Abstract
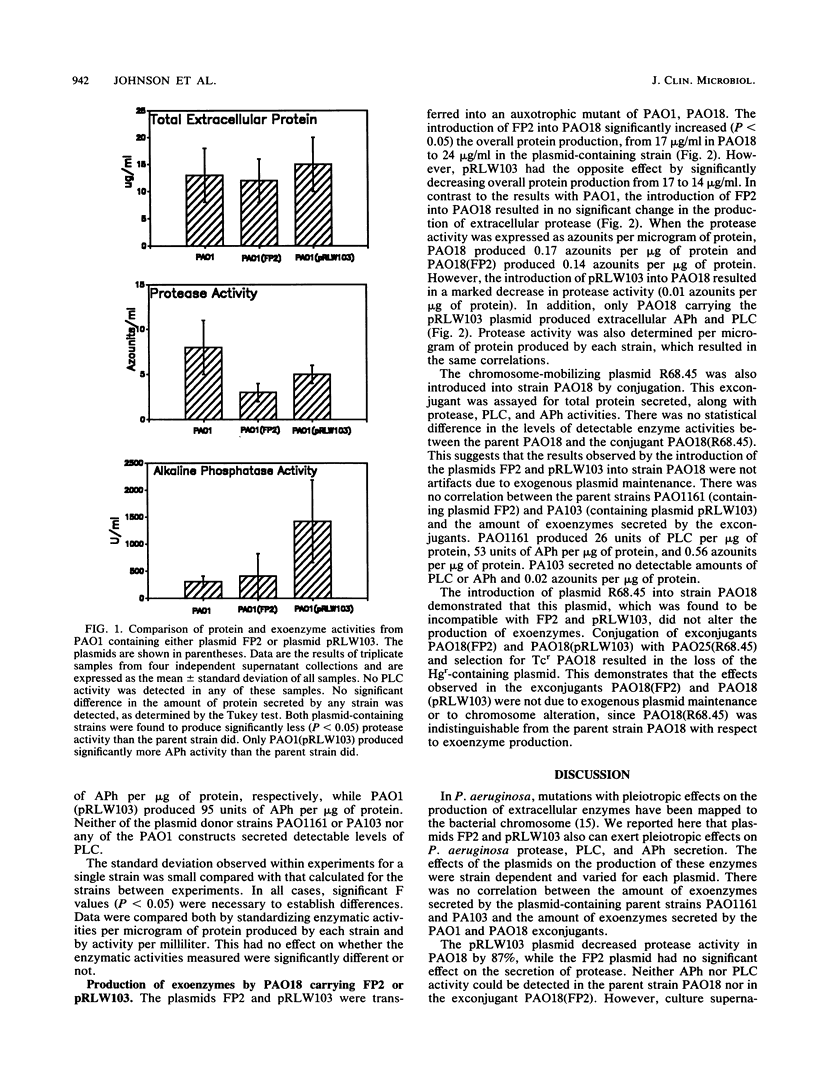
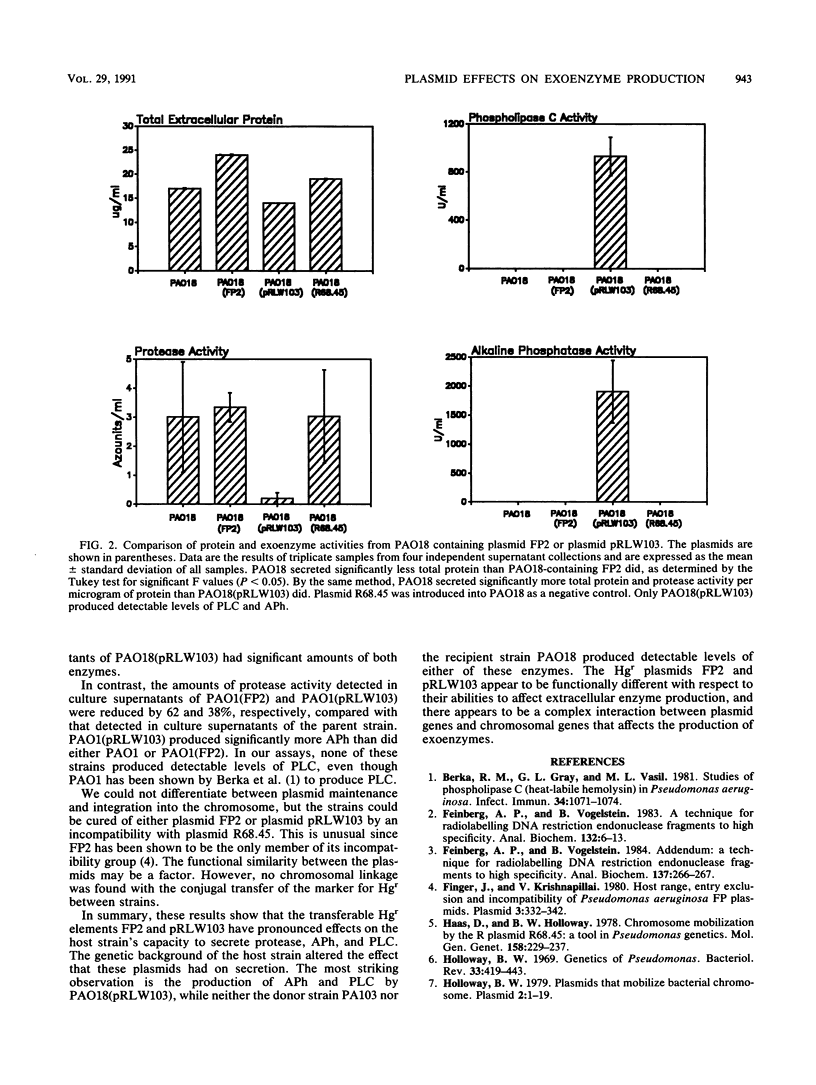
Plasmids encoding mercury resistance carried by Pseudomonas aeruginosa PAO1161 and PA103 were found to be involved in regulating the secretion of protease, phospholipase C, and alkaline phosphatase. Previously, mutations in Pseudomonas strains that caused pleiotropic effects on the production of extracellular enzymes were mapped to the bacterial chromosome. We show that pleiotropic changes in extracellular enzyme production can also be regulated by plasmids. In this study, the effects on secretion of exoenzymes by two mercury resistance plasmids, FP2 from PAO1161 and pRLW103 from PA103, were assayed in P. aeruginosa PAO1 and PAO18. The introduction of either plasmid into PAO1 resulted in a significant decrease in exoprotease production. Additionally, pRLW103 significantly increased the production of alkaline phosphatase by both strains. Phospholipase C was produced only in strain PAO18 containing the pRLW103 plasmid. FP2 had no effect on alkaline phosphatase or phospholipase C production in either strain and was found to decrease exoprotease secretion only in strain PAO1. The results indicate the P. aeruginosa mercury resistance plasmids vary in their ability to modify exoenzyme expression, and this ability is influenced by the host strain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berka R. M., Gray G. L., Vasil M. L. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect Immun. 1981 Dec;34(3):1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Finger J., Krishnapillai V. Host range, entry exclusion, and incompatibility of Pseudomonas aeruginosa FP plasmids. Plasmid. 1980 May;3(3):332–342. doi: 10.1016/0147-619x(80)90046-3. [DOI] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. Chromosome mobilization by the R plasmid R68.45: a tool in Pseudomonas genetics. Mol Gen Genet. 1978 Jan 17;158(3):229–237. doi: 10.1007/BF00267194. [DOI] [PubMed] [Google Scholar]
- Holloway B. W. Genetics of Pseudomonas. Bacteriol Rev. 1969 Sep;33(3):419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W. Plasmids that mobilize bacterial chromosome. Plasmid. 1979 Jan;2(1):1–19. doi: 10.1016/0147-619x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Jagger K. S., Bahner D. R., Warren R. L. Protease phenotypes of Pseudomonas aeruginosa isolated from patients with cystic fibrosis. J Clin Microbiol. 1983 Jan;17(1):55–59. doi: 10.1128/jcm.17.1.55-59.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnapillai V. A novel transducing phage. Its role in recognition of a possible new host-controlled modification system in Pseudomonas aeruginosa. Mol Gen Genet. 1972;114(2):134–143. doi: 10.1007/BF00332784. [DOI] [PubMed] [Google Scholar]
- Loutit J. S. Investigation of the mating system of Pseudomonas aeruginosa strain I. VI. Mercury resistance associated with the sex factor (FP). Genet Res. 1970 Oct 2;16(2):179–184. doi: 10.1017/s0016672300002408. [DOI] [PubMed] [Google Scholar]
- Snell K., Holder I. A., Leppla S. A., Saelinger C. B. Role of exotoxin and protease as possible virulence factors in experimental infections with Pseudomonas aeruginosa. Infect Immun. 1978 Mar;19(3):839–845. doi: 10.1128/iai.19.3.839-845.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes H. W., Krishnapillai V. Prevalence of Pseudomonas aeruginosa FP plasmids which enhance spontaneous and uv-induced mutagenesis. Mutat Res. 1978 Apr;50(1):19–28. doi: 10.1016/0027-5107(78)90056-8. [DOI] [PubMed] [Google Scholar]
- Warren R. L., Baker N. R., Johnson J., Stapleton M. J. Selective inhibition of the accumulation of extracellular proteases of Pseudomonas aeruginosa by gentamicin and tobramycin. Antimicrob Agents Chemother. 1985 Apr;27(4):468–472. doi: 10.1128/aac.27.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wretlind B., Pavlovskis O. R. Genetic mapping and characterization of Pseudomonas aeruginosa mutants defective in the formation of extracellular proteins. J Bacteriol. 1984 Jun;158(3):801–808. doi: 10.1128/jb.158.3.801-808.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


