Abstract
Autoimmune-prone nonobese diabetic mice deficient for B7-2 spontaneously develop an autoimmune peripheral neuropathy mediated by inflammatory CD4+ T cells that is reminiscent of Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy. To determine the etiology of this disease, CD4+ T cell hybridomas were generated from inflamed tissue–derived CD4+ T cells. A majority of T cell hybridomas were specific for myelin protein 0 (P0), which was the principal target of autoantibody responses targeting nerve proteins. To determine whether P0-specific T cell responses were sufficient to mediate disease, we generated a novel myelin P0–specific T cell receptor transgenic (POT) mouse. POT T cells were not tolerized or deleted during thymic development and proliferated in response to P0 in vitro. Importantly, when bred onto a recombination activating gene knockout background, POT mice developed a fulminant form of peripheral neuropathy that affected all mice by weaning age and led to their premature death by 3–5 wk of age. This abrupt disease was associated with the production of interferon γ by P0-specific T cells and a lack of CD4+ Foxp3+ regulatory T cells. Collectively, our data suggest that myelin P0 is a major autoantigen in autoimmune peripheral neuropathy.
Guillain-Barré syndrome (GBS) and chronic inflammatory demyelinating polyneuropathy (CIDP) are autoimmune diseases characterized by inflammatory demyelinating lesions of the peripheral nervous system (PNS) that cause devastating neurological deficits and paralysis. Multifocal demyelination associated with perivascular mononuclear cell infiltrates in the PNS is the pathological hallmark of GBS and CIDP, and autoreactive T and B cell responses are believed to be essential in both diseases (1). Defining self-antigens targeted by the autoimmune response can lead to a better understanding of the immunopathology of the disease and can potentially lead to antigen-specific tolerogenic therapies (2); however, progress has been limited by the paucity of animal models. We previously described the first spontaneous model of autoimmune disease of the PNS, called spontaneous autoimmune peripheral polyneuropathy, in autoimmune-prone nonobese diabetic (NOD) mice deficient for the co-stimulatory molecule B7-2 (NOD-B7-2KO mice) or mimicked many pathophysiological characteristics of GBS and CIDP (3). NOD-B7-2KO mice exhibit a progressive and generalized limb paralysis associated with severe demyelination and axonal damage caused by an autoimmune attack of the PNS (3). Inflammatory CD4+ T cells are essential for the development of autoimmune peripheral neuropathy, and NOD-B7-2KO mice deficient for IFN-γ were protected from disease (3, 4). In this report, we examined the antigen specificity of autoreactive T cells infiltrating the nerves of neuropathic mice and found that myelin protein 0 (P0) is a dominant self-antigen recognized by T cells and autoantibodies. We generated a TCR transgenic (TCR-Tg) mouse specific for a P0 epitope and demonstrated that these T cells were sufficient to induce a fulminant form of peripheral neuropathy.
RESULTS AND DISCUSSION
Generation of peripheral nerve–specific T cell hybridomas
We previously demonstrated that CD4+ T cells from neuropathic NOD-B7-2KO mice could transfer disease, suggesting that the site of infiltration and tissue destruction would be enriched in pathogenic autoreactive T cells specific for nerve antigens. CD4+ T cells were isolated from the infiltrated nerves of neuropathic NOD-B7-2KO mice (Fig. 1) and were expanded with anti-CD3 and anti-CD28 mAbs, and IL-2 by 20–100-fold after 2 wk in culture, as previously described (Fig. 1 B) (5).
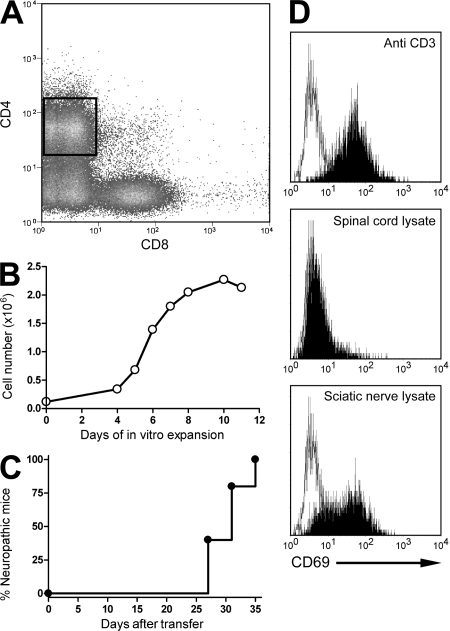
Figure 1.
Generation of peripheral nerve–specific T cell hybridoma. (A and B) CD4+ T cells were sorted from the nerves of neuropathic NOD-B7-2KO mice (A) and expanded in vitro (B). Data are representative of 15 sorts and expansions. (C) 2–5 × 106 expanded cells were transferred into NOD-SCID or NOD-RAGKO immunodeficient mice, and recipients were followed for the development of neuropathy (n = 5 mice from two independent experiments). (D) CD4+ T cell hybridomas were stimulated with APCs alone (open histogram) or in the presence of the indicated stimulus (shaded histograms), and CD69 up-regulation was assessed. Clone 2E7 is shown. Data are representative of four experiments.
Transfer of 2–5 × 106 expanded CD4+ T cells into immunodeficient recipients led to neuropathy within 5 wk (Fig. 1 C and not depicted). Expanded CD4+ T cells from nerves were fused with TCR− BW1100 cells (6) to generate antigen-specific T cell hybridomas. Stable CD4+ TCR+ T cell hybridomas, responsive to anti-CD3 stimulation, were tested further for their ability to be activated by protein lysates prepared from sciatic nerves. A small subset of CD4+ T cell hybridomas (11 out of 56) derived from the infiltrated nerves of neuropathic mice responded to sciatic nerve antigens as measured by up-regulation of CD69 (Fig. 1 D). 1 out of these 11 hybridoma responded to an antigen shared between the PNS and central nervous system (CNS; Table S1, available at http://www.jem.org/cgi/content/full/jem.20082113/DC1), supporting the previous finding that neuropathy in NOD-B7-2KO mice preferentially targeted the PNS without affecting CNS tissues (3). As expected, up-regulation of CD69 on T cell hybridomas in response to nerve lysate was inhibited in the presence of anti–MHC class II blocking mAbs (Fig. S1), confirming that the response of T cell hybridomas to nerve antigens was dependent on recognition of a peptide–MHC class II complex.
Antigen specificity of nerve-derived CD4+ T cell hybridomas
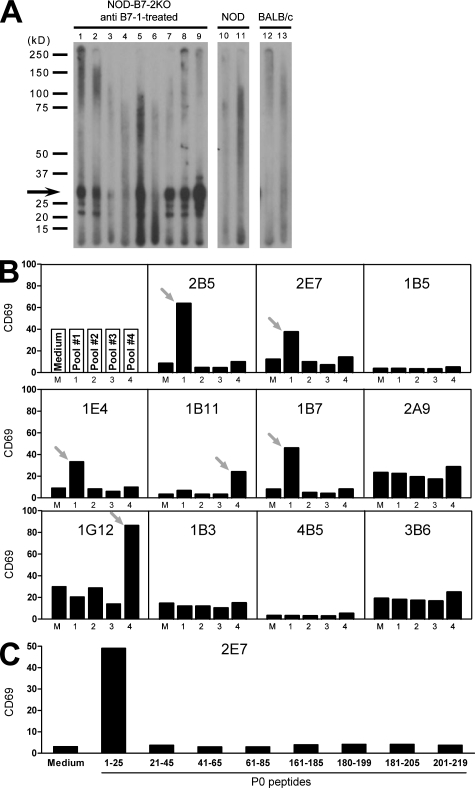
Neural proteins targeted by autoantibodies in NOD-B7-2KO mice were determined to identify potential targets of autoreactive T cells (7, 8). Protein extracts prepared from the sciatic nerve were immunoblotted with sera from neuropathic mice (4). The majority of sera (27 out of 50; 54%) from individual neuropathic T reg cell–depleted NOD-B7-2KO mice reacted strongly against an antigen migrating at ∼25–30 kD (Fig. 2 A). Immunoprecipitation was performed on columns containing protein G agarose coupled to sera from neuropathic animals, followed by peptide mass fingerprinting, and unequivocally identified the 28-kD protein P0, a major component of the myelin sheath expressed exclusively in the PNS but not in the CNS. 6 out of 11 hybridomas that were activated by peripheral nerve lysate up-regulated CD69 in the presence of P0 peptides, suggesting that P0 is a major autoantigen targeted by T cells in neuropathic mice (Fig. 2 B). Of these six P0-specific hybridomas, four recognized P0 peptides in the putatively extracellular portion of the protein (peptide pool #1), whereas the other two hybridomas were specific for P0 peptides located in the cytoplasmic portion of the protein (peptide pool #4; Fig. 2 B). Three out of the four extracellular P0-specific hybridomas were responsive to the same P0 peptide (amino acids 1–25), suggesting the presence of an immunodominant epitope in this portion of the P0 protein (Fig. 2 C and not depicted). We should emphasize that the four hybridomas specific for P0 1–25 were generated from distinct T cell clones, because the TCR Vα and Vβ combination was unique to each hybridoma (Table S1).
Figure 2.
Myelin P0 is targeted by the autoimmune response in NOD-B7-2KO mice. (A) Immunoblotting of sciatic nerve extracts with serum from individual anti–B7-1–treated NOD-B7-2KO neuropathic mice (lanes 1–9), nonneuropathic NOD mice (lanes 10 and 11), or BALB/c controls (lanes 12 and 13). Data are representative of five to nine independent experiments for each serum. The predominant 25–30-kD reactivity is indicated with an arrow. (B) Nerve-specific CD4+ T cell hybridomas were examined for reactivity to P0 as in Fig. 1 D, using pools of overlapping peptides spanning the P0 protein. Data are representative of two experiments. Arrows indicate reactive clones to a specific pool of peptide. (C) Activation of the 2E7 hybridoma by individual P0 peptides from pools #1 and #4. Data are representative of two experiments.
Generation of a myelin P0–specific TCR-Tg (POT) mouse
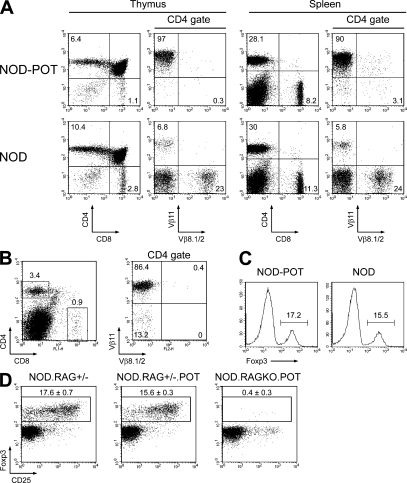
Next, we generated a POT mouse by cloning the rearranged TCR α (Vα9) and β (Vβ11) chains from hybridoma 2E7, which was strongly activated in response to peripheral nerve lysate and P0 immunodominant peptide 1–25 in vitro (Fig. 2 C). The POT mice showed no difference in the total cell numbers of the spleen cells or thymocytes as compared with non-Tg littermates (unpublished data). The percentages of CD4 and CD8 single-positive cells were decreased in the thymus in POT mice as compared with control littermates, and the CD4/CD8 ratio was slightly increased (4.9 ± 0.8% compared with 3.6 ± 0.2%, respectively; P < 0.05; Fig. 3 A, left). Similar data were observed in the spleen (Fig. 3 A, right). Analysis of TCR expression confirmed the expression of the Tg TCR and showed very efficient allelic exclusion in POT mice (Fig. 3 A, right). Indeed, >90% of CD4+ T cells were Vβ11+ in the spleen of POT mice versus <10% in non-Tg littermates (90.6 ± 5.4% in POT mice versus 6.5 ± 0.5% in controls). Conversely, POT splenic CD4+ T cells contained <2% Vβ8+ T cells, whereas Vβ8+ T cells represented >20% of CD4+ T cells in control littermates (1.2 ± 1.3% in POT mice versus 22.8 ± 1.2% in controls). These results suggested that T cells expressing the POT TCR were positively selected to generate a normal population of mature T cells in the periphery, similar to other TCR-Tg mice specific for autoantigens such as islet-specific BDC2.5 (9). Analysis of POT mice, crossed onto a RAGKO background to eliminate the potential influence of endogenous TCR α chains on the thymic selection, confirmed that TCR-Tg T cells were positively selected in the thymus, although the cell numbers were greatly reduced compared with RAG+/− or POT-RAG+/− mice (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20082113/DC1), suggesting a more effective negative selection induced by P0-expressing thymic stromal cells in this setting (10). Although the total spleen cell number was reduced in POT-RAGKO compared with POT mice, the majority of mature T cells from POT-RAGKO mice were CD4+Vβ11+ T cells, with few CD8+Vβ11+ T cells (a mean ratio of 10.6 ± 1.2% versus 3.7 ± 0.3% in POT-RAG+/− mice; Fig. 3 B and Fig. S2). Thus, P0-specific POT TCR-Tg T cells successfully survived thymic selection to generate a population of mature autoreactive T cells in the periphery even on a RAGKO background. Finally, we examined the presence of T reg cells and the activation status of TCR-Tg T cells in POT mice. There was no difference in the expression of CD69, CD62L, and CD44 on CD4+ T cells isolated from POT mice compared with non-Tg littermates (unpublished data), showing that POT T cells were not activated in the periphery. Furthermore, a similar percentage of CD4+ T cells expressed the T reg cell marker Foxp3 in POT mice compared with controls (Fig. 3 C). However, CD4+ Foxp3+ T reg cells could not be detected in the spleen and thymus of POT-RAGKO mice (Fig. 3 D; and Figs. S3 and S4), thus confirming that the thymic selection of T reg cells in POT mice involved TCRs using endogenous α chains.
Figure 3.
Phenotypic analysis of POT TCR-Tg mice. (A) Phenotyping of CD4+ and CD8+ T cells in the thymus (left) and spleen (right) of NOD-POT TCR-Tg mice (top) and transgene-negative littermates (bottom). (B) Phenotyping of CD4+ T cells in the spleens of NOD-RAGKO-POT TCR-Tg mice. (C) Foxp3 expression in the spleens of the indicated 12-wk-old mice (histograms are gated on CD4+ T cells). Numbers indicate percentages of Foxp3+ cells in the CD4 population. (A–C). (D) CD25 and Foxp3 expression among CD4+ cells in the spleens of the indicated 19-d-old mice. Percentages ± SD are indicated. Data are representative of two or more experiments.
Proliferation and cytokine production of P0-specific TCR-Tg T cells
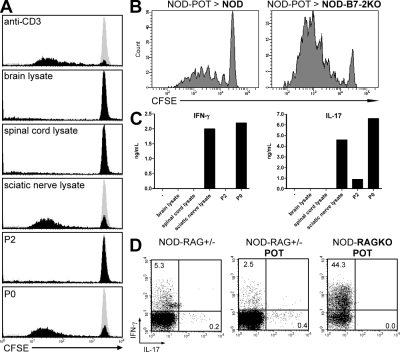
POT CD4+ T cells proliferated strongly in response to protein extracts prepared from sciatic nerves but not spinal cord or brain, confirming their strict PNS specificity (Fig. 4 A). Furthermore, POT T cells displayed a similarly strong proliferation when cultured with P0 protein (including the P0 peptide 1–25) but not with another myelin protein (P2), confirming the P0 specificity and demonstrating that the T cells are fully functional and not anergic in the periphery. We examined the capacity of activation of PNS-specific autoreactive T cells to proliferate in NOD (resistant) versus NOD-B7-2KO (susceptible) mice. POT T cells proliferated extensively in several lymph nodes in NOD-B7-2KO but not NOD recipients, including brachial, axillary, and inguinal lymph nodes (Fig. 4 B; and Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20082113/DC1), illustrating a clear dichotomy between NOD and NOD-B7-2KO mice, as the development of diabetes versus neuropathy directly correlates with the ability of islet versus nerve antigen-specific autoreactive T cells to proliferate in relevant lymph nodes (Fig. 4 B) (11). Finally, we detected high levels of IFN-γ and IL-17 in the culture supernatant of POT T cells upon in vitro stimulation in the absence of any skewing culture conditions (Fig. 4 C). Surprisingly, intracellular cytokine staining revealed that 40–50% of T cells isolated from POT-RAGKO mice produced IFN-γ but no IL-17 ex vivo, whereas only 2.5% of POT T cells on a RAG-sufficient background produced IFN-γ and <1% produced IL-17, similar to what was observed in NOD littermates (Fig. 4 D). This result suggested that cytokine production observed in a RAG-sufficient background was influenced by endogenous TCR α chains and/or regulatory cell populations that could limit the activation and differentiation of autoreactive POT T cells.
Figure 4.
Proliferation and cytokine production of P0-specific NOD-POT T cells. (A) Proliferation of CFSE-labeled NOD-POT T cells in the presence of APCs alone (gray histogram) or APCs and the indicated stimulus (black histograms). Histograms are gated on the CD4+ population. (B) Proliferation of CFSE-labeled NOD-POT spleen cells in NOD or NOD-B7-2KO recipients (22 wk of age) after 4 d (inguinal lymph nodes are shown). Histograms are gated on the Thy1.1+CD4+ population. (C) Production of IFN-γ (left) and IL-17 (right) in the culture supernatant of NOD-POT cells activated by the indicated stimulus. (D) Production of IFN-γ and IL-17 after stimulation of spleen cells from the indicated mice with PMA and ionomycin. Dot plots are gated on CD4+ T cells. Numbers indicate percentages of cells in the quadrants. Data are representative of two or more experiments.
Pathogenicity of P0-specific TCR-Tg T cells in vivo
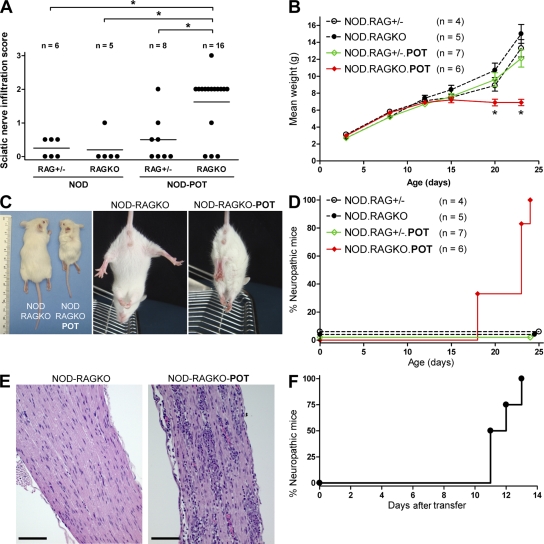
Although mononuclear infiltration could be detected in the nerves of some POT mice as early as 3 wk of age (Fig. 5 A), many POT mice did not show any sign of inflammation in sciatic nerves or clinically detectable peripheral neuropathy by 15–17 wk of age (Fig. 5 A and not depicted). However, adoptive transfer of in vitro–activated or CD25-depleted POT T cells led to peripheral nerve infiltration and induced neuropathy in immunodeficient recipients (unpublished data). These results suggested that the lack of spontaneous neuropathy in POT mice may have been a consequence of increased numbers of antigen-specific Tg+Foxp3+ T reg cells as compared with Tg-negative NOD mice. Thus, we examined T reg cell–deficient POT-RAGKO mice for tissue infiltration and clinical disease (Fig. 3 D; and Figs. S3 and S4). POT-RAGKO mice displayed a dramatic phenotype characterized by early weight loss and premature death by 3–5 wk of age (Fig. 5, B and C; and not depicted), and spontaneously developed a fulminant form of neuropathy that started as early as 18 d and affected all mice by 3–4 wk of age (Fig. 5, C and D). The spontaneous peripheral neuropathy induced by POT T cells led to motor dysfunction indicated by clasping of the limbs and difficulty walking (Fig. 5 C; and Videos 1–3, available at http://www.jem.org/cgi/content/full/jem.20082113/DC1), similar to what we originally observed in NOD-B7-2KO mice (3). Development of neuropathy was accompanied by a heavy infiltrate in the sciatic nerves of NOD-RAGKO-POT mice (Fig. 5, A and E), as well as myelin destruction (Fig. S6). The infiltration in the sciatic nerves of NOD-RAGKO-POT mice was strikingly higher than in their RAG-sufficient counterparts at 3–4 wk of age (P = 0.01 using a Mann-Whitney U test; Fig. 5 A), correlating with clinical disease. Indeed, adoptive transfer of 1.3 × 106 splenocytes from a 25-d-old neuropathic NOD-RAGKO-POT mouse induced severe neuropathy in all NOD-SCID recipients in <2 wk (Fig. 5 F). Finally, the peripheral neuropathy induced by P0-specific POT TCR-Tg T cells was extremely severe and led to death of the animals, usually within 24 h after clinical disease onset, either spontaneously in NOD-RAGKO-POT mice or after adoptive transfer of T cells from NOD-RAGKO-POT or NOD-POT mice (unpublished data).
Figure 5.
Spontaneous development of a fulminant form of neuropathy in NOD-RAGKO-POT mice. (A) Quantification of infiltration in the nerves of indicated mice at 3–4 wk of age. Horizontal bars indicate means. *, P < 0.05. (B) Weight in the indicated mice as a function of age. Results represent cumulated data from two different experiments. Data are means ± SEM. (C) Clasping phenotype in a representative NOD-RAGKO-POT mouse compared with the normal plantar reaction in a NOD-RAGKO littermate, and weight deficit in NOD-RAGKO-POT mice at 3–4 wk of age. (D) Neuropathy in the indicated mice as a function of age. (E) Intense infiltrate in the sciatic nerve of NOD-RAGKO-POT mice. Bars, 250 µm. (F) Adoptive transfer of neuropathy by spleen cells from NOD-RAGKO-POT mice into NOD-SCID recipients (n = 4). Data are representative of two independent transfers. Videos 1–3 are available at http://www.jem.org/cgi/content/full/jem.20082113/DC1.
Collectively, our data demonstrate that myelin P0 is a major autoantigen targeted by autoantibodies and autoreactive T cells in a spontaneous mouse model of peripheral neuropathy. Importantly, the development of an abrupt form of disease in NOD-RAGKO-POT mice reveals that P0-specific CD4+ T cells were sufficient to induce catastrophic damage in the PNS and suggests that P0-specific T cells could play a central role in inducing tissue destruction in autoimmune neuropathy. P0 is a major component of the PNS that represents >50% of the peripheral myelin protein content. Experimental autoimmune neuritis can be induced in rats and mice by immunization with P0 protein or peptides in adjuvant or by adoptive transfer of P0-specific T cell lines (1). However, these models involve active immunization and powerful adjuvants, which does not mirror the spontaneous disease in individuals genetically prone to autoimmunity. Reports of immune responses to P0 in GBS and CIDP patients have generated conflicting results that could be explained by different techniques and patient populations. Nevertheless, of all neural proteins tested, P0 has been the most consistent reactivity detected in the serum of patients with active GBS or CIDP (12–14). Khalili-Shirazi et al. initially reported proliferative responses to P0 protein or peptides in 9 out of 19 GBS patients (15). More recently, P0-specific T cell responses measured by proliferation or ELISPOT were not statistically different in GBS and CIDP patients as compared with nonautoimmune neuropathies (16), whereas another report described P0-specific IL-10 production in GBS patients (17), raising the possibility that immune responses to P0 may be regulatory rather than pathogenic. These findings were reminiscent of the glutamic acid decarboxylase 65-kD isoform (GAD65) autoantigen in type 1 diabetes. Similar to P0, autoantibodies to GAD were readily detected in type 1 diabetes patients and in the NOD mouse (18, 19), and GAD-specific T cells could adoptively transfer diabetes in the NOD mouse model (20). However, GAD-specific TCR-Tg or retrogenic mice revealed that GAD-specific T cells did not induce diabetes but rather exerted a protective effect (21). In contrast, our report of an aggressive form of peripheral neuropathy spontaneously occurring in P0-specific POT TCR-Tg mice strengthens the potential role of P0 as a central autoantigen involved in disease development and tissue damage in autoimmune neuropathy. While this paper was being reviewed, Kim et al. published a report (22) showing that autoimmune neuropathy in NOD-B7-2KO mice could be prevented by tolerance induction using a specific P0 peptide. Although the peptide specificity (P0 180–199) was different from the POT TCR epitope (P0 1–25), the epitope overlapped with two of our peripheral nerve–specific hybridoma clones (Fig. 2 B; and Table S2, available at http://www.jem.org/cgi/content/full/jem.20082113/DC1). In their report, the adoptive transfer of a P0-specific line only led to very mild disease (grade 1). The difference with our results may be because of the specificity differences or their use of a T cell line.
Our results suggest that neuropathy induced by P0-specific POT T cells involves mainly IFN-γ–producing Th1 cells in agreement with a central role of IFN-γ in the development of peripheral neuropathy in NOD-B7-2KO mice (4). Furthermore, elevated serum levels of IFN-γ have been reported in GBS patients, and the percentage of IFN-γ+ CD4+ T cells was markedly increased in the cerebrospinal fluid of CIDP patients (23, 24). Th17 cells have been suggested to play a major role in multiple sclerosis and its animal model experimental autoimmune encephalomyelitis (25). Although increased levels of IL-17 have been described in the spinal fluid of CIDP patients (24), it is still unclear whether Th17 cells are important for autoimmune peripheral neuropathies. We found that low levels of IL-17 were produced by POT T cells on a RAG-sufficient background in vitro. It was recently reported that Th1 and Th17 cells may have different abilities to infiltrate the CNS and cause experimental autoimmune encephalomyelitis depending on the level of local tissue inflammation (26). Thus, our report does not preclude a role for IL-17 in spontaneous autoimmune peripheral polyneuropathy and other PNS autoimmune diseases but suggests that IFN-γ is the dominant cytokine associated with peripheral neuropathy induced by P0-specific POT T cells.
Another important insight revealed by our report is the control of P0-specific T cells and, hence, the development of neuropathy by CD4+ Foxp3+ T reg cells. Indeed, the development of the fulminant form of neuropathy in POT-RAGKO mice was associated with a complete absence of CD4+ Foxp3+ T reg cells, suggesting that antigen-specific T reg cells are key to controlling the disease. Because the percentage of T reg cells has been shown to be lower in the peripheral blood of GBS and CIDP patients (27–29), our findings suggest that immunotherapy using P0-specific T reg cells may be an important future therapeutic option for these patients, as similar treatments are now being developed for several autoimmune diseases (2).
MATERIALS AND METHODS
Mice.
NOD and NOD-SCID mice were purchased from Taconic. NOD-B7-2KO (3), NOD-TCRαKO, and NOD-RAG2KO mice were bred in our facility. Neuropathy was assessed weekly (3). All mice were housed in a specific pathogen-free University of California, San Francisco (UCSF) facility. All animal experiments were approved by the Institutional Animal Care and Use Committee of UCSF.
Peripheral nerve–specific hybridomas.
Cells from sciatic nerves of neuropathic NOD-B7-2KO mice were prepared as previously described (3), stained with CD4-FITC and CD8-allophycocyanin. CD4+ CD8− cells were sorted on a cytometer (MoFlo; Dako) and expanded with anti-CD3– and anti-CD28–coated beads supplemented with 2,000 IU/ml rhIL-2, as previously described (5). The expanded CD4+ T cells were fused to the TCR-negative thymoma partner BW1100 (provided by E. Palmer, University Hospital Basel, Basel, Switzerland) using standard protocols. TCR-expressing hybridoma clones were tested for antigen specificity by incubating 50,000 cells with 0.2 × 106 APCs (NOD-TCRαKO spleen cells, irradiated NOD spleen cells, or NOD BM-derived DCs) with the stimulus indicated in the figures for 16–24 h, followed by staining with CD69-PE. Overlapping peptides spanning the sequence of human P0 (which is 94% homologous to mouse P0 at the amino-acid level) were provided by N. Gregson (King's College London, London, England, UK; Table S2).
Generation of POT TCR-Tg mice.
The full-length coding sequences for the TCR α and β chains were cloned by PCR from 2E7 hybridoma cDNA using the indicated primers (Table S3, available at http://www.jem.org/cgi/content/full/jem.20082113/DC1), and were subcloned into a CD2 and CD4 expression vector, respectively (30), allowing expression of the transgenes in both CD4 and CD8 T cells. Tg mice were generated by microinjection of CD2-TCRα and CD4-TCRβ constructs into NOD embryos. Vectors were provided by N. Killeen (UCSF, San Francisco, CA).
Statistical analysis.
All statistical analyses were performed using an unpaired two-tailed Mann-Whitney U test with Prism software (GraphPad Software, Inc.). P ≤ 0.05 was considered significant.
Online supplemental material.
Table S1 shows the characteristics of nerve-specific hybridomas. Tables S2 and S3 show the sequences of peptides and primers used in this report. Fig. S1 shows the inhibition of hybridoma activation by anti–MHC class II mAbs. Figs. S2–S4 show CD4, CD8, Vβ, CD25, and Foxp3 FACS analysis of thymus and spleen cells from NOD-RAGKO-POT mice. Fig. S5 shows the enhanced proliferation of NOD-POT cells in NOD-B7-2KO mice compared with NOD recipients. Fig. S6 shows sciatic nerve demyelination in NOD-RAGKO-POT mice. Videos 1 and 2 show a grid test to assess neuropathy in a NOD-RAGKO-POT mouse and a NOD-RAGKO littermate, respectively. Video 3 shows the clasping phenotype and weight deficit in a NOD-RAGKO-POT mouse compared with the normal plantar reaction in a NOD-RAGKO littermate. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082113/DC1.
Acknowledgments
The authors wish to thank H. Lu and N. Killeen for the vectors and microinjections, C. Austin for histology, S. Jiang for cell sorting, D. Vignali and A. Burton for discussions, N. Gregson for providing P0 peptides, and A. Weishaupt and R. Gold for providing P0 and P2 recombinant proteins.
This work was supported by National Institutes of Health grant R01 AI50834, a UCSF Research Evaluation and Allocation Committee grant, and a Neuropathy Association scientific research grant.
The authors have no conflicting financial interests.
References
- Kieseier B.C., Kiefer R., Gold R., Hemmer B., Willison H.J., Hartung H.P. 2004. Advances in understanding and treatment of immune-mediated disorders of the peripheral nervous system.Muscle Nerve. 30:131–156 [DOI] [PubMed] [Google Scholar]
- Miller S.D., Turley D.M., Podojil J.R. 2007. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease.Nat. Rev. Immunol. 7:665–677 [DOI] [PubMed] [Google Scholar]
- Salomon B., Rhee L., Bour-Jordan H., Hsin H., Montag A., Soliven B., Arcella J., Girvin A.M., Miller S.D., Bluestone J.A. 2001. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2–deficient NOD mice.J. Exp. Med. 194:677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour-Jordan H., Thompson H.L., Bluestone J.A. 2005. Distinct effector mechanisms in the development of autoimmune neuropathy versus diabetes in nonobese diabetic mice.J. Immunol. 175:5649–5655 [DOI] [PubMed] [Google Scholar]
- Tang Q., Henriksen K.J., Bi M., Finger E.B., Szot G., Ye J., Masteller E.L., McDevitt H., Bonyhadi M., Bluestone J.A. 2004. In vitro–expanded antigen-specific regulatory T cells suppress autoimmune diabetes.J. Exp. Med. 199:1455–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollob K.J., Palmer E. 1991. Physiologic expression of two superantigens in the BDF1 mouse.J. Immunol. 147:2447–2454 [PubMed] [Google Scholar]
- Steinman L. 1996. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system.Cell. 85:299–302 [DOI] [PubMed] [Google Scholar]
- Zhang L., Nakayama M., Eisenbarth G.S. 2008. Insulin as an autoantigen in NOD/human diabetes.Curr. Opin. Immunol. 20:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J.D., Wang B., Haskins K., Benoist C., Mathis D. 1993. Following a diabetogenic T cell from genesis through pathogenesis.Cell. 74:1089–1100 [DOI] [PubMed] [Google Scholar]
- Visan L., Visan I.A., Weishaupt A., Hofstetter H.H., Toyka K.V., Hunig T., Gold R. 2004. Tolerance induction by intrathymic expression of P0.J. Immunol. 172:1364–1370 [DOI] [PubMed] [Google Scholar]
- Bour-Jordan H., Salomon B.L., Thompson H.L., Szot G.L., Bernhard M.R., Bluestone J.A. 2004. Costimulation controls diabetes by altering the balance of pathogenic and regulatory T cells.J. Clin. Invest. 114:979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D., Giannopoulos K., Gray I., Gregson N., Makowska A., Pritchard J., Hughes R.A. 2005. Antibodies to peripheral nerve myelin proteins in chronic inflammatory demyelinating polyradiculoneuropathy.J. Peripher. Nerv. Syst. 10:174–180 [DOI] [PubMed] [Google Scholar]
- Khalili-Shirazi A., Atkinson P., Gregson N., Hughes R.A. 1993. Antibody responses to P0 and P2 myelin proteins in Guillain-Barre syndrome and chronic idiopathic demyelinating polyradiculoneuropathy.J. Neuroimmunol. 46:245–251 [DOI] [PubMed] [Google Scholar]
- Yan W.X., Archelos J.J., Hartung H.P., Pollard J.D. 2001. P0 protein is a target antigen in chronic inflammatory demyelinating polyradiculoneuropathy.Ann. Neurol. 50:286–292 [DOI] [PubMed] [Google Scholar]
- Khalili-Shirazi A., Hughes R.A., Brostoff S.W., Linington C., Gregson N. 1992. T cell responses to myelin proteins in Guillain-Barre syndrome.J. Neurol. Sci. 111:200–203 [DOI] [PubMed] [Google Scholar]
- Csurhes P.A., Sullivan A.A., Green K., Pender M.P., McCombe P.A. 2005. T cell reactivity to P0, P2, PMP-22, and myelin basic protein in patients with Guillain-Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy.J. Neurol. Neurosurg. Psychiatry. 76:1431–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowska A., Pritchard J., Sanvito L., Gregson N., Peakman M., Hayday A., Hughes R. 2008. Immune responses to myelin proteins in Guillain-Barre syndrome.J. Neurol. Neurosurg. Psychiatry. 79:664–671 [DOI] [PubMed] [Google Scholar]
- Tisch R., Yang X.D., Singer S.M., Liblau R.S., Fugger L., McDevitt H.O. 1993. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice.Nature. 366:72–75 [DOI] [PubMed] [Google Scholar]
- Kaufman D.L., Clare S.M., Tian J., Forsthuber T., Ting G.S., Robinson P., Atkinson M.A., Sercarz E.E., Tobin A.J., Lehmann P.V. 1993. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes.Nature. 366:69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekzer D., Wong F.S., Ayalon O., Millet I., Altieri M., Shintani S., Solimena M., Sherwin R.S. 1998. GAD-reactive CD4+ Th1 cells induce diabetes in NOD/SCID mice.J. Clin. Invest. 101:68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton A.R., Vincent E., Arnold P.Y., Lennon G.P., Smeltzer M., Li C.S., Haskins K., Hutton J., Tisch R.M., Sercarz E.E., et al. 2008. On the pathogenicity of autoantigen-specific T-cell receptors.Diabetes. 57:1321–1330 [DOI] [PubMed] [Google Scholar]
- Kim H.J., Jung C.G., Jensen M.A., Dukala D., Soliven B. 2008. Targeting of myelin protein zero in a spontaneous autoimmune polyneuropathy.J. Immunol. 181:8753–8760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohnoki K., Inoue A., Koh C.S. 1998. Elevated serum levels of IFN-gamma, IL-4 and TNF-alpha/unelevated serum levels of IL-10 in patients with demyelinating diseases during the acute stage.J. Neuroimmunol. 87:27–32 [DOI] [PubMed] [Google Scholar]
- Mei F.J., Ishizu T., Murai H., Osoegawa M., Minohara M., Zhang K.N., Kira J. 2005. Th1 shift in CIDP versus Th2 shift in vasculitic neuropathy in CSF.J. Neurol. Sci. 228:75–85 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Korn T., Oukka M., Kuchroo V.K. 2008. Induction and effector functions of T(H)17 cells.Nature. 453:1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor R.A., Prendergast C.T., Sabatos C.A., Lau C.W., Leech M.D., Wraith D.C., Anderton S.M. 2008. Cutting Edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis.J. Immunol. 181:3750–3754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L.J., Wang H.B., Zhang Y., Wang W.Z. 2007. Abnormality of circulating CD4(+)CD25(+) regulatory T cell in patients with Guillain-Barre syndrome.J. Neuroimmunol. 192:206–214 [DOI] [PubMed] [Google Scholar]
- Harness J., McCombe P.A. 2008. Increased levels of activated T-cells and reduced levels of CD4/CD25+ cells in peripheral blood of Guillain-Barre syndrome patients compared to controls.J. Clin. Neurosci. 15:1031–1035 [DOI] [PubMed] [Google Scholar]
- Chi L.J., Wang H.B., Wang W.Z. 2008. Impairment of circulating CD4+CD25+ regulatory T cells in patients with chronic inflammatory demyelinating polyradiculoneuropathy.J. Peripher. Nerv. Syst. 13:54–63 [DOI] [PubMed] [Google Scholar]
- Wang Q., Malherbe L., Zhang D., Zingler K., Glaichenhaus N., Killeen N. 2001. CD4 promotes breadth in the TCR repertoire.J. Immunol. 167:4311–4320 [DOI] [PubMed] [Google Scholar]