Abstract
We report a case of severe hypothyroidism in a cholangiocarcinoma patient with metastasis to the thyroid gland. A 58-year-old man was admitted for upper abdominal discomfort and multiple palpable neck nodules. Abdominal computed tomography (CT) demonstrated the presence of a 4.7-cm tumor in the right hepatic lobe, and core needle biopsy revealed it to be cholangiocarcinoma. Neck CT showed a diffuse, low attenuation thyroid gland, and fine-needle aspiration (FNA) demonstrated metastatic adenocarcinoma. Thyroid function tests were initially normal, but the size of the thyroid gland decreased and severe hypothyroidism developed after chemotherapy was implemented for cholangiocarcinoma. In a patient with malignant disease and a goiter, the possibility of a metastatic tumor involving the thyroid should be seriously considered. Metastatic thyroid cancer and thyroid dysfunction are probably infrequent, but diagnosis is important in the institution of appropriate therapy.
Keywords: Cholangiocarcinoma, Thyroid metastasis, Hypothyroidism
Introduction
Although metastatic disease is infrequently seen in the thyroid, both autopsy findings and clinical series indicate the problem is more common than generally thought. Autopsy series on cancer patients have yielded incidence rates ranging from 1.25% to 24%, and some investigators have suggested that the incidence of metastatic disease in the thyroid may have risen during the twentieth century (1,2). Recent reports suggest that the most common primary sites of thyroid metastasis are the kidney, lung, breast, and gastrointestinal tract (1-3). Currently there are several known cases of thyroid dysfunction caused by metastatic cancer infiltration (4,5). Most patients have presented with thyrotoxicosis followed by thyroid destruction. We report a patient who presented with a diffuse goiter and thyroid metastasis from cholangiocarcinoma. The patient developed severe hypothyroidism after undergoing chemotherapy.
Case Report
A 58-year-old man was admitted to the hospital with upper abdominal discomfort for one month, a palpable neck mass, and multiple neck nodules. The patient had been previously healthy and had no specific past medical history. Physical examination was positive for bilateral cervical lymphadenopathy and a diffuse goiter. Liver function tests were normal. Thyroid function tests were as follows: the free thyroxine (fT4) level was 1.51 ng/dL (normal range 0.8~1.71 ng/dL), the total triiodothyronine (T3) level was 0.948 ng/mL (normal range 0.6~1.6 ng/mL), and the serum thyrotropin (TSH) level was 0.445 uIU/mL (normal range 0.4~4.8 uIU/mL). Abdominal CT revealed a 4.7×3.6 cm-sized mass in the inferior segment of the right hepatic lobe (Fig. 1A). The hepatic tumor demonstrated mild peripheral enhancement during the arterial and portal venous phases, and showed strong enhancement of the central portion during the equilibrium phase. A fluorodeoxyglucose (FDG) positron emission tomography scan revealed increased uptake of F-18 FDG in the hepatic mass; the cervical, mediastinal, and intraabdominal lymph nodes; and the thyroid gland. An ultrasonography (US)-guided core needle biopsy was performed on the hepatic mass, and histopathological examination revealed adenocarcinoma with glandular or tubular structures within the sclerotic stroma (Fig. 1B, C). Cells were positive by immunohistochemical staining for CAM 5.2, but were negative for alpha-fetoprotein. Neck CT showed diffuse low attenuation in the thyroid gland (Fig. 2A). Cytology examination of FNAs obtained from the thyroid gland and cervical lymphadenopathy showed many atypical cellular clusters containing large, bizarre cells (Fig. 2B). Immunohistochemical staining was performed to differentiate primary thyroid cancer and metastatic cancer, and most of the tumor cells were found to be positive for CAM 5.2 and negative for TTF-1. The patient received 5-fluorouracil- and cisplatin-based systemic chemotherapy. After the third chemotherapy session, the TSH level was elevated to 82.34 µIU/mL, and the serum fT4 level was markedly suppressed to 0.1 ng/dL. Thyroid stimulating immunoglobulin (TSI) was negative, and the serum thyroglobulin and antimicrosomal antibody levels were normal. Follow-up neck CT demonstrated that the thyroid gland had significantly decreased in size. The patient complained of general weakness, fatigue, and poor appetite. He is currently undergoing follow-up observation and is receiving chemotherapy and thyroid hormone replacement.
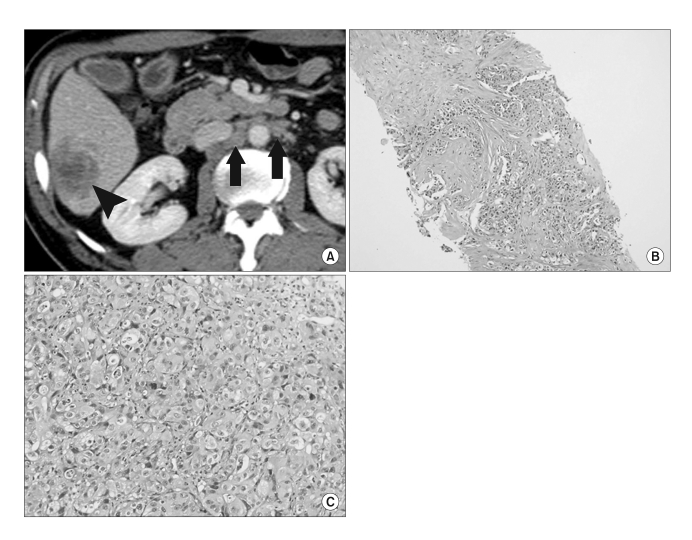
Fig. 1.
(A) A contrast-enhanced CT image demonstrates a poorly circumscribed, heterogeneously enhancing mass (black arrowhead) in the inferior segment of the right hepatic lobe. Metastatic lymphadenopathy (black arrows) is also seen in the retroperitoneum. (B) A low-power microscope field of a liver biopsy specimen demonstrates the presence of an adenocarcinoma showing glandular or tubular structures within a sclerotic stroma (hematoxylin and eosin (H & E) staining, ×40). (C) A high-power field shows that the tumor cells have round nuclei, prominent nucleoli, and abundant cytoplasm with vacuolation (H & E staining, ×200).
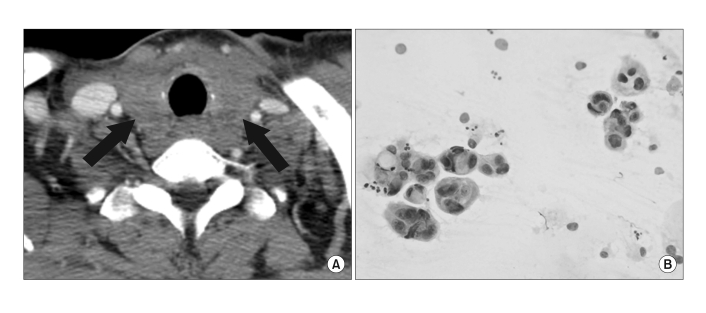
Fig. 2.
(A) An enhanced CT scan shows a low attenuation, mild, diffusely enlarged thyroid gland (black arrows). (B) Numerous atypical glandular structures are also noted. The cells have atypical nuclei and abundant vacuolated cytoplasm (Papanicolaou stain, ×400).
Discussion
Metastatic thyroid cancer may be more common than primary thyroid malignancies are, especially in patients who have a history of previous cancer (3). However, metastatic disease is found infrequently in the thyroid. Involvement by non-thyroid malignancies may arise through direct spread from adjacent structures, retrograde lymphatic spread, and hematogenous spread. A variety of presentations have been reported: from neck masses felt by the patient, to nodular goiters discovered on physical examination, to lesions discovered on imaging studies (1-3).
Numerous large autopsy series have reported the incidence of thyroid metastases in patients with known malignancy to be between 1.9% and 24%. Most clinical series have shown that renal cell carcinoma is the most common primary tumor related to symptomatic thyroid metastases, closely followed by breast and lung cancers (6-8). The pathological diagnosis of metastases to the thyroid gland may be difficult to make. FNA is often used to obtain tissue for analysis. However, it may be difficult to distinguish primary from metastatic disease if the cells are highly anaplastic (2). Positive immunohistochemical staining for thyroglobulin suggests the presence of primary thyroid malignancy. In the present case, FNA cytology showed cells with a morphology that resembled primary cholangiocarcinoma. Based on the morphology and immunohistochemical staining, it was reasonable to diagnose cholangiocarcinoma metastasis to the thyroid.
In the case of thyroid metastases, there exists the possibility of thyroid dysfunction due to thyroid destruction. Most reviewed cases have been characterized by transient thyrotoxicosis caused by massive metastasis of extrathyroid tumors. Malignant pseudothyroiditis has also been described in a few cases (5). In the present case, the fT4 level was normal, but the TSH level was already low-normal at the initial examination, and thyroid function tests showed hypothyroidism after 3 months. It is possible that a progressive destructive process in the thyroid gland followed a short episode of thyrotoxicosis, which might have otherwise gone unrecognized. We believe the tumor cells that metastasized to the thyroid from the cholangiocarcinoma destroyed the thyroid follicles, leading to severe hypothyroidism.
In summary, we have presented a case of severe hypothyroidism induced by thyroid metastasis from cholangiocarcinoma. In a patient with a malignant condition and a goiter, the possibility of a metastatic tumor involving the thyroid should be seriously considered. Metastatic thyroid cancer and thyroid dysfunction are probably infrequent, but accurate diagnosis is important in the implementation of appropriate therapy.
References
- 1.Kim TY, Kim WB, Gong G, Hong SJ, Shong YK. Metastasis to the thyroid diagnosed by fine-needle aspiration biopsy. Clin Endocrinol (Oxf) 2005;62:236–241. doi: 10.1111/j.1365-2265.2005.02206.x. [DOI] [PubMed] [Google Scholar]
- 2.Nakhjavani MK, Gharib H, Goellner JR, van Heerden JA. Metastasis to the thyroid gland. A report of 43 cases. Cancer. 1997;79:574–578. doi: 10.1002/(sici)1097-0142(19970201)79:3<574::aid-cncr21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Wood K, Vini L, Harmer C. Metastases to the thyroid gland: the royal marsden experience. Eur J Surg Oncol. 2004;30:583–588. doi: 10.1016/j.ejso.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson M, Ajmani SK, Mallette LE. Hyperthyroidism from thyroid metastasis of pancreatic adenocarcinoma. JAMA. 1977;238:1276–1278. [PubMed] [Google Scholar]
- 5.Miyakawa M, Sato K, Hasegawa M, Nagai A, Sawada T, Tsushima T, et al. Severe thyrotoxicosis induced by thyroid metastasis of lung adenocarcinoma: a case report and review of the literature. Thyroid. 2001;11:883–888. doi: 10.1089/105072501316973154. [DOI] [PubMed] [Google Scholar]
- 6.Hull OH. Critical analysis of two hundred twenty-one thyroid glands; study of thyroid glands obtained at necropsy in Colorado. AMA Arch Pathol. 1955;59:291–311. [PubMed] [Google Scholar]
- 7.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1,000 autopsied cases. Cancer. 1950;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Wychulis AR, Beahrs OH, Woolner LB. Metastasis of carcinoma to the thyroid gland. Ann Surg. 1964;160:169–177. doi: 10.1097/00000658-196408000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]