Abstract
The high vocal center (HVC) controls song production in songbirds and sends a projection to the robust nucleus of the archistriatum (RA) of the descending vocal pathway. HVC receives new neurons in adulthood. Most of the new neurons project to RA and replace other neurons of the same kind. We show here that singing enhances mRNA and protein expression of brain-derived neurotrophic factor (BDNF) in the HVC of adult male canaries, Serinus canaria. The increased BDNF expression is proportional to the number of songs produced per unit time. Singing-induced BDNF expression in HVC occurs mainly in the RA-projecting neurons. Neuronal survival was compared among birds that did or did not sing during days 31–38 after BrdUrd injection. Survival of new HVC neurons is greater in the singing birds than in the nonsinging birds. A positive causal link between pathway use, neurotrophin expression, and new neuron survival may be common among systems that recruit new neurons in adulthood.
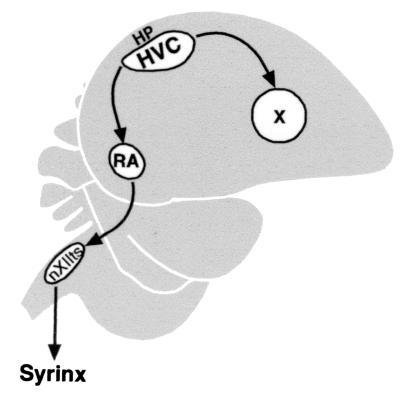
A set of interconnected brain nuclei referred to as the song system is responsible for song acquisition and production in songbirds. This system includes the high vocal center (HVC), which sends projections to two nuclei, the robust nucleus of the archistriatum (RA), and Area X (Fig. 1). The main motor pathway for song production runs from HVC to RA; RA in turn innervates mesencephalic and medullary nuclei that control respiratory muscles and the bird's vocal organ, the syrinx (1, 2). The projection from HVC to Area X is part of a separate forebrain circuit that is necessary for song learning but not for the production of learned song (3–6).
Figure 1.
Sagittal diagram showing the relationship of HVC to other song nuclei mentioned in the text. RA and nXIIts are part of the motor pathway that is necessary for song production. Area X (X) is part of the forebrain pathway necessary for song learning. The syrinx is the vocal organ of birds. The region of hippocampus (HP) above HVC was sampled as a nonsong system part of forebrain.
HVC continues to receive new neurons in adulthood, and a majority of these cells project to RA (7–10). The recruitment of new HVC neurons is part of a replacement process, with peaks in cell death in August (midsummer) and January (midwinter), when blood testosterone levels of adult male canaries are low. These peaks in cell death are followed by peaks in new neuron incorporation in October and March, when blood testosterone levels are high (11). Male canaries modify their song in adulthood by adding, dropping, and altering song syllables. Most of these changes occur during the summer and fall, with a secondary wave of change in winter. Song stability is maximal in the spring, when canaries breed and recruitment of new HVC neurons is at its lowest (12). It has been hypothesized that neuronal replacement in HVC provides a cellular basis for the song plasticity observed in adult canaries (11, 13).
The mechanism that regulates neuronal survival and turnover in HVC has been the focus of two previous studies. These studies suggested that the survival of new HVC neurons can be regulated by testosterone or a testosterone metabolite (14), and the testosterone effect is mediated by the brain-derived neurotrophic factor (BDNF) (15). Specifically, the increase in the recruitment of new HVC neurons after systemic testosterone treatment is blocked by infusing an antibody against BDNF into HVC. Moreover, the effect of testosterone could be mimicked by infusing BDNF into HVC, which is in line with the observation that the BDNF receptor TrkB is present in this nucleus (15). The present study extends these observations in two important ways. First, we show that the level of BDNF expression in HVC is closely related to how much a canary sings. Second, we show that singing increases the survival of new HVC neurons. Because testosterone is known to regulate singing in adult canaries (16–18), our results fit well with the earlier data. We also show that the changes in BDNF expression occur mostly in the class of HVC neurons that continue to be replaced in adulthood. Taken together, the earlier work and the results we report here suggest that singing and the attendant increase in BDNF expression help maintain the neural circuits involved in the production of learned song.
Materials and Methods
Animals and Song Behavior.
Adult male canaries (1–2 years old) from our colony of Belgian Waterslagers were used in all of the experiments. These birds were kept under the natural photoperiod of New York State. Singing experiments for gene expression analysis were carried out as described (19). Typically, 5–10 birds were placed in a quiet room and kept silent (by waving a hand at them when they tried to sing) for at least 2 h before a singing session began. Birds were stimulated to sing with conspecific song playbacks. Singing was quantified by counting the number of songs produced by each bird during a given period. Birds that sang 40–50 songs during a 1-h period were accepted as singing birds unless mentioned otherwise. The nonsinging birds naturally did not sing during this time. Singing and nonsinging birds were obtained from October to June, avoiding the summer months when canary song is more sporadic. We found no seasonal differences in BDNF expression in both singing and nonsinging birds, so the only variable we followed was whether a bird sang or not, and if it sang, how many songs it produced.
A song was defined by the onset of singing after a period of a least 5 sec of silence and counted as a single song for however long the bird continued to sing in an uninterrupted manner. The average song duration lasted approximately 10 sec (ranging from 5 to 30 sec), regardless of how many songs a bird sang. This was measured in a group of birds (10–15, housed singly but held communally in a same room); attention was focused on one bird at a time for 15 min; during this time, the number of songs produced by each bird was counted manually and the duration of each song was measured by using a stopwatch.
In Situ Hybridization and Quantification.
A BDNF cDNA fragment was isolated by screening a zebra finch forebrain cDNA library. Identity of this fragment (600 bp), as judged from its similarity to mammalian BDNF, was confirmed by sequence analysis. This fragment then was used to make sense or antisense riboprobes. In situ hybridization was carried out as described (19). Briefly, sections (10 μm) were fixed in 3% paraformaldehyde and hybridized with 106 cpm/section 35S-label BDNF probe at 65°C for 4 h [50% formamide, 2 × standard saline phosphate/EDTA (0.18 M NaCl/10 mM phosphate, pH 7.4/1 mM EDTA (SSPE), 400 μg/ml poly(A), 2 mg/ml tRNA, 1 mg/ml BSA, and 100 mM DTT]. Washing was conducted in 2 × SSPE containing 50% formamide, twice for 30 min each time, and then in 0.2 × SSPE, 30 min each for two times, all at 65°C. Slides were exposed to NTB-2 (Kodak) emulsion for 5–6 weeks. After development, sections were counterstained with cresyl violet and coverslipped. Images were captured with a charge-coupled device camera under a ×63 objective, and the number of exposed silver grains (size range of 2 to 8 pixels) was counted by using the National Institutes of Health image program. For each sample, three evenly spaced sagittal sections were quantified. Four non-overlapping, randomly picked rectangular areas (each included 50–80 cells) were analyzed for HVC. Areas of similar size were quantified in the hippocampus above HVC. Sense strand probe was always included in the hybridization reaction, and the nonspecific binding was subtracted.
Western Blot Analysis.
The singing birds sang a minimum of 40–50 songs/h during the first 3 h after lights went on in the morning and were allowed to sing for an additional 3–5 h before being killed. The nonsinging birds, for reasons of their own, did not sing during this time. HVC was punched out from brain sections with a glass pipette (0.75 mm in diameter) under a dissecting microscope. Tissues were homogenized (50 mM Tris, pH 7.5/150 mM NaCl/10% glycerol/1% NP-40/0.1% SDS/mixture of protease inhibitors, Sigma), incubated for 30 min on ice, and centrifuged (21,000 × g, 30 min). The supernatant was collected, and protein concentration was measured. Proteins were resolved on a 10–20% Tris-glycine/SDS gel. After transferring, the blot was incubated with an anti-BDNF polyclonal antibody made against a peptide sequence of recombinant human BDNF (1:500, Santa Cruz Biotechnology), then with a secondary antibody (1:2,000) and visualized with the enhanced chemiluminescence system (Amersham Pharmacia) combined with the ABC kit (Vector Laboratories). Quantification was done with National Institutes of Health image. The levels of BDNF protein found in the singing birds were expressed as a percentage of that in the nonsinging ones.
BDNF-Rhodamine Double-Labeling Experiment.
Canaries were injected with rhodamine-conjugated beads (Lumafluor, New York) into RA (240 nl) of one hemisphere and Area X (360 nl) of the other, with the right and left sides alternated. Birds were killed after singing 40–50 songs during a 1-h period. Brains were processed for in situ hybridization as described except that digoxigenin-labeled BDNF riboprobe was used and BDNF signal was detected with the TSA amplification system (NEN). Briefly, after hybridization sections were blocked (100 mM Tris, pH 7.5/150 mM NaCl/1% normal goat serum) and incubated with peroxidase conjugated anti-digoxigenin antibody (1:500, Boehringer Mannheim) overnight at 4°C. After washing (100 mM Tris, pH 7.5/150 mM NaCl), sections were incubated with biotinylated tyramide, washed again, and then incubated with Cy2-conjugated streptavidin (1:200, The Jackson Laboratory). Images were scanned with a confocal microscope (LSM510), at 585 nm (red) for rhodamine and 510 nm (green) for Cy2 with 2-μm photosectioning. The number of rhodamine-labeled cells and double-labeled cells (both rhodamine and BDNF positive) were counted by using the criterion that the neuronal soma had to be well labeled and defined with the markers used.
Neuronal Survival Experiment.
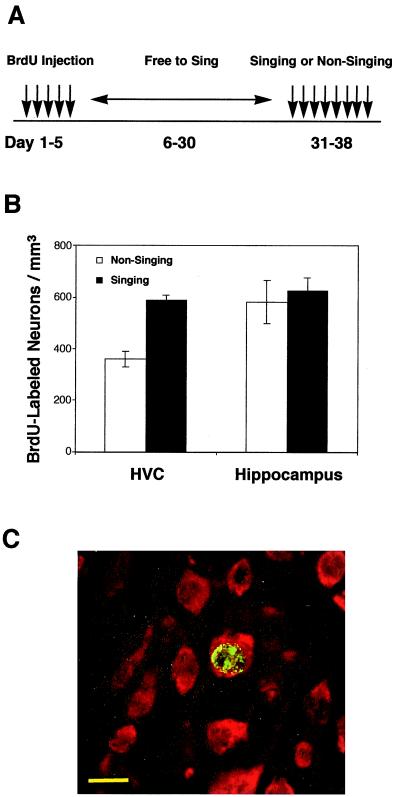
Canaries were injected with BrdUrd (10 mg/ml, in 50 μl of saline, Sigma) twice a day for 5 days in early October. The “singing experiment” (see Fig. 5A) began 25 days after the last injection and went on for 8 days. Birds were randomly divided into two groups. The singing group (n = 9) was stimulated to sing by exposing it to three 2-h sessions per day of conspecific song playbacks (song of a single bird, not related to experimental birds, singing on and off at its own tempo). The nonsinging birds (n = 5) were kept under similar conditions without hearing the playbacks. An investigator sat in the room where the nonsinging birds were held during the entire daylight hours of the 8-day period. Every time a bird started to sing, the investigator waved a hand at it to stop the singing. Birds were killed at the end of the 8-day period by an overdose of anesthesia (Nembutal) and perfused with 4% paraformaldehyde.
Figure 5.
Singing enhances the survival of new HVC neurons. (A) All birds received BrdUrd injections for 5 days and were free to sing from days 6 to 30. The singing group was stimulated to sing and the nonsinging group was prevented from singing from days 31 to 38. All birds were killed on day 38. (B) The number of BrdUrd-labeled neurons in the HVC of the singing group (n = 9) is significantly higher than that of the nonsinging group (n = 5), P < 0.005. No difference was found in the number of BrdUrd-labeled neurons in the hippocampus of the two groups, P = 0.736. Values correspond to mean ± SEM. (C) A confocal image showing neurons are labeled with the neuronal marker Hu (red), a neuron born during the 5-day injection period is labeled with BrdUrd (green in nucleus). (Scale bar: 10 μm.)
Brains were sectioned with a freezing microtome into 30-μm serial sagittal sections. Every fourth section (a total of 9–12 sections) containing HVC was processed for BrdUrd immunocytochemistry as described (20). Free-floating sections were treated with 0.6% H2O2/PBS for 30 min to block endogenous peroxidase. DNA was denatured by 2-h incubation in 50% formamide/2× SSC at 65°C, followed by 30-min incubation in 2 M HCl at 37°C, and 10 min in 0.1 M boric acid, pH 8.5. The sections then were blocked and incubated with anti-BrdUrd antibody (made in rat, 1:200, in 10% horse serum, 0.25% Triton/PBS) at 4°C overnight, then with biotinylated anti-rat secondary antibody at room temperature for 2 h, followed by standard ABC-diaminobenzidine (Vector Laboratories) staining. Sections then were incubated with anti-Hu antibody (made in mouse, 1:200) at 4°C overnight, followed by incubation with Cy3-conjugated anti-mouse antibody. Cells that were positive for both BrdUrd and the neuronal marker Hu (21) were identified and counted. A dark-field condenser was used to delineate the boundary of HVC, and HVC volume was calculated by multiplying the total area by the section thickness and section intervals. We also measured the nuclear diameters of BrdUrd-labeled HVC neurons (20 cells/individual) in the singing and nonsinging birds. The part of hippocampus immediately dorsal to HVC was sampled for the numbers of BrdUrd-labeled neurons. Quantification was performed by a person not aware of the experimental treatment of each group.
Results
Singing Induces BDNF Expression in HVC.
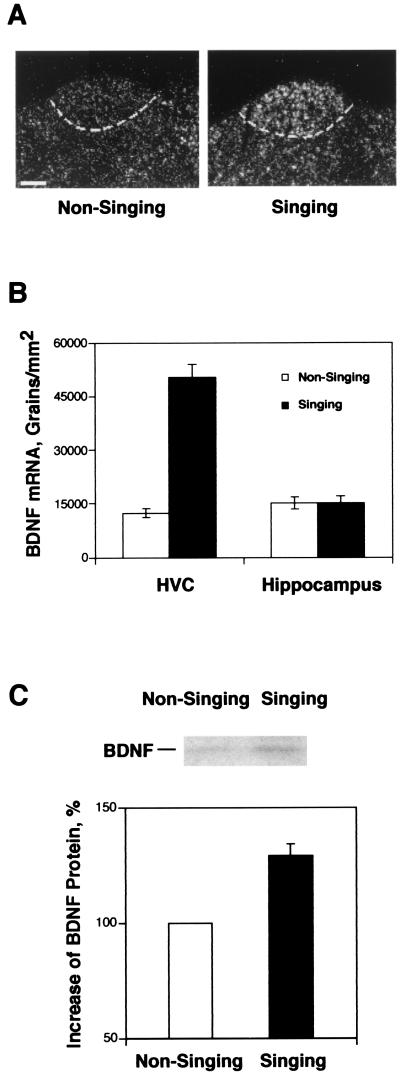
BDNF mRNA expression in the HVC of singing and nonsinging birds was studied by in situ hybridization. The singing birds (n = 6) had three times the level of BDNF mRNA expression in HVC as compared with the nonsinging ones (n = 4), P < 0.0001 (Fig. 2 A and B). BDNF mRNA expression also was found in some telencephalic regions that are not directly related to the song system. As an example of one of these areas, we quantified BDNF expression in the hippocampus above HVC, where we found no significant difference in BDNF expression between birds in the singing and nonsinging groups, P = 0.97.
Figure 2.
BDNF expression in the HVC of adult male canaries. (A) Dark-field images of in situ hybridization, showing BDNF mRNA expression in HVC of singing and nonsinging birds. (Scale bar, 200 μm.) (B) Quantitative analysis of BDNF mRNA expression in the HVC and hippocampus of nonsinging (n = 4) and singing birds (n = 6), P < 0.0001 for HVC and P = 0.97 for hippocampus, unpaired t test. Values are mean ± SEM. (C) Western blot analysis showing the amount of BDNF protein in the HVC of singing birds (n = 7) is 30% higher than that in the nonsinging birds (n = 6); P < 0.0001, unpaired t test.
We then did Western blot analysis to compare the levels of BDNF protein in the HVC of singing and nonsinging birds. A 14-kDa BDNF immunoreactive band similar to that of the recombinant human BDNF was detected in tissue homogenate made from HVC. The specificity of this band was confirmed by showing that preabsorption of the antibody with its corresponding peptide antigen completely abolished the antibody's ability to detect the 14-kDa band (data not shown). With this antibody, we show that (Fig. 2C) after birds sang for 6–8 h, BDNF protein levels in HVC increased by 30% compared with that of nonsinging birds (P < 0.0001, unpaired t test; n = 6 for nonsinging birds and n = 7 for singing birds). We conclude that singing-induced BDNF mRNA expression in HVC was followed by a modest, yet significant increase in BDNF protein in that same nucleus.
BDNF mRNA Expression in HVC Is Correlated to the Number of Songs Produced.
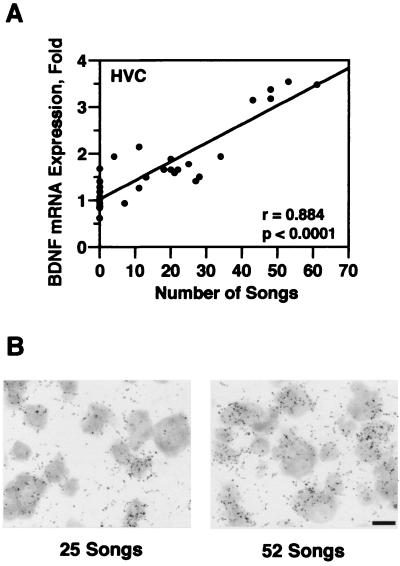
To determine whether singing-induced BDNF expression was an all-or-none phenomenon or a graded event, we next examined the relationship between the number of songs produced by each bird during a 30-min period and the level of BDNF expression in HVC. A total of 31 birds was exposed to song playbacks for 30 min; 12 of these birds remained silent and 19 responded by singing. The number of songs produced by each singing bird were counted. BDNF mRNA expression in HVC then was analyzed by in situ hybridization. Fig. 3A shows that a strong and significant correlation was found between the number of songs produced and BDNF mRNA expression. The more songs a bird sang, the higher the BDNF expression in its HVC (r = 0.884, P < 0.0001, regression analysis). Fig. 3B shows in situ hybridization autoradiography of two birds that sang 25 and 52 songs, respectively. The density of exposed silver grains reflects the relative level of BDNF mRNA expression.
Figure 3.
BDNF mRNA expression in HVC is correlated to the number of songs produced by each bird. (A) A strong and significant correlation was found between BDNF mRNA expression in HVC and the number of songs sung by each bird during a 30-min period (n = 31, r = 0.88, regression analysis; P < 0.001, ANOVA test). (B) In situ hybridization autoradiography of two birds that sang 25 and 52 songs, respectively. The density of silver grains reflects the relative level of BDNF mRNA expression.
BDNF Expression in HVC Occurs Primarily in the RA-Projecting Neurons.
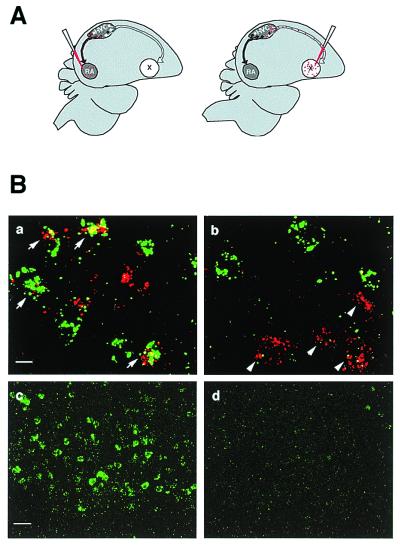
HVC contains one population of neurons that project to RA and another one that projects to Area X (Fig. 1A). We did a double-labeling experiment to determine in which population of these neurons BDNF is induced by singing. In this experiment, rhodamine (red) beads were injected into RA or Area X to retrogradely label the projecting neurons (Fig. 4A), and BDNF mRNA was detected by in situ hybridization with the aid of a fluorescence-conjugated antibody (green). As shown in Fig. 4B, many of the RA-projecting neurons were positive for BDNF (a), but only a minority of the Area X-projecting ones were BDNF positive (b). We quantified the percentage of each projecting neuron population that expressed BDNF after singing. As shown in Table 1, 73% ± 14% (SD) of the rhodamine-backfilled RA-projecting neurons were positive for BDNF mRNA expression, and 11% ± 5.7% (SD) of the rhodamine-backfilled Area X-projecting neurons expressed BDNF mRNA (n = 4, P < 0.05, Mann–Whitney U test). BDNF expression in the HVC of nonsinging birds was too low to be reliably quantified (compare Fig. 4B c and d). We conclude from this experiment that singing-induced BDNF mRNA expression in HVC occurs primarily in the RA-projecting neurons.
Figure 4.
BDNF mRNA is induced primarily in the RA-projecting neurons in HVC. (A) RA- and Area X-projecting neurons were retrogradely labeled with injections of rhodamine beads into the RA or Area X of each bird (n = 4). (B) The rhodamine signal (red) and the BDNF signal (green) were visualized with confocal microscope at 2-μm photosectioning. (a) Arrows point to neurons backfilled from RA that showed BDNF expression. (b) Arrows point to neurons backfilled from Area X that did not express BDNF. Lower-magnification view of HVC showing the level of BDNF expression was much higher in the singing birds (c) than in the nonsinging birds (d). (Scale bars: 10 μm for a and b; 50 μm for c and d.) See Table 1 for quantification.
Table 1.
Rhodamine-labeled projection neurons that express BDNF mRNA after singing
| Birds | RA-projecting cells
|
Area X-projecting cells
|
||||
|---|---|---|---|---|---|---|
| Double-labeled cells | Rhodamine-labeled cells | % | Double-labeled cells | Rhodamine-labeled cell | % | |
| Bird 1 | 28 | 45 | 62 | 2 | 34 | 6 |
| Bird 2 | 43 | 74 | 58 | 4 | 55 | 7 |
| Bird 3 | 79 | 99 | 80 | 4 | 28 | 14 |
| Bird 4 | 64 | 73 | 88 | 7 | 38 | 18 |
| Total | 214 | 291 | 73* | 17 | 155 | 11* |
This is an average of all four birds. P < 0.05 for both groups, Mann–Whitney test.
Singing-Related Neuronal Survival in HVC.
The experimental design of the neuronal survival study is shown in Fig. 5A. Adult male canaries received BrdUrd injections for 5 days. Twenty-five days after the last BrdUrd injection, birds were divided into singing and nonsinging groups and subjected to an 8-day singing experiment (as described in Materials and Methods). We then examined the volume of HVC, the nuclear diameter of the new neurons in HVC, and the number of new neurons in the HVC of the two groups. There was no significant difference in HVC volume between the singing and nonsinging groups (0.304 ± 0.01 mm3 for the singing group and 0.299 ± 0.018 mm3 for the nonsinging group; P = 0.779, unpaired t test). Similarly, there was no significant difference in the mean nuclear diameter of BrdUrd-labeled new neurons between the singing and nonsinging birds (7.9 ± 0.09 μm vs. 8.2 ± 0.37 μm, P = 0.34). However, as shown in Fig. 5B, the number of BrdUrd-labeled neurons in the HVC of the singing birds was significantly higher than that of the nonsinging birds (588 ± 20 BrdUrd+/Hu+ cells per mm3 in the singing group and 360 ± 29 BrdUrd+/Hu+ cells per mm3 in the nonsinging group, P < 0.005, unpaired t test). Besides HVC, new neurons were found in other forebrain regions, such as the hippocampus. However, we found no significant difference in the number of BrdUrd-labeled neurons in the hippocampus between the two groups (626 ± 50 BrdUrd+/Hu+ cells per mm3 for the singing group and 583 ± 85 BrdUrd+/Hu+ cells per mm3 for the nonsinging group; P = 0.736, unpaired t test). Fig. 5C shows an example of a BrdUrd+/Hu+ double-labeled neuron in HVC at high magnification.
Discussion
BDNF has been shown to play diverse roles in modulating the structure and function of the mammalian central nervous system during development and in adulthood (22–24). BDNF can function as an autocrine-paracrine survival factor in the central nervous system (25–27), regulates dendritic and axonal morphology (28–30), and affects synaptogenesis (31) and synaptic efficacy (32–35). In turn, BDNF gene expression is regulated by neural activities (36–39). These studies suggest that BDNF may provide a link between neural activity and the structural and functional plasticity of the nervous system. Here we report that singing enhances BDNF expression in the song nucleus HVC and the level of BDNF expression is closely related to the behavioral state of the birds. The more the birds sing, the higher the BDNF expression. The singing-induced BDNF expression occurs mostly in the kind of neurons that are necessary for song production and that continue to be replaced in adulthood.
Singing does not induce BDNF expression in hippocampus. This is not surprising because there is no evidence that hippocampus is involved in song behavior. HVC, however, is necessary for the production of learned song (1, 2), and there is a strong increase in neuronal firing in the HVC of birds when they sing (40, 41). BDNF expression is known to be regulated by neuronal depolarization (42, 43). It is likely that BDNF expression in HVC results from neuronal firing associated with song production. The singing bird also heard its own song. How do we know that the change in BDNF expression that accompanies singing results from the motor activity and not from hearing song? HVC neurons show little responses to playbacks of conspecific song in awake birds (44). Moreover, the expression of three activity-dependent genes (cFos, ZENK, and BDNF) does not increase in the HVC of birds that heard song but did not sing, and there is no difference in gene expression in the HVC of intact vs. deaf singing birds (refs. 19 and 45 and X.-C.L., unpublished results). These observations suggest that the increase in BDNF expression in the HVC of singing canaries resulted not from hearing song, but from song production.
Singing induced a 30% increase in BDNF protein in HVC. Although this increase seems modest when compared with the 3-fold increase in BDNF mRNA, a few caveats are in order. First, we do not know when the singing-induced rise in BDNF protein levels peaked in HVC. These levels were not significantly different from those in nonsinging birds 2–4 h after onset of singing (data not shown) but were significantly higher at 6–8 h and might have risen further. Second, BDNF protein has a relatively long half-life (46). We do not know whether BDNF protein produced during previous days contributed to the basal level seen in our nonsinging birds. Finally, and perhaps most importantly, it is known that much of the BDNF protein produced in one site can be carried away by anterograde transport (47, 48). If part of the BDNF protein produced in HVC was transported to RA or Area X, then the amount left in HVC would grossly underestimate the amount produced. Although the overall 30% increase in BDNF protein in HVC is modest, it can be considerably higher at a local, microanatomical level, which could have a significant impact on a specific set of neurons or synapses.
Singing enhanced the number of BrdUrd-labeled neurons in HVC, but, as for BDNF expression, there was no such effect in hippocampus. The hippocampus was chosen for this comparison because it shows adult neurogenesis (49), it is not known to be involved in song behavior, and at least in mammals, its neurogenesis is known to be sensitive to stress (50, 51). The absence of a difference in new neuron numbers in the hippocampus of singing vs. nonsinging birds suggests that our method of preventing canaries from singing (waving a hand at a bird that started to sing) did not induce systemic physiological changes that affected the number of new neurons in general. Rather, the effect of singing on new HVC neurons was functional and circuit specific, and probably related to the neuronal firing in HVC that accompanies song production.
The effect of singing on new HVC neuron numbers probably resulted from an effect on cell survival. The new neurons were born 25–30 days before the birds were separated into singing and nonsinging groups. Birds in the two groups received different treatment during the last 8 days of their life, from days 31 to 38 after the first BrdUrd injection. By that time probably all of the BrdUrd-labeled neurons had reached their destination, adopted a postmigratory phenotype, and were in the process of establishing connections (10). Recent work shows that, for reasons unknown, half of new HVC neurons born in adulthood disappear between their second and third week of life (10). Now we know that even after that initial wave of death, survival of the remaining new neurons is not guaranteed, but is probably activity-dependent.
Experience-dependent enhancement of new neuron numbers has been reported in other systems. For example, the number of new hippocampal neurons in the blackcapped chickadee (Parus atricapillus) is higher in free-ranging than in captive individuals; in the former, the abundance of these new cells is correlated with seasonal peaks in food caching (49). This work, however, did not discriminate between the effects of experience on production, migration, and survival of new neurons. Experiments in rodents have shown that training rats on learned tasks that require hippocampal participation enhances the survival of newly formed hippocampal cells (52); mice placed in an enriched environment and rats that ran in a running wheel also showed greater survival of new hippocampal neurons (53, 54). Our results with canaries extend the experience-dependent regulation of new neuron survival to another behavioral modality, singing. Our results are unique in that they show a relationship between the occurrence of a learned behavior, an increase in neurotrophin expression by neurons that produce this behavior, and an increase in the survival of new neurons of the latter type. Although our observations are drawn from a particular system, we suggest that a positive causal link between circuit usage, neurotrophin expression, and new neuron survival may be a common occurrence in neural circuits that continue to recruit new neurons in adulthood.
Acknowledgments
We thank J. Fawcett and R. Murphy for sharing unpublished result on BDNF protein, Q. Ting for advice on confocal analysis, and M. Grossman for rhodamine beads injection. We also thank A. Alvarez-Buylla, J. Conover, J. Hudspeth, J. Kirn, and C. Mello for critical reading of the manuscript. This work was supported by the Lee Memorial Fellowship and Public Health Service Grants DC03492 (to X.-C.L.) and MH18343 (to F.N.).
Abbreviations
- HVC
high vocal center
- RA
robust nucleus of the archistriatum
- BDNF
brain-derived neurotrophic factor
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140222497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140222497
References
- 1.Nottebohm F, Stokes T M, Leonard C M. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 2.Nottebohm F, Kelley D B, Paton J A. J Comp Neurol. 1982;207:344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- 3.Bottjer S W, Miesner E A, Arnold A P. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 4.Morrison R G, Nottebohm F. J Neurobiol. 1993;24:1045–1064. doi: 10.1002/neu.480240805. [DOI] [PubMed] [Google Scholar]
- 5.Scharff C, Nottebohm F. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brainard M S, Doupe A J. Nature (London) 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- 7.Goldman S A, Nottebohm F. Proc Natl Acad Sci USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paton J A, Nottebohm F. Science. 1984;225:1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- 9.Avarez-Buylla A, Kirn J R, Nottebohm F. Science. 1990;249:1444–1446. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- 10.Kirn J R, Fishman Y, Sasportas K, Nottebohm F. J Comp Neurol. 1999;411:487–494. [PubMed] [Google Scholar]
- 11.Kirn J R, O'loughlin B, Kasparian S, Nottebohm F. Proc Natl Acad Sci USA. 1994;91:7844–7848. doi: 10.1073/pnas.91.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nottebohm F, Nottebohm M E, Crane L. Behav Neural Biol. 1986;46:445–471. doi: 10.1016/s0163-1047(86)90485-1. [DOI] [PubMed] [Google Scholar]
- 13.Nottebohm F. Sci Am. 1989;260:74–79. doi: 10.1038/scientificamerican0289-74. [DOI] [PubMed] [Google Scholar]
- 14.Rasika S, Nottebohm F, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1994;91:7854–7858. doi: 10.1073/pnas.91.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasika S, Alvarez-Buylla A, Nottebohm F. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 16.Leonard S L. Proc Natl Acad Sci USA. 1939;41:229–230. [Google Scholar]
- 17.Shoemaker H H. Proc Natl Acad Sci USA. 1939;41:231–232. [Google Scholar]
- 18.Nottebohm F. Brain Res. 1980;189:429–436. doi: 10.1016/0006-8993(80)90102-x. [DOI] [PubMed] [Google Scholar]
- 19.Jarvis E D, Nottebohm F. Proc Natl Acad Sci USA. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn H G, Dickinson-Anson H, Gage F H. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barami K, Iversen K, Furneaux H, Goldman S A. J Neurobiol. 1995;28:82–101. doi: 10.1002/neu.480280108. [DOI] [PubMed] [Google Scholar]
- 22.Lo D C. Neuron. 1995;15:9779–9781. [Google Scholar]
- 23.Lewin G R, Yves-Alain B. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 24.Schuman E M. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- 25.Oppenheim R W, Yin Q W, Prevette D, Yan Q. Nature (London) 1992;360:755–759. doi: 10.1038/360755a0. [DOI] [PubMed] [Google Scholar]
- 26.Acheson A, Conover J C, Fandl J P, Dechiara T M, Russell M, Thadani A, Squinto S P, Yancopoulos G D, Lindsay R M. Nature (London) 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh A, Carnahan J, Greenberg M E. Science. 1994;263:1618–1622. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 28.McAllister A K, Lo D C, Katz L C. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 29.Cabelli R J, Hohn A, Shatz C J. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 30.Wilson Horch H, Kruttgen A, Portbury S D, Katz L C. Neuron. 1999;23:353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- 31.Causing C G, Gloster A, Aloyz R, Bamji S X, Chang E, Fawcett J, Kuchel G, Miller F D. Neuron. 1997;18:257–267. doi: 10.1016/s0896-6273(00)80266-4. [DOI] [PubMed] [Google Scholar]
- 32.Lohof A M, Ip N Y, Poo M-M. Nature (London) 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 33.Levine E S, Dreyfus C F, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1995;92:8074–8078. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang H, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 35.Patterson S L, Abel T, Deuel T A S, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 36.Isackson P J, Huntsman M M, Murray K D, Gall C M. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- 37.Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- 38.Castren E, Zafra F, Thoenen H, Lindholm D. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson S L, Grover L M, Schwartzkroin P A, Bothwell M. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 40.McCasland J S, Konishi M. Proc Natl Acad Sci USA. 1981;78:7815–7819. doi: 10.1073/pnas.78.12.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu A C, Margoliash D. Science. 1996;273:1871–1875. doi: 10.1126/science.273.5283.1871. [DOI] [PubMed] [Google Scholar]
- 42.Shieh P B, Hu S-C, Bobb K, Timmusk T, Ghosh A. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 43.Tao X, Finkbeiner S, Arnold D B, Shaywitz A J, Greenberg M E. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt M F, Konishi M. Nat Neurosci. 1999;1:513–518. doi: 10.1038/2232. [DOI] [PubMed] [Google Scholar]
- 45.Kimpo R R, Doupe A J. Neuron. 1997;18:315–325. doi: 10.1016/s0896-6273(00)80271-8. [DOI] [PubMed] [Google Scholar]
- 46.Nawa H, Carnahan J, Gall C. Eur J Neurosci. 1995;7:1527–1535. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 47.Altar C A, Cai N, Bliven T, Juhasz M, Conner J M, Acheson A L, Lindsey R M, Wiegand S J. Nature (London) 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 48.Johnson F, Hohmann S E, Distefano P, Bottjer S W. J Neurosci. 1997;17:2101–2111. doi: 10.1523/JNEUROSCI.17-06-02101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnea A, Nottebohm F. Proc Natl Acad Sci USA. 1994;91:11217–11221. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gould E, McEwen B S, Tanapat P, Galea L A, Fuche E. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McEwen B S. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 52.Gould E, Beylin A, Tanapat P, Reeves A, Shors T. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 53.Kempermann G, Kuhn H G, Gage F H. Nature (London) 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 54.Van Praag H, Kempermann G, Gage F H. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]