Abstract
The pore region of cyclic nucleotide–gated (CNG) channels acts as the channel gate. Therefore, events occurring in the cyclic nucleotide–binding (CNB) domain must be coupled to the movements of the pore walls. When Glu363 in the pore region, Leu356 and Thr355 in the P helix, and Phe380 in the upper portion of the S6 helix are mutated into an alanine, gating is impaired: mutant channels E363A, L356A, T355A, and F380A desensitize in the presence of a constant cGMP concentration, contrary to what can be observed in wild-type (WT) CNGA1 channels. Similarly to C-type inactivation of K+ channels, desensitization in these mutant channels is associated with rearrangements of residues in the outer vestibule. In the desensitized state, Thr364 residues in different subunits become closer and Pro366 becomes more accessible to extracellular reagents. Desensitization is also observed in the mutant channel L356C, but not in the double-mutant channel L356C+F380C. Mutant channels L356F and F380K did not express, but cGMP-gated currents with a normal gating were observed in the double-mutant channels L356F+F380L and L356D+F380K. Experiments with tandem constructs with L356C, F380C, and L356C+F380C and WT channels indicate that the interaction between Leu356 and Phe380 is within the same subunit. These results show that Leu356 forms a hydrophobic interaction with Phe380, coupling the P helix with S6, whereas Glu363 could interact with Thr355, coupling the pore wall to the P helix. These interactions are essential for normal gating and underlie the transduction between the CNB domain and the pore.
INTRODUCTION
CNG channels underlie sensory transduction in vertebrate photoreceptors and olfactory sensory neurons (Fesenko et al., 1985; Zimmerman et al., 1985; Nakamura and Gold, 1987) and require cyclic nucleotides (CNs) to open (Kaupp et al., 1989; Zagotta and Siegelbaum, 1996). The analysis of the amino acid sequence of the CNGA1 subunit indicates the existence of six transmembrane segments, usually referred to as S1, S2, S3, S4, S5, and S6 helices. Between the S5 and S6 helices there is a pore region, showing a significant sequence homology with the pore region of K+ channels (Becchetti et al., 1999; Liu and Siegelbaum, 2000); it suggests that the corresponding 3-D structure of CNG channels is similar to that of K+ channels, composed of a P helix and a hairpin. Next to the S6 helix, near the C terminal of the channel, there is the CN-binding (CNB) domain located at a distance of some tens of angstroms from the pore region (Matulef and Zagotta, 2003). The pore region in CNG channels also acts as the gate, and conformational changes occurring in the filter are indeed thought to underlie gating in these channels (Fodor et al., 1997; Becchetti et al., 1999; Becchetti and Roncaglia, 2000; Liu and Siegelbaum, 2000; Flynn and Zagotta, 2003; Contreras et al., 2008). Therefore, molecular events occurring in the CNB domain must be coupled to movements of the pore walls.
CNG channels open in the presence of a steady CN concentration, and their opening probability remains unaltered as long as CNs are present in the medium bathing the intracellular side of the membrane. Some years ago, it was shown that a point mutation in the pore region changed rather profoundly the gating of CNGA1 channels from bovine rods: when Glu363 was mutated into alanine, serine, or asparagine, mutant channels desensitized in the presence of a steady cGMP concentration (Bucossi et al., 1996). Given the significant homology between K+ and CNG channels (Becchetti et al., 1999; Liu and Siegelbaum, 2000), it is possible that desensitization of mutant channel E363A could originate from molecular mechanisms very similar to those underlying C-type inactivation in K+ channels (Liu et al., 1996; Contreras et al., 2008).
Here, we show that C-type inactivation of K+ channels and desensitization in CNGA1 channel E363A mutant are similarly associated with rearrangements of residues in the outer vestibule and in the P helix. The analysis of the presence or the absence of desensitization in different constructs from CNGA1 channels indicates that Leu356 has a strong hydrophobic interaction with Phe380, coupling the P helix and S6, and suggests that Glu363 could interact with Thr355, coupling the pore wall to the P helix. Our results show that these interactions are essential for normal gating: they underlie the transduction between the CNB domain and the pore, and their disruption is responsible for desensitization.
MATERIALS AND METHODS
Molecular biology
The CNGA1 channel from bovine rod consisting of 690 residues was used. Selected residues were replaced as described previously (Becchetti et al., 1999; Matulef et al., 1999) using the Quick Change Site-Directed Mutagenesis kit (Agilent Technologies). Point mutations were confirmed by sequencing using the sequencer LI-COR (4000L). cDNAs were linearized and transcribed to cRNA in vitro using the mMessage mMachine kit (Applied Biosystems).
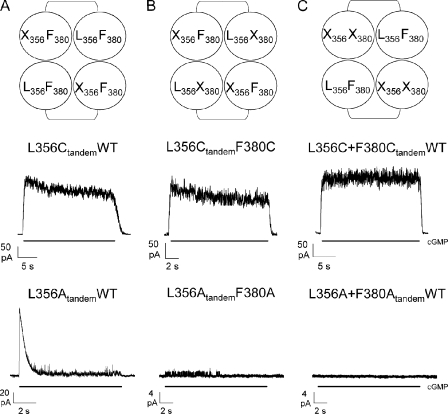
Tandem dimer constructs were generated by the insertion of one copy of the CNGA1 DNA into a vector pGEMHE already containing another copy of CNGA1 DNA. At the end of the cloning process, two copies of the CNGA1 DNA were connected by a 10–amino acid linker, GSGGTELGST (Rothberg et al., 2002), joining the C terminus of the first CNGA1 with the N terminus of the second one (see Fig. 5). This second subunit was made by replacing the ApaI restriction site GGGCCC at the end of the CNGA1 DNA without changing the amino acid GGTCCC and adding to the start codon a new ApaI restriction site, followed by a linker using a PCR reaction. Subunits were linked after HindIII/ApaI was cut.
Figure 5.
The interaction between L356 and F380 is within the same subunit. Diagrams representing subunit composition of the tandem L356XtandemWT (A, top row), the tandem L356XtandemF380X (B, top row), and the tandem L356X+F380XtandemWT (C, top row) in which X = C or A. Each subunit is represented by a circle, whereas the linker of 10 amino acids between two subunits of the tandem is represented by an arc (see Materials and methods). In the middle row, cGMP-activated currents in the presence of 1 mM cGMP at +60 mV in the tandem L356CtandemWT (A, middle row), in the tandem L356CtandemF380C (B, middle row), and in the tandem L356C+F380CtandemWT (C, middle row). In the bottom row, cGMP-activated currents in the presence of 1 mM cGMP at +60 mV in the tandem L356AtandemWT (A, bottom row), in the tandem L356AtandemF380A (B, bottom row), and in the tandem L356A+F380AtandemWT (C, bottom row).
Oocyte preparation and chemicals
Mutant channel cRNAs were injected into Xenopus laevis oocytes (Xenopus Express). Oocytes were prepared as described previously (Nizzari et al., 1993). Injected eggs were maintained at 18°C in a Barth solution supplemented with 50 µg/ml gentamycin sulfate and containing (in mM): 88 NaCl, 1 KCl, 0.82 MgSO4, 0.33 Ca(NO3)2, 0.41 CaCl2, 2.4 NaHCO3, and 5 TRIS-HCl, pH 7.4 (buffered with NaOH). During the experiments, oocytes were kept in a Ringer solution containing (in mM): 110 NaCl, 2.5 KCl, 1 CaCl2, 1.6 MgCl2, and 10 HEPES-NaOH, pH 7.4 (buffered with NaOH). Usual salts and reagents were purchased from Sigma-Aldrich, and 2-(trimethylammonium)ethyl] methanethiosulfonate bromide (MTSET) was purchased from Toronto Research Chemicals.
Recording apparatus
cGMP-gated currents from excised patches were recorded with a patch clamp amplifier (Axopatch 200; MDS Analytical Technologies) 2–6 d after RNA injection at room temperature (20–24°C). The perfusion system was as described previously (Sesti et al., 1995) and allowed a complete solution change in <1 s. Borosilicate glass pipettes had resistances of 2–5 MΩ in symmetrical standard solution. The standard solution on both sides of the membrane consisted of (in mM): 110 NaCl, 10 HEPES, and 0.2 EDTA, pH 7.4. The membrane potential was usually stepped from 0 to ±60 mV. We used Clampex 10.0, Clampfit 10.1, and SigmaPlot 9.0 for data acquisition and analysis. Data are usually given as mean ± SD. Currents were low-pass filtered at 2 kHz and digitally acquired at 5 kHz.
Application of sulfhydryl-specific reagents
In the inside-out patch clamp configuration, soon after patch excision, the cytoplasmic face of the plasma membrane was perfused with the same pipette-filling solution. In some experiments, 2.5 mM MTSET was added to the solution filling patch pipettes (Becchetti et al., 1999) to test the effect of sulfhydryl-specific reagents applied in the extracellular medium. In some selected experiments, the application, in the bath or in patch pipette solution, of 5 mM dithiothreitol (DTT) was used to reduce the formation of disulfide bonds.
RESULTS
The wild-type (WT) CNGA1 channel from bovine rods in the presence of a steady cGMP concentration of 1 mM does not desensitize (Fig. 1 A), and the amplitude of the cGMP-activated current remains stable for several minutes. We performed an extensive scanning mutagenesis from Val348 to Phe380 to find desensitizing mutant CNGA1 channels replacing the native residue with an alanine or cysteine. As the 3-D structure of CNGA1 channels is not identical (Mazzolini et al., 2008) to that of the construct where all native cysteines have been replaced by a residue not bearing a thiol group (CNGA1cys-free), we inserted alanine or cysteine residues in the CNGA1 background and not in the CNGA1cys-free background. The great majority of these mutant channels did not desensitize, but we found four mutant channels, which in the presence of a steady concentration of 1 mM cGMP desensitized: L356A, E363A, T355A, and F380A. As shown in Fig. 1, all these mutant channels to some extent desensitized: the cGMP-activated current at +60 mV in mutant channel L356A (Fig. 1 D) declined to 24.8 ± 5.6% (n = 6) of its original level within 3–5 s. A lower and slower desensitization was also observed in mutant channels E363A (Fig. 1 B), T355A (Fig. 1 C), and F380A (Fig. 1 E), where the cGMP-activated current declined to 51.0 ± 4.9% (n = 14), 55.6 ± 5.9% (n = 6), and 77.5 ± 6.6% (n = 13), respectively, of what was measured immediately after cGMP addition.
Figure 1.
Desensitized mutant channels. cGMP-activated currents in the presence of 1 mM cGMP at +60 mV in the WT (A) and mutant channels E363A (B), T355A (C), L356A (D), and F380A (E). cGMP was added as indicated by the solid horizontal black bars. (F) Sequence alignment between CNGA1, Shaker, Kv1.2, Kv2.1, KcsA, and NaK channels. The TVGYG signature of K+ channels is highlighted in yellow. Amino acids that become more accessible upon desensitization or C-type inactivation are highlighted in green. The sequence alignment was performed by using Clustal W (Thompson et al., 1994).
When cGMP was removed from the bathing medium for at least 1 min, 1 mM cGMP activated a current of amplitude similar to that initially observed (Bucossi et al., 1996). Desensitization was faster and stronger at negative voltages than at positive voltages (Bucossi et al., 1996), and at −60 mV the cGMP-activated current of mutant channels L356A, E363A, T355A, and F380A declined to 2.0 ± 0.5% (n = 3), 9.0 ± 4.4% (n = 6), 38.4 ± 2.0% (n = 5), and 52.5 ± 18% (n = 4), respectively, from the current initially observed.
Desensitization of mutant CNGA1 channels is reminiscent of C-type inactivation after a prolonged depolarization observed in the great majority of K+ channels (Kurata and Fedida, 2006). C-type inactivation has been recently investigated at a detailed molecular level in the KcsA channel (Cordero-Morales et al., 2006a,b, 2007; Chakrapani et al., 2007), and it is almost abolished in the KcsA channel E71A mutant (Cordero-Morales et al., 2007), suggesting that modifications of chemical interactions in the pore region control the degree of inactivation. These observations indicate that desensitization of mutant channels T355A, L356A, E363A, and F380A, illustrated in Fig. 1, could originate from the disruption and/or weakening of important chemical interactions necessary for the physiological operation of the channel. As the gate of CNG channels is thought to be located in the pore itself (Fodor et al., 1997; Becchetti et al., 1999; Becchetti and Roncaglia, 2000; Liu and Siegelbaum, 2000; Flynn and Zagotta, 2003; Contreras et al., 2008), the S6 helices must couple conformational rearrangements occurring in the CNB domain to movements of the pore walls. To identify the chemical interactions underlying this coupling, we analyzed similarities and differences between the amino acid sequence of CNGA1, Na+/K+, and K+ channels. The sequence alignment of CNG channels (Fig. 1 F), compatible with previous experimental results (Becchetti and Roncaglia, 2000; Liu and Siegelbaum, 2000) and Shaker K+ channel (Tempel et al., 1987), Kv1.2 (Long et al., 2005a,b), Kv2.1 (Long et al., 2007), KcsA (Doyle et al., 1998; Zhou et al., 2001), and NaK channel (Shi et al., 2006), shows three main features. First, the signature GYG of K+ channels is absent in CNGA1 channels (Heginbotham et al., 1992, 1994). Second, in CNGA1 channels there are several hydrophobic residues in the upper portion of the S6 (Phe380, Leu381, and Iso382) and in the P helix (Leu356 and Leu358), suggesting that the expected coupling between these two domains could be mediated by hydrophobic interactions between these residues. Third, Met448, Thr449, and Pro450 (in the green box of Fig. 1 F) of the K+ Shaker channel change their accessibility to chemical modification during C-type inactivation (Liu et al., 1996). The identification of residues in CNGA1 showing a similar behavior will identify conformational changes occurring during desensitization in mutant CNGA1 channels.
Conformational rearrangements during desensitization
A seminal investigation of C-type inactivation of K+ Shaker channels (Liu et al., 1996) has shown that C-type inactivation is associated to conformational changes occurring in the outer vestibule, during which some residues become more accessible to the reagents added to the extracellular side of the membrane (Liu et al., 1996). C-type inactivation is abolished in the KcsA channel E71A mutant (Cordero-Morales et al., 2006b, 2007), i.e., when a negative residue in the pore region has been neutralized and replaced with a smaller residue. Therefore, desensitization of mutant channel E363A, where the glutamate in the pore region has been neutralized, could share molecular mechanisms similar to those of C-type inactivation in K+ channels. Given the homology between CNGA1 and K+ channels, we asked whether desensitization in mutant channel E363A could be mediated by conformational changes similar to those occurring during C-type inactivation in K+ Shaker channels in which mutant channels M448C, T449C, and P450C change their accessibility to chemical modification in the inactivated state (Liu et al., 1996).
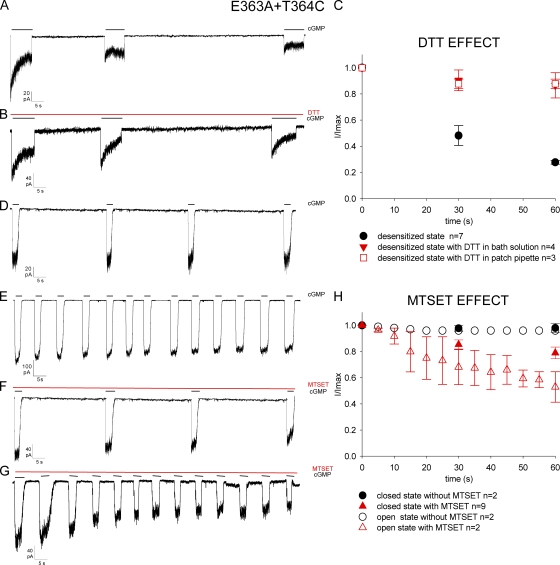
To verify this hypothesis, we engineered two desensitizing mutant channels, E363A+T364C and E363A+P366C, bearing a cysteine in positions similar to those of Shaker K+ channels, known to become more accessible to extracellular MTSET (Liu et al., 1996) in the inactivated state. In the K+ channel M448C mutant, the current flowing through open channels rapidly declined and did not recover upon termination of the prolonged depolarization. This phenomenon is due to cross-linking among exogenous cysteines coming very close when observed in the inactivated state.
To test accessibility of the pore in the closed, open, and desensitized states, we used a protocol similar to that described by Liu et al. (1996). As desensitization is more pronounced and faster at negative voltages, we analyzed the time course of the CNG current at −60 mV. Mutant channel E363A+T364C in the presence of a steady concentration of 1 mM cGMP rapidly desensitized, and the cGMP-activated current progressively and irreversibly declined to 18.7 ± 2.0% (n = 6) from its original level within 1 or 2 min (Fig. 2 A). Removal of cGMP for several minutes did not restore the initial amplitude of the cGMP-activated current. Because in Shaker K+ channel M448C mutant the rundown of the current was reduced and reverted by the application of the reducing agent DTT to the extracellular medium, we performed similar experiments in mutant channel E363A+T364C. As shown in Fig. 2 B, when 5 mM DTT was added to the solution filling the patch pipette, the decline of the cGMP-activated current in the mutant channel E363A+T364C was almost prevented. In fact, after 60 s the peak of the cGMP-activated current declined by ∼20% in the presence of DTT, but by ∼80% in its absence. The spontaneous decline of the cGMP-activated current in mutant channel E363A+T364C in the presence of a continuous application of 1 mM cGMP was greatly reduced by the presence of DTT both in the patch pipette and in the medium bathing the intracellular side of the patch membrane (Fig. 2 C).
Figure 2.
State-dependent modification of the mutant channel E363A+T364C. cGMP-activated current traces at +60 mV in three different conformations of mutant channel: desensitized (A), closed (D), and open (E) states. (B) Effect of 5 mM DTT added to patch pipette solution in desensitized conformation of channel. (F and G) Effect of 2.5 mM MTSET added to the extracellular medium in closed (F) and open (G) states. 1 mM cGMP, 5 mM DTT, and 2.5 mM MTSET was added as indicated by the solid horizontal black or red bars. (C) Comparison of 5 mM DTT added to bath solution (red solid triangle), in patch pipette (red open square), and in DTT absence (black solid circle) in desensitized state; the values represent the amplitude of the peak current (I) measured every 30 s and normalized (I/Imax) to peak current of control (Imax) at time zero. (H) State-dependent modification in the closed (black solid circle) and open (black open circle) states in the absence of 2.5 mM MTSET, and in closed (red solid triangle) and open (red open triangle) states in the presence of 2.5 mM MTSET in the extracellular medium. Single points of the plots represent the amplitude of the peak current (I) measured every 5 s in open state and 30 s in closed state and, as in C, normalized (I/Imax) to peak current of control (Imax) at time zero.
In contrast, when the patch was exposed to 1 mM cGMP for just 2 s every 30 s—and therefore the CNG channel was mostly in its closed state—the cGMP-activated current declined by <3% within 2 min (n = 5) (Fig. 2 D). When the patch was exposed to cGMP for 5 s every 10 s and CNG channels were more often in the open state, the cGMP-activated current declined by ∼5% within 2 min (n = 6) (Fig. 2 E). By analogy with the interpretation of the results obtained for Shaker K+ channel M448C mutant, it is concluded that in CNGA1 channel E363A mutant, Thr364 in different subunits in the desensitized state becomes closer; consequently, disulfide bonds form spontaneously, leading to an irreversible channel closure.
When 2.5 mM MTSET was present in the solution filling the patch pipette, corresponding to the extracellular side of the membrane of excised patches, the CNG current declined faster in the open (Fig. 2 F) than in the closed state (Fig. 2 G). Collected data from several experiments showed that in the presence of MTSET in the patch pipette, the cGMP-activated current within 60 s was reduced by 53 ± 5% (n = 6) and by 79 ± 1% (n = 9) in the open and in the closed state, respectively (Fig. 2 H).
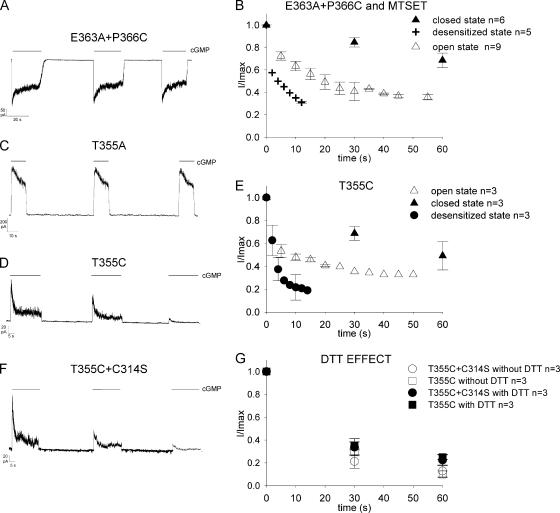
The CNG current in mutant channel E363A+P366C in the presence of 1 mM cGMP desensitized, but, contrary to what was observed for the mutant E363A+T364C, it recovered from desensitization after cGMP removal (Fig. 3 A). Therefore, we investigated the time course of blockage of the cGMP-activated current caused by 2.5 mM MTSET in the extracellular medium. As shown in Fig. 3 B, half blockage of the cGMP current was observed in ∼5 s in the desensitized state (50 ± 1.0%; n = 5) and in ∼20 and 60 s when the channel was in the open (49.0 ± 6%; n = 9) and closed state (68 ± 6%; n = 6), respectively. A very similar behavior was observed in Shaker K+ channel P450C mutant (Liu et al., 1996) during the analysis of C-type inactivation.
Figure 3.
Desensitization in mutant channels E363A+P366C, T355A, T355C, and T355C+C314S. cGMP-activated current traces in the desensitized state of four mutant channels: E363A+P366C (A) at −60 mV, T355A (C), T355C (D), and T355C+C314S (F) at +60 mV. 1 mM cGMP was added as indicated by the solid horizontal black bars. (B) State-dependent modification in closed (solid triangle), open (open triangle), and desensitized (plus) states of mutant channel E363A+P366C in the presence of 2.5 mM MTSET. The plot shows the state-dependent modification calculated from the amplitude of the peak current (I) every 30 s in the closed state and 5 s in the open state, whereas in the desensitized state the values of current were measured from the peak (I) to the steady state (I) every 2 s. The values were normalized (I/Imax) to the peak current of control (Imax) at time zero. (E) State-dependent modification in closed (solid triangle), open (open triangle), and desensitized (solid circle) states for mutant channel T355C in the absence of 2.5 mM MTSET. The values of current were measured as in B. (G) Comparison of mutant channels T355C and T355C+C314S in desensitizing state in the absence (black open circle and square) and in the presence (black solid circle and square) of 5 mM DTT applied to bath solution. Symbols represent the amplitude of the peak current (I) measured every 30 s for both mutant channels and normalized (I/Imax) to peak current of control (Imax) at time zero.
The results presented in Figs. 2 and 3 (A and B) show that desensitization in mutant channel E363A is associated with molecular rearrangements occurring in the outer vestibule during which residues in positions 364 and 366 become more accessible to reagents added to the extracellular medium, similarly to what occurs in K+ Shaker channels during C-type inactivation. These results suggest the structural equivalence of amino acids 364–366 in CNGA1 channels with amino acids 448–450 of Shaker K+ channels, rather than with amino acids 447–449, as suggested by the alignment of the relative sequences (Fig. 1 F).
To study changes of accessibility in the outer vestibule during desensitization of mutant channel L356A, we also constructed the double-mutant channels L356A+T364C and L356A+P366C. Unfortunately, both mutant channels did not express functional channels, and for this reason we could not verify whether the mutant channel L356A had changes of accessibility in the outer vestibule similar to those observed for mutant channel E363A.
The mutant channel T355A desensitizes (Fig. 1 C) and recovers from desensitization after cGMP removal (Fig. 3 C). In contrast, the mutant channel T355C desensitizes, but it does not recover from desensitization (Fig. 3 D). In fact, after exposure to 1 mM cGMP for 3 min, the maximal cGMP-activated current declined to 14 ± 3.4% (n = 8) from the original level and could not be resumed by several minutes of exposure to a solution without cGMP. The cGMP-activated current was halved in ∼60 s (49.2 ± 12%; n = 3) when the channel was kept in the closed state, but in 10 s (48.0 ± 3%; n = 3) and 3 s (39 ± 10%, n = 3) in the open and desensitized states, respectively, as shown in Fig. 3 E.
The irreversible decline of the cGMP-activated current of mutant channel T355C could be caused by the formation of disulfide bonds between the sulfur atoms of exogenous cysteines or by a cross-linkage between exogenous and endogenous cysteines. The CNGA1 channel contains seven native cysteines: Cys35, Cys169, Cys186, Cys314, Cys481, Cys505, and Cys573. Cys35 is located at the cytoplasmic side near the N terminal (Molday et al., 1991; Brown et al., 1998), and there are experimental evidences indicating that Cys35 interacts with Cys481 in the open state, but not in the closed state (Gordon et al., 1997; Rosenbaum and Gordon, 2002). Cys169 and Cys186 are located in the presumed S1 helix and therefore are expected to be far from the pore region. Cys481 is located in the C-linker region (Brown et al., 1998), and its role in channel function has been extensively studied. Modification of Cys481 in the open state with sulfhydryl reagents potentiates CNGA1 channels (Brown et al., 1998). Cys505 in the CNB domain is accessible to sulfhydryl reagents in the closed state, but not in the open state (Matulef et al., 1999). Cys573 is located near the C terminus of the CNGA1 channel. The only endogenous cysteine likely to be located near the pore region is Cys314 in the S5 transmembrane segment, and it is known to interact with residues at position 380 in the S6 domain (Nair et al., 2006). Consequently, we constructed the double-mutant channel T355C+C314S and verified that the current activated by 1 mM cGMP exhibited an irreversible decline, with a time course very similar to that noted for the single-mutant channel T355C, as shown in Fig. 3 F. Simultaneous application of DTT to the bathing medium did not modify or slow down the irreversible decline of the cGMP-activated current in both mutant channels T355C and T355C+C314S (Fig. 3 G). Similar results were observed when DTT was included in the patch pipette.
Therefore, the irreversible decline of the CNG current in mutant channel T355C—not observed in mutant channel T355A—is ascribed to the formation of disulfide bonds between exogenous cysteines of different subunits initiated by structural rearrangements of the residue in position 355 upon desensitization. The lack of the effect of DTT could be ascribed to a limited accessibility of the chemical environment around residues in position 355.
After the sequence alignment in Fig. 1 F, Thr355 corresponds to Val58 in the recently determined structure of the NaK channel (Shi et al., 2006), whose Cα-Cα distance in distinct subunits is larger than 16 Å. Therefore, Thr355's of CNGA1 channel in different subunits need to be closer to each other than Val58's in NaK channel. This suggests that the filter and the neighboring regions of CNGA1 channels are more flexible than corresponding domains of other known K+ channels.
Recovery from desensitization in double-mutant channels
If desensitization of these mutant channels is associated with the disruption of chemical interactions involving side chains of mutated residues, normal gating should be rescued when new interactions or chemical bonds are established between the same two residues. In particular, if desensitization in mutant channel L356A is caused by the disruption of the presumed hydrophobic interactions with Phe380, normal gating will be restored when the hydrophobic interaction is replaced by another strong chemical interaction between residues in positions 356 and 380.
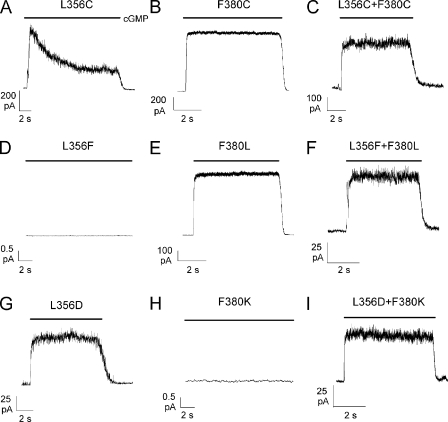
The mutant channel L356C desensitized and the cGMP-activated current declined to 27.0± 3.4% of the original value (n = 6), similar to what was observed with the mutant channel L356A (Fig. 4 A). The mutant channel F380C can be locked either in the open or closed state in the presence of oxidizing agents (Nair et al., 2006), but in their absence it has normal gating and does not desensitize. The double-mutant channel L356C+F380C was constructed, and it did not desensitize in the presence of a steady cGMP concentration (Fig. 4 C). However, application of DTT did not modify the observed behavior, and cGMP-activated current also did not desensitize after exposure to DTT for 20 min. The lack of effect of DTT could be explained if disulfide bonds between L356C and F380C are buried in the membrane and are poorly accessible to the bathing medium (not depicted). If Leu356 and Phe380 form a strong hydrophobic interaction necessary for normal gating, non-desensitizing functional channels should be observed in mutant channels where Leu356 and Phe380 were interchanged. The mutant channel L356F did not express (Fig. 4 D), but normal gating was observed in mutant channel F380L (Fig. 4 E). Mutant channel L356F presumably did not express because phenylalanine is much larger than leucine and could not fit properly in the restricted space between the P helix and S6, whereas leucine could substitute for phenylalanine for the formation of the hydrophobic interaction in mutant channel F380L. The double-mutant channel L356F+F380L (Fig. 4 F) expressed and had a normal gating. To confirm the notion that a strong interaction between side chains of residues in positions 356 and 380 is necessary for normal gating, we constructed mutant channels with a negatively charged residue in position 356 and a positively charged residue in position 380. The mutant channel F380K did not express (Fig. 4 H), and the mutant channel L356D (Fig. 4 G) did not desensitize. The double-mutant L356D+F380K (Fig. 4 I) expressed and did not desensitize.
Figure 4.
Recovery from desensitization in double-mutant channels. cGMP-activated currents in the presence of 1 mM cGMP at +60 mV in mutant channels L356C (A), F380C (B), L356C+F380C (C), L356F (D), F380L (E), L356F+F380L (F), L356D (G), F380K (H), and L356D+F380K (I). cGMP was added as indicated by the solid horizontal bars.
These results strongly suggest that the hydrophobic interaction between Leu356 in the P helix and Phe380 in the upper portion of S6 is essential for normal gating and underlies the coupling between the S6 and the P helix. As hydrophobic interactions are rather strong, we hypothesize that these interactions are present both in the open and closed state.
We attempted to obtain a similar experimental validation for the existence of chemical interactions between Thr355 and Glu363, but none of the constructed double-mutant channels, such as T355E+E363T and T355C+E363C, produced functional channels with a cGMP-gated current.
Having established the existence of a hydrophobic interaction between Leu356 and Phe380, we asked whether this interaction was between residues in the same subunit or in neighboring subunits. Hence, we made several tandem constructs: the tandem L356CtandemWT, where an exogenous cysteine is present in position 356 in two opposing subunits, and the tandem construct L356CtandemF380C, where two exogenous cysteines are introduced in position 356 of two opposing subunits and in position 380 of the other two subunits, respectively. These tandem constructs exhibited desensitization (Fig. 5, A and B, middle row), although to a lesser extent than mutant channel L356C; in particular, the cGMP-activated current at +60 mV declined to 79.0 ± 5.5% (n = 5) and to 80.0 ± 4.7% (n = 6) of its original level for the tandem L356CtandemWT and L356CtandemF380C, respectively. In contrast, the tandem construct L356C+F380CtandemWT with the two exogenous cysteines in positions 356 and 380 of the same two opposing subunits did not desensitize either at positive or at negative voltages (Fig. 5 C, middle row). The absence of desensitization in the tandem construct L356C+F380CtandemWT is caused by the presence of the native hydrophobic interaction between Phe380 and Leu356 in two subunits, which is substituted by a disulphide bond in the other two subunits. Under these conditions the coupling between S6 and the pore region necessary for normal gating is operational.
We have previously shown that mutant channel F380C in the presence of the oxidizing agent copper phenanthroline (CuP) could be locked in a specific configuration of the channel (Nair et al., 2006). When CuP is added in the presence of 1 mM cGMP, the mutant channel F380C is locked in the open state, and when CuP is added in the absence of cGMP, the channel is locked in the closed state. Therefore, we verified that none of the constructs, L356CtandemWT, L356CtandemF380C, and L356C+F380CtandemWT, was locked in the open state because the cGMP-activated current disappeared as soon as cGMP was removed from the bathing medium.
As shown in Fig. 4, the mutant channel F380C does not desensitize, whereas mutant channel F380A desensitizes (see Fig. 1). Therefore, we decided to analyze desensitization in tandem constructs made of two desensitizing channels, and we constructed the tandems L356AtandemWT, F380AtandemL356A, and L356A+F380AtandemWT. As shown in Fig. 5 A (bottom row), the construct L356AtandemWT desensitized almost as the simple mutant channel L356A. The construct F380AtandemL356A had a very low expression, and we could measure only single-channel openings, which desensitized very rapidly, usually in ∼8 s (Fig. 5 B, bottom row). Given the small amplitude of the cGMP-activated current, we could not establish whether desensitization was larger in the construct F380AtandemL356A. We could not measure any cGMP-activated current from the construct L356A+F380AtandemWT (Fig. 5 C, bottom row). The lack of functional channels in the construct L356A+F380AtandemWT could be caused by a closed-state inactivation or by the combination of a fast and deep desensitization of these channels.
These results, collectively, indicate that the interaction between residues in positions 356 and 380 occurs in the same subunit.
DISCUSSION
All native CNG channels from photoreceptors and olfactory sensory neurons do not desensitize in the presence of a steady CN concentration either cAMP or cGMP (Torre and Menini, 1994; Kaupp and Seifert, 2002). Similarly, functional channels composed of homomeric CNG channels, such as CNGA1 channels, do not desensitize (Karpen et al., 1988; Craven and Zagotta, 2006). Therefore, CNG channels under normal conditions do not desensitize. Here, we show that when Glu363, Thr355, Leu356, and Phe380 are individually mutated into an alanine, mutant channels desensitize (Fig. 1). The observed desensitization is attributed to the disruption of chemical interactions necessary for the normal operation of channel gating. Our results show that a strong interaction between residues in positions 380 and 356 is necessary for normal gating and that in its absence mutant channels desensitize. Therefore, desensitization in mutant channels L356A and F380A is attributed to the disruption of the hydrophobic interaction between Leu356 and Phe380.
Molecular mechanisms underlying desensitization in mutant channel E363A and likely in the other mutant channels T355A, L356A, and F380A appear to be very similar to those underlying C-type inactivation in K+ channels (Liu et al., 1996) involving a rearrangement of residues in the channel outer vestibule.
Desensitization of mutant channels E363A and T355A
The sequence alignment between CNGA1 and K+ channels, shown in Fig. 1 F, and experimental results (Becchetti et al., 1999; Liu and Siegelbaum, 2000; Contreras et al., 2008) indicate that the selectivity filter of CNGA1 channels is composed of residues from Thr360 to Thr364, with Thr360 and Thr364 facing the intracellular and extracellular vestibule, respectively. Therefore, the gate and the narrowest restriction of the filter are composed of Ile361 and Gly362.
We propose that desensitization of mutant channels E363A and T355A is caused by the disruption of important chemical interactions between Glu363 and Thr355 and/or other nearby charged or polar groups. As shown in Fig.1, the rate of desensitization in the two mutant channels is very similar, and it is possible that under normal conditions Glu363 and Thr355 in the same subunit interact as illustrated in Fig. 6 (A and B). We speculate that under these conditions the filter region loses a structural pillar and, after opening the pore walls, pinches off, leading to the narrowing of the pore as depicted in Fig. 6 B. In this framework, in the presence of cGMP, mutant channels undergo conformational rearrangements (Fig. 6 B) similar to those observed during C-type inactivation, in which residues in positions 364 and 366 become more accessible to reagents added to the extracellular medium.
Figure 6.
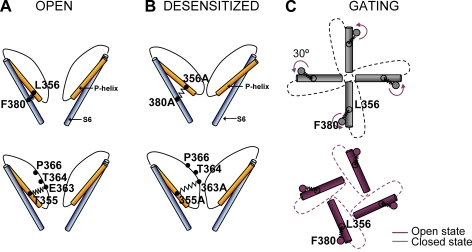
Illustrated representations of hypothesized molecular interactions between S6 and P helix during gating. (A) In the open (and closed) states of the WT CNGA1 channel, the hydrophobic interaction between Phe380 and Leu356 couples S6 and the P helix, and a presumed H-bond between Thr355 and Glu363 couples the P helix and the pore wall. (B) In mutant channels E363A, T355A, L356A, and F380A, these interactions are lost and, after opening, mutant channels desensitize. In mutant channel E363A, residues in positions 364 and 366 become more accessible to the reagents added to the extracellular medium. (C) A simplified model of gating in CNGA1 channels: in the closed state (gray) the tip of P helices occludes the pore lumen; during gating, the S6 helix rotates by 30° anticlockwise, the pore is not occluded (red), and ions can permeate through it. The hydrophobic interaction between Phe380 and Leu356 couples the rotation of S6 to the motion of the P helix.
Comparison of desensitization of mutant channel E363A and C-type inactivation of K+ channels
In Shaker K+ channels during C-type inactivation, three residues, identified as Met448, Thr449, and Pro450, become more accessible to chemical modifications caused by reagents in the extracellular medium (Liu et al., 1996). The Shaker K+ channel M448C mutant undergoes spontaneous disulfide cross-linking in a state-dependent manner, which becomes more prominent in the inactivated state. As shown in Fig. 2, the cGMP-activated current of the desensitizing mutant channel E363A+T364C has a state-dependent rundown very similar to that observed during C-type inactivation in Shaker K+ channel M448C mutant. A similar blockage by extracellular MTSET was observed in CNGA1 channel E363A+P366C mutant (Fig. 3 B) and in Shaker K+ channel P450C mutant.
Molecular rearrangements during C-type inactivation were recently investigated in the KcsA channel (Cordero-Morales et al., 2007), where the KcsA channel E71A mutant has a very reduced and almost absent inactivation. Crystallographic data suggest that upon channel activation, the formation of an H-bond between residues at positions Glu71 and Asp80 promotes a filter constriction along the permeation pathway, modifying K+-binding sites and presumably blocking ion conduction (Cordero-Morales et al., 2007). In contrast, in CNGA1 channels, the presumed interaction between Glu363 and Thr355 is essential for stabilizing the filter both in the closed and open states. These differences provide an explanation for why desensitization (Fig. 6 B) is observed in CNGA1 channel E363A mutant, whereas C-type inactivation is almost abolished in the KcsA channel E71A mutant.
Interactions between S6 and the P helix
The results shown in Figs. 4 and 5 suggest that desensitization in mutant channels L356A and F380A is caused by the abolition of the hydrophobic interactions between Leu356 and Phe380 in the same subunit. This conclusion is supported by the observation of lack of desensitization in the double-mutant channels L356F+F380L, L356C+F380C, and L356D+L380K, where strong molecular interactions are possible between residues in position 356 in the P helix and in position 380 in the upper portion of S6. The hydrophobic interaction between Leu356 and Phe380 provides the molecular coupling between S6 and the P helix (Fig. 6 A). The upper portion of S6 is rich in hydrophobic residues (Val376, Val377, Phe380, Leu381, and Val384), whereas Leu356 in the P helix is mostly flanked by polar residues. Therefore, when Phe380 is mutated, neighboring hydrophobic residues contribute to the coupling with Leu356, whereas in mutant channel L356C this coupling is lost. These observations provide a rationale that explains why desensitization is observed in mutant channel L356C and not in mutant channel F380C (Fig. 4 B). Desensitization in mutant channels L356A and F380A is attributed to the loss of a stabilizing mechanism necessary for normal gating.
A model of gating in CNGA1 channels
The existence of a strong coupling between the upper portion of S6 and the P helix provides a simple molecular mechanism for the gating. Several previous investigations have indicated that an anticlockwise rotation of ∼30–60° of the S6 domain (Johnson and Zagotta, 2001; Nair et al., 2006) occurs as a consequence of CN binding in CNB domains. We propose that this anticlockwise rotation is transmitted to the pore walls by the coupling between S6 and the P helix formed by the hydrophobic interactions between Phe380 and Leu356. As a consequence of these events, P helices rotate, leading to a widening of the pore walls and, therefore, to the channel opening, as illustrated in Fig. 6 C.
Flexibility of CNGA1 channels
Several experimental results (Becchetti et al., 1999; Liu and Siegelbaum, 2000; Contreras et al., 2008) indicate that the filter of CNGA1 channels is shorter than that of K+ channels. As a consequence, the network of interactions stabilizing the pore of CNGA1 channels is less extensive and the filter becomes more flexible. The flexibility of the filter of CNGA1 channels is likely to underlie the poor selectivity of CNG channels (Kaupp and Seifert, 2002; Craven and Zagotta, 2006) and the coupling between permeation and gating observed in these channels (Holmgren, 2003; Gamel and Torre, 2000).
Acknowledgments
We are extremely thankful to Anita Zimmerman for reading and commenting on the manuscript. We thank Manuela Lough for reading the manuscript.
This work was supported by a Human Frontier Science Program grant, a COFIN grant from the Italian Ministry, a grant from CIPE (GRAND FVG), and a FIRB grant from MIUR.
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper: CN, cyclic nucleotide; CNB, CN-binding; CuP, copper phenanthroline; DTT, dithiothreitol; MTSET, 2-(trimethylammonium)ethyl] methanethiosulfonate bromide; WT, wild-type.
References
- Becchetti A., Roncaglia P. 2000. Cyclic nucleotide-gated channels: intra- and extracellular accessibility to Cd2+ of substituted cysteine residues within the P-loop.Pflugers Arch. 440:556–565 [DOI] [PubMed] [Google Scholar]
- Becchetti A., Gamel K., Torre V. 1999. Cyclic nucleotide–gated channels. Pore topology studied through the accessibility of reporter cysteines.J. Gen. Physiol. 114:377–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R.L., Snow S.D., Haley T.L. 1998. Movement of gating machinery during the activation of rod cyclic nucleotide-gated channels.Biophys. J. 75:825–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucossi G., Eismann E., Sesti F., Nizzari M., Seri M., Kaupp U.B., Torre V. 1996. Time-dependent current decline in cyclic GMP-gated bovine channels caused by point mutations in the pore region expressed in Xenopus oocytes.J. Physiol. 493:409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrapani S., Cordero-Morales J.F., Perozo E. 2007. A quantitative description of KcsA gating I: macroscopic currents.J. Gen. Physiol. 130:465–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras J.E., Srikumar D., Holmgren M. 2008. Gating at the selectivity filter in cyclic nucleotide-gated channels.Proc. Natl. Acad. Sci. USA. 105:3310–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Morales J.F., Cuello L.G., Perozo E. 2006a. Voltage-dependent gating at the KcsA selectivity filter.Nat. Struct. Mol. Biol. 13:319–322 [DOI] [PubMed] [Google Scholar]
- Cordero-Morales J.F., Cuello L.G., Zhao Y., Jogini V., Cortes D.M., Roux B., Perozo E. 2006b. Molecular determinants of gating at the potassium-channel selectivity filter.Nat. Struct. Mol. Biol. 13:311–318 [DOI] [PubMed] [Google Scholar]
- Cordero-Morales J.F., Jogini V., Lewis A., Vasquez V., Cortes D.M., Roux B., Perozo E. 2007. Molecular driving forces determining potassium channel slow inactivation.Nat. Struct. Mol. Biol. 14:1062–1069 [DOI] [PubMed] [Google Scholar]
- Craven K.B., Zagotta W.N. 2006. CNG and HCN channels: two peas, one pod.Annu. Rev. Physiol. 68:375–401 [DOI] [PubMed] [Google Scholar]
- Doyle D.A., Morais C.J., Pfuetzner R.A., Kuo A., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity.Science. 280:69–77 [DOI] [PubMed] [Google Scholar]
- Fesenko E.E., Kolesnikov S.S., Lyubarsky A.L. 1985. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment.Nature. 313:310–313 [DOI] [PubMed] [Google Scholar]
- Flynn G.E., Zagotta W.N. 2003. A cysteine scan of the inner vestibule of cyclic nucleotide–gated channels reveals architecture and rearrangement of the pore.J. Gen. Physiol. 121:563–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor A.A., Gordon S.E., Zagotta W.N. 1997. Mechanism of tetracaine block of cyclic nucleotide–gated channels.J. Gen. Physiol. 109:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamel K., Torre V. 2000. The interaction of Na+ and K+ in the pore of cyclic nucleotide-gated channels.Biophys. J. 79:2475–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S.E., Varnum M.D., Zagotta W.N. 1997. Direct interaction between amino- and carboxyl-terminal domains of cyclic nucleotide-gated channels.Neuron. 19:431–441 [DOI] [PubMed] [Google Scholar]
- Heginbotham L., Abramson T., MacKinnon R. 1992. A functional connection between the pores of distantly related ion channels as revealed by mutant K+ channels.Science. 258:1152–1155 [DOI] [PubMed] [Google Scholar]
- Heginbotham L., Lu Z., Abramson T., MacKinnon R. 1994. Mutations in the K+ channel signature sequence.Biophys. J. 66:1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren M. 2003. Influence of permeant ions on gating in cyclic nucleotide–gated channels.J. Gen. Physiol. 121:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.P., Jr., Zagotta W.N. 2001. Rotational movement during cyclic nucleotide-gated channel opening.Nature. 412:917–921 [DOI] [PubMed] [Google Scholar]
- Karpen J.W., Zimmerman A.L., Stryer L., Baylor D.A. 1988. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies.Proc. Natl. Acad. Sci. USA. 85:1287–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp U.B., Seifert R. 2002. Cyclic nucleotide-gated ion channels.Physiol. Rev. 82:769–824 [DOI] [PubMed] [Google Scholar]
- Kaupp U.B., Niidome T., Tanabe T., Terada S., Bonigk W., Stuhmer W., Cook N.J., Kangawa K., Matsuo H., Hirose T. 1989. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel.Nature. 342:762–766 [DOI] [PubMed] [Google Scholar]
- Kurata H.T., Fedida D. 2006. A structural interpretation of voltage-gated potassium channel inactivation.Prog. Biophys. Mol. Biol. 92:185–208 [DOI] [PubMed] [Google Scholar]
- Liu J., Siegelbaum S.A. 2000. Change of pore helix conformational state upon opening of cyclic nucleotide-gated channels.Neuron. 28:899–909 [DOI] [PubMed] [Google Scholar]
- Liu Y., Jurman M.E., Yellen G. 1996. Dynamic rearrangement of the outer mouth of a K+ channel during gating.Neuron. 16:859–867 [DOI] [PubMed] [Google Scholar]
- Long S.B., Campbell E.B., MacKinnon R. 2005a. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel.Science. 309:897–903 [DOI] [PubMed] [Google Scholar]
- Long S.B., Campbell E.B., MacKinnon R. 2005b. Voltage sensor of Kv1.2: structural basis of electromechanical coupling.Science. 309:903–908 [DOI] [PubMed] [Google Scholar]
- Long S.B., Tao X., Campbell E.B., MacKinnon R. 2007. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment.Nature. 450:376–382 [DOI] [PubMed] [Google Scholar]
- Matulef K., Zagotta W.N. 2003. Cyclic nucleotide-gated ion channels.Annu. Rev. Cell Dev. Biol. 19:23–44 [DOI] [PubMed] [Google Scholar]
- Matulef K., Flynn G.E., Zagotta W.N. 1999. Molecular rearrangements in the ligand-binding domain of cyclic nucleotide-gated channels.Neuron. 24:443–452 [DOI] [PubMed] [Google Scholar]
- Mazzolini M., Nair A.V., Torre V. 2008. A comparison of electrophysiological properties of the CNGA1, CNGA1tandem and CNGA1cys-free channels.Eur. Biophys. J. 37:947–959 [DOI] [PubMed] [Google Scholar]
- Molday R.S., Molday L.L., Dose A., Clark-Lewis I., Illing M., Cook N.J., Eismann E., Kaupp U.B. 1991. The cGMP-gated channel of the rod photoreceptor cell characterization and orientation of the amino terminus.J. Biol. Chem. 266:21917–21922 [PubMed] [Google Scholar]
- Nair A.V., Mazzolini M., Codega P., Giorgetti A., Torre V. 2006. Locking CNGA1 channels in the open and closed state.Biophys. J. 90:3599–3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Gold G.H. 1987. A cyclic nucleotide-gated conductance in olfactory receptor cilia.Nature. 325:442–444 [DOI] [PubMed] [Google Scholar]
- Nizzari M., Sesti F., Giraudo M.T., Virginio C., Cattaneo A., Torre V. 1993. Single-channel properties of cloned cGMP-activated channels from retinal rods.Proc. Biol. Sci. 254:69–74 [DOI] [PubMed] [Google Scholar]
- Rosenbaum T., Gordon S.E. 2002. Dissecting intersubunit contacts in cyclic nucleotide-gated ion channels.Neuron. 33:703–713 [DOI] [PubMed] [Google Scholar]
- Rothberg B.S., Shin K.S., Phale P.S., Yellen G. 2002. Voltage-controlled gating at the intracellular entrance to a hyperpolarization-activated cation channel.J. Gen. Physiol. 119:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti F., Eismann E., Kaupp U.B., Nizzari M., Torre V. 1995. The multi-ion nature of the cGMP-gated channel from vertebrate rods.J. Physiol. 487:17–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N., Ye S., Alam A., Chen L., Jiang Y. 2006. Atomic structure of a Na+- and K+-conducting channel.Nature. 440:570–574 [DOI] [PubMed] [Google Scholar]
- Tempel B.L., Papazian D.M., Schwarz T.L., Jan Y.N., Jan L.Y. 1987. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila.Science. 237:770–775 [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice.Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre V., Menini A. 1994. Selectivity and single-channel properties of the cGMP-activated channel in amphibian retinal rods. Handbook of Membrane Channels: Molecular and Cellular Physiology. Peracchia C., editor Academic Press, San Diego, CA: 345–358 [Google Scholar]
- Zagotta W.N., Siegelbaum S.A. 1996. Structure and function of cyclic nucleotide-gated channels.Annu. Rev. Neurosci. 19:235–263 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Morais-Cabral J.H., Kaufman A., MacKinnon R. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution.Nature. 414:43–48 [DOI] [PubMed] [Google Scholar]
- Zimmerman A.L., Yamanaka G., Eckstein F., Baylor D.A., Stryer L. 1985. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments.Proc. Natl. Acad. Sci. USA. 82:8813–8817 [DOI] [PMC free article] [PubMed] [Google Scholar]