Abstract
Production of high-affinity pathogenic autoantibodies appears to be central to the pathogenesis of lupus. Because normal high-affinity antibodies arise from germinal centers (GCs), aberrant selection of GC B cells, caused by either failure of negative selection or enhanced positive selection by follicular helper T (TFH) cells, is a plausible explanation for these autoantibodies. Mice homozygous for the san allele of Roquin, which encodes a RING-type ubiquitin ligase, develop GCs in the absence of foreign antigen, excessive TFH cell numbers, and features of lupus. We postulated a positive selection defect in GCs to account for autoantibodies. We first demonstrate that autoimmunity in Roquinsan/san (sanroque) mice is GC dependent: deletion of one allele of Bcl6 specifically reduces the number of GC cells, ameliorating pathology. We show that Roquinsan acts autonomously to cause accumulation of TFH cells. Introduction of a null allele of the signaling lymphocyte activation molecule family adaptor Sap into the sanroque background resulted in a substantial and selective reduction in sanroque TFH cells, and abrogated formation of GCs, autoantibody formation, and renal pathology. In contrast, adoptive transfer of sanroque TFH cells led to spontaneous GC formation. These findings identify TFH dysfunction within GCs and aberrant positive selection as a pathway to systemic autoimmunity.
Systemic lupus erythematosus (SLE) is the prototypic systemic autoimmune disorder. With highly variable clinical manifestations and only 4 out of 11 criteria required to establish the diagnosis (1), multiple pathogenic pathways are likely to contribute to end-organ damage in this disease. Elucidating the different pathways that lead to lupus in particular subsets of patients and identifying biomarkers that flag the different pathways is essential to design more specific and effective therapies.
The formation of autoantibodies against cell nuclear components, including double-stranded DNA (dsDNA), ribonuclear proteins, and histones, is a consistent feature and therefore likely to be fundamental to the disease. This is supported by the observation that formation of autoantibodies precedes development of clinical manifestations of lupus (2), evidence that some of these antibodies contribute to end-organ damage, and the efficacy of B cell–depleting therapy with rituximab (3). Identification of defects that result in autoantibody formation is therefore of considerable importance in understanding the pathogenesis of lupus.
Numerous engineered and spontaneous defects in central and peripheral tolerance result in antinuclear antibodies (ANAs). However, the specificity and high affinity of the autoantibody response in lupus points to a defect in the response to self-antigen in the periphery. During T-dependent responses, activated B cells receive help from T cells in the T cell zones of secondary lymphoid tissues, and differentiate either extrafollicularly into short-lived plasma cells that produce low-affinity antibody, or enter the follicular pathway and form germinal centers (GCs) (4). Within this microenvironment, B cells undergo somatic hypermutation (SHM) and isotype switching, resulting in the generation of memory B cells and long-lived plasma cells that secrete high-affinity antigen-specific IgG antibodies (5, 6). Selection of mutated high-affinity GC B cells depends on restimulation with antigen arrayed on follicular dendritic cells and provision of help by follicular T helper (TFH) cells.
Because SHM has the potential to generate self-reactive antibodies (7), it has been long thought that aberrant selection within GCs represents a candidate pathway to the production of lupus-associated autoantibodies. Indeed, autoantibodies detected in SLE patients and mouse lupus models are generally high affinity and somatically mutated (7, 8). Exclusion of self-reactive B cells from GCs has been shown to be defective in SLE patients. Also, GCs have been shown to form spontaneously in several different mouse models of lupus (9), and these are rich in apoptotic cells displaying the antigenic targets of lupus autoimmunity (10, 11). Although SHM can occur outside GCs, this process is far less efficient (12, 13). Despite all of this circumstantial evidence, there is to date no definite proof that GCs and/or TFH cells are directly required for the production of lupus autoantibodies or end-organ damage. In contrast, extrafollicular affinity maturation of autoantibodies to dsDNA in MRLlpr mice (14, 15) and T-independent B cell activating factor of the TNF family–driven pathways to lupus have been demonstrated (16, 17).
Furthermore, the prevailing model is that within GCs, autoantibodies might arise because of defects in negative rather than positive selection, because GC B cells are programmed to undergo apoptosis by default if they do not receive T cell selection signals. This is consistent with evidence that centrocytes down-regulate apoptosis inhibitors such as Bcl-2 and Bcl-xL while up-regulating proapoptotic molecules such as Fas and Bim (18). Normally, a dedicated population of TFH cells is thought to provide help during selection of GC B cells (19, 20), and indeed a correlation between increased numbers of TFH cells and autoimmunity has been described in mouse models of lupus (21, 22), suggesting that defects in positive selection by TFH cells might indeed lead to lupus. Although recent evidence has suggested that Th17 cells may be responsible for aberrant selection of self-reactive GC B cells and autoantibody formation in BXD2 mice (23), direct evidence that TFH cells can drive autoimmunity has not been provided.
The sanroque strain was discovered from screening an ENU mutagenized mouse library for autoimmune regulators (24), and exhibits a lupus-like phenotype characterized by high-affinity anti-dsDNA antibodies, hypergammaglobulinemia, lymphanedopathy, splenomegaly, autoimmune thrombocytopenia, and glomerulonephritis with IgG-containing immune complex deposits. Both the autoimmunity and cellular characteristics of sanroque segregate with homozygosity for the san allele (M199R substitution) of Roquin (Roquinsan/san) (22). Roquin has been demonstrated to be a regulator of the stability T cell messenger RNAs. Development of autoimmunity in Roquinsan/san mice also correlates with spontaneous GC formation, which is largely driven by B cell–extrinsic factors (22). Roquinsan/san mice have a marked accumulation of T cells within the B cell follicles, and the TFH subset is overrepresented within the CD4+ cell compartment.
In this paper, we report that dysregulation of the GC response through excessive formation of TFH cells is responsible for autoimmunity in Roquinsan/san mice. Loss of one allele of Bcl6, the master transcriptional regulator of GCs (25, 26), significantly reduces spontaneous GC formation in Roquinsan/san mice and the lupus phenotype. Furthermore, deletion of Sap (Sh2d1a) from Roquinsan/san mice causes a dramatic reduction of TFH cells and IL-21. Sap is a small adaptor protein necessary for signaling through the signaling lymphocyte activation molecule family cell-surface receptors that regulates signals downstream of the TCR. Roquinsan/san Sap−/− CD4+ cells also express lower levels of ICOS than Roquinsan/san Sap+/+ cells. This results in abrogation of ANAs (including anti-dsDNA) and end-stage renal disease. These findings establish a causal pathway from the san allele of Roquin to excess TFH formation, aberrant GC formation, and positive selection of pathogenic high-affinity autoantibodies to illuminate a novel pathway of lupus pathogenesis.
RESULTS
Heterozygous Bcl6 deficiency reduces spontaneous GCs and attenuates autoimmunity in Roquinsan/san mice
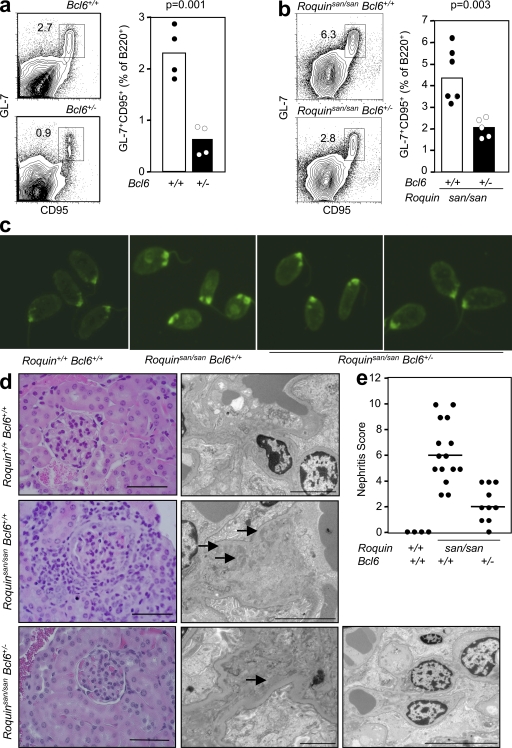
BCL6 has been shown to be the master transcriptional regulator of GC B cells (27). As BCL6 deficiency results in early mortality because of widespread inflammation, we investigated Bcl6+/− mice for possible defects in the GC response. 8 d after sheep red blood cell (SRBC) immunization, the percentage of GC cells was more than fivefold lower in mice heterozygous for Bcl6 deficiency (2.3 ± 0.59% vs. 0.43 ± 0.46%; P = 0.0011; Fig. 1 a).
Figure 1.
Heterozygosity for Bcl6 reduces the magnitude of the GC response in Roquin+/+ and Roquinsan/san mice and ameliorates the lupus-like phenotype of Roquinsan/san mice. (a) Flow cytometric contour plots (left) and graphical analysis (right) of B220+GL-7+CD95+ GC B cells in 10-wk-old wild-type (Bcl6+/+) and Bcl6+/− mice 8 d after SRBC immunization (P = 0.0011). Data are representative of four independent experiments (n = 4 per group). (b) Flow cytometric contour plots (left) and dot plots (right) showing B220+GL-7+CD95+ GC B cells from 10-wk-old naive Roquinsan/san Bcl6+/+ and Roquinsan/san Bcl6+/− mice. Data are representative of five independent experiments (n ≥ 4 per group). (c) Representative determination of serum IgG anti-dsDNA from 6-mo-old female Roquin+/+ Bcl6+/+, Roquinsan/san Bcl6+/+, and Roquinsan/san Bcl6+/− mice, determined by immunofluorescence staining of C. luciliae substrate. Data shown reflect the occurrence (n ≥ 6 mice per group); three out of six Roquinsan/san Bcl6+/− mice had low intensity staining (illustrated in the fourth panel from left), and three out of six were negative (illustrated in the third panel from left). (d) Representative images of kidney sections stained with H&E (left) or viewed under an electron microscope (right) from 6-mo-old mice of the indicated genotypes. Roquinsan/san animals show widespread mesangial proliferative lesions with moderate interstitial infiltrate. There are multiple electron-dense deposits (arrows). Histological changes in Roquinsan/san Bcl6+/− mice were mild, with occasional electron-dense deposits visible on electron microscopy in two individuals (far right). Images are representative (n ≥ 4 per group). Bars: (H&E) 100 µm in all panels; (electron microscopy) 5 µm in the Roquin+/+ Bcl6+/+ and Roquinsan/san Bcl6+/+ panels, 2 µm in the left Roquinsan/san Bcl6+/− panels, and 10 µm in the right Roquinsan/san Bcl6+/− panel. (e) Nephritis severity score of 6-mo-old female Roquin+/+, Roquinsan/san, and Roquinsan/san Bcl6+/− mice as determined by histological analysis according to the criteria given in Table S1 (available at http://www.jem.org/cgi/content/full/jem.20081886/DC1). Horizontal bars indicate medians. In a, b, and e, each symbol represents one mouse; p-values are indicated on the graphs, and the numbers in the plots represent percentages.
To determine whether Bcl6 heterozygosity could curtail the spontaneous GC response in Roquinsan/san mice, we compared the percentage of GC cells in unimmunized Roquinsan/san Bcl6+/+ and Roquinsan/san Bcl6+/− mice. As seen in wild-type mice, loss of one allele of Bcl6 caused a twofold reduction (4.4 ± 1.9% vs. 2.1 ± 0.5%; P = 0.0026) in the percentage of spontaneous GCs in Roquinsan/san mice (Fig. 1 b). Loss of one allele of Bcl-6 also reduced GC B cells after SRBC immunization in a cell-autonomous manner (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20081886/DC1).
We then tested whether reduction in the spontaneous GC response in Roquinsan/san Bcl6+/− mice was accompanied by reduced pathology. Serum dsDNA antibodies were present in 50% of Roquinsan/san Bcl6+/− mice tested compared with 100% of Roquinsan/san Bcl6+/+ mice (Fig. 1 c). Kidney pathology was also significantly reduced in Roquinsan/san Bcl6+/− mice (Fig. 1, d and e).
The lupus-like pathology of Roquinsan/san mice requires T cell activation
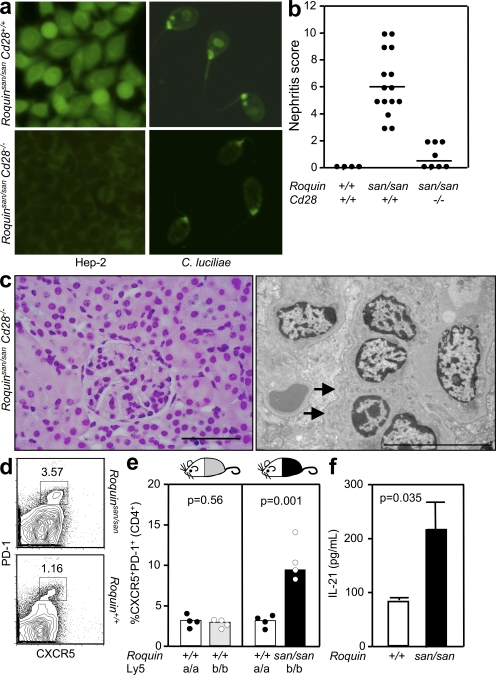
We have previously demonstrated that Roquin acts predominantly B cell extrinsically to induce spontaneous GC formation, and T cell intrinsically to prevent accumulation of activated/memory T cells and repress ICOS expression (22, 28). These findings suggest that the spontaneous GC reaction is driven by Roquin-mediated dysregulation of T cells. In a first attempt to confirm that Roquinsan/san lupus is T cell driven, we generated Roquinsan/san mice deficient in the major T cell co-stimulator CD28 (Cd28−/− mice). Failure to prime T cells in Roquinsan/san Cd28−/− mice abrogated the production of antinuclear IgG, including high-affinity anti-dsDNA antibodies (Fig. 2 a). Both interstitial nephritis and glomerular pathology, characterized by increased mesangial cellularity and dense deposits observed in 6-mo-old Roquinsan/san mice, were substantially reduced in age-matched Roquinsan/san Cd28−/− mice, with only very mild interstitial nephritis (Fig. 2, b and c). Less than half of the Roquinsan/san Cd28−/− mice examined had low-grade mesangial immune complex deposits (Fig. 2 c). Of note, Roquinsan/san Cd28−/− naive CD4+ T cells had a twofold decrease in ICOS expression (29).
Figure 2.
The autoimmune phenotype of Roquinsan/san mice requires T cell activation through CD28, and TFH cells are expanded cell autonomously. (a, left) Detection of IgG-ANA using Hep-2 slides in sera from 8-wk-old female Roquinsan/san and Roquinsan/san Cd28−/− mice (n = 5 per group). (right) Detection of anti-dsDNA IgG serum antibodies in 6-mo-old female Roquinsan/san and Roquinsan/san Cd28−/− mice determined by staining C. luciliae slides. Data are representative of three independent experiments (n ≥ 5 per group). (b) Score of nephritis severity in 6-mo-old female Roquin+/+ Roquinsan/san and Roquinsan/san Cd28−/− mice as determined by histological analysis defined by the criteria given in Table S1 (available at http://www.jem.org/cgi/content/full/jem.20081886/DC1). Each symbol represents one mouse. Horizontal bars indicate medians. (c) Representative images of kidney sections stained with H&E (left) or viewed under an electron microscope (right). Histology from Roquinsan/san Cd28−/− animals was much less severe, with normal H&E appearances and few electron-dense deposits (arrows) in the mesangium. Bars: (left) 100 µm; (right) 10 µm. (d) Representative flow cytometric contour plots of CD4+CXCR5+PD-1high TFH cells in 10-wk-old Roquinsan/san mice and control littermates. Data are representative of five independent experiments (n = 4 per group), and the numbers in the plots represent percentages. (e) Dot plots representing percentages of CD4+CXCR5+PD-1high TFH cells from SRBC-immunized chimeric mice generated by reconstituting sublethally irradiated mice with a 1:1 mix of either Roquin+/+.Ly5a/Roquin+/+.Ly5b (left) or Roquin+/+.Ly5a/ Roquinsan/san.Ly5b (right). Data are representative of three independent experiments (n = 4 per group). Each symbol represents the Ly5a or Ly5b population derived from one mouse. (f) ELISA was used to determine culture supernatant IL-21 levels from 24-h splenocyte cultures from Roquinsan/san mice and littermate controls in the presence of PMA and ionomycin. Error bars indicate means ± SEM. Data are representative of three independent tests where each sample was run in triplicate.
Expansion of the TFH subset in Roquinsan/san mice is T cell intrinsic
We have previously reported the similarity of the gene expression profiles of Roquinsan/san CD4+ T cells and TFH cells. Furthermore, Roquinsan/san T cells accumulate in the splenic GCs (22). To formally assess whether TFH cells, defined as CD4+CXCR5+PD-1high (30), are expanded in Roquinsan/san mice, we analyzed the percentage and total number of these cells in unimmunized mice and found more than a threefold increase of this subset in Roquinsan/san mice compared with littermate controls (Fig. 2 d). To determine whether this aberrant TFH cell accumulation is cell intrinsic, we generated mixed chimeras. Sublethally irradiated Roquin+/+Ly5a mice were reconstituted with a 1:1 mix of Roquin+/+Ly5a and Roquinsan/sanLy5b bone marrow cells. As a control, Roquin+/+Ly5a mice were reconstituted with a 1:1 mix of Roquin+/+Ly5a and Roquin+/+Ly5b bone marrow cells. 8 wk after reconstitution, mice were immunized with SRBCs, and the percentage of TFH cells derived from each type of donor marrow was determined by flow cytometry. In chimeras reconstituted with Roquin+/+Ly5a/Roquinsan/sanLy5b marrow, three times more Ly5b (Roquinsan/san) TFH cells than Ly5a cells were observed (P = 0.001), whereas in controls, an equivalent proportion of TFH cells arose from Ly5a and Ly5b cells (P = 0.56; Fig. 2 e). This indicates that Roquin acts in T cells to repress the formation and/or survival of TFH cells. Consistent with the observed increase in TFH cell numbers, IL-21 production was more than twofold higher in splenocyte cultures from Roquinsan/san mice (Fig. 2 f).
Although TFH cells are expanded in Roquinsan/san mice, it was important to exclude defects in other T cell subsets implicated in autoimmunity. CD4+CD25+FoxP3+ T reg cells have been shown to play a role in the regulation of lupus-associated autoantibodies (31). T reg cells are not reduced in number or function in Roquinsan/san mice (22). Quantification of FoxP3+CD25+CD4+ cells in the spleen confirmed previous observations: Roquinsan/san mice have approximately twofold more T reg cells than wild-type mice (18.9 ± 3.69% vs. 9.76 ± 1.03%, respectively; Fig. S2 a, available at http://www.jem.org/cgi/content/full/jem.20081886/DC1). This argues against a role for reduced T reg cell numbers in driving the T cell–mediated disease of Roquinsan/san mice. Another T helper subset, Th17 cells, have emerged as potent mediators of autoimmunity (31), and recent work suggests that they may be critical to maintain the spontaneous GCs of BXD2 mice (23). To test whether Th17 cell activity is dysregulated in Roquinsan/san mice, we analyzed IL-17 levels in splenocyte cell cultures after activation with PMA and ionomycin. IL-17 was detected at comparable levels in cultures from Roquin+/+ and Roquinsan/san mice (Fig. S2 b). In contrast, IL-21, a cytokine secreted by both TFH and Th17 cells (33, 34), was found at significantly higher levels in Roquinsan/san splenocyte cultures (Fig. 2 f). Collectively these data indicate that the TFH subset in Roquinsan/san mice is expanded in a cell-autonomous manner, whereas T reg and Th17 cells do not appear to be dysregulated in a way that has been previously described to result in autoimmunity.
SAP-deficient mice form reduced numbers of TFH cells
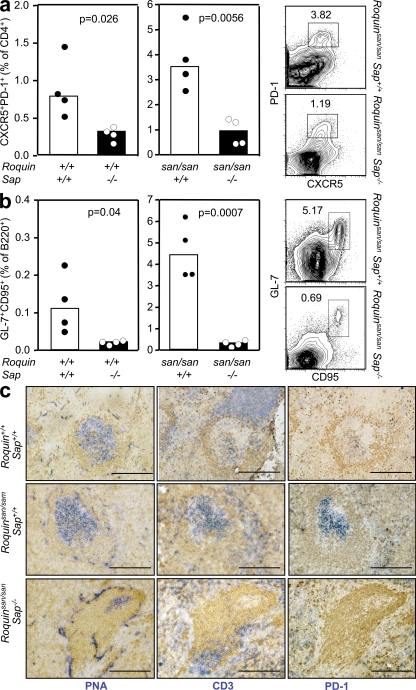
Having established that Roquin acts T cell intrinsically to dysregulate TFH cell numbers leading to the formation of abundant GCs, we hypothesized that disrupting the GC reaction through selective reduction of TFH cell numbers or function would attenuate systemic autoimmunity. SAP is an adaptor protein necessary for signaling through the signaling lymphocyte activation molecule family receptors that regulates signals downstream of the TCR. Mice lacking SAP cannot form GCs or generate immunological memory after immunization, a result of impaired CD4+ T cell help to GC B cells after antigen exposure (35–38). To date, it is not known whether SAP signaling is also required to maintain optimal TFH cell numbers. We enumerated CD4+CXCR5+PD-1high TFH cells in SAP-deficient unimmunized mice and wild-type littermates, and observed a twofold decrease in the number of basal TFH cells (Fig. 3 a, left). This was accompanied by a fourfold reduction in the number of background GC B cells (Fig. 3 b, right).
Figure 3.
Spontaneous GC and TFH formation are corrected by loss of SAP in Roquinsan/san mice. (a) CD4+CXCR5+PD-1high TFH cells in unimmunized 10-wk-old Roquinsan/san and Roquinsan/san Sap−/− mice (P = 0.0056). Representative flow cytometric contour plots are shown (right). Data are representative of four independent experiments (n = 4 per group). (b) B220+GL-7+CD95+ GC B cells in unimmunized 10-wk-old Roquinsan/san Sap+/+ and Roquinsan/san Sap−/− mice (P = 0.0007). Representative flow cytometric contour plots are shown (right). Data are representative of four independent experiments (n = 4 per group). (c) Photomicrographs of frozen spleen sections from unimmunized 6-mo-old mice of the indicated genotypes stained with IgD (brown; all panels), PNA (blue; left), TCRβ (blue; middle), and PD-1 (blue; right). Bars, 200 µm.
Roquinsan/san Sap−/− mice do not form excessive TFH cells or spontaneous GCs
Once we had established that SAP deficiency not only impairs CD4+ T cell help for GC B cells but also decreases TFH cell numbers, we generated Roquinsan/san Sap−/− mice to determine whether numerically and functionally defective TFH cells in Roquinsan/san mice could abrogate the spontaneous GC reactions. As observed in SAP-deficient mice, loss of SAP in Roquinsan/san mice also had an effect on TFH cell numbers: there was a fourfold reduction (0.94 ± 0.41% vs. 3.59 ± 1.99%) in the percentage of TFH cells in Roquinsan/san Sap−/− mice relative to Roquinsan/san mice (P = 0.0056; Fig. 3 a, middle). SAP deficiency also resulted in a 10-fold reduction in the percentage of GC B cells in Roquinsan/san mice (4.27 ± 1.93% vs. 0.43 ± 0.23%; P = 0.0007; Fig. 3 b, middle). Immunohistochemistry paralleled the flow cytometry data, showing reduced size and number of GCs, and a reduction of T cell numbers within PNA-positive follicles (Fig. 3 c). These findings indicate that loss of SAP corrects the aberrant formation of TFH cells and the inappropriate GC reaction phenotype of Roquinsan/san to levels comparable to Roquin+/+ mice.
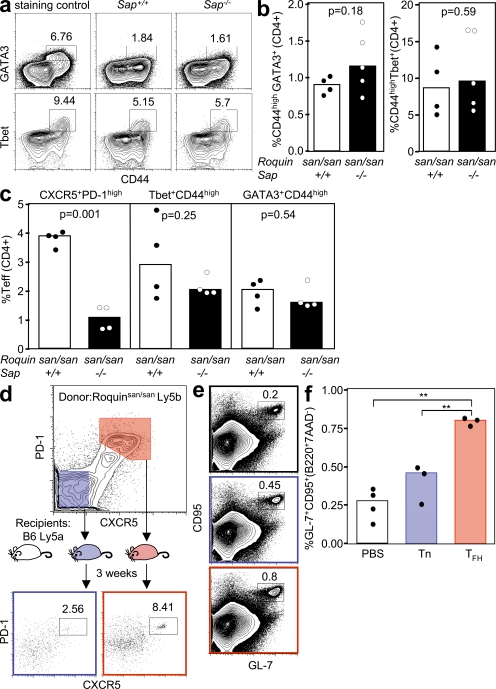
To test whether loss of SAP alters other T effector subsets in sanroque mice, we quantified Th1 and Th2 cells by flow cytometric staining for Tbet and Gata3, respectively. There were no statistically significant differences in the percentage of CD44highGATA3+CD4+ cells (0.91 ± 0.14% vs. 1.15 ± 0.41%; P = 0.18) or CD44highTbet+CD4+ cells (6.76 ± 10.6% vs. 8.66 ± 5.74%; P = 0.59) between Roquinsan/san Sap+/+ and Roquinsan/san Sap−/− mice in peripheral blood (Fig. 4, a and b). Lymph node CD44highGATA3+CD4+ cells (2.11 ± 1.68% vs. 1.59 ± 0.81%; P = 0.54) and CD44highTbet+CD4+ cells (2.89 ± 1.9% vs. 2.02 ± 0.64%; P = 0.25) between Roquinsan/san Sap+/+ and Roquinsan/san Sap−/− mice were slightly reduced, but these differences were not statistically significant (Fig. 4 c). In contrast, SAP deficiency caused an approximately fourfold reduction in Roquinsan/san lymph node TFH cells (3.93 ± 0.47% vs. 1.11 ± 0.38%; P = 0.001; Fig. 4 c). Collectively, these data indicate that TFH cells are the T helper subset whose generation is most severely impaired by SAP deficiency in Roquinsan/san mice.
Figure 4.
Th1 and Th2 cells are present in Roquinsan/san mice in the absence of SAP and TFH cells, but not non-TFH effector cells, induce a GC response in wild-type mice. (a) Representative flow cytometric contour plots and (b) graphical analysis of GATA3+CD44highCD4+ and Tbet+CD44highCD4+ cells in mice of the indicated genotypes. Data are representative of two independent experiments (n ≥ 4 per group). (c) Representative dot plots of lymph node CD4+PD-1highCXCR5+ (left), Tbet+CD44 highCD4+ (middle), and GATA3+CD44highCD4+ (right) cells. (d) Experimental outline for adoptive transfer of Roquinsan/san CD4+ CD45.2 CD44high PD-1high CXCR5+ or CD4+ CD45.2 CD44high PD-1− CXCR5− T cells into CD45.1 C57BL/6 mice. (e) Flow cytometric contour plots and (f) dot plots of B220+ GL-7+ CD95+ GC B cells from CD45.1 C57BL/6 recipients 3 wk after adoptive transfer of the indicated cell type. Data were generated from three mice per group (**, P > 0.001). In a, d, and e, the numbers in the plots represent percentages.
Transfer of Roquinsan/san TFH cells induces spontaneous GC reactions in wild-type mice
To test whether Roquinsan/san TFH cells are sufficient to induce GC reactions in unimmunized wild-type mice, CD45.2+ PD-1high CXCR5+ CD44high CD4+ TFH cells or CD45.2+ PD-1− CXCR5− CD44high CD4+ non-TFH T effector cells were adoptively transferred into CD45.1+ C57BL/6 mice. 3 wk after transfer, 8% of donor TFH cells retained their CXCR5high PD-1high phenotype. In mice receiving non-TFH san/san CD44high effectors, ∼3% of the transferred cells had also acquired a comparable TFH phenotype (Fig. 4 d). Adoptive transfer of Roquinsan/san TFH cells resulted in a threefold increase in the number of GC B cells compared with control mice injected with PBS alone (P = 0.003; Fig. 4, e and f). A small increase in the percentage of GC cells was also observed in the mice that received non-TFH effector cells, although this was not statistically significant (Fig. 4, e and f). These data suggest that Roquinsan/san TFH cells can drive a GC reaction in the absence of exogenous antigen.
SAP deficiency abrogates the lupus-like phenotype of Roquinsan/san mice
Having established that SAP deficiency prevents TFH cell accumulation and selectively impairs GC responses, we sought to determine whether SAP deficiency affects the serum autoantibodies and renal pathology of Roquinsan/san mice. Roquinsan/san Sap−/− mice had not developed ANAs by 8 wk of age (Fig. 5 a). Assessment of high-affinity anti-dsDNA IgG antibodies at 6 mo of age (by immunofluorescence using Crithidia luciliae substrate) revealed positive dsDNA antibodies in all Roquinsan/san mice evaluated, whereas only one out of six Roquinsan/san Sap−/− mice had detectable antibodies. Renal histology revealed that Roquinsan/san Sap−/− mice had only minor interstitial nephritis and thickening of the mesangial matrix, with no detection of immune complex deposition observed by electron microscopy (Fig. 5, b and c). These data suggest that abrogation of signaling through SAP greatly reduced the autoimmune manifestations of Roquinsan/san mice. In contrast, SAP deficiency conferred no significant reduction of splenomegaly, lymphadenopathy, and hypergammaglobulinemia present in Roquinsan/san mice, indicating that these defects are not mediated by excessive TFH cell formation and GC function (Fig. S3, a–c, available at http://www.jem.org/cgi/content/full/jem.20081886/DC1).
Figure 5.
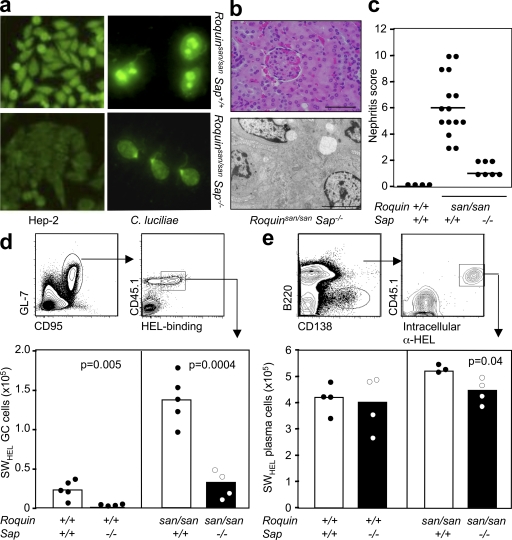
Serum autoantibodies and renal pathology in Roquinsan/san Sap−/− mice and reduction of the GC response in SAP-deficient Roquinsan/san mice is caused by B cell–extrinsic factors. (a, left) Representative staining of Hep-2 slides for detection of IgG ANA in the serum of 8-wk-old female Roquinsan/san and Roquinsan/san Sap−/− mice (n = 5 per group). (right) Detection of IgG anti-dsDNA serum antibodies in 6-mo-old female Roquinsan/san and Roquinsan/san Sap−/− mice determined by immunofluorescence staining using C. luciliae substrate. Data are representative of three independent experiments (n ≥ 5 per group). (b) Representative images of kidney sections stained with H&E (top) or viewed under an electron microscope (bottom) from 6-mo-old female Roquinsan/san Sap−/− mice. Roquinsan/san Sap−/− mice show slight mesangial expansion on H&E staining but no electron-dense deposits. Bars: (top) 100 µm; (bottom) 5 µm. (c) Score of nephritis severity in 6-mo-old female Roquin+/+, Roquinsan/san, and Roquinsan/san Sap−/− mice as determined by histological analysis according to the criteria given in Table S1 (available at http://www.jem.org/cgi/content/full/jem.20081886/DC1). Each symbol represents one mouse. Horizontal bars indicate medians. (d) Gating strategy for assessing the HEL-specific GC response (top) and graphic representation (bottom) of the total number of HEL-specific GC cells per spleen in mice with the indicated genotypes 7 d after cotransfer of SWHEL B cells and HEL2x-conjugated SRBCs. Data are representative of three independent experiments (n ≥ 4). Each symbol represents one mouse. (e) Gating strategy for determining HEL-specific extrafollicular plasma cells (top) and graphical representation (bottom) of the number of HEL-specific plasma cells 5 d after cotransfer into mice with the indicated genotypes. Data are representative of two independent experiments (n ≥ 3 per group). p-values are indicated on graphs.
Reduction of the GC response in Roquinsan/san Sap−/− mice is mainly caused by B cell–extrinsic factors
Several studies have shown that SAP acts T cell intrinsically to regulate GC responses (35, 37, 39), but some controversy still exists in the light of one study showing B cell–intrinsic SAP-mediated effects (40). To test whether the correction of the GC response observed in Roquinsan/san Sap−/− mice was caused by B cell–extrinsic factors, we transferred SWHEL B cells expressing intact SAP into Roquin+/+, Roquinsan/san, Sap−/−, and Roquinsan/san Sap−/− mice, in conjunction with hen egg lysozyme (HEL)2x–conjugated SRBCs.
Adoptively transferred SWHEL B cells formed HEL-specific GCs in all four cohorts. Significantly fewer GCs were observed in Sap−/− than in wild-type mice (23,958 ± 15,920 vs. 1,186 ± 1,019; P = 0.005; Fig. 5 d), confirming a B cell–extrinsic contribution of SAP to the GC response (34, 36, 38). In addition, SAP deficiency corrected the extreme GC formation observed in Roquinsan/san mice (124,528 ± 25,980 vs. 30,528 ± 18,100; P = 0.0004) to a response of similar magnitude to Roquin+/+ mice (Fig. 5 a). This suggests that the SAP-mediated effect in Roquinsan/san mice is also caused by B cell–extrinsic factors, and is most likely caused by SAP’s regulation of TFH cells.
To determine whether the loss of Sap in Roquinsan/san mice selectively affected the GC response or also impaired the extrafollicular response, we identified donor-derived extrafollicular plasma cells containing intracellular anti-HEL Ig at day 5 after immunization (Fig. 5 e). At this time point, GC-derived plasma cells cannot be detected (41). Although we observed a 4-fold decrease in SWHEL GC B cells, there was only a 1.3-fold decrease in the number of SWHEL extrafollicular plasma cells (53,198 ± 1,528 vs. 40,528 ± 7,548; P = 0.04) in Roquinsan/san Sap−/− recipients compared with Roquinsan/san Sap+/+ controls (Fig. 5 e). These data suggest that the reduction of the GCs in Roquinsan/san mice caused by Sap deficiency is mainly a result of B cell–extrinsic factors, and that this results in selective inhibition of the GC pathway.
Roquinsan/san Sap−/− mice have reduced expression of ICOS and IL-21
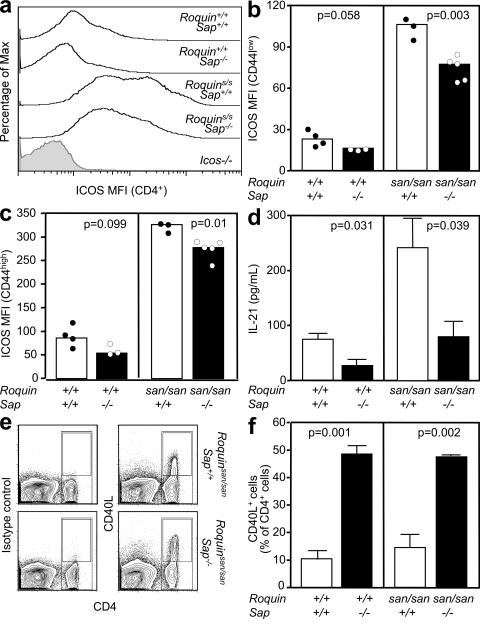
We have demonstrated that Roquinsan/san Sap−/− mice have fewer TFH cells, a correction of the spontaneous GC response, and a reduction in autoimmune pathology. To gain insight into the possible functional TFH defect brought about by SAP deficiency in Roquinsan/san mice, we quantified the changes in the expression of ICOS and IL-21, both of which are increased in Roquinsan/san CD4+ cells (22) and are shown to be essential for TFH cell formation, homeostasis, and function (33, 34, 42, 43). Loss of SAP reduced the levels of ICOS on both naive (CD44low) and activated/memory (CD44high) CD4+ T cells in Roquinsan/san mice and to an even greater extent on Roquin+/+ mice (Fig. 6, a–c). Despite this reduction, Roquinsan/san Sap−/− mice still expressed more than threefold higher levels of ICOS than Roquin+/+ Sap+/+ mice.
Figure 6.
TFH cell–associated molecules are decreased in Roquinsan/san Sap−/− mice. (a) Representative flow cytometric histograms and (b and c) graphical analysis showing ICOS mean fluorescence intensity (MFI) of splenic naive CD44low (b) and activated/memory CD44high (c) CD4+ T cells from 10-wk-old unimmunized mice of the indicated genotypes. Data are representative of three independent experiments. Each symbol represents one mouse. (d) ELISA quantification of IL-21 in supernatant from an overnight culture of splenocytes in the presence of PMA and ionomycin from mice of the indicated genotypes. Data are representative of three experiments. (e) Flow cytometric contour plots of CD40L expression on splenocytes, 5 h after stimulation with anti-CD3 and anti-CD28, derived from mice of the indicated genotypes. (left) Staining with an isotype control; (right) CD40L staining. (f) Histograms of the percentage of CD4+ cells that express CD40L (gated as shown in e) 5 h after CD3 and CD28 stimulation on splenocytes from mice with the indicated genotypes. In d and f, error bars indicate means ± SEM.
To determine whether loss of SAP had an effect on the production of IL-21, which was increased in Roquinsan/san mice, we compared IL-21 production from Roquin+/+ Sap+/+, Roquin+/+ Sap−/−, Roquinsan/san Sap+/+, and Roquinsan/san Sap−/− splenocytes. SAP deficiency reduced IL-21 production by both Roquinsan/san and Roquin+/+ splenocytes (Fig. 6 d). Remarkably, the levels of IL-21 found in Roquinsan/san Sap−/− cultures were comparable to those of wild-type (Roquin+/+ Sap+/+) mice. As previously reported (37), SAP deficiency resulted in higher CD40L expression upon activation in both Roquin+/+ and Roquinsan/san mice (Fig. 6, e and f).
IL-21 deficiency does not prevent autoimmunity in Roquinsan/san mice
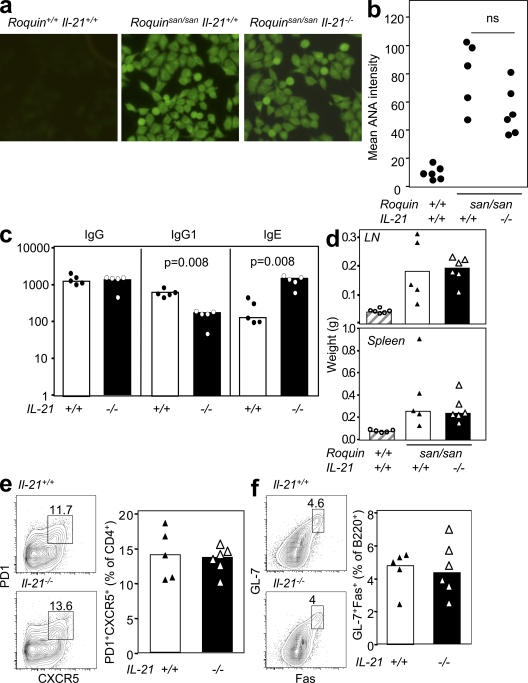
Given that Roquinsan/san mice produce high levels of IL-21 and given the recently described role for IL-21 in TFH cell generation after immunization (33, 34), we investigated whether IL-21 also controls autoantibody production, TFH cell accumulation, and spontaneous GC formation in these mice. To this end, we crossed Roquinsan/san mice to IL-21−/− mice on a C57BL/6 background and assessed ANA formation. There was no difference in the pattern or titer of ANAs produced by 10-wk-old Roquinsan/san IL-21+/+ and Roquinsan/san IL-21−/− mice (Fig. 7, a and b). Overall, IL-21 deficiency did not affect the hypergammaglobulinemia of Roquinsan/san mice (Fig. 7 c), and consistent with previous reports (44), IgG1 titers were reduced, whereas IgE titers were increased (Fig. 7 c). Likewise, loss of IL-21 did not correct the lymphadenopathy or the splenomegaly of Roquinsan/san mice (Fig. 7 d).
Figure 7.
Lack of IL-21 does not affect the phenotype, TFH cell accumulation, or GC formation of Roquinsan/san mice. (a) IgG ANAs in the serum of mice of the genotypes indicated, detected by immunofluorescence using Hep-2 substrate. Data are representative of three independent experiments (n ≥ 5 mice per group). (b) Score of ANA staining intensity by confocal microscopy from sera taken from mice of the indicated genotypes. Data are representative of three independent experiments (n ≥ 5 mice per group). (c) Basal serum total IgG, IgG1, and IgE measured by ELISA. Data are representative of two independent experiments (n = 5 mice per group). (d) Lymph node and spleen weight in grams for mice of the indicated genotypes. Data are representative of two independent experiments (n ≥ 5 per group). (e) Flow cytometric contour plots and dot plots of PD-1highCXCR5+CD4+ TFH cells and (f) GL-7+CD95+B220+ GC cells from mice of the indicated genotypes. Data are representative of two independent experiments (n ≥ 5 mice per group). In e and f, the numbers in the plots represent percentages.
We then investigated whether IL-21 influences TFH cell accumulation and spontaneous GC formation. There was no difference in either the number of TFH cells or GC cells between Roquinsan/san IL-21+/+ and Roquinsan/san IL-21−/− littermates, (Fig. 7, e and f). These data suggest that IL-21 does not play a role in spontaneous GC formation, TFH development, or accumulation in Roquinsan/san mice.
DISCUSSION
Elucidation of the defect that results in high-affinity antibodies to dsDNA is central to understanding lupus and, hence, the development of specific therapeutic interventions. In the Roquinsan/san model, a fundamental GC defect results in the production of autoantibodies. This pathway appears to be critical for disease development, because an impediment to GC formation conferred by halving the dose of Bcl6 is sufficient to attenuate the lupus phenotype. Our data are consistent with other evidence that aberrant T cell help can cause spontaneous GC formation and autoimmunity (9, 45). We show the expanded TFH cell subset, a consistent component of the Roquinsan/san phenotype, is responsible for excessive GC formation. In Roquinsan/san mice, GCs form in the absence of foreign antigen and yield ANAs. SAP deficiency reduces the size and activity of the TFH subset and abrogates autoantibody production and renal disease. These findings provide the in vivo cellular mechanism to link the defect in microRNA (miRNA)-mediated repression of TFH molecules conferred by the san allele of Roquin with the lupus phenotype of Roquinsan/san mice (22, 28). Moreover, they elucidate a mechanism of lupus that could be generalized to other defects of TFH homeostasis. Importantly, they provide evidence that defects in positive selection of GC B cells can cause autoimmunity.
In this paper, we demonstrate that Roquinsan/san acts T cell intrinsically to drive an accumulation of TFH cells that support a spontaneous GC response. Overexpression of ICOS is a plausible mechanism, because ICOS is important for the generation and survival of TFH cells (34, 42, 43), and Roquin normally acts to repress Icos miRNA (28). Previously, we have shown that this accounts for the lymphoproliferation, splenomegaly, and lymphadenopathy of Roquinsan/san mice (28). The data presented in this paper also show that CD28 signaling is a key contributor to the development of systemic autoimmunity in Roquinsan/san mice. CD28 is not only expressed on CD4+ cells, but it has also been found to be expressed by stromal cells (46) and plasma cells (47), and this expression can influence B cell development and antibody responses. Nevertheless, our previous data showing that the increased plasma cell and GC numbers, and B cell activation in sanroque are predominantly B cell extrinsic (22), together with the potent effects of SAP deficiency in the sanroque autoimmune phenotype, suggest that CD28 deficiency in T cells rather than in B cells or stromal cells is responsible for the observed effect. Thus, we infer that the sanroque lupus phenotype is T cell mediated. CD28 deficiency considerably decreased the abnormally high levels of ICOS normally found on Roquinsan/san T cells. This is likely to contribute to the abrogation of the lupus phenotype in a manner comparable to halving the gene dose of Icos (28). A nonmutually exclusive explanation is that although ICOS overexpression causes a lymphoproliferative syndrome, danger signals may also be required for the production of autoantibodies that cause end-organ damage. This is because ligands for CD28 are dependent on Toll-like receptor ligation, which typically occurs during infections and tissue damage, and potentially after apoptosis. Because mice in this study were housed under specific pathogen-free conditions, one explanation for the source of the danger signal in the san/san model might be the abundance of apoptotic cells in GCs, which overloads the normal nonimmunogenic modes of disposal (11). Apoptotic cells display self-antigens that are the target of the autoimmune response in lupus, and are ligands for TLR7 and/or TLR9 on antigen-presenting cells and/or B cells (48).
Our data indicate that the GC is central to the autoimmune phenotype of Roquinsan/san mice, because reduction of spontaneous GCs, conferred by halving the gene dose of the transcriptional regulator Bcl6, results in a proportionate amelioration of autoimmune pathology. This heterozygote phenotype is a novel finding. Although previous reports describe intact antibody responses in Bcl6+/− mice on day 11 after immunization (26), these are likely to derive predominantly from the extrafollicular response at this early time point. The heterozygote phenotype is consistent with the observation that GC differentiation is accompanied by only a twofold increase in Bcl6 miRNA expression (49). Although our work does not exclude an effect of halving the gene dose of Bcl6 on TFH cell formation, it does strongly suggest that the GC reduction seen in Bcl6+/− mice is a consequence of reduced B cell–expressed BCL6: in 50% Bcl6+/+/50% Bcl6+/− mixed bone marrow chimeras, GC B cells were only decreased in GC B cells deriving from Bcl6+/− bone marrow. Furthermore, transfer of Bcl6+/+ SWHEL B cells into Bcl6+/− recipient mice, providing the major source of TFH cells, resulted in normal donor-derived GC numbers after HEL-SRBC immunization.
SAP deficiency abrogates autoimmunity in Roquinsan/san mice. SAP deficiency affects several T cell subsets and natural killer cells (50, 51) but profoundly impairs the ability of T cells to provide help to B cells for GC formation (37–39). Our results demonstrate that SAP deficiency specifically reduces the development of CD4+ T cells with a TFH phenotype, leaving Th1 and Th2 cell formation largely intact. This, together with the evidence presented for the GC dependence of autoimmunity, as well as the observation that transfer of Roquinsan/san TFH cells induces spontaneous GC, places aberrant expansion of TFH cells at the root of spontaneous GC formation and autoimmunity in Roquinsan/san mice. This mechanism is consistent with another lupus model, B6.Sle1yaa, in which T cells have a transcriptional profile typical of TFH cells (21).
SAP deficiency has been shown to ameliorate autoimmunity in another lupus-prone strain, MRLlpr mice (52). These mice have been shown to also develop spontaneous GCs (9), although SHM of autoreactive B blasts has been shown to occur in the T zone areas rather than within GCs (16). SAP deficiency has been shown to eliminate the pathogenic CD4−CD8− T cells that account for the lymphadenopathy of MRLlpr mice (52), and there is evidence that autoimmunity in MRLlpr mice is T cell dependent (53). It will be interesting to examine whether dysregulated ectopically located TFH cells or an extrafollicular counterpart is contributing to autoimmunity in these mice. Finally, although sanroque SAP-deficient mice do not form spontaneous GCs, they still show hypergammaglobulinemia and very mild cell infiltrates in the kidney, suggesting that a non-TFH–mediated pathway contributes to these manifestations in Roquinsan/san mice.
Our data add to previous studies demonstrating that SAP deficiency acts B cell extrinsically, causing a profound and selective impairment of GC reactions (37) with little effect on extrafollicular plasma cell generation. The requirement for SAP for effective TFH cell function has previously been ascribed to dysregulated kinetics of ICOS up-regulation and increased expression of CD40L (37), which we confirmed in the present study. Our work also shows that SAP signaling is required to maintain elevated baseline levels of ICOS in naive sanroque T cells.
An intriguing finding of this study was the lack of any role for IL-21 in Roquinsan-induced lupus, TFH cell accumulation, and spontaneous GC formation. Although recent reports have shown that IL-21 is involved in TFH cell generation and optimal GC responses (33, 34), other groups have previously reported normal GC formation with an accumulation in IgG memory B cells in IL-21R−/− mice (54). A recent paper has also shown that IL-21 can be produced by extrafollicular T cells, which contribute to autoimmunity in MRLlpr mice (55). Furthermore, IL-21 has been shown to potently induce plasma cell generation from naive B cells (56), suggesting an important role for IL-21 in extrafollicular B cell responses. Even if IL-21 turns out to be critical for GC B cell selection, it is possible that other molecules overexpressed by sanroque TFH cells, such as ICOS, substitute for the effects normally mediated by IL-21.
The pathway to systemic autoimmunity identified in this paper highlights the importance of negative selection of self-reactive lymphocytes in GCs. Autoreactive B cells are a normal component of the naive peripheral B cell repertoire. In lupus patients, these cells enter GCs, whereas in healthy individuals, they are excluded (57). Other evidence indicates that in lupus, GCs can generate B cells that have antinuclear specificities from nonautoreactive precursors (7). Furthermore, GCs are increased in lupus (58). Regardless of their ontogeny, autoreactive centrocytes would normally fail to receive selection signals by TFH cells and would undergo apoptosis (59). Our data suggest that this process is perturbed by the san allele of Roquin.
According to the prevailing model for TFH selection of centrocytes, large numbers of GC B cells compete with each other for the limiting available T cell help (60) and for antigen on follicular dendritic cells. Centrocytes that have acquired higher affinity for antigen would scavenge more antigen from follicular dendritic cells, which would confer on them an avidity advantage in interactions with TFH cells compared with lower affinity B cells. Based on this model, we speculate that in Roquinsan/san mice, competition by B cells for T cell help is reduced by the expansion of TFH cells. Interactions between centrocytes and TFH cells are determined by affinity for MHC-peptide for the TCR modified by the action of accessory molecules. Loss of SAP in Roquinsan/san mice causes a reduction in ICOS overexpression, potentially increasing the antigen affinity required to reach the TFH activation threshold in Roquinsan/san Sap−/− mice.
Although the lupus phenotype of Roquinsan/san mice is Mendelian, apart from rare exceptions (61), lupus is a polygenic disorder. Nevertheless, it is plausible that one or more polymorphisms or mutations that affect TFH homeostasis, such as those that regulate IL-21, ICOS, or ICOSL expression, could contribute to lupus pathogenesis. Because TFH cells are present in peripheral blood, this is a testable hypothesis. Indeed, there is evidence that SLE patients have increased numbers of circulating CD4+ICOS+ cells in their peripheral blood compared with nonautoimmune individuals (62). Because TFH cells are the cells expressing the highest levels of ICOS (63, 64), it is possible that circulating CD4+ICOS+ cells reflect an excessive TFH response, and is consistent with recent data showing that a subset of SLE patients have an increased proportion of CD4+CD45RO+CXCR5+ICOShighPD-1high cells in their peripheral blood (a phenotype that correlates with higher titers of anti-dsDNA antibodies and more severe kidney damage; unpublished data). If confirmed in independent studies, the GC–TFH pathway elucidated in the Roquinsan/san model pathway would emerge as a novel and specific target for therapy.
MATERIALS AND METHODS
Mice and immunizations.
Roquinsan/san and C57BL/6 (B6) mice, all crossed to Sap−/−, Bcl6+/−, Cd28−/−, and IL-21−/− mice, and SWHEL mice, were housed in specific pathogen-free conditions at the Australian National University Bioscience Facility. IL-21 KO mice were generated at Lexicon Pharmaceuticals, Inc. and were provided by M. Smyth (Peter MacCallum Cancer Centre, Melbourne, Australia) and ZymoGenetics, Inc. These mice were backcrossed 10 generations onto the C57BL/6 background. SWHEL mice carry a Vk10-κ light chain transgene and a knocked in VH10 Ig heavy chain in place of the JH segments of the endogenous IgH gene that encode a high-affinity antibody for HEL (65). All animal procedures were approved by the Australian National University Animal Ethics and Experimentation Committee.
To generate thymus-dependent responses where indicated in the figures, 8–12-wk-old mice were immunized i.p. with 2 × 109 SRBCs (Institute of Medical and Veterinary Science Veterinary Services). For experiments involving SWHEL mice, 104 SWHEL B cells were transferred into recipients, which were simultaneously immunized i.v. with 2 × 108 SRBCs conjugated with mutant HEL2x using a protein conjugation kit (Invitrogen) (41).
Bone marrow chimeras.
Recipient C57BL/6-Ly5a mice were sublethally irradiated with 1,000 Rad and were reconstituted via i.v. injection with 2 × 106 donor bone marrow–derived hematopoetic stem cells.
Antibodies.
Antibodies and streptavidin conjugates for flow cytometry were from BD unless otherwise indicated: anti–mouse B220-PerCP, CD4-PerCP, ICOS-PE (eBioscience), FoxP3 (eBioscience), GL-7–FITC, GATA3-allophycocyanin, Tbet–PerCP Cy5.5, CD95-PE, CXCR5-biotin, PD-1–PE (eBioscience), CTLA-4–PE, CD25-allophycocyanin, streptavidin–PerCP Cy5.5, and CD40L-biotin. For immunohistochemistry, the primary antibodies and reagents used were rat anti–mouse IgD (SouthernBiotech), biotinylated anti–mouse TCRβ (BD), rat anti–mouse PD-1 (BioLegend), and PNA-biotin (Vector Laboratories); the secondary antibody used was rabbit anti–rat horseradish peroxidase (HRP; Dako).
Cell isolation, culture, and stimulation.
Single-cell suspensions were prepared from spleens of unimmunized and/or immunized mice. Single-cell suspensions were prepared in RPMI 1640 medium (JRH Biosciences) supplemented with 2 mM l-Glutamine (Invitrogen), 100 U penicillin-streptomycin (Invitrogen), 0.1 mM nonessential amino acids (Invitrogen), 100 mM Hepes (Sigma-Aldrich), 5 × 10−5 2-mercaptoethanol, and 10% fetal calf serum by sieving and gentle pipetting through 70-µm nylon mesh filters (Falcon; BD). Cells were then cultured for 24 h at 37°C/5% CO2 with 50 ng/ml PMA (Sigma-Aldrich) and 1 µM ionomycin (Sigma-Aldrich) in triplicate wells. Cell culture supernatants were aspirated for further analysis by ELISA. For CD40L expression, cells were cultured in triplicate wells for 5 h in 96-well plates (Costar; Corning) with platebound 1 µg/ml anti-CD3 (BD) and 10 µg/ml anti-CD28 (BD). Biotinylated anti-CD40L antibody was added 2 h before harvesting.
Flow cytometry.
For surface staining, single-cell suspensions were prepared as described in the previous section, and cells were maintained in the dark at 4°C throughout. Cells were washed twice in ice-cold FACS buffer (2% fetal calf serum, 0.1% NaN3 in PBS), and incubated with each antibody and conjugate layer for 30 min and washed thoroughly with FACS buffer between each layer. Intracellular staining was performed using the Cytofix/Cytoperm kit (BD) according to the manufacturer’s instructions. For detection of HEL-binding B cells, HEL was conjugated to Alexa Fluor 647 using the manufacturer’s instructions (Invitrogen). A FACSCalibur (BD) with CellQuest software (BD) was used for the acquisition of flow cytometric data, and FlowJo software (Tree Star, Inc.) was used for analysis.
Adoptive cell transfer experiments.
TFH cells (CD4+CD44highPD-1highCXCR5high) and non-TFH effector cells (CD4+CD44highPD-1int/loCXCR5−) from spleens and lymph nodes were prepared and stained as described in the previous paragraph and sorted on a FACSAria (BD). 5 × 106 sorted cells were resuspended in PBS and injected into the tail vein of unmanipulated CD45.1 C57BL/6 recipients. 3-wk after transfer, splenic donor (CD45.2) TFH cells and host GC cells were enumerated by flow cytometry.
ELISA.
Maxisorb plates (Thermo Fisher Scientific) were coated with 100 µg/ml of recombinant mouse IL-21R/Fc (R&D Systems). Serial supernatant dilutions were applied in quadruplicate, and IL-21 concentration was determined with 200 ng/ml of HRP-conjugated goat anti–mouse IL-21 IgG (R & D systems). Bound IL-21 was detected using phosphatase substrate tablets (Sigma-Aldrich). Plates were read at 405 nm using a microplate reader (Thermomax; MDS Analytical Technologies). The titers for supernatant samples were calculated according to the standard curve generated using twofold dilutions of recombinant mouse IL-21 (500-15 pg/ml; R&D Systems) and Prism software (GraphPad Software, Inc.). IL-17 ELISA was performed using the mouse IL-17A ELISA Ready-Set-Go! Kit (eBioscience) according to the manufacturer’s instructions.
For analysis of total IgG, IgG1, and IgE titers, Maxisorb plates were coated with goat anti–mouse κ light chain or anti–mouse IgE (BD). Serial serum dilutions were applied, and Ig concentration was determined with HRP-conjugated goat anti–mouse IgG, IgG1 (SouthernBiotech), or biotinlyated IgE (BD), followed by streptavidin-HRP. The enzyme bound to plates was developed using phosphatase substrate tablets. Plates were read at 405 nm using a Thermomax microplate reader. The titers for serum samples were calculated as the log serum concentration required to achieve 50% maximum OD.
Immunohistochemistry.
5-µm acetone-fixed frozen sections of spleen were air dried and washed in 0.1 M Tris-buffered saline (TBS), pH 7.6, and were stained with various antibodies for 45 min at room temperature in a moist chamber. After a further wash in TBS, secondary reagents, previously absorbed in 10% normal mouse serum, were added to the sections for 45 min. Where biotin-conjugated primary or secondary reagents were used, streptavidin alkaline phosphatase (Vector Laboratories) was added after a further wash in TBS and incubated for 20 min. HRP activity was detected using diaminobenzidine tetrahydrochloride solution (Sigma-Aldrich) and hydrogen peroxide. Alkaline phosphatase activity was detected using the AP Substrate Kit III (Vector Laboratories). Sections were mounted with IMMU-MOUNT (Thermo Shandon) and viewed under a microscope (model IX71; Olympus).
Renal pathology.
Kidneys were fixed in 10% neutral buffered formalin. 4-µm paraffin-embedded sections were dewaxed and stained with hematoxylin and eosin (H&E) and silver stains. For electron microscopy, kidneys were fixed in 2% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, at room temperature overnight. They were then washed in 0.1 M cacodylate buffer, treated with 0.2 mm of filtered 2% osmium tetroxide in 0.1 M cacodylate buffer, pH 7.4, at room temperature for 2 h, and washed in double-distilled H2O. Samples were stained with 0.2 mm of filtered 2% aqueous uranyl acetate for 30 min at room temperature, dehydrated in ethanol, and embedded in 100% TAAB medium mix, low viscosity resin. They were then mounted in orientation silicone molds and heated in an oven at 70°C for 8–12 h. The samples were examined on a transmission electron microscope (model 1011; JEOL) at 60 KeV. The images were captured using a digital camera (MegaView III) and the AnalySIS software package (Soft Imaging System; Olympus), and were subsequently scored using the criteria detailed in Table S1 (available at http://www.jem.org/cgi/content/full/jem.20081886/DC1).
ANA and dsDNA assessment.
Serum obtained by eye bleed from 8-wk-old mice was diluted in PBS 1:40 and used for indirect immunofluorescence on fixed Hep-2 slides (Antibodies, Inc.) for ANA detection, and serum from 6-mo-old mice was diluted 1:20 and used for indirect immunofluorescence on fixed C. luciliae slides (Antibodies, Inc.) for ANA anti-dsDNA antibody detection, respectively. Alexa Fluor 488 goat anti–mouse IgG (Invitrogen) was used to detect mouse antibodies. Autoantibodies were scored blind as negative or positive on a scale of 1–3 based on the intensity of fluorescence. Relative levels of ANAs were estimated by viewing the slides using a confocal microscope (TCS SP5; Leica) at 20× magnification and a fixed laser power, and measuring the fluorescence of five randomly selected 1,250-µm2 regions of Hep2 cells, compared with five regions where cells were absent. The sample-specific mean background was subtracted from the sample-specific mean fluorescence to give an estimation of fluorescent intensity. These results concurred with a blinded manual scoring of fluorescence for the same samples.
Statistical analysis.
Data were analyzed using a two-tailed Student’s t test using Prism software.
Online supplemental material.
Fig. S1 illustrates that the loss of one allele of Bcl6 results in B cell–intrinsic defects in GC formation. Fig. S2 a shows that CD4+FoxP3+ T reg cells are expanded twofold in Roquinsan/san mice relative to Roquin+/+ mice. Fig. S2 b demonstrates that Roquinsan/san splenocytes produce equivalent levels of IL-17 to Roquin+/+ splenocytes. Fig. S3 (a and b) shows that Sap deficiency does not correct the splenomegaly and lymphadenopathy in Roquinsan/san mice. Fig. S3 c demonstrates that hyper-IgG in Roquinsan/san mice is not corrected in the absence of Sap. Table S1 describes the scoring strategy used to assess the severity of mouse nephritis. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081886/DC1.
Acknowledgments
We thank C. Goodnow for helpful discussions of this work, A. Prins for kidney sectioning, T. Chan for preparing HEL2X-conjugated SRBCs for SWHEL experiments, M. Townsend for cutting frozen spleen sections, and M. Smyth and ZymoGenetics, Inc. for providing us with the IL-21−/− mice.
This work was funded by a Viertel Senior Medical Research Fellowship and National Health and Medical Research Council grants 316956 and 427620 to C.G. Vinuesa.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: ANA, antinuclear antibody; dsDNA, double-stranded DNA; GC, germinal center; H&E, hematoxylin and eosin; HEL, hen egg lysozyme; miRNA, microRNA; SHM, somatic hypermutation; SLE, systemic lupus erythematosus; SRBC, sheep red blood cell; TFH, follicular helper T.
References
- Hochberg M.C. 1997. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus.Arthritis Rheum. 40:1725. [DOI] [PubMed] [Google Scholar]
- Arbuckle M.R., McClain M.T., Rubertone M.V., Scofield R.H., Dennis G.J., James J.A., Harley J.B. 2003. Development of autoantibodies before the clinical onset of systemic lupus erythematosus.N. Engl. J. Med. 349:1526–1533 [DOI] [PubMed] [Google Scholar]
- Rahman A., Isenberg D.A. 2008. Systemic lupus erythematosus.N. Engl. J. Med. 358:929–939 [DOI] [PubMed] [Google Scholar]
- MacLennan I.C., Toellner K.M., Cunningham A.F., Serre K., Sze D.M., Zuniga E., Cook M.C., Vinuesa C.G. 2003. Extrafollicular antibody responses.Immunol. Rev. 194:8–18 [DOI] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G., Rajewsky K., Weiss U. 1991. Intraclonal generation of antibody mutants in germinal centers.Nature. 354:389–392 [DOI] [PubMed] [Google Scholar]
- MacLennan I.C. 1994. Germinal centers.Annu. Rev. Immunol. 12:117–139 [DOI] [PubMed] [Google Scholar]
- Diamond B., Katz J.B., Paul E., Aranow C., Lustgarten D., Scharff M.D. 1992. The role of somatic mutation in the pathogenic anti-DNA response.Annu. Rev. Immunol. 10:731–757 [DOI] [PubMed] [Google Scholar]
- Behar S.M., Lustgarten D.L., Corbet S., Scharff M.D. 1991. Characterization of somatically mutated S107 VH11-encoded anti-DNA autoantibodies derived from autoimmune (NZB × NZW)F1 mice.J. Exp. Med. 173:731–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzina I.G., Atamas S.P., Storrer C.E., daSilva L.C., Kelsoe G., Papadimitriou J.C., Handwerger B.S. 2001. Spontaneous formation of germinal centers in autoimmune mice.J. Leukoc. Biol. 70:578–584 [PubMed] [Google Scholar]
- Casciola-Rosen L.A., Anhalt G., Rosen A. 1994. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes.J. Exp. Med. 179:1317–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanayama R., Tanaka M., Miyasaka K., Aozasa K., Koike M., Uchiyama Y., Nagata S. 2004. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice.Science. 304:1147–1150 [DOI] [PubMed] [Google Scholar]
- Weller S., Braun M.C., Tan B.K., Rosenwald A., Cordier C., Conley M.E., Plebani A., Kumararatne D.S., Bonnet D., Tournilhac O., et al. 2004. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire.Blood. 104:3647–3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Kim J., Jang Y.S., Chung G.H. 2006. Germinal center-independent affinity maturation in tumor necrosis factor receptor 1-deficient mice.J. Biochem. Mol. Biol. 39:586–594 [DOI] [PubMed] [Google Scholar]
- Shlomchik M.J., Aucoin A.H., Pisetsky D.S., Weigert M.G. 1987. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse.Proc. Natl. Acad. Sci. USA. 84:9150–9154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M.J., Marshak-Rothstein A., Wolfowicz C.B., Rothstein T.L., Weigert M.G. 1987. The role of clonal selection and somatic mutation in autoimmunity.Nature. 328:805–811 [DOI] [PubMed] [Google Scholar]
- William J., Euler C., Christensen S., Shlomchik M.J. 2002. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers.Science. 297:2066–2070 [DOI] [PubMed] [Google Scholar]
- Groom J.R., Fletcher C.A., Walters S.N., Grey S.T., Watt S.V., Sweet M.J., Smyth M.J., Mackay C.R., Mackay F. 2007. BAFF and MyD88 signals promote a lupuslike disease independent of T cells.J. Exp. Med. 204:1959–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama T., Tanahashi M., Kobayashi Y., Yamakawa Y., Maeda M., Inaba T., Kiriyama M., Fukai I., Fujii Y. 2002. The expression of Bcl-2 family proteins (Bcl-2, Bcl-x, Bax, Bak and Bim) in human lymphocytes.Immunol. Lett. 81:107–113 [DOI] [PubMed] [Google Scholar]
- Liu Y.J., Joshua D.E., Williams G.T., Smith C.A., Gordon J., MacLennan I.C. 1989. Mechanism of antigen-driven selection in germinal centers.Nature. 342:929–931 [DOI] [PubMed] [Google Scholar]
- Vinuesa C.G., Tangye S.G., Moser B., Mackay C.R. 2005. Follicular B helper T cells in antibody responses and autoimmunity.Nat. Rev. Immunol. 5:853–865 [DOI] [PubMed] [Google Scholar]
- Subramanian S., Tus K., Li Q.Z., Wang A., Tian X.H., Zhou J., Liang C., Bartov G., McDaniel L.D., Zhou X.J., et al. 2006. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus.Proc. Natl. Acad. Sci. USA. 103:9970–9975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa C.G., Cook M.C., Angelucci C., Athanasopoulos V., Rui L., Hill K.M., Yu D., Domaschenz H., Whittle B., Lambe T., et al. 2005. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity.Nature. 435:452–458 [DOI] [PubMed] [Google Scholar]
- Hsu H.C., Yang P., Wang J., Wu Q., Myers R., Chen J., Yi J., Guentert T., Tousson A., Stanus A.L., et al. 2008. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice.Nat. Immunol. 9:166–175 [DOI] [PubMed] [Google Scholar]
- Vinuesa C.G., Goodnow C.C. 2004. Illuminating autoimmune regulators through controlled variation of the mouse genome sequence.Immunity. 20:669–679 [DOI] [PubMed] [Google Scholar]
- Dent A.L., Shaffer A.L., Yu X., Allman D., Staudt L.M. 1997. Control of inflammation, cytokine expression, and germinal center formation by BCL-6.Science. 276:589–592 [DOI] [PubMed] [Google Scholar]
- Ye B.H., Cattoretti G., Shen Q., Zhang J., Hawe N., de Waard R., Leung C., Nouri-Shirazi M., Orazi A., Chaganti R.S., et al. 1997. The BCL-6 proto-oncogene controls germinal-center formation and Th2-type inflammation.Nat. Genet. 16:161–170 [DOI] [PubMed] [Google Scholar]
- Shaffer A.L., Yu X., He Y., Boldrick J., Chan E.P., Staudt L.M. 2000. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control.Immunity. 13:199–212 [DOI] [PubMed] [Google Scholar]
- Yu D., Tan A.H., Hu X., Athanasopoulos V., Simpson N., Silva D.G., Hutloff A., Giles K.M., Leedman P.J., Lam K.P., et al. 2007. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA.Nature. 450:299–303 [DOI] [PubMed] [Google Scholar]
- Linterman M.A., Rigby R.J., Wong R., Silva D., Withers D., Anderson G., Verma N.K., Brink R., Hutloff A., Goodnow C.C., Vinuesa C.G. 2009. Roquin differentiates the specialized functions of duplicated T cell co-stimulatory receptor genes Cd28 and Icos.Immunity. In press [DOI] [PubMed] [Google Scholar]
- Haynes N.M., Allen C.D., Lesley R., Ansel K.M., Killeen N., Cyster J.G. 2007. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation.J. Immunol. 179:5099–5108 [DOI] [PubMed] [Google Scholar]
- Seo S.J., Fields M.L., Buckler J.L., Reed A.J., Mandik-Nayak L., Nish S.A., Noelle R.J., Turka L.A., Finkelman F.D., Caton A.J., Erikson J. 2002. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells.Immunity. 16:535–546 [DOI] [PubMed] [Google Scholar]
- Stockinger B., Veldhoen M. 2007. Differentiation and function of Th17 T cells.Curr. Opin. Immunol. 19:281–286 [DOI] [PubMed] [Google Scholar]
- Vogelzang A., McGuire H.M., Yu D., Sprent J., Mackay C.R., King C. 2008. A fundamental role for interleukin-21 in the generation of T follicular helper cells.Immunity. 29:127–137 [DOI] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Hwang D., Yang X.O., Kang H.S., Ma L., Wang Y.H., Watowich S.S., Jetten A.M., Tian Q., Dong C. 2008. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages.Immunity. 29:138–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S., Kersh E.N., Cannons J., Schwartzberg P.L., Ahmed R. 2003. SAP is required for generating long-term humoral immunity.Nature. 421:282–287 [DOI] [PubMed] [Google Scholar]
- Hron J.D., Caplan L., Gerth A.J., Schwartzberg P.L., Peng S.L. 2004. SH2D1A regulates T-dependent humoral autoimmunity.J. Exp. Med. 200:261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannons J.L., Yu L.J., Jankovic D., Crotty S., Horai R., Kirby M., Anderson S., Cheever A.W., Sher A., Schwartzberg P.L. 2006. SAP regulates T cell–mediated help for humoral immunity by a mechanism distinct from cytokine regulation.J. Exp. Med. 203:1551–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCausland M.M., Yusuf I., Tran H., Ono N., Yanagi Y., Crotty S. 2007. SAP regulation of follicular helper CD4 T cell development and humoral immunity is independent of SLAM and Fyn kinase.J. Immunol. 178:817–828 [DOI] [PubMed] [Google Scholar]
- Veillette A., Zhang S., Shi X., Dong Z., Davidson D., Zhong M.C. 2008. SAP expression in T cells, not in B cells, is required for humoral immunity.Proc. Natl. Acad. Sci. USA. 105:1273–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra M., Barrington R.A., Abadia-Molina A.C., Okamoto S., Julien A., Gullo C., Kalsy A., Edwards M.J., Chen G., Spolski R., et al. 2005. Defective B cell responses in the absence of SH2D1A.Proc. Natl. Acad. Sci. USA. 102:4819–4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus D., Phan T.G., Chan T.D., Gardam S., Basten A., Brink R. 2006. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation.J. Exp. Med. 203:1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaller L., Burger J., Draeger R., Grimbacher B., Knoth R., Plebani A., Durandy A., Baumann U., Schlesier M., Welcher A.A., et al. 2006. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells.J. Immunol. 177:4927–4932 [DOI] [PubMed] [Google Scholar]
- Akiba H., Takeda K., Kojima Y., Usui Y., Harada N., Yamazaki T., Ma J., Tezuka K., Yagita H., Okumura K. 2005. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo.J. Immunol. 175:2340–2348 [DOI] [PubMed] [Google Scholar]
- Ozaki K., Spolski R., Feng C.G., Qi C.F., Cheng J., Sher A., Morse H.C., 3rd, Liu C., Schwartzberg P.L., Leonard W.J. 2002. A critical role for IL-21 in regulating immunoglobulin production.Science. 298:1630–1634 [DOI] [PubMed] [Google Scholar]
- Munthe L.A., Corthay A., Os A., Zangani M., Bogen B. 2005. Systemic autoimmune disease caused by autoreactive B cells that receive chronic help from Ig V region-specific T cells.J. Immunol. 175:2391–2400 [DOI] [PubMed] [Google Scholar]
- Gray Parkin K., Stephan R.P., Apilado R.G., Lill-Elghanian D.A., Lee K.P., Saha B., Witte P.L. 2002. Expression of CD28 by bone marrow stromal cells and its involvement in B lymphopoiesis.J. Immunol. 169:2292–2302 [DOI] [PubMed] [Google Scholar]
- Delogu A., Schebesta A., Sun Q., Aschenbrenner K., Perlot T., Busslinger M. 2006. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells.Immunity. 24:269–281 [DOI] [PubMed] [Google Scholar]
- Krieg A.M., Vollmer J. 2007. Toll-like receptors 7, 8, and 9: linking innate immunity to autoimmunity.Immunol. Rev. 220:251–269 [DOI] [PubMed] [Google Scholar]
- Vinuesa C.G., Cook M.C., Cooke M.P., Maclennan I.C., Goodnow C.C. 2002. Analysis of B cell memory formation using DNA microarrays.Ann. NY Acad. Sci. 975:33–45 [DOI] [PubMed] [Google Scholar]
- Griewank K., Borowski C., Rietdijk S., Wang N., Julien A., Wei D.G., Mamchak A.A., Terhorst C., Bendelac A. 2007. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development.Immunity. 27:751–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols K.E., Hom J., Gong S.Y., Ganguly A., Ma C.S., Cannons J.L., Tangye S.G., Schwartzberg P.L., Koretzky G.A., Stein P.L. 2005. Regulation of NKT cell development by SAP, the protein defective in XLP.Nat. Med. 11:340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori H., Furukawa H., Mori S., Ito M.R., Terada M., Zhang M.C., Ishii N., Sakuma N., Nose M., Ono M. 2006. A signal adaptor SLAM-associated protein regulates spontaneous autoimmunity and Fas-dependent lymphoproliferation in MRL-Faslpr lupus mice.J. Immunol. 176:395–400 [DOI] [PubMed] [Google Scholar]
- Steinberg A.D., Roths J.B., Murphy E.D., Steinberg R.T., Raveche E.S. 1980. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice.J. Immunol. 125:871–873 [PubMed] [Google Scholar]
- Ettinger R., Kuchen S., Lipsky P.E. 2008. The role of IL-21 in regulating B-cell function in health and disease.Immunol. Rev. 223:60–86 [DOI] [PubMed] [Google Scholar]
- Odegard J.M., Marks B.R., Diplacido L.D., Poholek A.C., Kono D.H., Dong C., Flavell R.A., Craft J. 2008. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity.J. Exp. Med. 205:2873–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger R., Sims G.P., Robbins R., Withers D., Fischer R.T., Grammer A.C., Kuchen S., Lipsky P.E. 2007. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells.J. Immunol. 178:2872–2882 [DOI] [PubMed] [Google Scholar]
- Cappione A., III, Anolik J.H., Pugh-Bernard A., Barnard J., Dutcher P., Silverman G., Sanz I. 2005. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus.J. Clin. Invest. 115:3205–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammer A.C., Slota R., Fischer R., Gur H., Girschick H., Yarboro C., Illei G.G., Lipsky P.E. 2003. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions.J. Clin. Invest. 112:1506–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C.C., Sprent J., Fazekas de St Groth B., Vinuesa C.G. 2005. Cellular and genetic mechanisms of self tolerance and autoimmunity.Nature. 435:590–597 [DOI] [PubMed] [Google Scholar]
- Allen C.D., Okada T., Cyster J.G. 2007. Germinal-center organization and cellular dynamics.Immunity. 27:190–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingsby J.H., Norsworthy P., Pearce G., Vaishnaw A.K., Issler H., Morley B.J., Walport M.J. 1996. Homozygous hereditary C1q deficiency and systemic lupus erythematosus. A new family and the molecular basis of C1q deficiency in three families.Arthritis Rheum. 39:663–670 [DOI] [PubMed] [Google Scholar]
- Hutloff A., Buchner K., Reiter K., Baelde H.J., Odendahl M., Jacobi A., Dorner T., Kroczek R.A. 2004. Involvement of inducible costimulator in the exaggerated memory B cell and plasma cell generation in systemic lupus erythematosus.Arthritis Rheum. 50:3211–3220 [DOI] [PubMed] [Google Scholar]
- Hutloff A., Dittrich A.M., Beier K.C., Eljaschewitsch B., Kraft R., Anagnostopoulos I., Kroczek R.A. 1999. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28.Nature. 397:263–266 [DOI] [PubMed] [Google Scholar]
- Rasheed A.U., Rahn H.P., Sallusto F., Lipp M., Muller G. 2006. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression.Eur. J. Immunol. 36:1892–1903 [DOI] [PubMed] [Google Scholar]
- Phan T.G., Amesbury M., Gardam S., Crosbie J., Hasbold J., Hodgkin P.D., Basten A., Brink R. 2003. B cell receptor-independent stimuli trigger immunoglobulin (Ig) class switch recombination and production of IgG autoantibodies by anergic self-reactive B cells.J. Exp. Med. 197:845–860 [DOI] [PMC free article] [PubMed] [Google Scholar]