Abstract
Background and Purpose
Results of our recent pilot clinical trial suggest that the efficacy of thrombolytic therapy in acute ischemic stroke may be enhanced by the coadministration of high-dose albumin. Here, we explored the microvascular hemodynamic effects of this combined therapy in a laboratory model of cortical arteriolar thrombosis.
Methods
We studied the cortical microcirculation of physiologically monitored rats in vivo by two-photon laser-scanning microscopy after plasma-labeling with fluorescein-dextran. We induced focal thrombosis in 30- to 50-µm cortical arterioles by laser irradiation and measured arteriolar flow velocity by repeated line-scanning. At 30 minutes post-thrombosis, we treated animals with the thrombolytic agent, reteplase, which was coadministered with either human albumin, 2 g/kg, or with saline control.
Results
Baseline arteriolar flow velocity averaged 3.8±0.7 mm/s, was immediately reduced by thrombosis to 22% to 25% of control values, and remained unchanged before treatment. Subthrombolytic doses of reteplase combined with saline led to a median increase in flow velocity to 37% of control distal to the thrombus (P=nonsignificant versus pretreatment). By contrast, reteplase combined with albumin therapy resulted in a prompt, highly significant increase of median flow velocity to 58% of control levels (P=0.013 versus reteplase+saline), which remained significantly higher than the reteplase+saline group at multiple time-points over the subsequent hour.
Conclusions
The beneficial effect of subthrombolytic doses of reteplase on microvascular hemodynamics distal to a cortical arteriolar thrombosis is markedly enhanced by the coadministration of high-dose albumin therapy; these results have important clinical implications for the management of patients with acute ischemic stroke.
Keywords: albumin, thrombolysis, microcirculation, flow velocity, two-photon microscopy, thrombotic stroke
High-dose human albumin (ALB) therapy is highly neuroprotective in experimental models of focal cerebral ischemia1–3 and has been shown to be safe and to exhibit strong preliminary suggestions of efficacy in a recently completed pilot clinical trial of patients with acute ischemic stroke.4,5 These results have led to a large randomized multicenter phase III trial of this therapy, which is currently in progress—the ALIAS (Albumin in Acute Stroke) trial.6
An important component of albumin’s protective effect is thought to be exerted via intravascular mechanisms, which include hemodilution; salutary interactions with vascular endothelium7; platelet antiaggregatory effects8; antagonism of erythrocyte sedimentation9 and neutrophil binding to endothelium10; and local release of bound nitric oxide.11 Using in vivo confocal microscopy in rats with middle cerebral artery occlusion, we showed that ALB reverses postischemic thrombotic phenomena in the venous microcirculation.12 In a recent study using two-photon laser-scanning microscopy in vivo,13 we validated a model of laser-induced focal cortical arteriolar thrombosis in the rat and demonstrated that ALB therapy leads to improved microvascular hemodynamics.
Previous experimental studies have not examined the role of albumin when administered in conjunction with thrombolytic therapy. Positive evidence bearing on this issue, however, emerged from the ALIAS Pilot Clinical Stroke Trial, in which subjects who received thrombolysis (IV tissue plasminogen activator [tPA]) plus high-dose albumin were twice as likely to attain a favorable neurological outcome at 3 months as tPA-treated subjects who received lower-dose albumin therapy.5
In the present report, we have extended our use of the model of laser-induced focal arteriolar thrombosis13 in order to explore the microvascular pathophysiology of albumin therapy when combined with the thrombolytic agent, reteplase. Reteplase (Retavase, PDL BioPharma) is a nonglycosylated recombinant plasminogen activator variant derived from human tissue-type plasminogen activator and having a 4-fold longer half-life than tPA, permitting bolus injection. 14,15 It is cleared from the plasma in a biphasic manner, with a half-life of about 1 minute in the α-phase (28% cleared) and 20 to 28 minutes in the β-phase (72% cleared).16 The findings of the present study show that the addition of albumin to subthrombolytic doses of reteplase results in a robust improvement of microvascular hemodynamics distal to an arteriolar thrombosis.
Materials and Methods
Preparation
Twenty-one male Sprague-Dawley rats (weight 334 to 444 g; Charles River Laboratories, Inc; Wilmington, Mass) were studied. Animals had free access to food and water before the experiments. All study protocols were approved by the Animal Care and Use Committee of the University of Miami, Fla. Animals were anesthetized with isoflurane (3.5% for induction, 1% for maintenance), 65% nitrous oxide and a balance of oxygen. They were orotracheally intubated (14-ga shielded IV catheter, BD Insyte Autoguard), immobilized with pancuronium bromide (0.6 mg/kg IV), and mechanically ventilated. A rectal probe was inserted to measure rectal temperature, which was maintained at 37.0°C with the use of a heating pad. The right femoral artery and vein were catheterized for continuous blood pressure–monitoring, periodic arterial blood gas and plasma glucose measurements, and drug administration. Blood gases were maintained within normal limits by ventilatory adjustments.
After catheterization, the animal was placed in a stereotactic frame and the head was immobilized. A 4×4-mm cranial window (intact dura) was produced over the right or left fronto-parietal cortex as previously described13; its anterior and posterior margins were delimited by the coronal suture and lambdoid suture, respectively, the medial margin by the sagittal suture, and the lateral limit by the curve of the cranium from the horizontal to the vertical plane. The animal and stereotactic frame were then transferred to the stage of the two-photon microscope. The cranial defect was filled with warmed artificial cerebrospinal fluid, and coils placed around the microscope’s objective lens were circulated with warmed water whose temperature was regulated so as to help maintain cranial temperature.13
The two-photon laser-scanning microscopy system consisted of a Coherent Chameleon Ultra system with 690- to 1040-nm tuning range; a Bio-Rad (now Zeiss) Radiance 2100 multiphoton laser-scanning system controlled by LaserSharp2000 software; and an Olympus BX51 WIF upright fixed-stage microscope fitted with high numeric-aperture, long working-distance water-immersion lenses. The details of this system are described in our previous publication.13
Thrombus Induction, Data Acquisition, and Treatment
The cortical microvasculature was imaged after plasma-labeling with fluorescein isothiocyanate-dextran (70 kDa; ≈0.7 mg/kg IV). In each animal, a medium-sized cortical arteriole (30- to 50-µm diameter, subsurface depth 10 to 20 µm) was selected for study as previously descibed.13 The selected arteriole was imaged via z-scans at 1-µm steps, and flow velocity was measured along the vessel’s central longitudinal axis by repetitive line-scanning.13,17
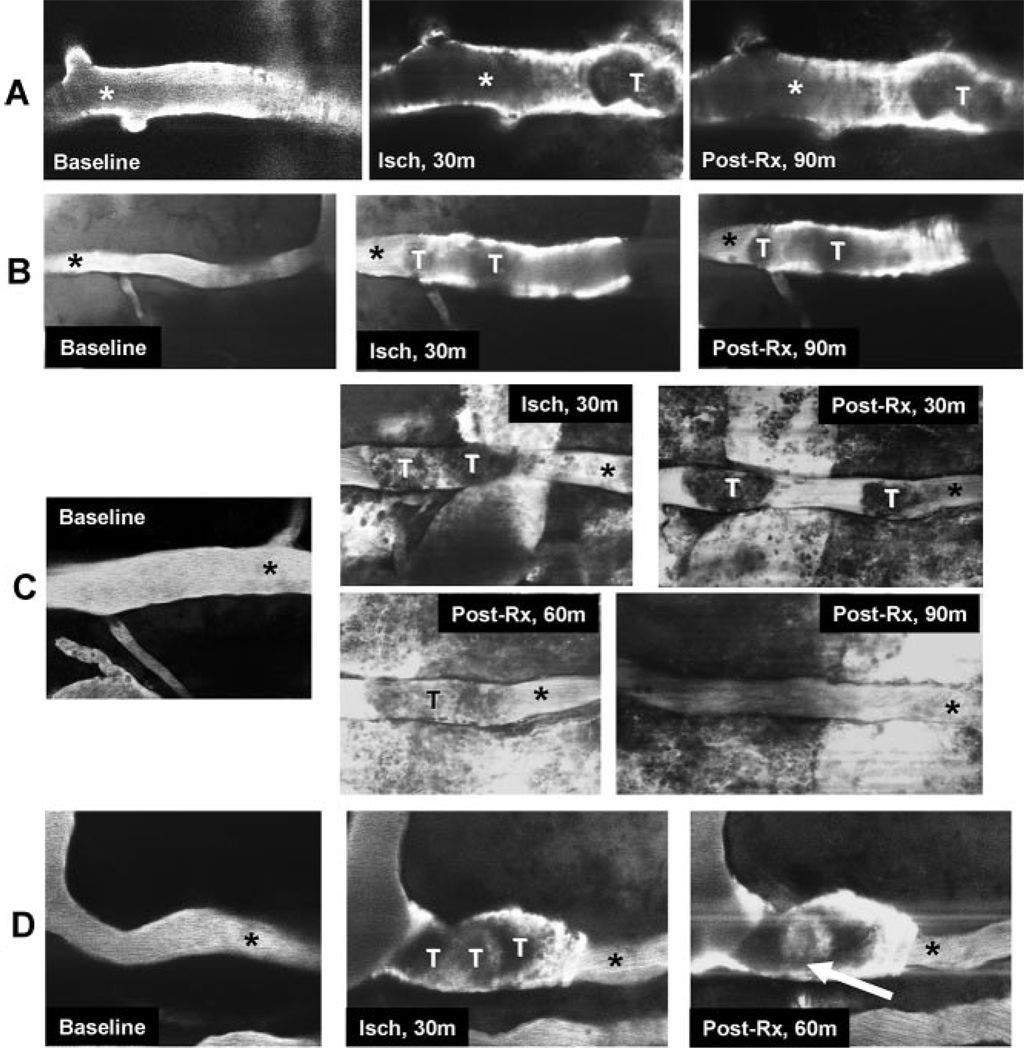
Following baseline measurements, localized arteriolar injury was induced by delivering pulses of laser-irradiation to a ≈35×70-µm rectangular field positioned on the arteriole according to the method of Nishimura et al13,18 (irradiation duration ≈5 minutes; wavelength 800 nm; intensity 264 watts). This method, established in our previous report,13 gives rise to a thrombus occupying the entire luminal cross-section. The onset of thrombosis was signaled by dilatation of the irradiated segment, the emergence of bright fluorescence along the vessel wall, and by a nonfluorescent mass within the irradiated arteriole (Figure 1). The laser pulse energy was held beneath the level needed to induce dye extravasation and vessel rupture.18 If vascular rupture occurred, the animal was not used.
Figure 1.
Representative experiments in which 30- to 50-µm cortical arterioles were visualized by two-photon laser-scanning microscopy after IV administration of FITC-dextran, showing the effects of laser-induced thrombosis and treatment paradigms. Thrombosis is evident as an interruption of the FITC dye column. The thrombosed vascular segments are variably dilated, and there is focal plasma extravasation into the vessel wall, suggesting vascular injury (20× objective). Panel A: Reteplase, 1.84 g/kg, plus ALB. Panel B: Reteplase, 1.84 g/kg, plus saline; the thrombus is bifid. Panel C: Reteplase, 1.84 g/kg, plus ALB. At 30 minutes post-treatment, the thrombus is divided into two segments separated by plasma column; later, there is partial lysis of the thrombus. Panel D: Reteplase, 1.84 g/kg, plus ALB. At 60 minutes post-treatment, incipient separation of the thrombus from the vessel wall is apparent (arrow). Abbreviations: Baseline indicates before thrombosis; Isch, after thrombus induction, before treatment; Post-Rx, after administration of reteplase along with either ALB or saline; T, thrombus. *site of flow-velocity measurement, downstream from thrombus site.
In our previous study,13 we assessed the therapeutic effects of ALB versus saline control, but we did not administer a thrombolytic agent. By contrast, in the present experiment, we wished to determine whether ALB might augment the effect of thrombolytic therapy. Accordingly, at 30 minutes after induction of thrombosis, all rats with successful induction of arteriolar thrombosis were treated with reteplase, 1.84 to 3.68 mg/kg, IV. (Pilot dose-finding studies established that reteplase in this dose-range favorably influenced microvascular hemodynamics but, by itself, was subthrombolytic as judged by direct imaging of the thrombus.) In addition, all animals were randomly allocated to treatment with either 25% human ALB, 2.5 g/kg (Baxter Bioscience), or a comparable volume of isotonic saline. This treatment (IV infusion over 3 minutes) was also begun at 30 minutes after vascular injury. In the reteplase+ALB group, the reteplase dose was 1.84 g/kg in n=10 rats, 2.76 g/kg in 1 rat, and 3.68 g/kg in 1 rat. In the reteplase+saline group, the reteplase dose was 1.84 g/kg in n=7 rats and 3.68 g/kg in n=2 rats.
Microvascular flow velocity in the arteriolar segment immediately downstream to thrombosis was determined in all rats by successive line-scans beginning 10 minutes before injury and at 5-minute intervals throughout the subsequent 120 minutes.13 Z-scan images of the arteriole and its injury-site were obtained immediately before injury, at 30 minutes postinjury, and at 30, 60, and 90 minutes after treatment.
Statistical Analysis
Comparison of flow velocities in the 2 treatment groups was conducted by two-way repeated-measures ANOVA. In this analysis, a weighting factor was introduced to adjust for the unequal numbers of rats receiving lower-dose (1.84 mg/kg) reteplase in the two treatment groups. Differences at P<0.05 were considered significant.
Results
Physiological Variables
These are shown in the Table; there were no differences between groups for any physiological variable except for the expected decline in hematocrit (on average, by 25.5%) due to ALB-induced hemodilution.1,2 Temperatures measured in the artificial–cerebrospinal fluid pool superficial to the craniotomy averaged 36.5±0.9°C.
Table 1.
Physiological Variables
| Reteplase+Albumin, n=8–12 |
Reteplase+Saline, n=6–9 |
|
|---|---|---|
| Baseline | ||
| MAP, mm Hg | 114±12 | 107±22 |
| PO2, mmHg | 100±9 | 97±12 |
| PCO2, mmHg | 38.2±2.9 | 39.5±2.8 |
| pH, units | 7.50±0.03 | 7.48±0.04 |
| Glucose, mg/dL | 204±43 | 203±45 |
| Hematocrit, % | 46±2 | 46±2 |
| Rectal temperature, °C | 36.7±0.3 | 36.8±0.2 |
| 30 minutes post-thrombosis | ||
| MAP, mm Hg | 105±14 | 98±14 |
| PO2, mmHg | 124±14 | 126±12 |
| PCO2, mmHg | 36.9±2.6 | 37.6±2.8 |
| pH, units | 7.48±0.04 | 7.45±0.05 |
| Glucose, mg/dL | 205±50 | 215±46 |
| Hematocrit, % | 46±3 | 46±2 |
| Rectal temperature, °C | 36.9±0.3 | 36.9±0.4 |
| End of post-Rx period | ||
| MAP, mm Hg | 100±13 | 94±9 |
| PO2, mmHg | 126±20 | 120±14 |
| PCO2, mmHg | 37.8±1.8 | 38.8±2.4 |
| pH, units | 7.46±0.04 | 7.44±0.03 |
| Glucose, mg/dL | 193±28 | 210±27 |
| Hematocrit, % | 35±2* | 47±2 |
| Rectal temperature, °C | 36.9±0.2 | 36.9±0.4 |
MAP indicates mean arterial pressure.
Values are mean±SD.
P<0.0001, Student t test.
Microvascular Diameter
Baseline arteriolar diameter for the series was 31.7±9.0 µm (mean±SD). Successful thrombus induction was signaled by dark focal interruptions of intravascular FITC fluorescence and bright fluorescence accumulations in the overlying walls of the immediately distal arteriole (Figure 1). The diameter of the thrombosed arteriole increased to 40.8±19.1 µm (mean % increase, 34%) when measured 30 minutes post-thrombosis and remained unchanged throughout the post-treatment period; at 90 minutes post-treatment, mean diameter was 39.4±8.4 µm. At no time did arteriolar diameter differ by treatment group (P=nonsignificant, Student t test).
Arteriolar Flow-Velocity Measurements
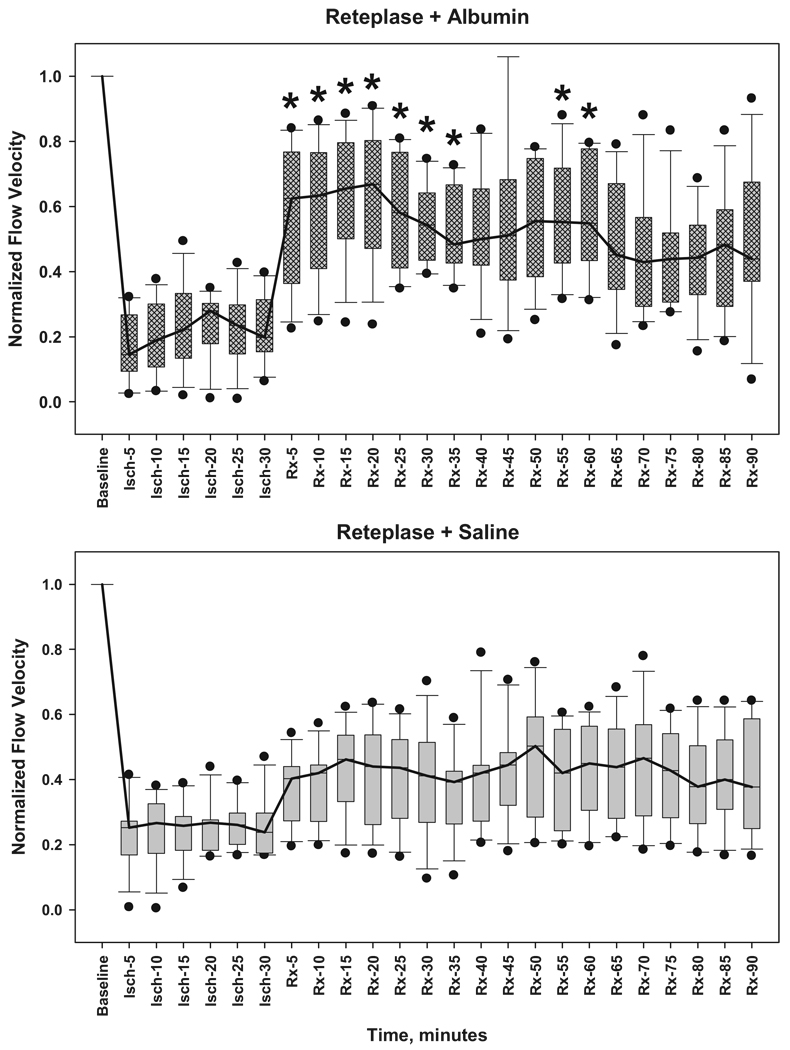
At baseline before induction of thrombosis, mean arteriolar flow velocity was similar in the reteplase+ALB and reteplase+saline groups: ALB, 3.8±0.7 mm/s (mean±SD, n=12); saline, 3.5±0.4 mm/s (n=9). Subsequent flow-velocity measurements in each rat were normalized to that animal’s baseline value. After induction of arteriolar thrombosis, median flow velocity distal to the thrombus declined immediately (ALB group, 22±2% of control; saline group, 25±1% of control; P=nonsignificant) and remained at these levels before treatment (Figure 2).
Figure 2.
Arteriolar flow velocity, normalized to each animal’s baseline velocity value, measured at baseline, during 30-minute thrombosis before treatment (Isch-5 through -30), and up to 90 minutes after treatment with either 2.5 g/kg of 25% human albumin (Rx-5 through -90) (upper panel) or isotonic saline control (lower panel). All animals received reteplase. Bars span the 25th to 75th percentiles; whiskers denote the 10% and 90% percentiles; black dots represent outliers; lines connect median values. Median flow velocity declined to 22% to 25% of control values within 5 minutes of thrombus induction. Reteplase+ALB administration was followed promptly by statistically significant median flow-velocity increments of 52% to 64% of control during the subsequent 60 minutes. By contrast, reteplase+saline led to median increments of 37% to 47%, which did not attain statistical significance. *different from corresponding reteplase+saline value, repeated-measures ANOVA followed by Student-Newman-Keuls comparisons.
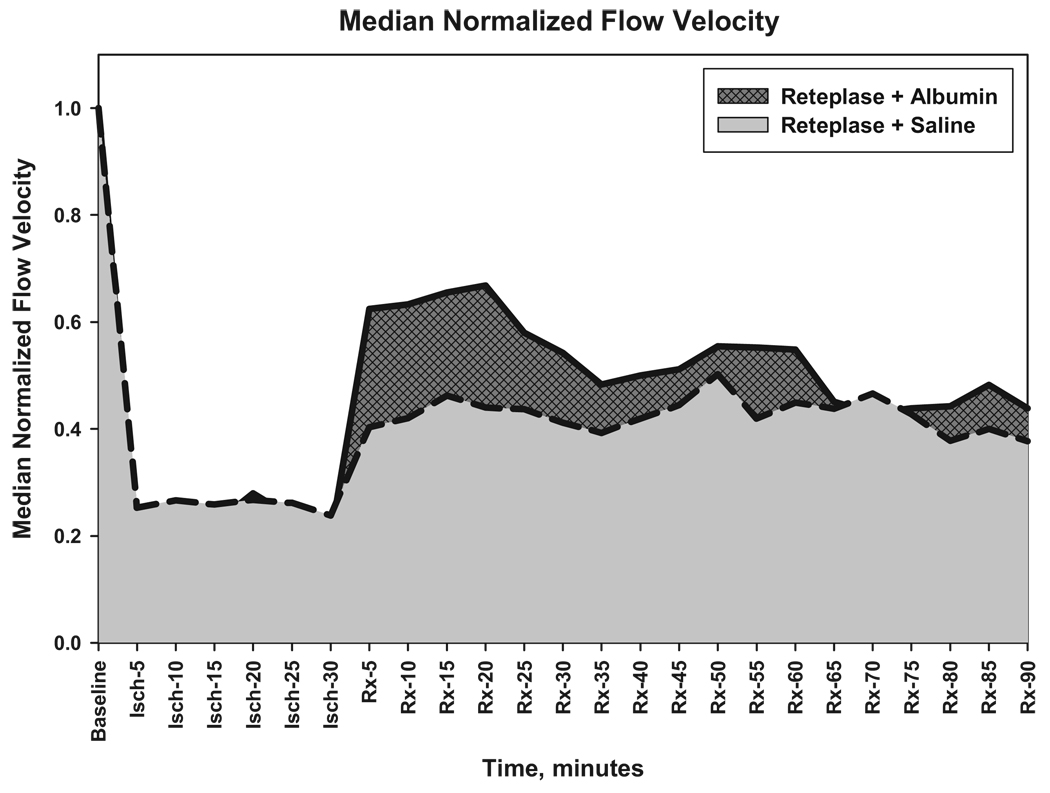
After treatment at 30 minutes of thrombosis, flow velocity responded differently in the two treatment groups. In rats receiving reteplase+ALB, median flow velocity increased promptly to 58% of control whereas animals receiving reteplase+saline exhibited a lesser increase (to 37% of control; Figure 2). Flow-velocities in the reteplase+ALB group exceeded those in the reteplase+saline group at multiple time-points during the first hour post-treatment (Figure 2). Comparison of post-treatment flow-velocity data by two-way repeated-measures ANOVA revealed a highly significant effect of treatment (reteplase+ALB versus reteplase+saline; F(1,22)=7.235, P=0.013) and a significant time×treatment group interaction (P=0.037). The latter effect denotes the greater early flow-velocity improvement observed in the reteplase+ALB group. The incremental increase in arteriolar flow velocity attributable to ALB coadministration is evident from the plot shown in Figure 3.
Figure 3.
Median normalized flow velocities in the reteplase+ALB and reteplase+saline groups, illustrating the increment in flow-velocity conferred by ALB therapy during the first post-treatment hour.
Thrombus Morphology
Consistent with the subthrombolytic reteplase dose-range chosen, frank thrombolysis was rarely observed in this series. By two-photon laser-scanning microscopy, the arteriolar thrombi appeared as irregular masses filling the entire affected lumen, with the adjacent downstream vessel wall showing evidence of fluorescein uptake (Figure 1, panel A). In typical experiments (comprising about half of each treatment group), the thrombus failed to change morphologically during the 90-minute post-treatment observation period (example in Figure 1, panel A), whereas in other cases the thrombus appeared to diminish slightly in size over time. Some thrombi were dumbbell-shaped (example in Figure 1, panel B), and instances were observed in which the thrombus tended over time to divide into separate fragments separated by a plasma column (panel C), or in which the thrombus appeared to separate somewhat from the arterial wall (panel D).
Discussion
The model of laser-induced cortical arteriolar thrombosis used here and validated in our previous study,13 conducted in anesthetized and physiologically regulated animals, results in a consistent subocclusive thrombotic lesion, likely attributable to primary vascular-wall injury which triggers the endogenous clotting cascade.18 This lesion, in turn, gives rise to a high-grade downstream perfusion decrement. In our previous validation study,13 we showed (in the absence of a thrombolytic agent) that high-dose human ALB therapy leads to a prompt, sustained improvement in distal flow velocity; we speculated that ALB may have loosened the thrombus’s texture or the extent of its endothelial attachment. In the present study, we have extended these findings by showing, in rats treated with sublytic doses of the thrombolytic agent, reteplase, that the coadministration of high-dose ALB markedly improves distal microvascular hemodynamics compared to animals receiving equivalent doses of reteplase+saline control (Figure 2 and Figure 3).
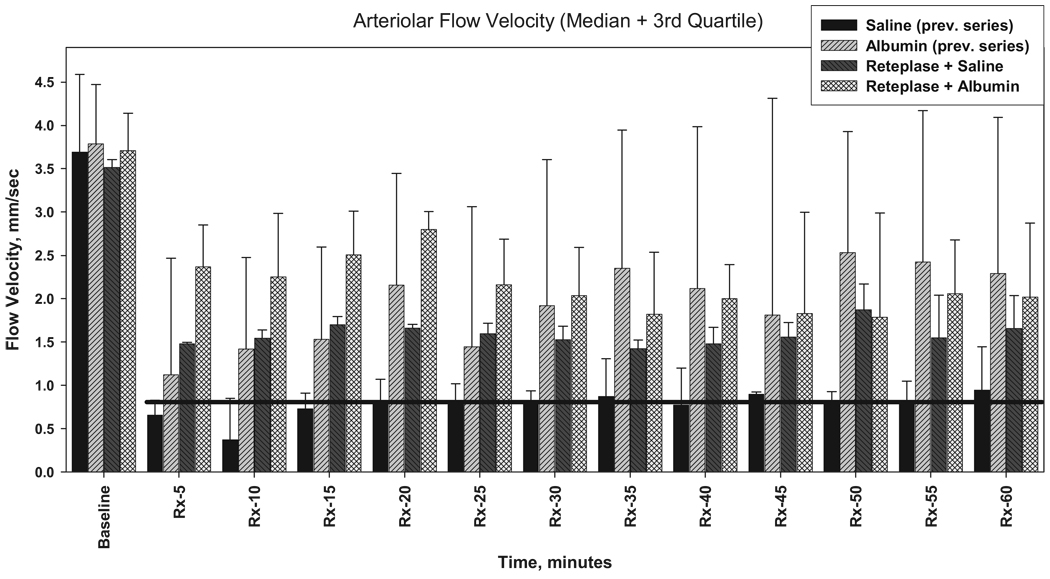
Because our previous experiments13 were not conducted contemporaneously with the present study, statistical comparability of the two sets of results cannot be assured. Nonetheless, it is of interest to view the two data-sets in a single graphic (Figure 4). Remarkably, median baseline flow velocities in all 4 treatment groups are highly similar, attesting to the reproducibility of the model. It is apparent that saline treatment alone fails to improve flow velocity (Figure 4)13 whereas the other 3 treatment groups all exhibit early post-treatment perfusion increments. As demonstrated in the present study, the addition of ALB to subthrombolytic reteplase doses results in a highly significant flow-velocity improvement in the first post-treatment hour (Figure 2 and Figure 3). Interestingly, the side-by-side comparisons of Figure 4 suggest that the effect of reteplase+saline on microvascular perfusion may be generally comparable to that of ALB when administered alone, ie, without reteplase.13
Figure 4.
Arteriolar flow velocities in rats receiving laser-induced arteriolar thrombosis and subsequent treatment paradigms. The groups receiving ALB or saline (without reteplase) are replotted from our previous study.13 The reteplase+ALB and reteplase+saline groups are from the present study. Bars represent median values; whisker represents third quartile. The black horizontal line denotes the median flow velocity in the saline-alone group. Relative to this group, all other treatments are associated with incremental flow-velocity improvements.
The present results lend strong support to the speculative hypothesis arising from the efficacy analysis of our recent ALIAS Pilot Clinical Trial.4,5 In that trial, an open-label dose-escalation study of ALB therapy in 82 patients with acute ischemic stroke, 42 of these subjects received standard-of-care IV tPA therapy according to the National Institute of Neurological Disorders and Stroke (NINDS) guidelines.19 Twenty of these 42 subjects received lower (putatively, subtherapeutic) ALB doses (0.34 to 1.03 g/kg), whereas the other 22 received higher-dose ALB therapy (1.37 to 2.05 g/kg). The likelihood of good clinical outcome at 3 months in the tPA+higher-dose ALB group (ie, the relative benefit) was 2.9-fold greater than in the tPA+lower-dose ALB group (95% CI, 1.3 to 6.5).5 Thus, these pilot-trial results suggested that ALB administration appeared to enhance the efficacy of thrombolytic therapy.5 The present experimental results lend further credence to this possibility. We are currently conducting a large multicenter, randomized, placebo-controlled phase III trial (the ALIAS Phase III Trial), in which the neuroprotective effects of high-dose ALB therapy versus saline placebo are being separately evaluated in thrombolytic and nonthrombolytic cohorts (target enrollment, n=900 in each cohort).6 This design is expected to shed light on the hypothesis that thrombolytic therapy is enhanced by ALB coadministration.
Although IV tPA has been shown to be beneficial19,20 and is accepted as a standard of care for hyperacute ischemic stroke, it is not a panacea. In the survey of Alexandrov and Grotta21 of consecutive stroke patients with an M1 or M2 middle cerebral artery occlusion treated with IV tPA within 3 hours, transcranial Doppler–monitoring over the first 2 hours showed complete recanalization in only 30%, partial recanalization in 48%, and none in 22%. Reocclusion occurred in 34% of patients with any degree of initial recanalization and accounted for two-thirds of deterioration after initial improvement. 21 Thus, clinical deterioration after successful thrombolysis is not only attributable to hemorrhagic transformation (which occurs in only ≈6% to 7% of patients) but also appears to be related to continued vascular occlusive events in large and small vessels.21,22 Small vessels—arterioles, capillaries and venules—are particularly vunerable to injury after ischemia.12 Microvascular endothelial cells appear to be a target site for inflammatory cells and mediators to initiate thrombosis and prevent normalization of tissue perfusion. These observations underscore the need for adjunctive therapeutic approaches to enhance the benefit of thrombolysis.
In summary, the present study supports the view that albumin coadministration confers additional benefit in the context of thrombolytic therapy for acute ischemic stroke. Possible mechanisms include the enhancing of perfusion in relation to subocclusive microvascular lesions, maintaining vascular patency after successful thrombolysis, or preventing post-thrombolytic reocclusion. These possibilities are deserving of continued study.
Acknowledgments
PDL BioPharma kindly supplied us with Retavase (reteplase).
Sources of Funding
This study was supported by National Institutes of Health (NIH) grants NS46295 and NS05820.
Footnotes
Disclosures
None.
References
- 1.Belayev L, Busto R, Zhao W, Clemens JA, Ginsberg MD. Effect of delayed albumin hemodilution on infarction volume and brain edema after transient middle cerebral artery occlusion in rats. J Neurosurg. 1997;87:595–601. doi: 10.3171/jns.1997.87.4.0595. [DOI] [PubMed] [Google Scholar]
- 2.Belayev L, Zhao W, Pattany PM, Weaver RG, Huh PW, Lin B, Busto R, Ginsberg MD. Diffusion-weighted magnetic resonance imaging confirms marked neuroprotective efficacy of albumin therapy in focal cerebral ischemia. Stroke. 1998;29:2587–2599. doi: 10.1161/01.str.29.12.2587. [DOI] [PubMed] [Google Scholar]
- 3.Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32:553–560. doi: 10.1161/01.str.32.2.553. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg MD, Hill MD, Palesch YY, Ryckborst KJ, Tamariz D. The ALIAS Pilot Trial: a dose-escalation and safety study of albumin therapy for acute ischemic stroke. I. Physiological responses and safety results. Stroke. 2006;37:2100–2106. doi: 10.1161/01.STR.0000231388.72646.05. [DOI] [PubMed] [Google Scholar]
- 5.Palesch YY, Hill MD, Ryckborst KJ, Tamariz D, Ginsberg MD. The ALIAS Pilot Trial: a dose-escalation and safety study of albumin therapy for acute ischemic stroke. II. Neurological outcome and efficacy-analysis. Stroke. 2006;37:2107–2114. doi: 10.1161/01.STR.0000231389.34701.b5. [DOI] [PubMed] [Google Scholar]
- 6.Ginsberg MD, Palesch YY, Hill MD. The ALIAS (ALbumin In Acute Stroke) Phase III randomized multicentre clinical trial: design and progress report. Biochem Soc Trans. 2006;34:1323–1326. doi: 10.1042/BST0341323. [DOI] [PubMed] [Google Scholar]
- 7.Schnitzer JE, Oh P. Albondin-mediated capillary permeability to albumin: differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J Biol Chem. 1994;269:6072–6082. [PubMed] [Google Scholar]
- 8.Tokumura A, Yoshida J, Maruyama T, Fukuzawa K, Tsukatani H. Platelet aggregation induced by ether-linked phospholipids. 1. Inhibitory actions of bovine serum albumin and structural analogues of platelet activating factor. Thromb Res. 1987;46:51–63. doi: 10.1016/0049-3848(87)90206-4. [DOI] [PubMed] [Google Scholar]
- 9.Reinhart WH, Nagy C. Albumin affects erythrocyte aggregation and sedimentation. Eur J Clin Invest. 1995;25:523–528. doi: 10.1111/j.1365-2362.1995.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 10.Lang JD, Jr, Figueroa M, Chumley P, Aslan M, Hurt J, Tarpey MM, Alvarez B, Radi R, Freeman BA. Albumin and hydroxyethyl starch modulate oxidative inflammatory injury to vascular endothelium. Anesthesiology. 2004;100:51–58. doi: 10.1097/00000542-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Keaney JFJ, Simon DI, Stamler JS, Jaraki O, Scharfstein J, Vita JA, Loscalzo J. NO forms an adduct with serum albumin that has endothelium–derived relaxing factor-like properties. J Clin Invest. 1993;91:1582–1589. doi: 10.1172/JCI116364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belayev L, Pinard E, Nallet H, Seylaz J, Liu Y, Riyamongkol P, Zhao W, Busto R, Ginsberg MD. Albumin therapy of transient focal cerebral ischemia: in vivo analysis of dynamic microvascular responses. Stroke. 2002;33:1077–1084. doi: 10.1161/hs0402.105555. [DOI] [PubMed] [Google Scholar]
- 13.Nimmagadda A, Park H-P, Prado R, Ginsberg MD. Albumin therapy improves local vascular dynamics in a rat model of primary microvascular thrombosis: a two-photon laser-scanning microscopy study. Stroke. 2008;39:198–204. doi: 10.1161/STROKEAHA.107.495598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganguly K, Krasik T, Medinilla S, Bdeir K, Cines DB, Muzykantov VR, Murciano JC. Blood clearance and activity of erythrocyte-coupled fibrinolytics. J Pharmacol Exp Ther. 2005;312:1106–1113. doi: 10.1124/jpet.104.075770. [DOI] [PubMed] [Google Scholar]
- 15.Martin U, Kaufmann B, Neugebauer G. Current clinical use of reteplase for thrombolysis: a pharmacokinetic-pharmacodynamic perspective. Clin Pharmacokinet. 1999;36:265–276. doi: 10.2165/00003088-199936040-00002. [DOI] [PubMed] [Google Scholar]
- 16.Kuiper J, van de BH, Martin U, van Berkel TJ. Uptake, internalization and degradation of the novel plasminogen activator reteplase (BM 06.022) in the rat. Thromb Haemost. 1995;74:1501–1510. [PubMed] [Google Scholar]
- 17.Kleinfeld D, Mitra PP, Helmchen F, Denk W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci U S A. 1998;95:15741–15746. doi: 10.1073/pnas.95.26.15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimura N, Schaffer CB, Friedman B, Tsai PS, Lyden PD, Kleinfeld D. Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: three models of stroke. Nat Methods. 2006;3:99–108. doi: 10.1038/nmeth844. [DOI] [PubMed] [Google Scholar]
- 19.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 20.Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 21.Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59:862–867. doi: 10.1212/wnl.59.6.862. [DOI] [PubMed] [Google Scholar]
- 22.Alexandrov AV, Hall CE, Labiche LA, Wojner AW, Grotta JC. Ischemic stunning of the brain: early recanalization without immediate clinical improvement in acute ischemic stroke. Stroke. 2004;35:449–452. doi: 10.1161/01.STR.0000113737.58014.B4. [DOI] [PubMed] [Google Scholar]