Abstract
Bevacizumab is a monoclonal antibody that targets vascular endothelial growth factor (VEGF), and it has shown promise as a clinical agent against metastatic colorectal cancer, and particularly in combination with chemotherapy. Bowel perforation is a known risk that's associated with bevacizumab use, but the etiology is unknown. Here we report on two cases of metastatic colorectal cancer in which the patients suffered from intestinal perforation after chemotherapy with bevacizumab. For the first case, a 47 year-old man had rectal cancer with concurrent liver and lung metastasis. He underwent chmotherapy with 5-fluorouracil, irinotecan and bevacizumab. Fever and abdominal pain developed seven days later, and rectal perforation was identified upon exploration 13 days later. For the second case, a 48 year-old woman had sigmoid colon cancer with peritoneal and ovary metastases. After seven days of chemotherapy with 5-fluorouracil, oxaliplatin and bevacizumab, exploratory surgery revealed a perforation at the ileum.
Keywords: Bevacizumab, Intestinal perforation, Colorectal neoplasms
INTRODUCTION
Bevacizumab (Avastin®, Genentech Inc., South San Francisco, CA) is a monoclonal antibody that targets vascular endothelial growth factor (VEGF). It has shown promise as a clinical agent against metastatic colorectal cancer, and particularly in combination with chemotherapy (1). This drug inhibits tumor angiogenesis, which is an important process in tumor growth and development. The reported side effects of bevacizumab include hypertension, proteinuria, thromboembolism, delayed wound healing, gastrointestinal bleeding and bowel perforation (2~4). Although bowel perforations have rarely occurred at a frequency of less than 2%, this complication is very often fatal (3,4). Herein we report on two cases of bowel perforations that occurred after administering bevacizumab combined with chemotherapy in metastatic colorectal cancer patients.
CASE REPORT
1) Case 1
A 47-year-old man was diagnosed with rectal adenocarcinoma and concurrent liver and lung metastasis. The primary tumor was located in the rectum 15 cm above the anal verge. He was admitted to our hospital for chemotherapy. Upon admission, he had no signs of colon obstruction. He was treated with bevacizumab at 5 mg/kg every two weeks in combination with irinotecan at 125 mg/m2, 5-fluorouracil at 500 mg/m2 and leucovorin at 20 mg/m2 once weekly. At the third week of the first cycle, bevacizumab was administered with irinotecan, 5-fluorouracil and leucovorin. Seven days after the initiation of bevacizumab combination chemotherapy, the patient presented with fever and abdominal pain, and after thirteen days the patient complained of aggravated abdominal pain.
Physical examination revealed a diffuse tenderness that was most prominent in the lower abdomen, rebound tenderness and guarding. The bowel sounds were decreased. The patient's vital signs were notable for a heart rate of 100 beats per minute and a blood pressure of 120/80 mmHg. His body temperature was 38.7℃, while the prominent laboratory finding was a hematocrit of 31.0%. The white blood count was 7,900/mm3.
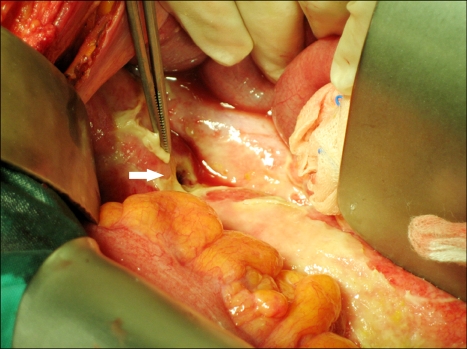
Emergency exploration was performed, which revealed a large amount of dark brownish fluid throughout the abdominal cavity, suggesting perforation as the cause of the patient's peritonitis. A rectal perforation was identified at the sacral promontory level, and so a Hartmann procedure was performed (Fig. 1). The patient suffered from ileus postoperatively. He improved with conservative management and then he was discharged.
Fig. 1.
Operation findings. Rectal perforation was identified at the sacral promontory level, which was the primary tumor site in case 1.
2) Case 2
A 48-year-old woman was admitted for sigmoid colon cancer with concurrent peritoneal and ovary metastasis. One year previously, she had undergone sigmoidectomy for sigmoid colon cancer and then postoperative adjuvant chemotherapy at another hospital. She was diagnosed with peritoneal and ovary recurrence seven months later. At admission to our hospital, she had signs of intestinal obstruction. Bevacizumab was administered by intravenous infusion at 5 mg/kg in combination with oxaliplatin at 85 mg/m2 by 2 hour-infusion, 5-fluorouracil at 400 mg/m2 by bolus infusion and this was followed by 600 mg/m2 continuous infusion of 5-fluorouracil for 22 hours and leucovorin at 20 mg/m2.
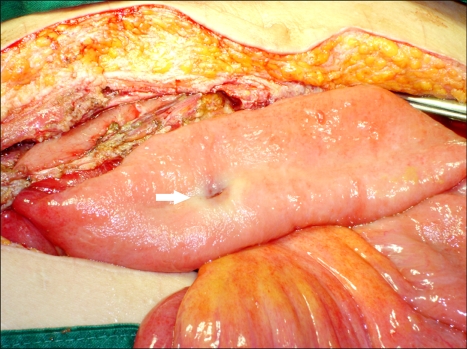
After 7 days, the patient presented with abdominal pain. Physical examination revealed diffuse tenderness, rebound tenderness and guarding. The blood pressure was 90/60 mmHg and the body temperature was 38℃. The white blood count was 7,700/mm3. Emergency exploratory surgery was performed under the impression of peritonitis; the surgery revealed a significant amount of dark greenish fluid throughout the abdominal cavity. An ileal perforation was identified 150 cm distal from the Treitz ligament (Fig. 2). The proximal bowel was moderately distended and it showed wall thickening, and the distal loop of the bowel was adhered to a huge recurrent pelvic mass that surrounded the peritoneum. Small bowel segmental resection and end-type ileostomy were performed. The patient was discharged without any postoperative complication.
Fig. 2.
Operation findings. An ileal perforation was identified in case 2. The proximal intestine was moderate distended and it showed wall thickening.
DISCUSSION
Bevacizumab is a recombinant humanized monoclonal antibody that targets vascular endothelial growth factor (VEGF), and bevacizumab has been demonstrated to have activity against metastatic colorectal cancer in combination with chemotherapy. In a phase II trial for the treatment of colorectal cancer, the addition of bevacizumab to fluorouracil (FU) plus leucovorin (LV) increased the response rate, the median time to progression and the median duration of survival (2,3). In a phase III trial sponsored by Genentech Inc., the addition of bevacizumab to irinotecan/FU/LV (IFL) for the first-line treatment of metastatic colorectal cancer was associated with a significant prolongation in survival and the progression-free survival and an increased response rate (4).
In an initial trial of bevacizumab in combination with FU/LV or IFL, the most common side effects were hypertension, proteinuria and diarrhea. Less common, but serious complications such as bleeding, thromboembolic events and bowel perforation occurred with a frequency of less than 2% (3,4). Among the complications, bowel perforation in patients undergoing treatment with bevacizumab remains a rare, but lethal complication.
The commonly suggested predisposing factors for perforation are peptic ulcer disease, diverticulitis, chemotherapy-induced colitis, a history of abdominal radiation, abdominal carcinomatosis and bowel obstruction (5~7). Saif et al(8) observed that the incidence of gastrointestinal perforation seemed to be higher in patients with intact primary tumors, a recent history of sigmoidoscopy or colonoscopy, or previous adjuvant radiotherapy. For our first case, the patient had an intact primary tumor in the rectum. For our second case, the patient had signs of bowel obstruction before bevacizumab combination therapy. The perforation site was located at the proximal intestinal portion of the obstructive lesion.
The pathophysiologic mechanism by which bevacizumab leads to bowel perforation is unknown. There are several possible mechanisms (9). One possibility is that the perforations are tumor-related. In this case, subsequent chemotherapy with bevacizumab may lead to necrosis of the tumor in the bowel serosa and so predispose the patient to developing a bowel perforation. Another possibility is that bevacizumab could limit the blood flow to the splanchnic vasculature via thrombosis or vasoconstriction. A marginal blood supply to an area of the bowel could potentially lead to bowel infarction and then perforation. Finally, it is possible that delayed wound healing after chemotherapy with bevacizumab may result in leakage from the anastomotic site after the bowel operation. Scappaticci et al(5) suggested that the use of bevacizumab within 28 days of operation could increase the risk of wound healing complications after primary cancer surgery in colorectal cancer patients. For the first of our two present cases, we assumed that tumor necrosis produced the rectal perforation. The perforation site was located on the primary tumor of the rectum. For the second case, although the pathogenesis of the patient's intestinal perforation was not entirely understood, we presumed that intestinal obstruction was the predisposing factor for bevacizumab-induced intestinal perforation.
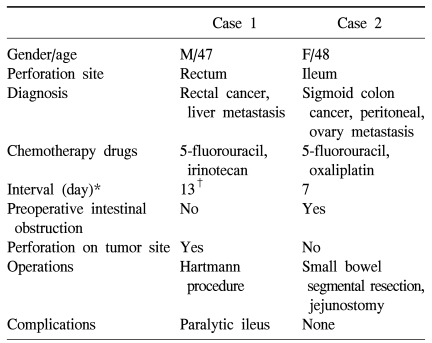
The reported locations of perforation have included the stomach, small bowel and large bowel (3,4,7,10). The large bowel is the most common site of bowel perforation after bevacizumab combination chemotherapy. In our cases, we did not observe that the risk of bowel perforation was related to the length of treatment with bevacizumab. The bowel perforations developed after a second cycle of bevacizumab combination chemotherapy in the first case, but after just the first cycle in the second case. After 7~8 days of bevacizumab combination chemotherapy, signs of peritonitis such as abdominal pain and tenderness appeared in both patients (Table 1).
Table 1.
Review of the cases of intestinal perforation after chemotherapy with bevacizumab
*interval between chemotherapy and operation, †abdominal pain and fever developed at the 7th day after chemotherapy.
Bowel perforations, whether induced by bevacizumab alone or as a result of combination chemotherapy, are frequently fatal (3,4). Careful observation is imperative for those patients who are administered bevacizumab alone or in combination chemotherapy. Especially, our present report suggests that careful observation is necessary for those patients who have an intact primary tumor or intestinal obstruction within 7~10 days after bevacizumab administration. If patients present with acute abdominal pain and they are suspected to have bowel perforation, then resuscitation and early surgical intervention should be the treatment of choice.
References
- 1.Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 2.Kabbinavar F, Hurwitz H, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 3.Chen HX, Mooney M, Boron M, Vena D, Mosby K, Grochow L, et al. Phase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: an NCI Treatment Referral Center Trial TRC-0301. J Clin Oncol. 2006;24:3354–3360. doi: 10.1200/JCO.2005.05.1573. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Scappaticci FA, Fehrenbacher L, Cartwright T, Hainsworth JD, Heim W, Berlin J, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173–180. doi: 10.1002/jso.20301. [DOI] [PubMed] [Google Scholar]
- 6.Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005;69(suppl 3):25–33. doi: 10.1159/000088481. [DOI] [PubMed] [Google Scholar]
- 7.Lordick F, Geinitz H, Theisen J, Sendler A, Sarbia M. Increased risk of ischemic bowel complications during treatment with bevacizumab after pelvic irradiation: report of three cases. Int J Radiat Oncol Biol Phys. 2006;64:1295–1298. doi: 10.1016/j.ijrobp.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol. 2007;14:1860–1869. doi: 10.1245/s10434-006-9337-9. [DOI] [PubMed] [Google Scholar]
- 9.Han ES, Monk BJ. What is the risk of bowel perforation associated with bevacizumab therapy in ovarian cancer? Gynecol Oncol. 2007;105:3–6. doi: 10.1016/j.ygyno.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Heinzerling JH, Huerta S. Bowel perforation from bevacizumab for the treatment of metastatic colon cancer: incidence, etiology, and management. Curr Surg. 2006;63:334–337. doi: 10.1016/j.cursur.2006.06.002. [DOI] [PubMed] [Google Scholar]