Abstract
Lack of immunological tolerance against self-antigens results in autoimmune disorders. During onset of autoimmunity, dendritic cells (DCs) are thought to be critical for priming of self-reactive T cells that have escaped tolerance induction. However, because DCs can also induce T cell tolerance, it remains unclear whether DCs are required under steady-state conditions to prevent autoimmunity. To address this question, we crossed CD11c-Cre mice with mice that express diphtheria toxin A (DTA) under the control of a loxP-flanked neomycin resistance (neoR) cassette from the ROSA26 locus. Cre-mediated removal of the neoR cassette leads to DTA expression and constitutive loss of conventional DCs, plasmacytoid DCs, and Langerhans cells. These DC-depleted (ΔDC) mice showed increased frequencies of CD4 single-positive thymocytes and infiltration of CD4 T cells into peripheral tissues. They developed spontaneous autoimmunity characterized by reduced body weight, splenomegaly, autoantibody formation, neutrophilia, high numbers of Th1 and Th17 cells, and inflammatory bowel disease. Pathology could be induced by reconstitution of wild-type (WT) mice with bone marrow (BM) from ΔDC mice, whereas mixed BM chimeras that received BM from ΔDC and WT mice remained healthy. This demonstrates that DCs play an essential role to protect against fatal autoimmunity under steady-state conditions.
The adaptive immune system can respond to a huge variety of pathogens as a result of a broad repertoire of antigen receptors on T and B cells generated by genomic recombination during development of these cells. To avoid autoimmune reactions, self-reactive lymphocytes have to be deleted or rendered tolerant. Normal polyclonal and self-tolerant T cell repertoires depend on positive and negative selection of developing T cells in the thymus. Positive selection is mediated by thymic cortical epithelial cells, whereas negative selection can occur in the cortex or in the medulla and is induced by both BM–derived cells and medullary thymic epithelial cells (1–5). It has been demonstrated that thymic DCs are very efficient in mediating negative selection of developing thymocytes (5–9). Furthermore, peripheral DCs can migrate to the thymus and contribute to negative selection (9, 10). However, because B cells (11), and perhaps other cells of hematopoietic origin, could also be involved in negative selection, it remains unclear whether a selective lack of DCs would result in impaired clonal deletion and release of self-reactive T cells into the periphery. Self-reactive T cells that escaped clonal deletion in the thymus need to be further controlled by peripheral tolerance mechanisms to prevent tissue damage (12). Under steady-state conditions, DCs are thought to play an important role in peripheral tolerance induction by various mechanisms, including production of soluble factors like IL-10, TGF-β or indoleamine 2,3-dioxygenase (13–15), induction of T reg cells (16–18), and initiation of abortive T cell proliferation resulting in clonal deletion of autoreactive T cells (19, 20). However, it remains unclear whether DCs are required to protect from spontaneous onset of autoimmunity. To address this important question, we generated constitutively DC-depleted mice. These mice rapidly developed spontaneous autoimmunity, which demonstrates for the first time that DCs are essential to maintain a self-tolerant immune system.
RESULTS
Efficient ablation of DCs in CD11c-Cre/R–diptheria toxin A (DTA) mice
To determine the role of DCs for maintenance of self-tolerance, we bred mice that selectively express the Cre recombinase in DCs (CD11c-Cre mice) (21) with a strain carrying the diphtheria toxin α chain (DTA) under control of a loxP-flanked stop cassette in the ubiquitously expressed ROSA26 locus (R-DTA mice) (22). As a consequence, DTA is expressed directly in DCs causing their constitutive elimination.
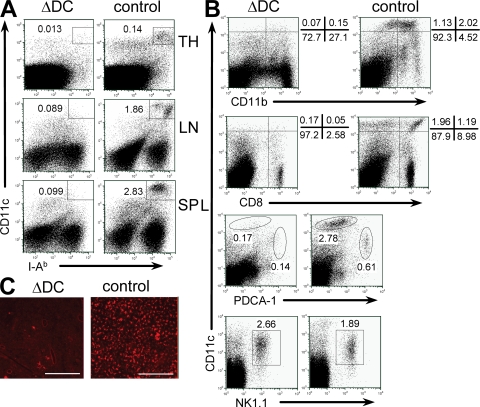
CD11c-Cre/R-DTA mice (ΔDC mice, for short) lack >90% of DCs in thymus, spleen, and LNs (Fig. 1 A). Ablation affected all major DC subsets, including myeloid, lymphoid, and plasmacytoid DCs, whereas the recently described interferon-producing killer DC population (IKDC; CD11clo NK1.1+B220+) (23, 24) was not affected (Fig. 1 B). Furthermore, only few remaining Langerhans cells were detectable in epidermal sheaths of the ear from ΔDC mice (Fig. 1 C). DCs can efficiently present foreign antigens and prime naive T cells. To determine whether ΔDC mice are impaired in generating a primary immune response, we analyzed the efficiency of CD4 T cell priming in ΔDC mice by adoptive transfer of OVA-specific TCR transgenic CD4 T cells (OT-II) followed by vaccination with MVA-OVA (25), a modified vaccinia virus Ankara which encodes chicken ovalbumin complementary DNA. At the peak of T cell expansion, 4 d after vaccination, total cell counts of transferred OT-II cells in the spleen of ΔDC mice were fourfold lower as compared with control mice (Fig. 2 A). In addition, OT-II cells in ΔDC mice were only partially activated, which is indicated by inefficient down-regulation of the surface marker CD62L (Fig. 2 B). Low expansion of OT-II cells was also observed when DC-depleted OT-II cells were transferred, indicating that remaining DCs or other APCs were able to induce a weak CD4 T cell proliferation (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20082394/DC1). Indeed, purified B cells, macrophages, or deletion-resistent DCs were all able to stimulate OT-II cell proliferation in vitro (Fig. S2). We further observed only a weak OVA-specific response of CD8 T cells upon MVA-OVA immunization of ΔDC mice (Fig. 2, C and D). To determine the requirement of DCs for generation of an efficient immune response against gastrointestinal nematodes, we infected ΔDC mice with the helminth Nippostrongylus brasiliensis. Adult worms were efficiently cleared from WT mice, whereas ΔDC mice could not eliminate the parasites (Fig. 2 E). Furthermore, N. brasiliensis-infected ΔDC mice showed impaired recruitment of eosinophils to the lung (Fig. 2 F). These immunization and infection experiments clearly demonstrate that DCs are required for efficient priming of naive T cells and execution of protective immune responses, which is consistent with their role as major APCs of the immune system. Next, we addressed the question of whether DCs are required for maintenance of immunological tolerance against self-antigens.
Figure 1.
Efficiency of DC depletion in ΔDC mice. (A) Single cell suspensions of thymus (TH), spleen (SP), and mesenteric LN from ΔDC and control mice were stained for CD11c and MHC class II (I-Ab). (B) Staining of DC subsets in the spleen of ΔDC or control mice. Dot plots are gated on Gr-1− cells. The CD11c versus NK1.1 plots are additionally gated on B220+ cells. (C) Epithelial layers of the ears of indicated mice were stained for I-Ab to detect Langerhans cells. Original magnification was 80×. The results are representative of two to three independent experiments. Bars, 300 µm.
Figure 2.
Impaired immune response in ΔDC mice. (A) Frequency of OT-II cells in the spleen after adoptive transfer and before (open bars) or 4 d after (filled bars) MVA-OVA immunization of ΔDC or control mice. Pooled results are from two separate experiments. n = 4; *, P < 0.028. (B) Transferred OT-II cells (Thy1.1+) are less activated (CD62Lneg) in ΔDC as compared with control mice. (C) Kinetics of expansion of Kb-OVA257-264 pentamer-specific CD8 T cells in ΔDC mice (open circles) or controls (filled circles) after immunization with MVA-OVA. n = 3 ΔDC mice and 2 controls. Error bars show SD. (D) Dot plots are gated on CD8+ cells and show staining of Kb-OVA257-264 pentamers versus CD62L. (E) Number of adult worms in the small intestine of four ΔDC mice or controls on day 10 after N. brasiliensis infection from two independent experiments. The filled circles show worm counts from individual mice. The bars show the means. (F) Frequency of CD4 T cells (CD4+Siglec-F−) and eosinophils (CD4−Siglec-F+) in dispersed total lung tissue of ΔDC mice and controls. Dot plots are representative of four mice per group.
Impaired negative selection of CD4 T cells in ΔDC mice
DCs can mediate clonal deletion of self-reactive T cells in the thymus (5–10). To determine whether the reduced number of DCs in ΔDC mice results in impaired negative selection, we analyzed the frequency of different thymocyte populations in ΔDC and control mice. ΔDC mice showed 30% higher frequencies and total numbers of CD4 single-positive (SP) thymocytes, whereas CD8 SP cells were not increased (Fig. 3, A and B; and Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20082394/DC1). The TCR expression level on SP thymocytes was comparable between ΔDC and control mice (Fig. S3). Furthermore, the expression levels of CD5, CD24, and CD69 on CD4 or CD8 SP thymocytes were comparable between ΔDC and control mice, indicating normal thymocyte maturation (unpublished data). The increase of CD4 SP cells was not a result of recirculation of peripheral CD4 T cells because ΔDC mice showed no significant increase of Qa2+CD24lo cells (Fig. S4) (26, 27). We generated BM chimeras to determine whether the increased frequency of CD4 SP cells is linked to hematopoietic cells and whether it can be prevented by coadministration of BM from WT mice. Lethally irradiated WT mice received either BM from ΔDC mice or WT mice (single chimeras) or a 1:1 mixture of BM from ΔDC and WT donors (mixed chimeras). 9 wk after reconstitution, the frequency of CD4 SP cells in ΔDC→WT chimeras was 21%, as compared with 11% in mixed ΔDC + WT→WT chimeras or WT→WT chimeras (Fig. 3 C). The ratio of CD4 SP/CD8 SP thymocytes was significantly increased in ΔDC mice and ΔDC→WT chimeras as compared with WT mice or mixed chimeras (Fig. S5). This supports the concept that DCs contribute mainly to clonal deletion of CD4 but not CD8 T cells (28), although thymic epithelial cells can also mediate negative selection of both T cell subsets (29, 30).
Figure 3.
T cell development in ΔDC mice. (A and B) Frequency of SP thymocytes in ΔDC mice (filled bars) compared with controls (open bars). Pooled results are from three independent experiments. n = 6; *, P = 0.001. The filled circles show results from individual mice. The bars show the means. (C) Flow cytometric analysis of thymocytes from indicated BM chimeras. (D) Frequency of Vβ3+, Vβ11+, Vβ12+, and Vβ2+ cells among CD4 SP thymocytes from 5-wk-old ΔDC mice (filled bars) or R-DTA control mice (open bars) relative to C57BL/6 controls. Pooled results are from two independent experiments. n = 3; *, P = 0.01. (E) Frequency of T reg cells in the thymus of ΔDC or control mice. Dot plots are gated on CD4+CD8− thymocytes and representative of two independent experiments.
To further substantiate these findings, we analyzed superantigen (sAg)-induced deletion of CD4 SP cells in ΔDC mice. To this end, CD11c-Cre mice on C57BL/6 background were crossed once with R-DTA mice on a BALB/c background carrying the endogenous retroviruses Mtv-6, 8, and 9, which encode sAgs that cause deletion of Vβ3+ (Mtv-6) or Vβ11+/Vβ12+ (Mtv-8/9) thymocytes. Because only Mtv-6 is expressed by thymic DCs (31), we expected impaired deletion of Vβ3+ CD4 SP thymocytes in ΔDC mice, whereas deletion of Vβ11+ and Vβ12+ thymocytes should not be affected. Indeed, ∼60% of Vβ3+ CD4 SP cells was deleted in DC-sufficient mice whereas only 25% was deleted in ΔDC mice (Fig. 3 D). In contrast, negative selection of Mtv-8/9–responsive Vβ11+ and Vβ12+ cells appeared to be DC independent. It has been estimated that 50–60% of initially positively selected CD4 T cells are subsequently deleted by hematopoietic cells (29). As we detected only a 30% increase of CD4 SP cells in ΔDC mice, it remains possible that other cell types, including thymic B cells, also participate in negative selection (11). We found no difference in the frequency of natural T reg cells in the thymus of ΔDC mice as compared with control mice (Fig. 3 E), which supports previous observations demonstrating that intrathymic natural T reg cell development occurs independently of MHC class II expression on BM-derived cells (32, 33).
ΔDC mice develop severe pathology
ΔDC mice were born at the expected Mendelian ratio but they appeared smaller in size, had a hunched posture, and their body weight was reduced by ∼30% as compared with controls at 6 wk of age (Fig. 4 A). 40% of the mice died within 8 wk of age (Fig. 4 B). Macroscopic analysis of peripheral lymphoid organs of 6–8-wk-old ΔDC mice revealed that spleen and LNs were enlarged (Fig. 4 C). Small intestine and colon were inflamed and the fat pads were missing (Fig. 4 D). Histological analysis of colon, small intestine, kidney, and liver revealed the presence of cellular infiltrates (Fig. 4 E).
Figure 4.
Phenotypic characterization of ΔDC mice. (A) Body weight of ΔDC mice (filled circles) and controls (open circles) between 4 and 15 wk of age. n = 3–6 per time point. Error bars show SD. (B) Survival of ΔDC mice (thick line; n = 12) and controls (thin line; n = 10). (C) Size of spleen (SP), inguinal LN (ILN), and mesenteric LN (MLN) of indicated mice on a metric scale. (D) Picture of an 8-wk-old ΔDC mouse and a negative littermate demonstrating splenomegaly, intestinal inflammation, and lack of the fat pad in the ΔDC mouse. The green line indicates the small intestine and the blue line indicates the large intestine. SP, spleen; FP, fat pad. (E) Histology of tissue sections from indicated organs from 8-wk-old ΔDC or control mice demonstrating cellular infiltrates in tissues from ΔDC mice. Bars, 400 µm. The experiment has been repeated with similar results.
To determine whether splenomegaly was caused by selective expansion of a distinct cell type, we analyzed the main leukocyte populations in the spleen by flow cytometry. The total number of CD4 T cells, CD8 T cells, and B cells was not significantly different between ΔDC and control mice (Fig. 5 A). However, the number of F4/80+ macrophages was increased twofold and Gr-1+ cells increased more than 20-fold (Fig. 5 A). Further analysis revealed that the expanded Gr-1+ population consisted mainly of Gr-1hi cells, which represent neutrophils with segmented nuclei (Fig. S6, A and B, available at http://www.jem.org/cgi/content/full/jem.20082394/DC1). These cells could also be found in peripheral organs like kidney, liver, small intestine, and colon of ΔDC mice (Fig. S6 C). To exclude the possibility that unspecific expression of DTA in nonhematopoietic cells was responsible for these phenotypic alterations and to determine whether pathology can be prevented in the presence of DCs, we generated single ΔDC→WT and mixed ΔDC + WT→WT BM chimeras. Importantly, reduced body weight and neutrophilia was only observed in single chimeras and not in mixed chimeras, suggesting that DCs originating from WT BM provide protection from spontaneous development of pathology (Fig. 5 C).
Figure 5.
Expansion of Gr-1+ cells in ΔDC mice. (A) Cell counts of indicated populations in the spleen of 6–8-wk-old ΔDC mice (filled bars) or controls (open bars). Pooled results are from four independent experiments. n = 3–5; *, P = 0.02; **, P = 0.001. (B) Body weight and number of Gr-1+ cells in the spleen of ΔDC→WT (hatched gray bars) or ΔDC + WT→WT (hatched white bars) BM chimeras. Pooled results are from two independent experiments. n = 3–5; *, P = 0.001; **, P = 0.011. The filled circles show results from individual mice. The bars show the means.
High frequency of IL-17A– and IFN-γ–producing CD4 T cells in ΔDC mice
Because ΔDC mice seem to be impaired in negative selection of CD4 T cells, we considered it likely that pathology was caused by autoreactive CD4 T cells that left the thymus and could not be controlled by peripheral tolerance mechanisms. Flow cytometric analysis of peripheral T cells from 6–8-wk-old ΔDC mice revealed that CD4 T cells (but not CD8 T cells) showed an increased frequency of activated cells (CD62LloCD44hi) (Fig. 6 A). The frequency of IFN-γ– and IL-17A–producing cells was increased ∼10-fold in ΔDC as compared with control mice, which indicates that CD4 T cells had differentiated toward a Th1 or Th17 effector phenotype, respectively (Fig. 6, B and C). It has been shown that Th17 cells are associated with autoimmune disorders (34) and promote neutrophilia (35), which is consistent with the high number of neutrophils in ΔDC mice. Interestingly, the spontaneous increase of Th1 and Th17 cells occurred despite an almost normal frequency of peripheral T reg cells (Fig. 6 B). Analysis of the TCR-Vβ repertoire revealed no major differences between ΔDC and control mice, which demonstrates that the effector T cell populations in ΔDC mice did not result from oligoclonal T cell expansion (Fig. S7, available at http://www.jem.org/cgi/content/full/jem.20082394/DC1).
Figure 6.
Phenotype of T cells in ΔDC mice. (A) Frequency of activated (CD44hiCD62Llo) T cells in the spleen of indicated mice. Bars show the mean frequency of activated T cells from 6–8-wk-old ΔDC mice (filled bars) or control mice (open bars). Pooled results are from three independent experiments. n = 5; *, P = 0.0003. (B and C) Intracellular cytokine staining of PMA/ionomycin restimulated CD4 T cells and CD25/Foxp3 staining of untreated CD4 T cells isolated from mesenteric LNs (B) or peripheral organs (C) of indicated mice. Bars show the mean frequency of indicated T cell subsets. Pooled results are from three independent experiments. n = 4; *, P = 0.02; **, P = 0.01. The filled circles show results from individual mice.
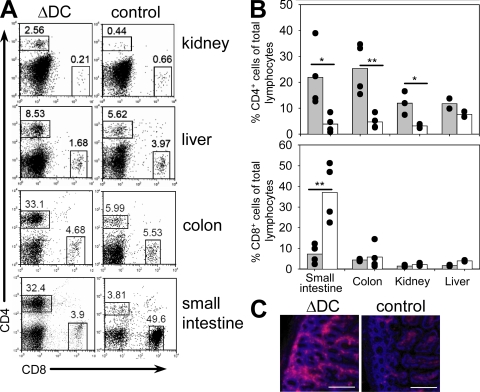
Next, we determined the infiltration of T cells into various peripheral tissues. Significantly increased frequencies of CD4 T cells were observed in small intestine, colon, and kidney of ΔDC as compared with control mice, which suggests that pathology might be caused by tissue infiltration of autoreactive CD4 T cells (Fig. 7). The frequency of T reg cells among total lymphocytes in the small intestine was increased in ΔDC as compared with control mice and, therefore, the inflammatory immune response does not seem to be caused by a local lack of T reg cells in ΔDC mice (Fig. S8, available at http://www.jem.org/cgi/content/full/jem.20082394/DC1). We also observed a significant reduction of CD8 T cells in the small intestine of ΔDC mice, which could at least partially be explained by infiltration of large numbers of CD4 T cells resulting in a relative decrease of other lymphocyte populations. In addition, DCs in the small intestine might be required for survival or recruitment of CD8 T cells. Increased CD4 T cell infiltration was also observed in single ΔDC→WT BM chimeras as compared with mixed ΔDC + WT→WT BM chimeras, providing further evidence that DCs are required to prevent spontaneous CD4 T cell activation and infiltration of peripheral tissues (Fig. S9).
Figure 7.
Infiltration of CD4 T cells in peripheral organs. (A and B) Frequency of CD4 and CD8 T cells in indicated organs of 6–8-wk-old ΔDC or control mice. Pooled results are from four independent experiments. n = 4; *, P < 0.05; **, P < 0.01. The filled circles show results from individual mice. The bars show the means. (C) Immunofluorescent analysis of CD4 T cell infiltrations in the lamina propria of the small intestine of indicated mice. Bars, 200 µm.
Hyperimmunoglobulinemia and generation of autoantibodies in ΔDC mice
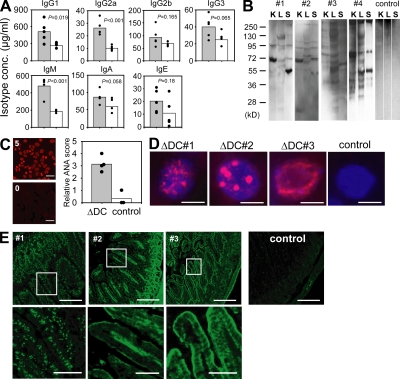
Autoimmunity is often associated with high levels of immunoglobulins and generation of autoantibodies. To determine whether a similar activation of the humoral immune response occurred in ΔDC mice despite the near complete ablation of DCs, we measured the concentration of immunoglobulin isotypes in the serum. All isotypes were elevated with the largest increase observed for IgM and IgG2a (Fig. 8 A). To determine whether ΔDC mice generated autoantibodies, we performed Western blot analysis where protein extracts from kidney, liver, and spleen of Rag-deficient mice was probed with serum from four individual ΔDC mice and one negative littermate as control. Several distinct bands could be observed that varied between different tissues (Fig. 8 B). Individual mice showed only partially overlapping patterns, indicating that autoantigens from individual mice recognized different self-antigens. Staining of HEp-2 cells with serum from ΔDC and control mice revealed the presence of antinuclear antibodies (ANAs) in ΔDC mice (Fig. 8 C). To further define whether ANAs from individual mice recognize different nuclear antigens, we stained tissue sections from the liver of Rag-deficient mice with sera from ΔDC mice. We observed a variety of staining patterns of subnuclear structures with sera from individual mice, which complements the result of the Western blot analysis (Fig. 8 D). Because intestinal inflammation was observed in most ΔDC mice, we further analyzed the staining pattern of autoantibodies directed against intestinal antigens. Tissue sections from the small intestine of Rag-deficient mice were stained with serum from three individual ΔDC mice. The sera stained either nuclei (mouse #1), the lamina propria (mouse #2), or the epithelial layer (mouse #3), whereas no staining was observed with serum from negative littermates (Fig. 8 E). Collectively, ΔDC mice generated a variety of autoantibodies directed against nuclear and tissue-specific antigens with specificities that differed between individual mice.
Figure 8.
Hyperimmunoglobulinemia and autoantibodies in ΔDC mice. (A) Immunoglobulin isotype concentrations in the serum of ΔDC mice (filled bars) or controls (open bars). n = 5 from two independent experiments. (B) Western blot analysis showing the staining pattern of autoantibodies present in the serum of four individual ΔDC mice and one control on total cell extracts of kidney (K), liver (L), and spleen (S) from Rag-deficient mice. (C) ANAs were scored between 0 (negative control serum from WT mice) and 5 (positive control serum from MRL/lpr mice) by determining the staining intensity on Hep2 cells. Filled bar, serum from ΔDC mice; open bar, serum from control mice. Bars, 10 µm. The filled circles show results from individual mice. The bars show the means. (D) Different staining pattern of nuclear structures by ANAs (red) from three individual ΔDC mice and a negative littermate control on liver sections from Rag-deficient mice. DAPI staining is shown in blue. Original magnification was 480×. Bars, 5 µm. (E, top) Staining pattern of autoantibodies from three individual ΔDC mice (#1–3) and a negative littermate on sections from the small intestine of Rag-deficient mice. Bars, 200 µm. (E, bottom) Magnified view of the areas indicated in the top. Bars, 40 µm.
DISCUSSION
In this paper, we demonstrate for the first time that constitutively DC-depleted mice develop spontaneous severe autoimmune disease. It seems counterintuitive that lack of DCs, the most potent APC type, results in autoimmunity. Indeed, transiently DC-depleted adult mice have not been reported to develop such pathological disorders (36), which suggests that either presence of DCs in the young animal is sufficient to promote long-term immune tolerance or that spontaneous priming and expansion of self-reactive T cells in the periphery can only occur when DCs are depleted for a prolonged period of time. The same strain of CD11cCre mice that we used in this study to generate ΔDC mice has also been crossed to another strain of conditional ROSA26-DTA mice that encodes DTA and the neomycin resistance gene behind a loxP-flanked LacZ cassette (37). These mice developed a myeloid proliferative syndrome indicated by an increased frequency of Gr-1+ cells in a manner similar to that in ΔDC mice (38). However, they remained healthy and no signs of autoimmunity were reported. This apparent discrepancy may be explained by the fact that plasmacytoid DCs and Langerhans cells were not ablated in these mice so that immune tolerance may still be functional. Both plasmacytoid DCs and Langerhans cells have been shown to provide powerful tolerogenic signals to prevent activation of alloreactive or allergen-specific T cells, so it seems likely that they also control self-reactive T cells (39–42).
Negative selection by DCs in the thymus is a very efficient process. Using reaggregate cultures, it has been demonstrated that central tolerance against alloantigens can be achieved by only one DC per 200 thymocytes (6). Furthermore, deletion of peptide MHC-specific TCR transgenic cells was still efficient when the frequency of thymic APCs was lowered to ∼1% of normal numbers (8). Therefore, the potential requirement of DCs for negative selection can only be observed in mice with almost complete depletion of thymic DCs. The efficient ablation of thymic DCs in ΔDC mice may result in release of autoreactive T cells into the periphery where they could be primed by the few remaining DCs or other APCs. Importantly, autoimmunity was not caused by unspecific DTA expression in nonhematopoietic cells or by bystander toxicity of DTA released from dieing DCs because autoimmunity could be induced by reconstitution of WT mice with BM from ΔDC mice but not by cotransfer of BM from WT and ΔDC mice. The role of DCs in regulation of autoimmunity remains controversial. It has been shown that extending the lifespan of DCs can also result in autoimmunity in some (43, 44) but not all transgenic models (45).
Why does autoimmunity develop when the life span of DCs is shortened or prolonged? When DC life span is prolonged, they might increase their expression of costimulatory molecules which could lead to priming of self-reactive T cells (43). Our results indicate that if DC life span is too short so that they cannot build up a normal-sized pool of DCs, tolerance induction of CD4 T cells is impaired. This can occur at two levels: in the thymus (impaired negative selection) and in the periphery (lack of tolerogenic DCs). We observed that ΔDC mice have significantly more CD4 SP thymocytes as compared with control mice. In addition, ΔDC mice show less efficient deletion by DC-restricted endogenous retroviral sAgs. Both observations are consistent with the view that DCs play an important role in negative selection of CD4 T cells. Mice that express MHC class II only on thymic cortical epithelial cells generate autoreactive CD4 T cells, yet these mice do not develop spontaneous autoimmunity as a result of lack of MHC class II expression on APCs (3). Transfer of these autoreactive T cells into nonirradiated WT mice results in only mild autoimmunity, which is different from the severe phenotype of ΔDC mice we describe in this paper (46). Therefore, it seems likely that in addition to their important role in negative selection, DCs might be required to maintain peripheral tolerance. This assumption is further supported by our recent observation that autoreactive T cells accumulated in mice with DCs that were defective for uptake of apoptotic cells (47).
It remains unclear at the moment whether the activated CD4 T cells were primed by the few remaining DCs or by other hematopoietic MHC class II+ APCs, such as B cells or macrophages, or whether they recognize MHC class II directly on tissue cells such as enterocytes (48–50). This latter possibility might explain why the intestine is particularly vulnerable to fatal autoimmune attack. However, it also remains possible that the barrier function of the intestines is compromised in ΔDC mice, which might result in a local inflammatory response by commensal gut flora and subsequently initiate autoimmunity. Marguerat et al. (50) have shown that ulcerative colitis develops in MHC class II KO→WT BM chimeras at 6–8 wk after reconstitution with heavy T cell infiltration into the lamina propria, which supports the possibility that antigen presentation by nonhematopoietic cells might be sufficient for priming of autoreactive T cells. In addition, conditional ablation of MHC class II in hematopoietic and endothelial cells resulted in a similar increase of CD4 SP thymocytes to that described in this paper for ΔDC mice (51). However, peripheral T cells were not spontaneously activated, suggesting that priming of autoreactive T cells requires MHC class II expression on hematopoietic or endothelial cells.
It has been demonstrated that mice lacking integrin αvβ8 on DCs have reduced frequencies of T reg cells in the colon and develop late onset of autoimmune colitis (15). Furthermore, depletion of T reg cells results in increased numbers and spontaneous activation of DCs under steady-state conditions and fatal autoimmunity (52). These results suggested that T reg cells prevent autoimmunity by modulation of DC activation. This model does not apply to the situation described in this paper because peripheral T reg cells were reduced only by 20–30% in spleen and LNs of ΔDC mice and autoimmunity developed despite (and as a result of) the absence of DCs. However, it remains possible that the repertoire of T reg cells is altered or that a critical subpopulation of T reg cells is missing in ΔDC mice so that some autoreactive T cells may escape the control by T reg cells. The spontaneously autoreactive T cells observed in ΔDC mice illustrate that DCs play an important role for tolerance induction at multiple levels throughout T cell development and homeostasis. Thymic DCs remove thymocytes with a high TCR avidity for self-antigens. As a result, peripheral T cells express TCRs with relatively low avidity for self-antigens but they probably still need to be kept under control by peripheral DCs to avoid autoimmunity.
Collectively, our results demonstrate for the first time that DCs play a central role in preventing spontaneous autoimmunity under steady-state conditions and suggest a functional coevolution between the T cell compartment and DCs. The result of this development is the efficient establishment of immune tolerance against self-antigens by DCs. In the absence of DCs, self-reactive CD4 T cells can become prevalent and induce devastating autoimmunity.
MATERIALS AND METHODS
Mice.
R-DTA mice were generated by insertion of the diphtheria toxin α subunit (DTA) into the genomic ROSA26 locus behind a loxP-flanked STOP cassette by homologous recombination in ES cells (129/Sv background) (22). Therefore, cells were immediately killed when they expressed the Cre recombinase. R-DTA mice were backcrossed four generations to C57BL/6 background. CD11c-Cre/R-DTA mice (ΔDC mice) were generated by crossing R-DTA mice (22) to CD11c-Cre mice (provided by B. Reizis, Columbia University, New York, NY) (21). Unless indicated otherwise, these mice were maintained on a mixed 129/Sv × C57BL/6 background. Control mice were negative littermates from this breeding. Rag-deficient mice on a C57BL/6 background were originally obtained from The Jackson Laboratory. Mice were housed according to institutional guidelines and used at 6–8 wk of age unless otherwise indicated. The animal experiments were approved by the Regierung von Oberbayern.
BM chimeras.
2 × 106 BM cells from indicated mice were injected into lethally (1,200 rad) irradiated recipient mice. Chimeras were kept with antibiotica containing drinking water (2 g/liter neomycin sulfate and 100 mg/liter polymyxin B). Mice were analyzed 9–10 wk after reconstitution.
OT-II transfer and MVA-OVA vaccination.
Total splenocytes from OT-II/Thy1.1 mice containing 1.5 × 106 TCR transgenic cells were transferred into ΔDC or control mice that were injected 1 d later i.v. with 107 infectious units of MVA-OVA (25). 4 d later, splenocytes were analyzed by staining with anti-CD4, anti-Thy1.1, and anti-CD62L to determine the frequency and activation status of transferred OT-II cells. To monitor the expansion of endogenous OVA-specific CD8 T cells, peripheral blood samples of a separate set of mice were stained with anti-CD8 and Kb-OVA257-264 pentamers (ProImmune) at different days after immunization with 107 infectious units of MVA-OVA.
N. brasiliensis infection.
Third-stage larvae (L3) of N. brasiliensis were washed extensively in sterile 0.9% saline at 37°C, and 500 organisms were injected s.c. into mice. Mice were provided with antibiotics-containing water (2 g/liter neomycin sulfate and 100 mg/liter polymyxin B sulfate; Sigma-Aldrich) for the first 5 d after infection.
Flow cytometry.
Single cell suspensions were prepared by collagenase digestion (small intestine, colon, and kidney), by liberase CI and DNase I digestion (Roche; Fig. 1), or by mechanical dispersion, incubated with anti-CD16/CD32 blocking antibody (2.4G2) for 5 min at room temperature, and stained with the corresponding antibody mixtures on ice. The following monoclonal antibodies were purchased from Invitrogen, unless otherwise indicated: PE–Alexa Fluor 700–labeled anti-CD4, APC- or FITC-labeled anti-CD8, biotinylated anti-CD11b (eBioscience), PE- or APC-labeled anti-CD11c (BD), APC-labeled anti-F4/80 (eBioscience), PE-labeled anti–Siglec-F (BD), PE-labeled anti-CD25 (eBioscience), PE-labeled anti-CD44, FITC-labeled anti-CD62L, Alexa Fluor 647–labeled anti-B220, biotinylated anti–Gr-1, biotinylated anti–MHC class II (clone M5/114.15.2; eBioscience), biotinylated anti-NK1.1 (clone PK136; eBioscience), FITC-labeled anti-TCR screening panel (BD), PE-Cy5.5–labeled streptavidin, and PE- or APC-labeled streptavidin (SouthernBiotech). CFSE staining was performed as previously described (53). Intracellular staining for Foxp3 was performed with the anti–mouse/rat Foxp3 staining set (eBioscience). Intracellular cytokine staining was performed with FITC-labeled anti–IFN-γ (XMG1.2; eBioscience), PE-labeled anti-IL-4 (BVD6-24G2; Invitrogen), and Alexa Fluor 647–labeled anti–IL-17A (eBioTC11-18H10.1; eBioscience) after cells had been stimulated for 4 h with 1 µg/ml ionomycin and 40 ng/ml PMA with Brefeldin A added at 5 µg/ml for the last 2 h. Cells were analyzed on a FACSCalibur instrument (BD).
Histology.
Cryosections of tissues fixed in 4% paraformaldehyde were stained with a Hemacolor staining kit for microscopy (Merck) according to manufacturer's instructions. Staining for Langerhans cells in the ear was performed as previously described (54), except that biotinylated anti–I-A/I-E (M5/114) and Alexa Fluor 555–labeled streptavidin were used for detection. CD4+ T cells in the small intestine were detected by staining 5-µm cryosections with biotinylated anti-CD4 (RM4-5; Invitrogen) followed by Cy3-conjugated streptavidin (Jackson ImmunoResearch Laboratories) for detection. ANAs were detected using HEp-2 cells fixed on glass slides (EUROIMMUN AG). The staining pattern of autoantibodies was determined by staining of tissue sections of the liver or small intestine from Rag-deficient mice with serum from ΔDC or control mice followed by goat anti–mouse Cy3. Pictures were acquired with a 10×/0.40 U Plan SApo or a 60×/1.35 U Plan SApo objective on a microscope (BX41; Olympus) equipped with a camera (F-View II; Olympus) and cell^F software (Olympus). Original magnification was 80 or 480×, respectively.
Western blot.
Tissue samples were homogenized on ice in lysis buffer containing 50 mM Tris, pH 8.0, 150 mM NaCl, 1% CHAP, and a protease inhibitor cocktail (Complete mini tablets). The lysates were centrifuged for 10 min at 18,600 g. The supernatants were mixed with sample buffer (62.5 mM Tris-HCL, pH 6.8, 25% glycerol, 2% SDS, and 5% β-mercaptoethanol), separated by a 10% SDS-PAGE, and 0.2 µm were electrophoretically transferred onto an Immun-Blot PVDF membrane (Bio-Rad Laboratories). The membranes were blocked with 5% nonfat dried milk in PBS for 1 h, washed with PBS/0.05% Tween20, and incubated for 1 h in a 1:100 dilution of sera from individual mice. After washing with PBS/Tween20, the bound antibodies were reacted with donkey anti–mouse IgG HRP (1:3,000; Jackson ImmunoResearch Laboratories) for 1 h and revealed with a chemiluminescence reagent (Western Lightning; PerkinElmer).
ELISA.
Immunoglobulin isotypes were determined using a commercial ELISA kit (SouthernBiotech). Serum IgE levels were analyzed using the mAb B1E3 for coating and the biotinylated mAb EM95 for detection.
Statistical analysis.
P-values were calculated with Student's t test using SigmaPlot software (SPSS Inc.).
Online supplemental material.
Fig. S1 shows the proliferation of transferred OT-II cells in ΔDC mice. Fig. S2 shows in vitro stimulation of OT-II cells with different APCs from ΔDC mice. Fig. S3 shows total number and TCR expression level on SP thymocytes. Fig. S4 shows the frequency of mature and immature CD4 SP thymocytes. Fig. S5 shows the ratio of CD4/CD8 SP thymocytes in single and mixed chimeras. Fig. S6 describes the phenotype of expanded Gr-1+ cells. Fig. S7 shows the TCR-Vβ repertoire in ΔDC and control mice. Fig. S8 shows the frequency of Foxp3+ cells in the small intestine of ΔDC and control mice. Fig. S9 shows T cell infiltration in peripheral organs of single and mixed chimeras. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082394/DC1.
Acknowledgments
We thank C. Ried for technical assistance, A. Bol and W. Mertl for animal husbandry, and L. Klein and R. Obst for helpful comments on the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft with an Emmy Noether grant to D. Voehringer (Vo944/2-2), with an SFB 455 grant to T. Brocker, and with an SFB 456 grant to I. Drexler.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: ANA, antinuclear antibody; DTA, diptheria toxin A; sAg, superantigen; SP, single positive.
References
- Anderson G., Moore N.C., Owen J.J., Jenkinson E.J. 1996. Cellular interactions in thymocyte development.Annu. Rev. Immunol. 14:73–99 [DOI] [PubMed] [Google Scholar]
- Gallegos A.M., Bevan M.J. 2004. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation.J. Exp. Med. 200:1039–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer T.M., DeKoning J., Markowitz J.S., Lo D., Glimcher L.H. 1996. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex.Nature. 383:81–85 [DOI] [PubMed] [Google Scholar]
- Volkmann A., Zal T., Stockinger B. 1997. Antigen-presenting cells in the thymus that can negatively select MHC class II-restricted T cells recognizing a circulating self antigen.J. Immunol. 158:693–706 [PubMed] [Google Scholar]
- McCaughtry T.M., Baldwin T.A., Wilken M.S., Hogquist K.A. 2008. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla.J. Exp. Med. 205:2575–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P., Guerder S. 1989. Does T-cell tolerance require a dedicated antigen-presenting cell? Nature. 338:74–76 [DOI] [PubMed] [Google Scholar]
- Brocker T., Riedinger M., Karjalainen K. 1997. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo.J. Exp. Med. 185:541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager M., Power M.O., Pircher H., Fisher A.G. 1999. Intrathymic deletion of MHC class I-restricted cytotoxic T cell precursors by constitutive cross-presentation of exogenous antigen.Eur. J. Immunol. 29:1477–1486 [DOI] [PubMed] [Google Scholar]
- Proietto A.I., van Dommelen S., Zhou P., Rizzitelli A., D'Amico A., Steptoe R.J., Naik S.H., Lahoud M.H., Liu Y., Zheng P., et al. 2008. Dendritic cells in the thymus contribute to T-regulatory cell induction.Proc. Natl. Acad. Sci. USA. 105:19869–19874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonasio R., Scimone M.L., Schaerli P., Grabie N., Lichtman A.H., von Andrian U.H. 2006. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus.Nat. Immunol. 7:1092–1100 [DOI] [PubMed] [Google Scholar]
- Kleindienst P., Chretien I., Winkler T., Brocker T. 2000. Functional comparison of thymic B cells and dendritic cells in vivo.Blood. 95:2610–2616 [PubMed] [Google Scholar]
- Steinman R.M., Hawiger D., Nussenzweig M.C. 2003. Tolerogenic dendritic cells.Annu. Rev. Immunol. 21:685–711 [DOI] [PubMed] [Google Scholar]
- Akbari O., DeKruyff R.H., Umetsu D.T. 2001. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen.Nat. Immunol. 2:725–731 [DOI] [PubMed] [Google Scholar]
- Munn D.H., Sharma M.D., Lee J.R., Jhaver K.G., Johnson T.S., Keskin D.B., Marshall B., Chandler P., Antonia S.J., Burgess R., et al. 2002. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase.Science. 297:1867–1870 [DOI] [PubMed] [Google Scholar]
- Travis M.A., Reizis B., Melton A.C., Masteller E., Tang Q., Proctor J.M., Wang Y., Bernstein X., Huang X., Reichardt L.F., et al. 2007. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice.Nature. 449:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar M.V., Steinman R.M., Krasovsky J., Munz C., Bhardwaj N. 2001. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells.J. Exp. Med. 193:233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonuleit H., Schmitt E., Schuler G., Knop J., Enk A.H. 2000. Induction of interleukin 10–producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells.J. Exp. Med. 192:1213–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnke K., Qian Y., Knop J., Enk A.H. 2003. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells.Blood. 101:4862–4869 [DOI] [PubMed] [Google Scholar]
- Steinman R.M., Nussenzweig M.C. 2002. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance.Proc. Natl. Acad. Sci. USA. 99:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger D., Inaba K., Dorsett Y., Guo M., Mahnke K., Rivera M., Ravetch J.V., Steinman R.M., Nussenzweig M.C. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo.J. Exp. Med. 194:769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton M.L., Smith-Raska M.R., Reizis B. 2007. Notch–RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen.J. Exp. Med. 204:1653–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D., Liang H.E., Locksley R.M. 2008. Homeostasis and effector function of lymphopenia-induced “memory-like” T cells in constitutively T cell-depleted mice.J. Immunol. 180:4742–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb J., Chaput N., Menard C., Apetoh L., Ullrich E., Bonmort M., Pequignot M., Casares N., Terme M., Flament C., et al. 2006. A novel dendritic cell subset involved in tumor immunosurveillance.Nat. Med. 12:214–219 [DOI] [PubMed] [Google Scholar]
- Chan C.W., Crafton E., Fan H.N., Flook J., Yoshimura K., Skarica M., Brockstedt D., Dubensky T.W., Stins M.F., Lanier L.L., et al. 2006. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity.Nat. Med. 12:207–213 [DOI] [PubMed] [Google Scholar]
- Kastenmuller W., Gasteiger G., Gronau J.H., Baier R., Ljapoci R., Busch D.H., Drexler I. 2007. Cross-competition of CD8+ T cells shapes the immunodominance hierarchy during boost vaccination.J. Exp. Med. 204:2187–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell F., Jenkins M., Dinh Q., Fowlkes B.J. 1991. The majority of CD4+8- thymocytes are functionally immature.J. Immunol. 147:1779–1785 [PubMed] [Google Scholar]
- Sprent J., Kishimoto H. 2002. The thymus and negative selection.Immunol. Rev. 185:126–135 [DOI] [PubMed] [Google Scholar]
- Gallegos A.M., Bevan M.J. 2006. Central tolerance: good but imperfect.Immunol. Rev. 209:290–296 [DOI] [PubMed] [Google Scholar]
- van Meerwijk J.P., Marguerat S., Lees R.K., Germain R.N., Fowlkes B.J., MacDonald H.R. 1997. Quantitative impact of thymic clonal deletion on the T cell repertoire.J. Exp. Med. 185:377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Kosaka H., Gao E.K. 1992. T cell tolerance after bone marrow transplantation in mice.Bone Marrow Transplant. 10:5–9 [PubMed] [Google Scholar]
- Moore N.C., Anderson G., McLoughlin D.E., Owen J.J., Jenkinson E.J. 1994. Differential expression of Mtv loci in MHC class II-positive thymic stromal cells.J. Immunol. 152:4826–4831 [PubMed] [Google Scholar]
- Aschenbrenner K., D'Cruz L.M., Vollmann E.H., Hinterberger M., Emmerich J., Swee L.K., Rolink A., Klein L. 2007. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells.Nat. Immunol. 8:351–358 [DOI] [PubMed] [Google Scholar]
- Ribot J., Enault G., Pilipenko S., Huchenq A., Calise M., Hudrisier D., Romagnoli P., van Meerwijk J.P. 2007. Shaping of the autoreactive regulatory T cell repertoire by thymic cortical positive selection.J. Immunol. 179:6741–6748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Oukka M., Kuchroo V.K. 2007. T(H)-17 cells in the circle of immunity and autoimmunity.Nat. Immunol. 8:345–350 [DOI] [PubMed] [Google Scholar]
- Smith E., Zarbock A., Stark M.A., Burcin T.L., Bruce A.C., Foley P., Ley K. 2007. IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice.J. Immunol. 179:8274–8279 [DOI] [PubMed] [Google Scholar]
- Bennett C.L., Clausen B.E. 2007. DC ablation in mice: promises, pitfalls, and challenges.Trends Immunol. 28:519–525 [DOI] [PubMed] [Google Scholar]
- Brockschnieder D., Pechmann Y., Sonnenberg-Riethmacher E., Riethmacher D. 2006. An improved mouse line for Cre-induced cell ablation due to diphtheria toxin A, expressed from the Rosa26 locus.Genesis. 44:322–327 [DOI] [PubMed] [Google Scholar]
- Birnberg T., Bar-On L., Sapoznikov A., Caton M.L., Cervantes-Barragan L., Makia D., Krauthgamer R., Brenner O., Ludewig B., Brockschnieder D., et al. 2008. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome.Immunity. 29:986–997 [DOI] [PubMed] [Google Scholar]
- de Heer H.J., Hammad H., Soullie T., Hijdra D., Vos N., Willart M.A., Hoogsteden H.C., Lambrecht B.N. 2004. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen.J. Exp. Med. 200:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochando J.C., Homma C., Yang Y., Hidalgo A., Garin A., Tacke F., Angeli V., Li Y., Boros P., Ding Y., et al. 2006. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts.Nat. Immunol. 7:652–662 [DOI] [PubMed] [Google Scholar]
- Hadeiba H., Sato T., Habtezion A., Oderup C., Pan J., Butcher E.C. 2008. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease.Nat. Immunol. 9:1253–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Ginhoux F., Collin M. 2008. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells.Nat. Rev. Immunol. 8:935–947 [DOI] [PubMed] [Google Scholar]
- Stranges P.B., Watson J., Cooper C.J., Choisy-Rossi C.M., Stonebraker A.C., Beighton R.A., Hartig H., Sundberg J.P., Servick S., Kaufmann G., et al. 2007. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity.Immunity. 26:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Wang Y.H., Wang Y., Huang L., Sandoval H., Liu Y.J., Wang J. 2006. Dendritic cell apoptosis in the maintenance of immune tolerance.Science. 311:1160–1164 [DOI] [PubMed] [Google Scholar]
- Nopora A., Brocker T. 2002. Bcl-2 controls dendritic cell longevity in vivo.J. Immunol. 169:3006–3014 [DOI] [PubMed] [Google Scholar]
- Laufer T.M., Fan L., Glimcher L.H. 1999. Self-reactive T cells selected on thymic cortical epithelium are polyclonal and are pathogenic in vivo.J. Immunol. 162:5078–5084 [PubMed] [Google Scholar]
- Luckashenak N., Schroeder S., Endt K., Schmidt D., Mahnke K., Bachmann M.F., Marconi P., Deeg C.A., Brocker T. 2008. Constitutive crosspresentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo.Immunity. 28:521–532 [DOI] [PubMed] [Google Scholar]
- Li X.C., Almawi W., Jevnikar A., Tucker J., Zhong R., Grant D. 1995. Allogeneic lymphocyte proliferation stimulated by small intestine-derived epithelial cells.Transplantation. 60:82–89 [DOI] [PubMed] [Google Scholar]
- Westendorf A.M., Templin M., Geffers R., Deppenmeier S., Gruber A.D., Probst-Kepper M., Hansen W., Liblau R.S., Gunzer F., Bruder D., Buer J. 2005. CD4+ T cell mediated intestinal immunity: chronic inflammation versus immune regulation.Gut. 54:60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguerat S., MacDonald H.R., Kraehenbuhl J.P., van Meerwijk J.P. 1999. Protection from radiation-induced colitis requires MHC class II antigen expression by cells of hemopoietic origin.J. Immunol. 163:4033–4040 [PubMed] [Google Scholar]
- Shimoda M., Mmanywa F., Joshi S.K., Li T., Miyake K., Pihkala J., Abbas J.A., Koni P.A. 2006. Conditional ablation of MHC-II suggests an indirect role for MHC-II in regulatory CD4 T cell maintenance.J. Immunol. 176:6503–6511 [DOI] [PubMed] [Google Scholar]
- Kim J.M., Rasmussen J.P., Rudensky A.Y. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice.Nat. Immunol. 8:191–197 [DOI] [PubMed] [Google Scholar]
- Weston S.A., Parish C.R. 1990. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy.J. Immunol. Methods. 133:87–97 [DOI] [PubMed] [Google Scholar]
- Larsen C.P., Steinman R.M., Witmer-Pack M., Hankins D.F., Morris P.J., Austyn J.M. 1990. Migration and maturation of Langerhans cells in skin transplants and explants.J. Exp. Med. 172:1483–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]