Abstract
Prostaglandins, particularly prostaglandin E2 (PGE2), play an important role during inflammation. This is exemplified by the clinical use of cyclooxygenase 2 inhibitors, which interfere with PGE2 synthesis, as effective antiinflammatory drugs. Here, we show that PGE2 directly promotes differentiation and proinflammatory functions of human and murine IL-17–producing T helper (Th17) cells. In human purified naive T cells, PGE2 acts via prostaglandin receptor EP2- and EP4-mediated signaling and cyclic AMP pathways to up-regulate IL-23 and IL-1 receptor expression. Furthermore, PGE2 synergizes with IL-1β and IL-23 to drive retinoic acid receptor–related orphan receptor (ROR)-γt, IL-17, IL-17F, CCL20, and CCR6 expression, which is consistent with the reported Th17 phenotype. While enhancing Th17 cytokine expression mainly through EP2, PGE2 differentially regulates interferon (IFN)-γ production and inhibits production of the antiinflammatory cytokine IL-10 in Th17 cells predominantly through EP4. Furthermore, PGE2 is required for IL-17 production in the presence of antigen-presenting cells. Hence, the combination of inflammatory cytokines and noncytokine immunomodulators, such as PGE2, during differentiation and activation determines the ultimate phenotype of Th17 cells. These findings, together with the altered IL-12/IL-23 balance induced by PGE2 in dendritic cells, further highlight the crucial role of the inflammatory microenvironment in Th17 cell development and regulation.
Prostaglandins, prostaglandin E2 (PGE2) in particular, play an important role in the regulation of inflammatory responses. PGE2 is a key mediator of pyrexia, hyperalgesia, and arterial dilation, which increases blood flow to inflamed tissues and, in combination with enhanced microvascular permeability, results in edema. The relevance of this pathway in promoting inflammation is supported by the clinical use of cyclooxygenase inhibitors, which interfere with prostaglandin synthesis and are used as effective antiinflammatory agents (1). However, PGE2 can also exert antiinflammatory properties and is a negative regulator of neutrophil, monocyte, and lymphocyte function, particularly of Th1 cells that produce IFN-γ (2). This apparent paradox has puzzled many investigators for decades. The interplay among PGE2, IL-23, and IL-1β biology may now provide an explanation of this paradox.
Th17 cells have been recognized as a unique subset of effector T cells that are distinct from the Th1 and Th2 subsets (3–6), and they have been implicated as potent effectors of autoimmune disorders, such as multiple sclerosis, psoriasis, arthritis, and inflammatory bowel disease (IBD) (7–10). We and others have previously reported that IL-23 and IL-1β are crucial factors during development of human Th17 cells (9, 11, 12). In addition, IL-23 and the IL-23–dependent Th17 cell population play essential roles in chronic inflammation and autoimmunity (13).
PGE2 has been shown to exacerbate inflammation and disease severity in murine models of IBD and collagen-induced arthritis through the IL-23–IL-17 pathway (14, 15). These effects have been attributed to actions of PGE2 on innate cells, as PGE2 enhances the production of IL-23 and IL-1β in macrophages and DCs, while down-regulating IL-12 production (16). A recent report has shown that PGE2, together with IL-23, favors the expansion of human Th17 cells from PBMCs, and that PGE2 enhances IL-17 production induced by IL-23 from memory CD4+ cells (17). However, the molecular mechanism of PGE2-mediated signaling during human Th17 cell development has not yet been examined.
In this study, we show that PGE2 acts directly on both human and murine T cells to enhance Th17 development and effector cytokine production. In human T cells, PGE2 acts via the prostaglandin receptor EP2- and EP4-mediated signaling and cAMP pathways to up-regulate IL-23 and IL-1 receptor expression. Furthermore, PGE2 synergizes with IL-1β and IL-23 to drive retinoic acid receptor–related orphan receptor (ROR)-γt, IL-17, IL-17F, CCL20, and CCR6 expression, which is consistent with the previously reported Th17 phenotype (8, 18). While enhancing Th17 cytokine expression mainly through EP2, PGE2 differentially regulates IFN-γ production and inhibits production of the antiinflammatory cytokine IL-10 in both naive and memory Th17 cells predominantly through EP4. Hence, the combination of inflammatory cytokines and noncytokine immunomodulators, such as PGE2, during differentiation and activation determines the ultimate phenotype of Th17 cells. These findings, together with the altered IL-12/IL-23 balance induced by PGE2 in dendritic cells, further highlight the crucial role of the inflammatory microenvironment in Th17 cell development and regulation.
RESULTS
PGE2 up-regulates IL-23 and IL-1 receptor expression on naive T cells via EP2, EP4, and cAMP signaling
To study the effects of PGE2 on T cells, we first analyzed the expression of the PGE2 receptors EP1, EP2, EP3, and EP4. Human naive CD4+CD45RA+ T cells were isolated from peripheral blood of healthy donors, as previously described (9). The purity of these naive T cell populations was routinely >99.5% (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20082293/DC1). Freshly isolated naive T cells constitutively expressed high levels of EP2 and EP4 mRNA, whereas EP1 and EP3 mRNA expression was low or nonexistent (Fig. 1 A). Cell surface expression of EP2 and EP4 protein was confirmed by flow cytometric analysis (Fig. 1 B). Activation and culture of naive T cells led to a two- to threefold up-regulation of EP2 and EP4 transcripts (Fig. S2 A). This up-regulation was not affected by the addition of Th1–IL-12– or Th17–IL-23– and/or IL-1β–polarizing culture conditions. EP1 and EP3 mRNA levels remained low after activation and culture under these same conditions. These results indicate that EP2 and EP4 constitute the major PGE2 receptors on naive and activated T cells.
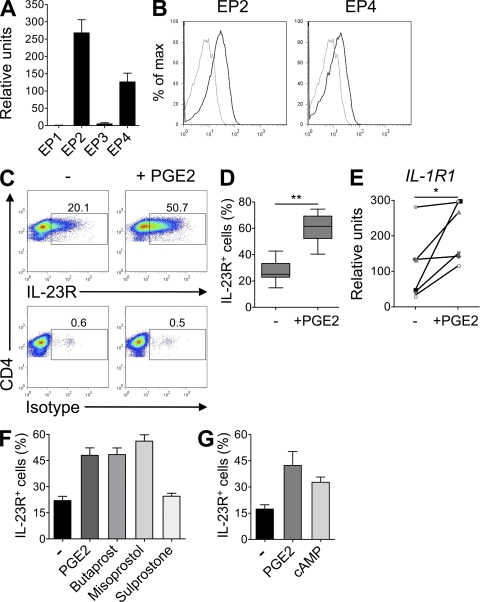
Figure 1.
PGE2 up-regulates IL-23R and IL-1R1 expression on cultured human naive CD4+ T cells. (A) Real-time PCR analysis of EP1, EP2, EP3, and EP4 gene expression in human naive CD4+ T cells. Mean + SEM of eight donors. Relative amplification efficiencies of EP primer sets fell within standard Taqman assay specifications. (B) Isotype (gray) or EP2 and EP4 (black) surface staining. (C–G) Naive CD4+ T cells were activated with anti-CD3/CD28/CD2 beads and cultured for 11 d in the presence or absence of PGE2, dibutyryl-cAMP, or specific EP receptor agonists. (C–D) Flow cytometric quantification of IL-23R in CD3+ CD4+ T cells restimulated for 48 h. (D) Box and whiskers of eight independent experiments are shown. **, P < 0.01. (E) Real-time PCR analysis of IL-1R1 gene expression in T cells restimulated for 24 h. Results from six different donors are shown. *, P < 0.05. (F and G) Flow cytometric quantification of IL-23R in CD3+ CD4+ T cells restimulated for 48 h. Mean + SEM of four independent experiments.
IL-23 and IL-1β have been shown to drive the development of human Th17 cells, which are characterized by a specific signature cytokine profile consisting of IL-17, IL-17F, IL-22, CCL20/MIP3α, and IFN-γ (9, 11, 12). To investigate a potential role of PGE2 in Th17 differentiation, we next examined the effects of PGE2 on the expression of IL-23 and IL-1 receptors. Interestingly, activation and culture of naive T cells in the presence of PGE2 resulted in a strong and reproducible up-regulation of IL-23R expression (Fig. 1, C and D). IL-1R1 gene expression was also increased in response to PGE2 (Fig. 1 E). Butaprost, an EP2 selective agonist, and misoprostol, a nonselective agonist with the highest affinity for EP4 and EP3, increased IL-23R expression comparable to PGE2. In contrast, the EP1/EP3 agonist sulprostone had no effect (Fig. 1 F), indicating that the effects of PGE2 on IL-23R expression were specifically mediated through EP2 and EP4 receptors. The addition of the intracellular cAMP analogue dibutyryl-cAMP mimicked the effect of PGE2 on IL-23R expression (Fig. 1 G), suggesting that the effect of PGE2 was dependent on cAMP formation. These results suggest a mechanism by which PGE2 could affect the Th17 pathway through up-regulation of IL-23 and IL-1 receptor expression.
PGE2 promotes development of human Th17 cells
As PGE2 enhanced both IL-23 and IL-1β receptor expression from activated naive T cells, we determined whether PGE2 could modulate the effects of IL-1β and IL-23 during the development of Th17 cells. Activation and culture of naive T cells with PGE2 alone slightly increased IL-17 production in few donors (Fig. 2 A). However, PGE2 significantly enhanced IL-17 production induced by IL-23 and/or IL-1β (Fig. 2 A). The combination of IL-23, IL-1β, and PGE2 (IL-23–IL-1β–PGE2) did not lead to further increase in IL-17 production over that induced by PGE2 and IL-1β. The addition of IL-23 to IL-23–IL-1β–PGE2–treated cells compared with IL-23–IL-1β cultures resulted in an enhanced phosphorylation of STAT3, which is in line with the up-regulated expression of IL-23R in the presence of PGE2 (Fig. S2 B), and indicates that the enhanced IL-23R expression is functional. The increase in IL-17 production induced by IL-1β and IL-23 in response to PGE2 was dose dependent (Fig. S2 C). In addition, the proportion of IL-17+ and IL-17+/IFN-γ+ producers was increased in IL-23–IL-1β–PGE2 cultures compared with IL-23–IL-1β–treated cells, whereas IFN-γ single producers were present at similar levels (Fig. S2 D). Furthermore, the increased IL-17 production was more strongly controlled by EP2 than EP4 signaling because the EP2 agonist butaprost could mimic the effect of PGE2 to a similar extent, whereas misoprostol was not able to induce such a strong induction of IL-17 production (Fig. 2 B). The increase in IL-17 secretion mediated through activation of the cAMP pathway was consistent with the observed EP2 or EP4 signaling (Fig. 2 C). The effects of dibutyryl-cAMP on IL-17 production were consistent and dose dependent (Fig. S2 E). The differential increase of IL-17 production in response to EP2 or EP4 may be caused by the more robust stimulation of intracellular cAMP formation by EP2 compared with EP4, as previously reported (19).
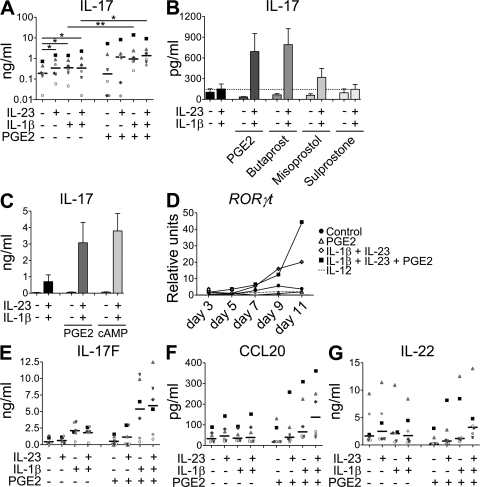
Figure 2.
PGE2, together with IL-1β and IL-23, enhances Th17 cell development predominantly via EP2 signaling and cAMP pathways. Human naive CD4+ T cells were activated with anti-CD3/CD28/CD2 beads and cultured for 11 d in the presence of IL-23, IL-1β, PGE2, dibutyryl-cAMP, and/or EP receptors agonists. (A–C) IL-17 production in cell-free supernatants of T cells restimulated for 48 h. (D) Real-time PCR of RORγt gene expression in T cells after indicated time of culture. (E–G) IL-17F (E), CCL20 (F), and IL-22 (G) production in cell-free supernatants of T cells restimulated for 48 h. Data from six different donors are shown in A, E, F, and G; horizontal bars represent median values. Results in B show mean + SEM of three independent experiments. Data in C show mean + SEM of five independent experiments. Data in D are representative of two independent experiments. *, P < 0.05; **, P < 0.01.
The development of Th17 cells is dependent on the induction of the Th17-specific transcription factor RORγt (20). A time course of RORγt expression during T cell polarization confirmed that after 11 d of culture, IL-23–IL-1β–PGE2–derived Th17 cells expressed higher levels of RORγt transcripts compared with IL-23–IL-1β control cultures (Fig. 2 D), which is in agreement with the up-regulated IL-17 production by these cells. Th17 cells also produce IL-17F, IL-22, and CCL20/MIP3α. A similar profile of IL-23–IL-1β–PGE2–dependent regulation was observed for IL-17F and CCL20 expression (Fig. 2 E, F). In contrast, IL-22 and IL-26 expression was decreased in the presence of PGE2 alone; however, their expression was not altered by the addition of PGE2 to cultures containing IL-23 and IL-1β (Fig. 2 G and Fig. S2 F). These results indicated that although IL-22 and IL-26 are an integral part of Th17 biology, their expression is subject to multiple levels of regulation. All the observations on Th17 cytokine production were confirmed at the level of gene expression (Fig. S2 F). These results thus indicate that PGE2 can act directly on naive T cells and enhance Th17 development mainly through the EP2–cAMP pathway.
PGE2 induces IL-17 production in T cell–APC co-cultures
PGE2 pretreatment of APCs promotes Th17 differentiation in mice by altering the IL-12/IL-23 balance (14–16, 21). In addition, PGE2 enhanced IFN-γ and TLR7 induced IL-23 production (22). It was therefore of interest to determine the effects of PGE2 on T cells in the presence of APCs. Like T cells, human monocytes mainly expressed EP2 and EP4 transcripts (Fig. 3 A). Activation of monocytes through the TLR2 pathway by peptidoglycan resulted in the production of IL-23 and IL-1β (Fig. 3 B). However, when monocytes were activated with PGE2 in the absence of TLR ligands or cytokines, we did not detect any IL-23 or IL-1β production (Fig. 3 B). In line with these results, addition of PGE2 to co-culture of naive T cells with monocytes in the absence of exogenously added cytokines did not induce IL-17 production. Interestingly, in the presence of monocytes, the combination of IL-23 and IL-1β failed to induce IL-17 production in most donors; however, addition of PGE2 overcame this suppressive effect and resulted in IL-17 production (Fig. 3 C). These results indicate that PGE2 was essential for IL-17 production induced by IL-23 and IL-1β in this T cell–APC co-culture system.
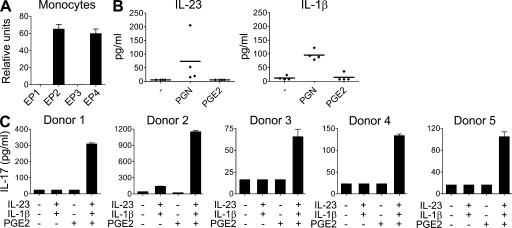
Figure 3.
PGE2 is required for IL-23–IL-1β–induced IL-17 production in a monocyte-naive T cell co-culture system. (A) Real-time PCR analysis of EP1, EP2, EP3, and EP4 gene expression in human CD14+ monocytes. Mean + SEM of four donors. (B) Human CD14+ monocytes were stimulated in the presence of peptidoglycan or PGE2 for 24 h. IL-1β and IL-23 production was assessed in cell-free supernatants. Results from four independent donors are shown. (C) Human naive CD4+ T cells were co-cultured with monocytes (1:1), activated with anti-CD3 antibody, and cultured for 11 d in the presence of IL-23, IL-1β, and/or PGE2. IL-17 production in cell-free supernatants of T cells restimulated for 48 h was measured by ELISA. Results from five independent donors are shown.
PGE2 inhibits Th17 production of IL-10 and IFN-γ predominantly through EP4 signaling
Recent evidence suggests that IL-10 restrains the pathogenicity of Th17 cells in mice (23, 24), prompting us to also examine the production of IL-10 in human Th17 cells. IL-23, but not IL-1β, increased IL-10 production in activated naive T cell cultures (Fig. 4 A). IL-10 production remained below levels that were observed with IL-12 (unpublished data). Addition of PGE2 strongly suppressed the production of IL-10 in both control and Th17-inducing conditions (Fig. 4 A). This effect was mainly mediated through EP4 signaling as shown by the addition of the EP4 agonist misoprostol (Fig. 4 B). Interestingly, although dibutyryl-cAMP inhibited IL-10 production in control cultures, it failed to down-regulate IL-10 production in IL-23–IL-1β–treated cells (Fig. 4 C), suggesting that additional signaling pathways are involved in response to PGE2 and EP4 stimulation (19). These results further corroborate the finding that down-regulation of the antiinflammatory IL-10 pathway by PGE2 might further exacerbate Th17-mediated inflammation.
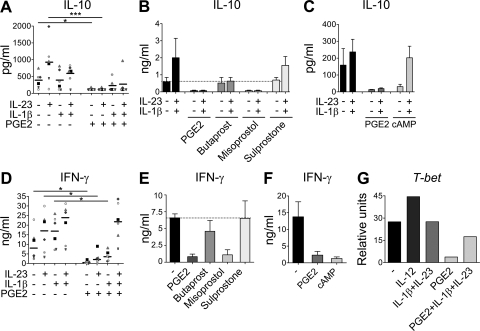
Figure 4.
PGE2 inhibits IL-10 and IFN-γ production predominantly via EP4 signaling and cAMP pathways. Human naive CD4+ T cells were activated with anti-CD3/CD28/CD2 beads and cultured for 11 d in the presence of IL-23, IL-1β, PGE2, dibutyryl-cAMP, and/or EP receptor agonists. (A–C) IL-10 production in cell-free supernatants of T cells restimulated for 48 h. (D–F) IFN-γ production in cell-free supernatants of T cells restimulated for 48 h. (G) Real-time PCR of T-bet mRNA expression in T cells after 11 d of culture. Data from six different donors are shown in A and D; horizontal bars represent median values. Results in B and E show mean + SEM of three independent experiments. Data in C and F show mean + SEM of five independent experiments. Data in G are representative of two independent experiments. *, P < 0.05; ***, P < 0.001.
PGE2 suppressed IFN-γ production from activated naive T cells cultured in the absence of additional cytokines (Fig. 4 D), in accordance with the established dogma (25–27). We previously reported that IL-23 enhanced IFN-γ production by PHA blasts (28) and that human Th17 cells, differentiated in the presence of either IL-1β or IL-23, are able to produce IFN-γ (9). The production of IFN-γ by Th17 cells differentiated by IL-1β or IL-23 was strongly inhibited by PGE2 (Fig. 4 D). The inhibitory effect of PGE2 on IFN-γ production was predominantly mediated through EP4 signaling because it could be mimicked by the addition of the EP4 agonist misoprostol (Fig. 4 E) and through elevation of cAMP (Fig. 4 F). However, when Th17 cells were generated by the combination of IL-1β and IL-23, PGE2 was not able to inhibit IFN-γ secretion, indicating that PGE2 could not effectively interfere with the efficacy of the combined signal transduction pathways. Accordingly, PGE2 inhibited T-bet mRNA expression in activated T cells differentiated with either IL-1β or IL-23, whereas T-bet levels were only slightly reduced in IL-23–IL-1β–PGE2–cultured cells (Fig. 4 G). These results indicate that the ability of PGE2 to inhibit IFN-γ production is dependent on the maturation state of the T cells and cytokine milieu. Overall, these data indicate that PGE2 modulates the Th17 phenotype to a high IL-17–, IL-17F–, CCL20-, low IFN-γ–, low IL-10–producing cell that could be more pathogenic.
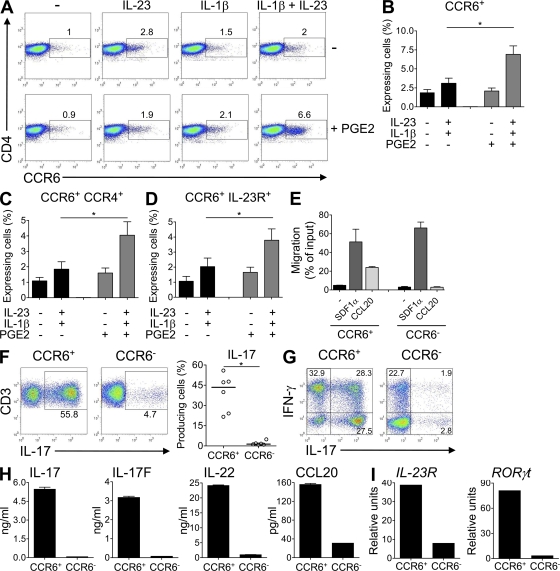
PGE2 enhances maturation of developing Th17 cells
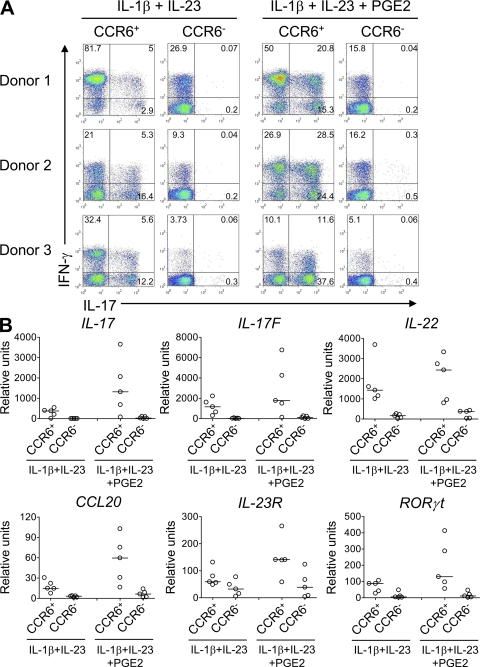
In addition to its effects on cytokine and cytokine receptor expression, the combined activity of PGE2, IL-1β, and IL-23 led to an enhanced maturation of developing Th17 cells. Memory Th17 cells isolated from human blood express CCR6 (8, 18), the receptor for CCL20 and β-defensins. We observed that only the combined presence of IL-23, IL-1β, and PGE2 led to an enhanced up-regulation of CCR6 expression in naive T cells (Fig. 5, A and B), as well as an increase of CCR6+CCR4+ and CCR6+IL-23R+ cells (Fig. 5, C and D). CCR6 expressed by IL-23–, IL-1β–, and PGE2-treated T cells was functional, as CCR6+ T cells, but not CCR6− T cells, had chemotactic activities in response to its ligand CCL20. Both T cell populations migrated equally well toward SDF1α (Fig. 5 E), a ligand of CXCR4 that is expressed on T cells. Reactivation of sorted CCR6+ T cells isolated from IL-23–IL-1β–PGE2–driven Th17 cells revealed that >50% were IL-17–producing cells, whereas few IL-17 producers were present in the CCR6− T cell population (Fig. 5 F). Both IL-17+ IFN-γ+ and IL-17+ IFN-γ− T cells were present at similar percentages in the CCR6+ T cell population, whereas the CCR6− T cell fraction contained predominantly IFN-γ only producers (Fig. 5 G). The CCR6+ T cells also expressed higher levels of the other Th17 cytokines IL-17F, IL-22, CCL20, as well as of IL-23R and RORγt transcripts (Fig. 5, H and I). A similar Th17 profile was observed in sorted CCR6+ T cells isolated from IL-23–IL-1β–driven Th17 cells in the absence of PGE2 (Fig. 6, A and B). Strikingly, CCR6+ T cells from IL-23–IL-1β–treated cells in the presence of PGE2 expressed higher levels of all these Th17 markers (Fig. 6 B). Interestingly, the proportion of IL-17+, IL-17+/IFN-γ+, but not IFN-γ+–producing cells, was increased in IL-23–IL-1β–PGE2 cultures (Fig. 6 A), demonstrating that PGE2 not only quantitatively enhanced the number of IL-17 producers but also increased their intrinsic capacity to produce IL-17. Collectively, these data indicate that PGE2 also enhanced maturation of Th17 cells.
Figure 5.
CCR6 expression defines Th17 cytokine producers and is up-regulated in the presence of IL-1β, IL-23, and PGE2. Human naive CD4+ T cells were activated with anti-CD3/CD28/CD2 beads and cultured for 11 d in the presence of IL-23, IL-1β, and/or PGE2. (A) Flow cytometric quantification of CCR6 in CD3+ CD4+ T cells restimulated for 48 h. Results are representative of three independent experiments. (B–D) Flow cytometric quantification of CCR6+ (B), CCR6+CCR4+ (C), or CCR6+IL-23R+ (D) in CD3+ CD4+ T cells restimulated for 48 h. *, P < 0.05. Results represent mean + SEM of nine independent experiments. (E) CD4+CCR6+ and CD4+CCR6− T cells from IL-1β, IL-23, and PGE2-treated T cells were sorted and added to inserts that were placed in wells containing CCL20 or SDF-1α, forming an upper and lower chamber separated by a membrane bearing 5-µm pores. Responding cells that migrated to the lower chamber were harvested and counted by flow cytometry. Results represent mean + SEM of two independent donors and are expressed as the percentage of migration compared with the 100% migration control. (F–I) Naive T cells were activated with anti-CD3/CD28/CD2 beads and cultured for 11 d in the presence of IL-1β, IL-23, and/or PGE2. After reactivation, CD4+CCR6+ and CD4+CCR6− T cells were sorted and cultured for 7 d in the presence of IL-2. (F and G) Intracellular IL-17 and IFN-γ staining after stimulation with PMA/ionomycin. Results from six independent donors are shown in F. *, P < 0.05. Data in G are representative of six independent experiments. (H) Production of IL-17, IL-17F, IL-22, and CCL20 in cell-free supernatants of T cells restimulated for 24 h. (I) Real-time PCR analysis of IL-23R and RORγt gene expression in T cells restimulated 24 h. Results in H and I are representative of four independent experiments.
Figure 6.
PGE2 enhances maturation of Th17 cells. Human naive CD4+ T cells were activated with anti-CD3/CD28/CD2 beads and cultured for 11 d with IL-1β and -23, in the presence or absence of PGE2. After reactivation, CD4+CCR6+ and CD4+CCR6− T cells were sorted from both cultures and cultured for 7 d in the presence of IL-2. (A) Intracellular IL-17 and IFN-γ staining after stimulation with PMA/Ionomycin. Results from three independent donors are shown. (B) Real-time PCR analysis of IL-17, IL-17F, IL-22, CCL20, IL-23R, and RORγt in T cells restimulated for 24 h. Results from five donors are shown; horizontal bars represent median values.
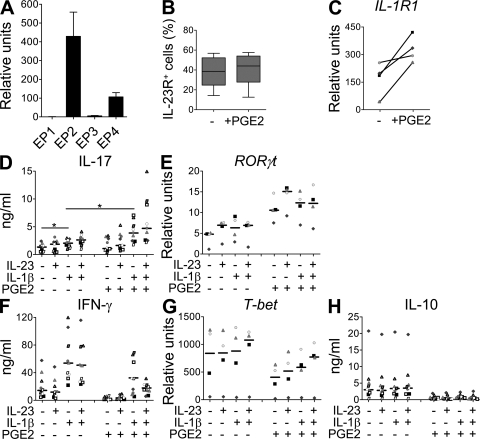
PGE2 regulates memory Th17 cell function
As activated/memory T cells constitute a major cell population found in inflamed tissue, we next assessed whether PGE2 could affect the function of mature Th17 cells in short-term cultures. We previously identified in situ Th17 cells as part of the CD4+CD45RO+ memory T cell subset that expresses the IL-23R in PBMCs of normal donors (9). Like naive T cells, memory T cells mainly expressed EP2 and EP4 (Fig. 7 A) and could respond to PGE2. Consistent with their Th17 memory phenotype, PGE2 stimulation did not further enhance the percentage of IL-23R–positive cells or the level of IL-23R expression (Fig. 7 B). However, PGE2 did up-regulate the expression of the IL-1R1 mRNA on the memory T cells (Fig. 7 C), and also modulated cytokine production and transcription factor profiles. The activation of memory T cells with IL-1β enhanced IL-17 production, and the combination of both IL-1β and IL-23 led to a higher IL-17 up-regulation, as recently described (29). The presence of PGE2 alone or with IL-23 did not enhance IL-17 production in most donors (Fig. 7 D). However, PGE2 enhanced IL-17 production induced by IL-1β and the combination of PGE2, IL-1β, and IL-23 induced an even stronger IL-17 up-regulation that correlated with enhanced expression of RORγt (Fig. 7 E). Interestingly even PGE2 alone induced RORγt expression, but not IL-17 production. Similar results were recently described for TGF-β (30), and are consistent with the requirements for additional (transcription) factors to fully induce IL-17 production. Similar to naive T cells, PGE2 down-regulated IFN-γ, T-bet, and IL-10 expression by activated memory T cells cultured without cytokines or with IL-1β or IL-23 (Fig. 4, F–H). Furthermore, PGE2 inhibited IFN-γ, IL-10, and T-bet expression even in the presence of both IL-1β and IL-23 (Fig. 4, F–H). Thus PGE2 will not only affect the phenotype and function of differentiating Th17 cells but also the function of mature effector Th17 cells.
Figure 7.
PGE2 modulates memory Th17 functions.(A) Real-time PCR analysis of EP1, EP2, EP3, and EP4 gene expression in human memory CD4+ T cells. Mean + SEM of three donors. (B–H) Memory CD4+ T cells were activated with anti-CD3/CD28/CD2 beads and cultured for 3 d in the presence or absence of IL-23, IL-1β, and/or PGE2. (B) Flow cytometric quantification of IL-23R. Box and whiskers of five independent experiments are shown. (C) Real-time PCR analysis of IL-1R1 gene expression. Results from four different donors are shown. (D) IL-17 production in cell-free supernatants. (E) Real-time PCR of RORγt gene expression. (F) IFN-γ production in cell-free supernatants. (G) Real-time PCR of T-bet gene expression. (H) IL-10 production in cell-free supernatants. Data from four (E and G) or nine (D, F, and H) independent donors are shown; horizontal bars represent median values. *, P <0.05.
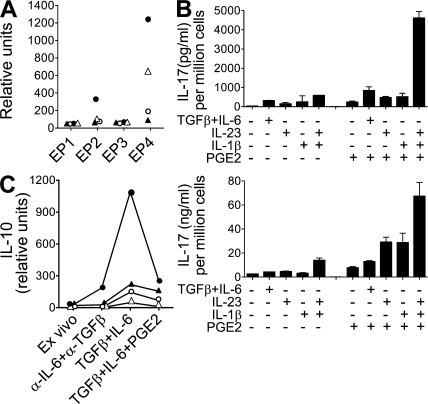
PGE2 regulates murine Th17 function
We also assessed whether PGE2 could directly affect IL-17 production from mouse CD4+ T cells. Similar to human cells, murine naive and memory CD4+ T cells expressed EP2 and EP4 genes, whereas EP1 and EP3 mRNA transcripts were not detected (Fig. 8 A). The combination of TGF-β and IL-6 (TGF-β–IL-6) is crucial for the initial development of Th17 cells in mice (31–33), whereas IL-1β and IL-23 are important for the maturation, expansion, and effector function of this subset (24, 34). Stimulation of purified naive CD4+CD44loCD62L+ T cells with anti-CD3 and -CD28 antibodies in the presence of TGF-β–IL-6 led to IL-17 production, as previously described. Restimulation of these newly activated T cells in the presence of IL-1β or IL-23 maintained IL-17 production. Furthermore, addition of PGE2 during this restimulation step greatly enhanced IL-17 production, particularly in combination with both IL-1β and IL-23 (Fig. 8 B). A similar profile was observed with stimulation of purified CD4+CD44hiCD62Llo memory T cells (Fig. 8 B). Once again, it is the combination of PGE2, IL-1β, and IL-23 that induced the highest level of IL-17 production. The effect of PGE2 on IL-10 production by naive and memory T cells in several mouse strains was also examined. As expected, activation of these cells in the presence of TGF-β–IL-6 induced the production of IL-10, which was inhibited by the addition of PGE2 (Fig. 8 C). These results suggest that the direct effects of PGE2 on Th17 cells are comparable in human and murine biology.
Figure 8.
PEG2 regulates murine Th17 cell functions. (A) Real-time PCR analysis of EP1, EP2, EP3, and EP4 gene expression in purified mouse CD4+ T cells. Closed triangle symbols represent T cells from draining lymph nodes of CFA-primed SJL mice. Open triangle symbols represent naive CD4+CD44loCD62Lhi from lymph nodes and spleen of C57BL/6 mice. Closed circle symbols represent memory CD4+CD44hiCD62Llo from C57BL/6 mice. Open circle symbols represent naive CD4+CD44loCD62Lhi from BALB/c DO11.10 OVA-TCR transgenic mice. (B, top) FACS-purified naive CD4+ T cells (CD44loCD25lo) from SJL mice were activated with anti-CD3/CD28 beads in the presence of TGF-β and IL-6 for 3 d. Cells were then restimulated for 5 d in the presence of the indicated combinations of cytokines with or without PGE2. (B, bottom) Memory CD4+ T cells (CD44hiCD25lo) were activated with anti-CD3/CD28 beads in the presence of cytokines with or without PGE2 for 4 d. IL-17 production in cell-free supernatants was assessed by ELISA and expressed as values normalized to the final number of cells per well. Results are representative of two experiments. (C) Real-time PCR analysis of IL-10 gene expression in purified mouse CD4+ T cells. Closed triangle symbols represent T cells from draining lymph nodes of CFA-primed SJL mice. Cells were cultured for 12 h with indicated cytokines with or without PGE2. Cells cultured for 2 d showed similar results (not depicted). Open triangles represent naive CD4+CD44loCD62Lhi from lymph nodes and spleen of C57BL/6 mice cultured for 1 d. Filled circles represent memory CD4+CD44hiCD62Llo from C57BL/6 mice cultured for 1 d. Open circles represent naive CD4+CD44loCD62Lhi from BALB/c DO11.10 OVA-TCR transgenic mice cultured for 2 d. SJL and BALB/c data are representative of two experiments/mouse strain, whereas results from naive and memory C57BL/6 cells are from one experiment.
DISCUSSION
Cyclooxygenase 2 (COX2) inhibitors inhibit prostaglandin synthesis, including PGE2, and are used in human to treat chronic inflammatory diseases such as osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis (35, 36). In addition, COX2 inhibitors have been shown to reduce the severity of experimental autoimmune encephalomyelitis and collagen-induced arthritis in mice (37, 38). These autoimmune disease models are associated with exacerbated Th17 responses, which are driven by the presence of proinflammatory cytokines such as IL-1 and IL-23 (13) In this context, Chizzolini et al. recently reported that another inflammatory mediator, PGE2, enhanced IL-17 production from memory CD4+ T cells in total human PBMC cultures (17). Here, we specifically assessed the PGE2-mediated effects on T cell development and function. We showed that PGE2 signals via EP2 and EP4 receptors and the cAMP–PKA pathway to promote human naive T cell differentiation to Th17 cells (proposed model in Fig. 9). PGE2 up-regulated the expression of IL-23R and IL-1R on differentiating naive T cells and, in combination with Th17-driving cytokines, enhanced STAT3 phosphorylation and induced a qualitative and quantitative shift in function and phenotype toward a strong proinflammatory/pathogenic Th17 cell. PGE2 enhanced the percentage of CCR6-positive, in vitro–matured Th17 cells that expressed IL-17A, IL-22, IL-17F, CCL20, RORγt, and IL-23R, and it significantly up-regulated these functional markers of the Th17 phenotype (9, 11, 12). This enhanced Th17 functionality was also observed when mature CD45RO+ memory T cell populations were activated by IL-1β and PGE2. Although previous studies have reported that PGE2 has antiproliferative effects on T cells, we did not observe a strong inhibition of proliferation when naive T cells were cultured in the presence of IL-23 and IL-1β (unpublished data). There are two plausible explanations that may reconcile PGE2's apparent antiproliferative effect and our pro-Th17 cell development findings: (a) in the presence of IL-1β and IL-23, PGE2 may favor the development and/or expansion of Th17 cells, while down-regulating proliferation of non-Th17 cells; (b) PGE2 may act on “non-Th17 cells” and convert them into IL-17 producers, while suppressing overall T cell proliferative responses, resulting in an “apparent” increase in the number of Th17 cells. Furthermore, in addition to these direct effects of PGE2 on T cells, PGE2 was also essential for the induction of IL-17 production from naive T cells in an APC-dependent manner. We demonstrate that PGE2 inhibits IFN-γ and IL-10 production by Th17 cells and thus regulates their pathogenic potential. The opposing effects of PGE2 on increased IL-17 production and reduced IFN-γ and IL-10 production are mediated by differential signaling through EP2 and EP4 receptors, which is unique in PGE2 signal transduction (Fig. 9). Finally, we show that the PGE2 effects on Th17 function can be extended from human to murine biology. Overall, we show that the proinflammatory activity of PGE2 is mediated through the IL-23–Th17 pathway.
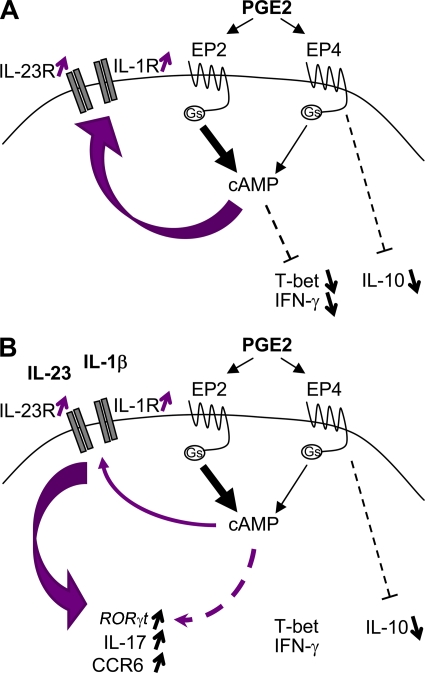
Figure 9.
Proposed model for PGE2 effects during Th17 cell development. (A) Binding of PGE2 to EP2 and EP4 enhances intracellular cAMP formation and signaling, leading to up-regulation of IL-23R and IL-1R1 expression, as well as inhibition of the expression of T-bet and production of IFN-γ. PGE2 binding to EP4 also induces concomitant signaling pathways independent of cAMP, such as PI3K or ERK, leading to inhibition of IL-10 production. (B) The presence of IL-23, IL-1β, and PGE2 leads to an up-regulation of RORγt, IL-17, and CCR6 expression and inhibition of IL-10. Gs, stimulatory guanine nucleotide binding protein.
Our results provide new mechanistic insights regarding PGE2-mediated signaling in Th17 cells. We show that EP2 and EP4, but not EP1 or EP3, are the receptors mediating PGE2 effects on CD4+ T cells. In addition, PGE2 activities are likely mediated through the cAMP–PKA pathway, as the effects of PGE2 can be mimicked in the presence of the cAMP analogue dibutyryl cAMP. These findings are in line with the previously reported induction of the cAMP–PKA pathway by EP2 and EP4 (19, 39). Furthermore, we show that stimulatory and inhibitory activities of PGE2 on cytokine production seem to be mediated by specific PGE2 receptors, as enhanced IL-17 production is preferentially mediated by EP2 signaling, whereas inhibition of IL-10 and IFN-γ production occurs mainly through EP4 signaling. In addition, the inhibition of IL-10 production induced by cAMP was not observed in the presence of IL-1β and IL-23, suggesting the induction of additional signaling pathway(s) in response to EP4 stimulation. It has been shown that EP4, but not EP2, can also induce a concomitant activation of phosphatidyl inositol 3 kinase and extracellular signal-regulated kinase pathways (39). However, addition of phosphatidyl inositol 3 kinase and MAP kinase inhibitors to our cultures completely inhibited all cytokine production by T cells (unpublished data), so it was not possible to formally test this hypothesis. It is of interest to note that PGE2 and the cAMP pathway have been shown to up-regulate IL-10 expression in monocytic cells through a cAMP-responsive element (CRE) in the IL-10 promoter. However, the cAMP-induced PKA-dependent phosphorylation of the CRE-binding protein 1 does not seem to have an important regulatory role in T cells, which is also reflected by their lack of responsiveness to catecholamine-induced, cAMP-dependent IL-10 production (40, 41).
Human CD4+ memory T cells producing IL-17 were first identified from peripheral blood or gut of healthy and Crohn's patients (8), and increasing efforts have been made to characterize this cell subset. Two groups independently identified CCR6 as a chemokine receptor selectively expressed on Th17 cells (8, 18). CCR6 is involved in the recruitment of pathogenic T cells in multiple sclerosis, rheumatoid arthritis, Crohn's disease, and psoriasis (8, 42–44), and Th17 cells are increasingly recognized as crucial mediators of those diseases (7, 9, 10, 45, 46). Therefore, the up-regulated CCR6 expression in IL-23–IL-1β–PGE2–driven Th17 cells is consistent with the generation of cells bearing the phenotype of pathogenic effector Th17 cells. In addition, the higher expression of Th17 cytokines in CCR6+ cells isolated from cells treated with a combination of IL-23, IL-1β, and PGE2 compared with IL-23–IL-1β–treated cells suggest a stronger maturation of Th17 cells in the presence of PGE2. It has been recently shown that human IL-17–producing cells are contained within the CD161+ fraction of circulating or tissue-infiltrating T cells and that these cells originate from CD161+CD4+ T cells of umbilical cord blood (47). Additional data also suggest that such CD161+ Th17 cells are crucial mediators of intestinal inflammation during Crohn's disease (48).
IL-10 was originally described as an immunosuppressive cytokine produced by Th2 cells. However, it is now recognized that all T cell subsets can produce this cytokine as a feedback response to suppress immune pathologies (23, 49). Indeed, we have previously shown that TGF-β induced IL-10 production even in the presence of IL-6 and/or IL-23, and that IL-10 can restrain the pathogenicity of Th17 cells in a mouse model of CNS autoimmune encephalomyelitis (24). As shown in this study, human Th17 cells stimulated in the presence of IL-1β and/or IL-23 produced IL-10. Thus, the ability of PGE2 to strongly inhibit IL-10 production is crucial to enhancement of Th17-mediated inflammation and pathogenicity.
PGE2 is also an important regulator of Th1 cells, particularly through the inhibition of IFN-γ production (25, 26, 50). We show here that PGE2 also inhibited IFN-γ production by Th17 cells generated in the presence of IL-1β or IL-23; however, the signaling pathways induced by both cytokines prevented this inhibition and led to the development of both IL-17+/IFN-γ−, as well as IL-17+/IFN-γ+ producers. These results are in accordance with the phenotype of Th17 cells observed by Chizzolini et al. when culturing PBMCs in the presence of IL-23 and PGE2 (17). Interestingly, ex vivo Th17 cells producing IL-17 alone or both IL-17 and IFN-γ have been identified in the blood and gut of Crohn's patients (8). Our results support the idea that the presence of IL-23, IL-1β, and PGE2 would lead to the development of Th17 cells bearing a phenotype/cytokine profile close to that found during disease.
Our results show that PGE2 directly acts on murine Th17 cells to enhance IL-17 production. This extends previous studies demonstrating that in the mouse system, PGE2's pro-Th17 effects were mediated through enhanced DC and macrophage production of IL-23 and IL-1β (14, 15, 21). Hence, the mechanisms of PGE2-mediated expansion of pathogenic Th17 cells are likely through both T cell–dependent and myeloid cell–dependent pathways.
In the mouse culture system, it is well established that the combination of TGF-β and IL-6 is necessary and sufficient to promote both in vitro (and likely in vivo) development of IL-17–producing CD4+ T cells. It is important to note that IL-6 is a major driver of IL-1R1 and IL-23R in murine T cells. Our assessment of PGE2's influence on murine T cell differentiation toward Th17 cells indicated that PGE2 cannot replace TGF-β and/or IL-6 for induction of Th17 cells in a 3-d naive CD4+ T cell assay (unpublished data). This was not unexpected, as TGF-β/IL-6 can promote 30–70% of IL-17–producing CD4+ T cells in our culture system. PGE2 has more profound effects on differentiating Th17 cells in our human culture system. Although we did not observe a TGF-β/IL-6–dependent effect on naive human T cells, we found that PGE2 induced IL-1R and IL-23R in naive CD4+ T cells, which enhanced the development of Th17 cells induced by IL-23 and IL-1β. Although TGF-β is clearly required for the generation of murine Th17 cells, its role during the development of the human counterpart is still controversial. In this regard, Santarlasci et al. recently suggested that TGF-β was not critical for human Th17 cell development; instead, TGF-β would indirectly favor expansion of Th17 cells through inhibition of Th1 cell development (51).
In conclusion, our study highlights the role of the inflammatory microenvironment as a critical factor in Th17 development and regulation. Our findings also shed light on a paradox where the important role of PGE2 in mediating inflammation remained controversial in respect to its effects on T helper cell function and cytokine production.
MATERIALS AND METHODS
Human cell cultures.
Buffy coats were obtained from normal healthy human volunteers participating in the Stanford Medical School Blood Center blood donation program in accordance with the Declaration of Helsinki. Human protocols were approved by the Schering Plough Institutional Biosafety Committee. CD14+ monocytes were isolated from normal peripheral blood using CD14 microbeads (Miltenyi Biotech). Naive CD4+CD45RO− T cells were isolated and cultured as previously described (9). Memory CD4+CD45RA− T cells were isolated using the memory T cell isolation kit, human (Miltenyi Biotech), according to the manufacturer's instructions. T cells were activated in the presence of beads coated with anti-CD3/CD28/CD2 antibodies (1 bead:10 cells). For co-culture experiments, monocytes and naive T cells were cultured at a 1:1 ratio in the presence of 0.2 µg/ml anti-CD3 mAb (Spv-T3b; DNAX). Where indicated, 50 ng/ml hIL-23 (DNAX), 50 ng/ml hIL-1β (R&D Systems), 10 µM PGE2 (Sigma-Aldrich), 10 µM butaprost (EP2 agonist), 35 µM misoprostol (EP4,EP3>EP1>EP2 agonist), 10 µM sulprostone (EP1/EP3 agonist; Cayman Chemical), or 100 µM of dibutyryl-cAMP (Alexis Biochemicals) were added.
Mouse cell cultures.
Handling of mice and experimental procedures were approved by and conducted in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines of the Schering-Plough Biopharma Institutional Animal Care and Use Committee. Lymph nodes and spleen cells from naive SJL/J, C57BL/6 (JAX Laboratories), or BALB/c DO11.10 OVA TCR transgenic mice (maintained at the Schering-Plough Biopharma mouse facility) were enriched for CD4+ cells using magnetic purification (Miltenyi Biotech), according to the manufacturer's instructions. Naive CD4+CD44loCD25lo and memory CD4+CD44hiCD25lo T cells were then purified by FACS. Memory cells were stimulated for 4 d with CD3/CD28 T cell expander beads (Dynal) in the presence of different cytokines (5 ng/ml IL-1β; 20 ng/ml IL-23) and/or 10 µM PGE2. Cells were then counted using a Vicell counter and supernatants were analyzed by ELISA. Naive cells were cultured for 3 d with CD3/CD28 T cell expander beads (Dynal) and 10 ng/ml TGF-β and 100 ng/ml IL-6 (R&D Systems). Cells were washed and stimulated for an additional 5 d with different cytokines before cell number was assessed and supernatant cytokine was analyzed by ELISA. Cytokine levels are expressed as values normalized to the final number of cells per well.
Chemotaxis assays.
Migration assays were performed in 5-µM pore transwells, as previously described (52, 53), using 100 nM CCL20 or 50 nM SDF1α (R&D Systems). The migration was carried out for 2 h at 37°C, and then a known number of counting beads was added to each well. Beads and cells were easily distinguishable by flow cytometry when plotting side scatter versus forward scatter, allowing the determination of the cell/bead ratio. The cell/bead ratio obtained from cells added directly to the wells was considered as 100% migration.
Cell sorting.
CD4+CCR6+ and CD4+CCR6− cell subsets were purified by cell sorting using anti-CCR6 and -CD4 antibodies (BD). Cell sorting was done with a FACSAria instrument (BD).
Cell surface receptor and intracellular cytokine staining.
For analysis of cell surface proteins, cells were stained with anti-CD4, -CD3, -CD45RA, -CCR6, -CCR4 (BD), -EP2, -EP4 (Cayman Chemical), and/or –IL-23R (BAF1400; R&D Systems) antibodies. For intracellular staining, cells were stimulated for 5 h with PMA, ionomycin (Sigma-Aldrich), and GolgiStop (BD), and permeabilized and stained with anti–IL-17 (eBioscience) and anti–IFN-γ (BD) antibodies using the Cytofix/Cytoperm Plus kit (BD). For STAT3 phosphorylation analysis, cells were stimulated for 15 min in the presence of IL-23. Cells were fixed, permeabilized, and stained with anti-PSTAT3 (tyrosine 705) antibody (BD) following the manufacturer's instructions (BD). Data were acquired on a LSR II or a Canto II cytometer and analyzed with FlowJo software (Tree Star, Inc.).
ELISA and electrochemiluminescence assay.
IL-17F electrochemiluminescence assay and IFN-γ ELISA were performed as previously described (9). Human IL-17, IL-22, CCL20, IL-10, IL-1β, and mouse IL-17 ELISA kits were obtained from R&D Systems. IL-23 electrochemiluminescence assay was developed in-house with biotinylated anti–IL-23p19 and –IL-23p40. The lower limit of quantitation of this assay was 6 pg/ml and had no cross-reactivity to IL-12p40 up to 4 µg/ml or to IL-12p40/p35 heterodimer up to 5 µg/ml, which were the highest concentrations tested.
Real-time quantitative PCR.
Real-time quantitative PCR was performed as previously described (9).
Statistics.
Wilcoxon signed-rank test or one-way ANOVA (for multiple groups) with a Friedman test was used for statistical analysis. P values of 0.05 or less were considered significant, and all data are represented as mean + the SEM.
Online supplemental material.
Fig. S1 depicts the purity of human naive CD4+CD45RA+ T cells after isolation. Fig. S2 shows the enhancing effects of PGE2 and dibutyryl-cAMP on Th17 cytokines expression in Th17 cells polarized in the presence of IL-1β and IL-23. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082293/DC1.
Acknowledgments
We thank Bela Desai, Steve Jungers, and Fran Shen for cell sorting; Deepa Prabhavalkar and Wolfgang Seghezzi for IL-23 assay; Eddie Bowman for chemotaxis assays; and Jennifer Louten for helpful discussions.
Schering-Plough Biopharma is a division of Schering-Plough Corporation. All authors were employed by Schering-Plough at the time of these studies. The authors have no further conflicting financial interests.
Footnotes
Abbreviations used: COX2, cyclooxygenase 2; CRE, cAMP-responsive element; IBD, inflammatory bowel disease; PGE2, prostaglandin E2; ROR, retinoic acid receptor–related orphan receptor.
References
- Flower R.J. 2003. The development of COX2 inhibitors.Nat. Rev. Drug Discov. 2:179–191 [DOI] [PubMed] [Google Scholar]
- Harris S.G., Padilla J., Koumas L., Ray D., Phipps R.P. 2002. Prostaglandins as modulators of immunity.Trends Immunol. 23:144–150 [DOI] [PubMed] [Google Scholar]
- Weaver C.T., Hatton R.D., Mangan P.R., Harrington L.E. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages.Annu. Rev. Immunol. 25:821–852 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Oukka M., Kuchroo V.K. 2007. T(H)-17 cells in the circle of immunity and autoimmunity.Nat. Immunol. 8:345–350 [DOI] [PubMed] [Google Scholar]
- Dong C. 2006. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells.Nat. Rev. Immunol. 6:329–333 [DOI] [PubMed] [Google Scholar]
- McKenzie B.S., Kastelein R.A., Cua D.J. 2006. Understanding the IL-23-IL-17 immune pathway.Trends Immunol. 27:17–23 [DOI] [PubMed] [Google Scholar]
- Kebir H., Kreymborg K., Ifergan I., Dodelet-Devillers A., Cayrol R., Bernard M., Giuliani F., Arbour N., Becher B., Prat A. 2007. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation.Nat. Med. 13:1173–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Fili L., Ferri S., Frosali F., et al. 2007. Phenotypic and functional features of human Th17 cells.J. Exp. Med. 204:1849–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N.J., Boniface K., Chan J.R., McKenzie B.S., Blumenschein W.M., Mattson J.D., Basham B., Smith K., Chen T., Morel F., et al. 2007. Development, cytokine profile and function of human interleukin 17-producing helper T cells.Nat. Immunol. 8:950–957 [DOI] [PubMed] [Google Scholar]
- Hirota K., Yoshitomi H., Hashimoto M., Maeda S., Teradaira S., Sugimoto N., Yamaguchi T., Nomura T., Ito H., Nakamura T., et al. 2007. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model.J. Exp. Med. 204:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Rodriguez E.V., Napolitani G., Lanzavecchia A., Sallusto F. 2007. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells.Nat. Immunol. 8:942–949 [DOI] [PubMed] [Google Scholar]
- Chen Z., Tato C.M., Muul L., Laurence A., O'Shea J.J. 2007. Distinct regulation of interleukin-17 in human T helper lymphocytes.Arthritis Rheum. 56:2936–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy M.J., Cua D.J. 2007. The link between IL-23 and Th17 cell-mediated immune pathologies.Semin. Immunol. 19:372–376 [DOI] [PubMed] [Google Scholar]
- Sheibanie A.F., Khayrullina T., Safadi F.F., Ganea D. 2007. Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis.Arthritis Rheum. 56:2608–2619 [DOI] [PubMed] [Google Scholar]
- Sheibanie A.F., Yen J.H., Khayrullina T., Emig F., Zhang M., Tuma R., Ganea D. 2007. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23→IL-17 axis.J. Immunol. 178:8138–8147 [DOI] [PubMed] [Google Scholar]
- Sheibanie A.F., Tadmori I., Jing H., Vassiliou E., Ganea D. 2004. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells.FASEB J. 18:1318–1320 [DOI] [PubMed] [Google Scholar]
- Chizzolini C., Chicheportiche R., Alvarez M., de Rham C., Roux-Lombard P., Ferrari-Lacraz S., Dayer J.M. 2008. Prostaglandin E2 (PGE2) synergistically with interleukin-23 (IL-23) favors human Th17 expansion.Blood. 112:3696–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Rodriguez E.V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells.Nat. Immunol. 8:639–646 [DOI] [PubMed] [Google Scholar]
- Regan J.W. 2003. EP2 and EP4 prostanoid receptor signaling.Life Sci. 74:143–153 [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells.Cell. 126:1121–1133 [DOI] [PubMed] [Google Scholar]
- Khayrullina T., Yen J.H., Jing H., Ganea D. 2008. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells.J. Immunol. 181:721–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner M., Stilper A., Morhart P., Holter W. 2008. Plasticity of dendritic cell function in response to prostaglandin E2 (PGE2) and interferon-gamma (IFN-gamma).J. Leukoc. Biol. 83:883–893 [DOI] [PubMed] [Google Scholar]
- Jankovic D., Trinchieri G. 2007. IL-10 or not IL-10: that is the question.Nat. Immunol. 8:1281–1283 [DOI] [PubMed] [Google Scholar]
- McGeachy M.J., Bak-Jensen K.S., Chen Y., Tato C.M., Blumenschein W., McClanahan T., Cua D.J. 2007. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology.Nat. Immunol. 8:1390–1397 [DOI] [PubMed] [Google Scholar]
- Snijdewint F.G., Kalinski P., Wierenga E.A., Bos J.D., Kapsenberg M.L. 1993. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes.J. Immunol. 150:5321–5329 [PubMed] [Google Scholar]
- Katamura K., Shintaku N., Yamauchi Y., Fukui T., Ohshima Y., Mayumi M., Furusho K. 1995. Prostaglandin E2 at priming of naive CD4+ T cells inhibits acquisition of ability to produce IFN-gamma and IL-2, but not IL-4 and IL-5.J. Immunol. 155:4604–4612 [PubMed] [Google Scholar]
- Hilkens C.M., Snijders A., Snijdewint F.G., Wierenga E.A., Kapsenberg M.L. 1996. Modulation of T-cell cytokine secretion by accessory cell-derived products.Eur. Respir. J. Suppl. 22:90s–94s [PubMed] [Google Scholar]
- Oppmann B., Lesley R., Blom B., Timans J.C., Xu Y., Hunte B., Vega F., Yu N., Wang J., Singh K., et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12.Immunity. 13:715–725 [DOI] [PubMed] [Google Scholar]
- Liu H., Rohowsky-Kochan C. 2008. Regulation of IL-17 in human CCR6+ Effector memory T cells.J. Immunol. 180:7948–7957 [DOI] [PubMed] [Google Scholar]
- Manel N., Unutmaz D., Littman D.R. 2008. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat.Nat. Immunol. 9:641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells.Nature. 441:235–238 [DOI] [PubMed] [Google Scholar]
- Mangan P.R., Harrington L.E., O'Quinn D.B., Helms W.S., Bullard D.C., Elson C.O., Hatton R.D., Wahl S.M., Schoeb T.R., Weaver C.T. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage.Nature. 441:231–234 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells.Immunity. 24:179–189 [DOI] [PubMed] [Google Scholar]
- Sutton C., Brereton C., Keogh B., Mills K.H., Lavelle E.C. 2006. A crucial role for interleukin (IL)-1 in the induction of IL-17–producing T cells that mediate autoimmune encephalomyelitis.J. Exp. Med. 203:1685–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton J.E., Keating G.M. 2007. Celecoxib: a review of its use in the management of arthritis and acute pain.Drugs. 67:2433–2472 [DOI] [PubMed] [Google Scholar]
- Geusens P., Lems W. 2008. Efficacy and tolerability of lumiracoxib, a highly selective cyclo-oxygenase-2 (COX2) inhibitor, in the management of pain and osteoarthritis.Ther. Clin. Risk Manag. 4:337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J., Shu Y.Y., Zhu Y.N., Fu Y.F., Tang W., Zhong X.G., Wang H., Yang Y.F., Ren J., Wang M.W., Zuo J.P. 2007. COX-2 inhibitors ameliorate experimental autoimmune encephalomyelitis through modulating IFN-gamma and IL-10 production by inhibiting T-bet expression.J. Neuroimmunol. 186:94–103 [DOI] [PubMed] [Google Scholar]
- Anderson G.D., Hauser S.D., McGarity K.L., Bremer M.E., Isakson P.C., Gregory S.A. 1996. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis.J. Clin. Invest. 97:2672–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino H., Salvi S., Regan J.W. 2005. Differential regulation of phosphorylation of the cAMP response element-binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2.Mol. Pharmacol. 68:251–259 [DOI] [PubMed] [Google Scholar]
- Niho Y., Niiro H., Tanaka Y., Nakashima H., Otsuka T. 1998. Role of IL-10 in the crossregulation of prostaglandins and cytokines in monocytes.Acta Haematol. 99:165–170 [DOI] [PubMed] [Google Scholar]
- Riese U., Brenner S., Docke W.D., Prosch S., Reinke P., Oppert M., Volk H.D., Platzer C. 2000. Catecholamines induce IL-10 release in patients suffering from acute myocardial infarction by transactivating its promoter in monocytic but not in T-cells.Mol. Cell. Biochem. 212:45–50 [PubMed] [Google Scholar]
- Kohler R.E., Caon A.C., Willenborg D.O., Clark-Lewis I., McColl S.R. 2003. A role for macrophage inflammatory protein-3 alpha/CC chemokine ligand 20 in immune priming during T cell-mediated inflammation of the central nervous system.J. Immunol. 170:6298–6306 [DOI] [PubMed] [Google Scholar]
- Ruth J.H., Shahrara S., Park C.C., Morel J.C., Kumar P., Qin S., Koch A.E. 2003. Role of macrophage inflammatory protein-3alpha and its ligand CCR6 in rheumatoid arthritis.Lab. Invest. 83:579–588 [DOI] [PubMed] [Google Scholar]
- Homey B., Dieu-Nosjean M.C., Wiesenborn A., Massacrier C., Pin J.J., Oldham E., Catron D., Buchanan M.E., Muller A., deWaal Malefyt R., et al. 2000. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis.J. Immunol. 164:6621–6632 [DOI] [PubMed] [Google Scholar]
- Murphy C.A., Langrish C.L., Chen Y., Blumenschein W., McClanahan T., Kastelein R.A., Sedgwick J.D., Cua D.J. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation.J. Exp. Med. 198:1951–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation.J. Exp. Med. 201:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmi L., De Palma R., Santarlasci V., Maggi L., Capone M., Frosali F., Rodolico G., Querci V., Abbate G., Angeli R., et al. 2008. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor.J. Exp. Med. 205:1903–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschek M.A., Boniface K., Sadekova S., Grein J., Murphy E.E., Turner S.P., Raskin L., Desai B., Faubion W.A., de Waal Malefyt R., et al. 2009. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation.J. Exp. Med. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.O., Flavell R.A. 2008. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10.Immunity. 28:468–476 [DOI] [PubMed] [Google Scholar]
- Kuroda E., Sugiura T., Zeki K., Yoshida Y., Yamashita U. 2000. Sensitivity difference to the suppressive effect of prostaglandin E2 among mouse strains: a possible mechanism to polarize Th2 type response in BALB/c mice.J. Immunol. 164:2386–2395 [DOI] [PubMed] [Google Scholar]
- Santarlasci V., Maggi L., Capone M., Frosali F., Querci V., De Palma R., Liotta F., Cosmi L., Maggi E., Romagnani S., Annunziato F. 2009. TGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells.Eur. J. Immunol. 39:207–215 [DOI] [PubMed] [Google Scholar]
- Campbell J.J., Qin S., Bacon K.B., Mackay C.R., Butcher E.C. 1996. Biology of chemokine and classical chemoattractant receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells.J. Cell Biol. 134:255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E.P., Campbell J.J., Soler D., Dong Z., Manlongat N., Picarella D., Hardy R.R., Butcher E.C. 2000. Developmental switches in chemokine response profiles during B cell differentiation and maturation.J. Exp. Med. 191:1303–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]