the evaluation of therapeutic effectiveness.
Docetaxel showed approximately 17~24% response rate as monotherapy and 37~46% as combination therapy, and the synergic effect with cisplatin, etc. has been reported numerously (8-11). It is excellent effectiveness in gastric cancer. It has been widely used in the gastric cancer and side effects is also different with 5FU. We conducted this study to evaluate the effectiveness as well as side effects of the combination chemotherapy of LV5FU and docetaxel for the first time.
MATERIALS AND METHODS
1) The study subjects
The subject patients were inoperable or recurrent gastric adenocarcinoma patients diagnosed histologically at the Choong Ang University Hospital and the Choong Ang University Yong San Hospital. Their age was between 18 years and 80 years, Karnofsky scale was higher than 70 points, and in the test performed within one week before the initiation of therapy, leukocyte was higher than 3,000/mm3, neutrophil was higher than 1,500/mm3, and platelet was higher than 100,000/mm3. The subjects were all patients regardless of the presence or absence of measurable lesion. Consent for this study was obtained from all patients prior to the therapy. Patients with other medical disorders that could not be controlled by appropriate drugs were excluded.
2) Administration method
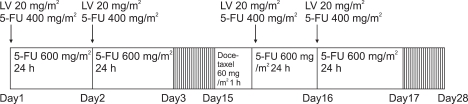
The therapeutic mode used in our study was the bolus injection of 20 mg/m2 LV and 400 mg/m2 5-FU followed by continuous infusion of 600 mg/m2 5-FU mixed with 1,000 mL for 24 hours on the day 1, 2, 15, and 16, and on the day 15, 60 mg/m2 docetaxel mixed with 300 mL 5% dextrose was injected for 1 hour continuously immediately prior to the administration of LV and 5-FU. It was repeated at 4 weeks interval (Fig. 1). To prevent anaphylaxis, 30 minutes prior to the administration of docetaxel, 50 mg ranitidine, 2 mg clemastine, and 20 mg dexamethasone were injected. The treatment was continued unless the patient refused, or non-acceptable toxicity was developed. Based on the Common Terminology Criteria for Adverse Events v3.0 (CTCAE) of the National Cancer Institute (NCI), if 3~4 degrees hematological side effects were developed more than 2 times, the dose of docetaxel was reduced to 75%.
Fig. 1.
Infusion schedule of bimonthly low-dose LV and 5-FU bolus plus continuous infusion with docetaxel.
On the day 8 of drug administration and on the day 7 after the administration of docetaxel, blood test was performed, and if neutrophils were less than 1,000/mm3, 400µg GM-CSF was administered and for lower than 500/mm3, 150µg G-CSF was administered.
3) Determination of therapeutic effectiveness and evaluation of side effects
Prior to each cycle of chemotherapy and prior to the administration of docetaxel, comprehensive physical examination, chest X-ray, complete blood count, and general chemistry test were performed. For the evaluation of response, only on patients with measurable lesions, abdominal computed tomography was performed at every 2 cycles.
In regard to therapeutic response, according to the evaluation criteria of the Response Evaluation Criteria In Solid Tumors (RECIST), it was divided to four types, complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), and side effects was analyzed based on the CTCAE v3.0 of NCI (12).
4) Statistical analysis
The primary end point of this study was the measurement of the response rate to the combination therapy of docetaxel/5-FU/leucovorin in inoperable or recurrent gastric adenocarcinoma patients, and the secondary end point was the measurement of the overall survival and the progression-free survival. It was an one-sided study, and it was assumed that by treating with the combination therapy, 35% response rate would be shown, and considering the response rate of conventional therapy as 15%, at the α value 0.05 and power=0.8, minimum 24 patients are required, and considering 10% drop-out rate, the number of minimal recruited patients was 26 cases.
The overall survival was the period from the day of the initiation of chemotherapy to the day of death, the progression-free survival was the period from the day of the initiation of therapy to the day of the detection of progression or the day of death, and the response duration was the period from the day of the detection of response to the day of the detection of progression or the day of death. Each median value was calculated by the application of Kaplan-Meier method, and as a statistics program, the SPSS 12.0 was used.
RESULTS
1) Characteristic of patients
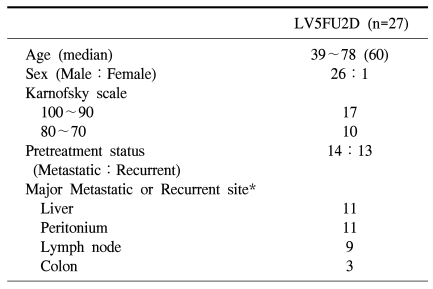
The subject patients from November 2004 to March 2007 were total 27 cases, and their pretreatment characteristic is shown in Table 1. The age of patients was from 39~78 years, the median value was 60 years. Among 27 patients, 26 patients (96.3%) were the male, cases recurred after radical resection were 13 patients (48.1%), and inoperable cases at the time of diagnosis were 14 patients (51.9%). As the site of metastasis or recurrence, the liver and the peritoneum were most prevalent, followed by the order of lymph nodes and the colon.
Table 1.
Patients characteristics
*Some patients had more than one site of metastatic or recurrent site.
2) The effectiveness of therapy
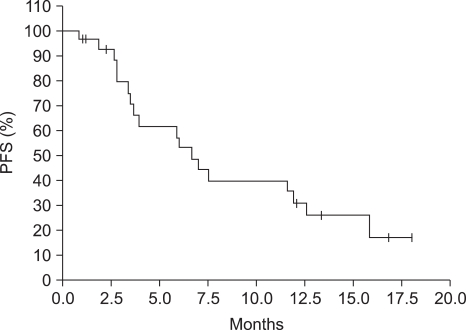
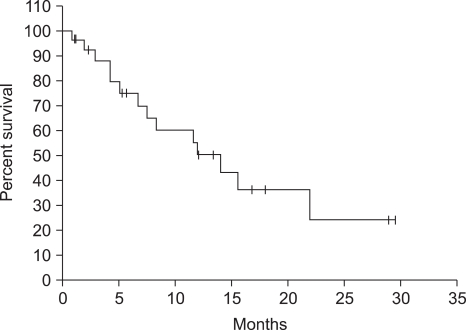
In all total 27 patients, more than 1 time chemotherapy was administered, and total 141 times (average 5.2 times, maximum15 times) was administered. Lesions were measurable in 19 patients, complete response was 2 patients (10.5%), partial response was 5 patients (26.3%), and the response rate was 36.8% (Table 2). The median follow up duration was 7.5 months (95% confidence level: 2.9~12.0 months), the median response duration was 8.1 months (95% confidence level, 4.0~12.1 months), the median progression-free survival was 6.7 months (95% confidence level: 5.0~8.5 months) (Fig. 2), and the median overall survival was 11.9 months (95% confidence level: 4.8~19.1 months) (Fig. 3).
Table 2.
Objective response to treatment of LV5FU2D patients
Fig. 2.
Kaplan-Meier plot of progression-free survival of LV5FU2D patients. The median progression-free survival was 6.7 months.
Fig. 3.
Kaplan-Meier plot of overall survival of LV5FU2D patients. The median overall survival was 11.9 months.
3) Side effects
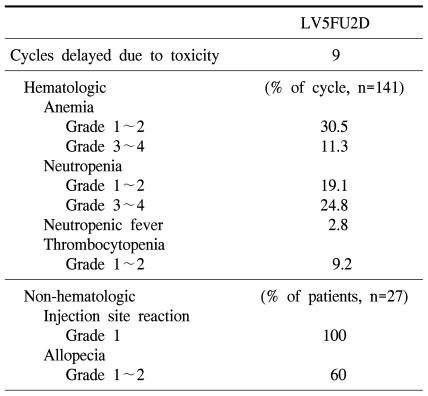
Among total 27 patients, docetaxel was reduced to 75% in 4 patients due to neutropenia. The dose intensity of docetaxel was 88.2%, and leucovorin as well as 5-FU were reduced in none of patients. Among total 141 times chemotherapy, cases whose treatment was delayed for more than one week due to side effects were developed 9 times, and as the causatives, neutropenia was 5 times, stomatitis was 3 times, and general weakness was 1 time. As 3~4 degrees hematologic toxicity, anemia was 16 times (11.3%), neutropenia was 35 times (24.8%), and neutropenic fever was 4 times (2.8%). As non-hematologic toxicity, 1 degree phlebitis was developed in the injection site in all patients, and 1~2 degrees alopecia was developed in 60% (Table 3).
Table 3.
Toxicities
DISCUSSION
In inoperable or recurrent gastric cancer patients, palliative chemotherapy has been proven to improve survival by recent meta-analysis, nonetheless, the types of standard therapy are controversial (3). In comparison with monotherapy, although short, approximately one month of significant prolongation of survival was shown in combination chemotherapies, and considering the short survival period of advanced gastric cancer, it may be regarded to be significant. Among them, the 3 drug combination regimen, containing 5-FU, cisplatin, and anthracyclin was shown to be most effective. The representative regimen is the combination of epirubicin, cisplatin, and 5-FU (ECF), and the European Society for Medical Oncology (ESMO) also recommends the ECF regimen. It is controversial whether it is the standard chemotherapy for gastric cancer (13). Most clinical studies engaging 3 drugs regimen were performed on young patients without cardiac diseases or renal diseases, and it has not been elucidated whether it could be the standard therapy for the elderly or patients with associated diseases, and the consequent increase of side effects should be considered (14).
In advanced gastric cancer patients, the drug that has been studied most as a chemotherapeutic agent is 5-FU. However, attempts to maximize 5-FU therapy have been focused on colon cancer in basic sciences as well as clinical studies, and the representatives are the variation of the injection methods and biochemical modulation. Recently, in colon cancer, in comparison with bolus injection, infusion has been shown to be better regarding survival (15). The major action mechanism of the infusion and bolus injection of 5-FU is different from each other, and in cases applied the both modes in combination, the improvement of progression-free survival was observed and thus the superiority of the combination method has been proven. In gastric cancer, studies on it are not sufficient (16).
As biochemical modulation, in colon cancer, low doses of LV also showed effectiveness comparable to high doses and less side effects. It was not proven in gastric cancer (17). Docetaxel showed a superior response rate as monotherapy as well as combination therapy in advanced gastric cancer, and in a recent phase III clinical study, when it was added to 5-FU and cispplatin, statistically significant improvement of survival was observed. It is a chemotherapeutic agent approved by the FDA for advanced gastric cancer. However, the shortcoming is significant increase of toxicity due to docetaxel, and particularly, the neutropenia and neutropenic fever. Thus in the phase III studies conducted already, the use of granulocyte colony-stimulating factor (G-CSF) was recommended (18). Until now, in advanced gastric cancer, the appropriate administration mode of docetaxel is controversial because of its side effects. In our study, considering such side effects, instead of 75 mg/m2 at 3 weeks interval that has been used in other clinical studies, the dose was decreased and 60 mg/m2 was administered at 4 weeks interval. 3~4 degrees neutropenia was developed in 24.8% cases, netropenic fever was developed 2.8%, and relatively acceptable side effects were observed. In addition, the response rate was 36.8%, and in comparison with 37~46% of other studies of the docetaxel combination therapy, a similar effectiveness was shown (8-11,18).
Recently, to enhance the compliance of patients, interests have been paid on the possibility of the replacement of 5-FU with capecitabine, TS-1 and other oral fluoropyrimidine. In addition, in several clinical studies on colon cancer, the replacement of 5-FU with capecitabine has been reported to be not inferior, and thus it is anticipated that by the use of oral fluoropyrimidine, hospitalization or the necessity of the central venous catheter would be decreased (19,20). In future, studies on the possibility of the replacement of the LV5FU2 regimen based on intravenous injection used in our study with oral fluoropyrimidine are required.
CONCLUSION
The combination chemotherapy that docetaxel was added to the LV5FU2 regimen, engaging a low dose LV at 2 weeks interval and the bolus IV of 5-FU followed by continuous IV was effective on inoperable or recurrent gastric cancer patients and also side effects were acceptable. In future, it is required to investigate the possibility of replacement of the LV5FU2 regimen with oral fluoropyrimidine.
References
- 1.Shin HR, Jung KW, Won YJ, Park JG 139 KCCR-affiliated Hospital. 2002 Annual Report of the Korea Central Cancer Registry: Based on Registered Data from 139 Hospitals. Cancer Res Treat. 2004;36:103–114. doi: 10.4143/crt.2004.36.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dupont JB, Jr, Lee JR, Burton GR, Cohn I., Jr Adenocarcinoma of the stomach: review of 1,497 cases. Cancer. 1978;41:941–947. doi: 10.1002/1097-0142(197803)41:3<941::aid-cncr2820410323>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 4.Sobrero AF, Aschele C, Bertino JR. Fluorouracil in colorectal cancer--a tale of two drugs: implications for biochemical modulation. J Clin Oncol. 1997;15:368–381. doi: 10.1200/JCO.1997.15.1.368. [DOI] [PubMed] [Google Scholar]
- 5.Poon MA, O'Connell MJ, Moertel CG, Wieand HS, Cullinan SA, Everson LK, et al. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989;7:1407–1418. doi: 10.1200/JCO.1989.7.10.1407. [DOI] [PubMed] [Google Scholar]
- 6.Bouche O, Raoul JL, Bonnetain F, Giovannini M, Etienne PL, Lledo G, et al. Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone de Cancerologie Digestive Group Study--FFCD 9803. J Clin Oncol. 2004;22:4319–4328. doi: 10.1200/JCO.2004.01.140. [DOI] [PubMed] [Google Scholar]
- 7.Sobrero AF, Aschele C, Guglielmi AP, Mori AM, Melioli GG, Rosso R, et al. Synergism and lack of cross-resistance between short-term and continuous exposure to fluorouracil in human colon adenocarcinoma cells. J Natl Cancer Inst. 1993;85:1937–1944. doi: 10.1093/jnci/85.23.1937. [DOI] [PubMed] [Google Scholar]
- 8.Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Cabral Filho S, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol. 2005;23:5660–5667. doi: 10.1200/JCO.2005.17.376. [DOI] [PubMed] [Google Scholar]
- 9.Einzig AI, Neuberg D, Remick SC, Karp DD, O'Dwyer PJ, Stewart JA, et al. Phase II trial of docetaxel (Taxotere) in patients with adenocarcinoma of the upper gastrointestinal tract previously untreated with cytotoxic chemotherapy: the Eastern Cooperative Oncology Group (ECOG) results of protocol E1293. Med Oncol. 1996;13:87–93. doi: 10.1007/BF02993858. [DOI] [PubMed] [Google Scholar]
- 10.Ridwelski K, Gebauer T, Fahlke J, Kroning H, Kettner E, Meyer F, et al. Combination chemotherapy with docetaxel and cisplatin for locally advanced and metastatic gastric cancer. Ann Oncol. 2001;12:47–51. doi: 10.1023/a:1008328501128. [DOI] [PubMed] [Google Scholar]
- 11.Sulkes A, Smyth J, Sessa C, Dirix LY, Vermorken JB, Kaye S, et al. Docetaxel (Taxotere) in advanced gastric cancer: results of a phase II clinical trial. EORTC Early Clinical Trials Group. Br J Cancer. 1994;70:380–383. doi: 10.1038/bjc.1994.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham D, Jost LM, Purkalne G, Oliveira J. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of gastric cancer. Ann Oncol. 2005;16(Suppl 1):i22–i23. doi: 10.1093/annonc/mdi812. [DOI] [PubMed] [Google Scholar]
- 14.Pye JK, Crumplin MK, Charles J, Kerwat R, Foster ME, Biffin A. One-year survey of carcinoma of the oesophagus and stomach in Wales. Br J Surg. 2001;88:278–285. doi: 10.1046/j.1365-2168.2001.01655.x. [DOI] [PubMed] [Google Scholar]
- 15.Meta-analysis Group In Cancer. Cancer M-aGI. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16:301–308. doi: 10.1200/JCO.1998.16.1.301. [DOI] [PubMed] [Google Scholar]
- 16.de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol. 1997;15:808–815. doi: 10.1200/JCO.1997.15.2.808. [DOI] [PubMed] [Google Scholar]
- 17.Jager E, Heike M, Bernhard H, Klein O, Bernhard G, Lautz D, et al. Weekly high-dose leucovorin versus low-dose leucovorin combined with fluorouracil in advanced colorectal cancer: results of a randomized multicenter trial. Study Group for Palliative Treatment of Metastatic Colorectal Cancer Study Protocol 1. J Clin Oncol. 1996;14:2274–2279. doi: 10.1200/JCO.1996.14.8.2274. [DOI] [PubMed] [Google Scholar]
- 18.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 19.Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282–2292. doi: 10.1200/JCO.2001.19.8.2282. [DOI] [PubMed] [Google Scholar]
- 20.Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19:4097–4106. doi: 10.1200/JCO.2001.19.21.4097. [DOI] [PubMed] [Google Scholar]