Abstract
Resting antigen-experienced memory B cells are thought to be responsible for the more rapid and robust antibody responses after antigen reencounter, which are the hallmark of memory humoral responses. The molecular basis for the development and survival of memory B cells remains largely unknown. We report that phospholipase C (PLC) γ2 is required for efficient formation of germinal center (GC) and memory B cells. Moreover, memory B cell homeostasis is severely hampered by inducible loss of PLC-γ2. Accordingly, mice with a conditional deletion of PLC-γ2 in post-GC B cells had an almost complete abrogation of the secondary antibody response. Collectively, our data suggest that PLC-γ2 conveys a survival signal to GC and memory B cells and that this signal is required for a productive secondary immune response.
Humoral memory is characterized by recall immune responses, which are more rapid than the primary response, and by production of higher serum titers of antigen-specific antibodies, mostly of the IgG isotype. The prevailing view is that antigen-specific B cells are maintained as a pool of memory B cells after clonal expansion during the primary immune response (1–4). Most memory B cells have been thought to originate from the germinal center (GC) reaction. In the GC, the combined processes of somatic hypermutation and selection based on the affinity of the B cell receptor (BCR) for the antigen are responsible for the generation of high-affinity antibody variants that ultimately differentiate into long-lived plasma cells or long-lived memory B cells (5, 6). The GC is also a preferential site of antibody class switching. In the GC reaction, de novo–generated antigen-specific memory B cells are thought to acquire intrinsically different traits from their naive predecessors, accounting for faster and heightened secondary responses. Thus, understanding the mechanism by which memory B cells are generated and maintained, as well as the intrinsic functional differences between naive and memory B cells, is of fundamental interest to reveal the basis of immunological memory.
The analysis of gene-targeted mice lacking the cytoplasmic tail of the IgG1 or IgE BCR has revealed its essential function in secondary responses (7, 8). In response to T cell–dependent antigens, mice harboring the tailless IgG1 had ∼25-fold fewer IgG1-expressing B cells, presumably reflecting a reduced number of GC and memory B cells and raising the possibility that the IgG1 cytoplasmic tail is involved in the generation and/or maintenance of memory B cells or their direct precursors. Two non–mutually exclusive models have been proposed to explain the function of the IgG1 tail (9). First, it may be required for efficient BCR-mediated internalization and, hence, presentation of antigen to T cells (10). As T cells facilitate productive IgG1 memory responses, inefficient antigen presentation by mutant B cells could lead to defective proliferation of GC B cells and, consequently, diminished generation of memory B cells. Second, the IgG1 tail may contribute to memory responses by modifying the BCR signal, for example by transmitting survival signals to memory B cells and/or their direct precursors (11, 12).
To define the signaling molecules required for the establishment and maintenance of memory B cells, we focused on the function of phospholipase C (PLC) γ2 because this enzyme is well recognized as an important component of the BCR signaling pathway (13, 14). Indeed, PLC-γ2–deficient mice show a differentiation block between the immature and mature B cell stages owing to defective BCR signaling (15, 16). However, given the expression of PLC-γ2 in several immune cell types (17, 18) and the premature block in B cell development in conventional PLC-γ2 KO mice, these mice are not ideal for analyzing the role of PLC-γ2 in a B cell–intrinsic manner during T cell–dependent antibody responses. Thus, we used conditional mice in which PLC-γ2 function was specifically inactivated in GC B cells and in an inducible manner. We show in this paper that PLC-γ2 is required for the efficient generation and maintenance of memory B cells, probably through the delivery of a prosurvival signal.
RESULTS
Recall responses are severely impaired in mice with conditional deletion of PLC-γ2 in GC B cells
For conditional ablation of PLC-γ2 in GC B cells, we crossed PLC-γ2 conditional (flox) mice (15) to Cγ1-Cre mice (19) to generate PLC-γ2f/f–Cγ1-CreKI/wt, which we designated PLC G1KO mice. In Cγ1-Cre mice, Cre recombinase is expressed predominantly in GC B cells activated to express the Cγ1 germline transcript before switching to IgG1. Cre-mediated deletion of the PLC-γ2 gene in Cγ1-Cre mice is expected to occur mainly in the GC B cells of mice immunized with T-dependent antigens. We found that B cell development was normal in PLC G1KO mice and that there were no major changes in basal serum Ig levels except for those of IgG1 and IgG3, which were 8.2- and 3.2-fold decreased, respectively, compared with control mice (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20082100/DC1). This phenotype enabled us to analyze the intrinsic roles of PLC-γ2 in B cells during the immune response, including recall responses.
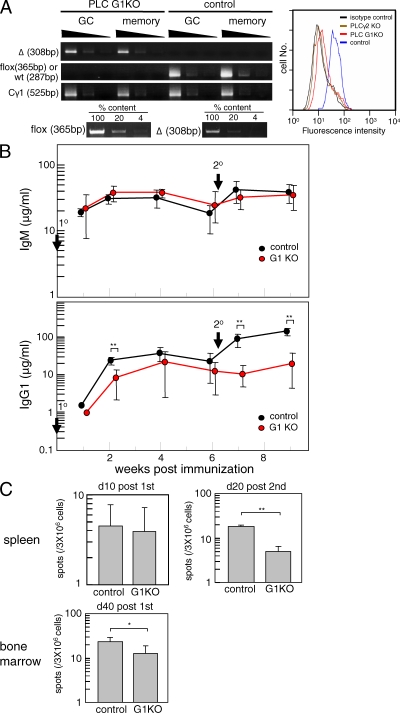
When PLC G1KO mice were immunized with the T cell–dependent antigen 4-hydroxy-3-nitrophenylacetyl (NP)–chicken γ-globulin (CGG) adsorbed on alum, the PLC-γ2 gene was efficiently deleted in the majority of NIP+IgG1+CD38dull GC B cells and NIP+IgG1+CD38hi memory B cells on day 10 after the primary immunization (Fig. 1 A, left). Flow cytometric determination of PLC-γ2 protein in IgG1+ B cells of PLC G1KO mice showed a 10-fold reduction when compared with control B cells (Fig. 1 A, right). There was a modest reduction in antigen-specific IgG1 levels in the PLC G1KO mice after primary immunization (Fig. 1 B), confirming previous studies showing a modest impairment in primary IgG1 responses in PLC-γ2–null mice (16). Consistent with this result, only a slight decrease was observed in the number of anti-NP IgG1 spot-forming cells in the spleen on day 10 after primary immunization (Fig. 1 C). Later, on day 40 after primary immunization, anti-NP IgG1 spot-forming cells could be detected in the BM of PLC G1KO mice, although their numbers were reduced (Fig. 1 C). There was no significant difference in the affinity maturation anti-NP IgG1 antibodies in PLC G1KO and control animals (Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20082100/DC1). Furthermore, accumulation of mutations in the VH186.2 gene of GC B cells isolated from NP-CGG–immunized mice, including the W33L mutation that leads to a 10-fold increase in affinity for the antigen, was unaffected upon acute PLC-γ2 ablation in GC B cells (Fig. S2 B).
Figure 1.
The secondary IgG1 response is impaired in PLC G1KO mice. (A) PLC G1KO and control mice were immunized with NP-CGG, and the deletion efficiency of the PLC-γ2 gene in GC and memory B cells was assessed by semiquantitative PCR (left). To confirm the sensitivity of the PCR, PLC-γ2f/f genomic DNA was diluted with PLC-γ2−/− genomic DNA to achieve the indicated percentages and PCR was performed under the same conditions. The sensitivity for detecting the deleted allele was examined reciprocally. Depletion of PLC-γ2 in IgG1+ B cells from immunized PLC G1KO mice was assessed by intracellular staining (right). These data are representative of four repeated experiments and each experimental group contains at least three mice. (B) PLC G1KO and control mice were immunized with NP-CGG on day 0 (1°) and boosted 42 d later (2°). Serum anti-NP titers were determined by ELISA every 2 wk. Each group consisted of at least five mice, and representative data from three repeated experiments are shown. Error bars indicate the SD for each group. **, P < 0.01 (C) The frequency of anti-NP IgG1-forming cells in spleen was enumerated by ELISPOT assay on days 10 and 20 after primary and secondary immunization, respectively (top). The frequency of anti-NP IgG1-forming cells in the BM on day 40 after primary immunization was assessed similarly (bottom). Error bars indicate the SD for each group. These data are representative of three repeated experiments and each experimental group contained at least four mice. **, P < 0.01; *, P < 0.05. The absolute number of antibody-forming cells was relatively small as a result of the suppressive effects of Cγ1-Cre that have been described previously (19).
In contrast, rechallenge of PLC G1KO mice 6 wk after the primary immunization failed to induce the efficient secondary anti-NP IgG1 response that was clearly detected in the control mice (Fig. 1 B). To examine whether the defective recall response was a result of reduced levels of antibody production by individual cells or of a reduced number of antibody-forming cells, we performed ELISPOT assays. The number of NP-specific IgG1-forming cells in the spleen of PLC G1KO mice was fourfold reduced compared with control mice on day 20 after the secondary immunization, indicating that the observed reduction in IgG1 production was at least partly a result of an impaired formation of antibody-secreting cells (Fig. 1 C).
PLC-γ2 is required for efficient generation of GC and memory B cells
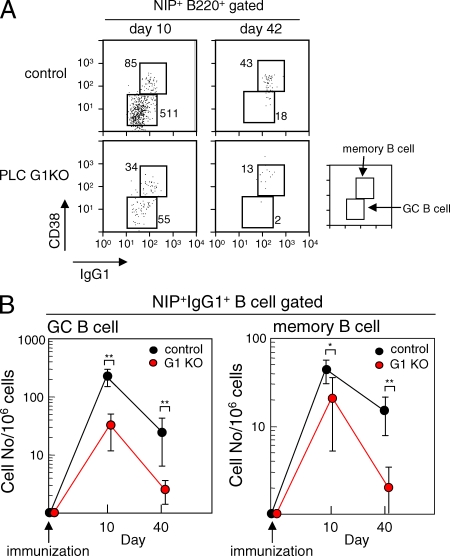
Having demonstrated the importance of PLC-γ2 in recall IgG1 responses, we wished to address the underlying mechanism. We first analyzed the frequency of NP-specific IgG1+ GC and memory B cells in the spleen on days 10 and 42 after the primary immunization. Although GC and memory responses in control mice were lower than those previously observed (20), probably because of the immunization protocol and/or genetic background, the numbers of both NIP+IgG1+CD38dull GC B cells and NIP+IgG1+CD38hi memory B cells were reduced in PLC G1KO mice (Fig. 2 B). Immunohistochemical analysis also revealed an impairment of GC formation in these mice (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20082100/DC1).
Figure 2.
The frequency of NP-specific IgG1+ GC B cells and memory B cells is diminished in PLC G1KO mice. (A) Representative data depicting analysis of the frequency of GC B and memory B cells. Spleen cells were stained as described in Materials and methods and 2.5 × 106 cells were analyzed in each panel. The numbers of GC and memory cells per 2.5 × 106 cells are indicated. (B) The frequency of NIP binding IgG1+ memory and GC B cells was examined on days 10 and 40 after the primary immunization. The frequency per 106 cells is indicated. Each group consisted of at least five mice, and representative data from three repeated experiments are shown. Error bars indicate the SD for each group. **, P < 0.01; *, P < 0.05.
These results indicate that the defective secondary IgG1 response in PLC G1KO mice depends at least in part on the impaired development of memory B cells from PLC-γ2–deficient GC B cells. A non–mutually exclusive alternative is that memory B cells, once generated, might have a reduced life span in PLC G1KO mice (see subsequent section).
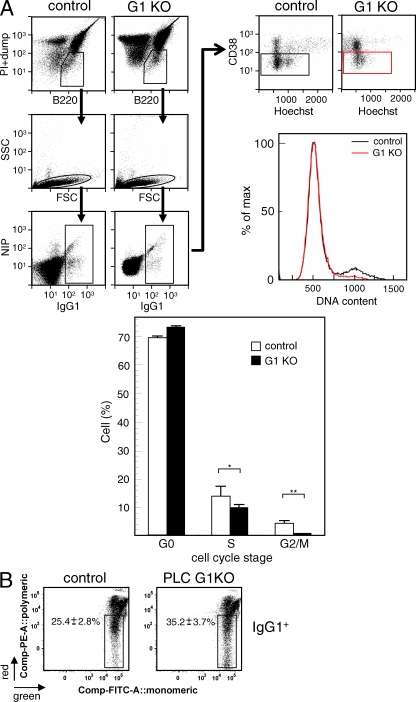
The cellular basis for the decreased number of GC B cells in PLC G1KO mice was further assessed by analyzing the cell cycle status and the percentage of apoptotic cells. As demonstrated in Fig. 3 A, Hoechst 33342 staining revealed that the percentage of PLC G1KO GC B cells in S phase was slightly reduced, and there was an even more pronounced reduction in G2/M phases, suggesting that depletion of PLC-γ2 affected cell division by GC B cells. We next analyzed the frequency of GC B cells that had recently triggered the apoptotic program by assessing the disruption of mitochondrial membrane potential. As shown in Fig. 3 B, the frequency of IgG1+ apoptotic GC B cells, in which the fluorochrome JC-1 remained monomeric in the cytosol because of disrupted mitochondrial membrane potential, was higher in PLC G1KO mice. Collectively, these results suggest that the decrease in the number of GC B cells in PLC G1KO mice is a result of the combined effects of impaired proliferation and survival.
Figure 3.
Cell cycle progression and survival of GC B cells is impaired in PLC G1KO mice. (A) Cell cycle analysis of GC IgG1+ B cells was performed 9 d after the primary immunization by staining with Hoechst 33342 (bottom). Typical FACS profiles and the gating strategy are also shown (top). Dead cells (PI), non-B cells, and IgM+ cells (dump) were excluded as described in Materials and methods (top left). IgG1+ B cells were further analyzed for CD38 expression levels and fluorescence intensity of Hoechst 33342 (top right). A typical DNA histogram of CD38dullIgG1+ GC B cells is also shown (top right). Each group consisted of at least five mice, and representative data from three repeated experiments are shown. Error bars indicate the SD for each group. **, P < 0.01; *, P < 0.05. (B) Early apoptosis in GC IgG1+ B cells was measured 12 d after the primary immunization. Apoptotic cells were detected by measuring the frequency of the cells in which the mitochondrial membrane potential was disrupted by staining with fluorochrome JC-1 as described in Materials and methods. Apoptotic cells remained green single positive as a result of the inability of the dye to multimerize in mitochondria with disrupted membrane potential. In healthy cells, a portion of the JC-1 dye was taken up into the intact mitochondria where it formed red multimers, changing the fluorescence of the cell from green to red and green double positive. The numbers in each panel indicate the mean ± SD. The data are representative of five repeated experiments, and each experimental group contains at least three mice.
T cell–dependent immune responses depend on BCR-mediated antigen recognition, internalization, and presentation to cognate T cells. IgG1+ GC and memory B cells in PLC G1KO mice up-regulated surface expression of the costimulatory molecule CD86 (Fig. S4 A, available at http://www.jem.org/cgi/content/full/jem.20082100/DC1) in a similar fashion to control B cells. Furthermore, we obtained data suggesting that IgM-stimulated PLC-γ2−/− B cells are capable of presenting antigen to helper T cells (not depicted).
PLC-γ2 is required for maintenance of memory B cells
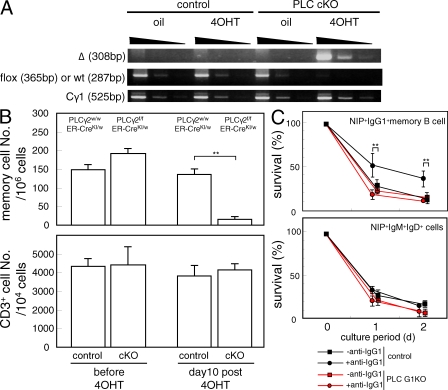
In addition to insufficient generation of memory cells from GC B cells, deficiency in the maintenance of memory B cells in PLC G1KO mice could contribute to the defective secondary responses observed in these mice. To address this issue, we conditionally deleted the PLC-γ2 gene using the tamoxifen-inducible Cre-ERT2 transgene (PLC-γ2 conditional KO [cKO] mice). To minimize the effects of PLC-γ2 loss on memory B cell formation, we delayed the administration of 4-hydroxy tamoxifen (4OHT) to day 30 after primary immunization when active GC reactions have subsided. As shown in Fig. 4 B, memory B cell formation proceeded normally in PLC-γ2 cKO mice before 4OHT treatment. In contrast, on day 10 after 4OHT treatment, when the PLC-γ2 gene in IgG1+ cells was efficiently deleted (Fig. 4 A) the frequency of NIP+IgG1+ memory B cells was markedly reduced in PLC cKO mice, whereas it remained unchanged in mice harboring only the Cre-ERT2 gene (Fig. 4 B). Importantly, T cell numbers were unchanged after this treatment. Thus, although our data cannot completely rule out the possibility that deletion of PLC-γ2 in non-B cells affects the maintenance of memory B cells, it is more likely that memory B cells require PLC-γ2 in an intrinsic manner for their maintenance.
Figure 4.
Inducible deletion of PLC-γ2 in immunized mice after the establishment of memory B cells. 30 d after the primary immunization, the PLC cKO mice were injected with sunflower oil alone or with oil containing 4OHT, as described in Materials and methods, to conditionally ablate the PLC-γ2 gene. (A) Deletion efficiency of the PLC-γ2 gene in IgG1+ spleen B cells was examined by semiquantitative PCR. The PCR conditions were the same as in Fig. 1 A. The result is representative of three repeated experiments, and each experimental group contains at least five mice. (B) The frequency of NIP binding IgG1+ memory B cells was analyzed before and 10 d after the 4OHT treatment. Each group consisted of at least five mice, and representative data from three repeated experiments are shown. Error bars indicate the SD for each group. **, P < 0.01. (C) Spleen B cells from immunized PLC G1KO mice and control mice were cultured with or without 10 µg/ml of anti-IgG1 antibody for 2 d as described in Materials and methods. The frequency of NIP binding IgG1+ memory cells and NIP binding IgM+ IgD+ cells was analyzed on days 0, 1, and 2. Error bars indicate the SD in each group. The results are representative of three repeated experiments. **, P < 0.01.
When splenic memory IgG1+ B cells from immunized control mice were stimulated in vitro with anti-IgG1 F(ab′)2 fragments, we observed a significant enhancement in cell recovery as compared with untreated cells. In contrast, treatment with anti-CD40 Ab or B cell activating factor belonging to the TNF family (BAFF) had no significant effect on the survival of memory B cells (unpublished data). The BCR-mediated survival effect was lost in memory B cells isolated from immunized PLC G1KO mice (Fig. 4 C). These results support a model in which PLC-γ2 contributes to maintenance of memory cells in a B cell–intrinsic manner and suggest that the BCR is the receptor that utilizes PLC-γ2 to generate a survival signal and is therefore required for maintenance of humoral memory.
DISCUSSION
Previous studies have shown that PLC-γ2 is required for transition from the immature to the mature B cell stage (15, 16, 21). In this paper, we have shown that during T cell–dependent immune responses, PLC-γ2 is needed for efficient generation of GC and memory B cells in a B cell–intrinsic manner. Moreover, maintenance of memory B cells also requires PLC-γ2 function. Despite the reduced numbers of GC B cells, after the primary immunization only a modest reduction in antigen-specific IgG1 levels was observed in PLC-γ2–insufficient mice, suggesting that PLC-γ2 has no direct significant role in differentiation of GC B to plasma cells.
In vivo evidence suggests the importance of CD40, BAFF-R, and BCR for efficient generation and/or maintenance of GC B cells (7, 22–24). Thus, defects in proliferation and/or survival of PLC-γ2–deficient GC IgG1+ B cells could be accounted for by defective signals emanating from these receptors. We made repeated attempts to perform in vitro GC B cell survival experiments to identify the defective receptor in PLC-γ2–insufficient cells. However, GC B cells, as reported previously (25–27), promptly undergo apoptosis thereby preventing this line of experimentation. Extrapolating from what is known about naive B cells (21), we speculate that not only BCR but also CD40 and BAFF-R use PLC-γ2 to confer survival and proliferative activity on GC B cells. In addition, considering that CD40 and BAFF-R can induce such cellular outcomes independently of antigen affinity, one possible explanation for the minor effects of PLC-γ2 depletion on affinity maturation might be that PLC-γ2 in CD40 and/or BAFF-R signaling, rather than BCR signaling, more dominantly affects the in vivo phenotype. Because the majority of memory B cells are thought to be derived from GC B cells, the defective generation of GC B cells observed in PLC-γ2–insufficient mice would be expected to result in a decrease in memory B cell numbers.
A unique feature of memory B cells is their prolonged life span; however, little is known about mechanisms required for memory B cell maintenance. Several extrinsic factors, including antigen and cytokines, are known to affect the development and survival of memory T cells (28–31). In contrast, several observations favor the idea that memory T cell and B cell survival is independent of antigen receptor occupancy, although the issue is still being debated (32, 33). For instance, using an elegant in vivo system in which BCR specificity could be changed after memory B cells had been generated, Maruyama et al. (33) found that memory B cells that could no longer bind the immunizing antigen had a lifespan comparable to that of memory B cells that had not switched receptor specificity. However, even though antigen occupancy is apparently not required for maintenance of memory B cells, the available data do not exclude the possibility that a BCR signal, presumably similar to that needed for the survival of naive B cells (34), is required for maintenance of memory B cells. Indeed, one of the explanations for the defective memory responses observed in mice harboring the tailless IgG1 BCR is that the cytoplasmic tail of IgG1 heavy chain contributes to maintenance and/or generation of memory B cells. In this regard, our results support this scenario and we propose that survival receptors, including the BCR, use PLC-γ2 for the maintenance of memory B cells. Additional studies are underway to define the essential upstream receptors and the downstream targets of PLC-γ2 that support the longevity of memory B cells.
Recall responses have been studied in several mutant mice defective in BCR signaling. Similar to our findings, the GC reaction in the primary response was diminished and the frequency of antigen-specific memory B cells was reduced 10-fold in Btk-deficient mice (35). However, despite the drastic reduction in the number of Btk-deficient memory B cells, secondary exposure to antigen resulted in levels of serum IgG1 similar to those in the WT, which is in marked contrast to our results in PLC G1KO mice. The normal secondary IgG1 response in Btk-deficient mice could be the net outcome of positive and negative roles exerted by Btk in humoral immunity. Although Btk is a critical upstream regulator for PLC-γ2 activation in the context of BCR signaling, it may have additional PLC-γ2–independent negative roles in B cell responses. Considering the fact that Btk is expressed in other type of immune cells, particularly in the myeloid lineage (36), it is also possible that Btk plays a positive role in B cells but a negative role in non-B cells that are required for productive secondary humoral immune responses. Indeed, it was recently demonstrated that Btk plays a role in TLR-induced IL-10 production, which in turn leads to inhibition of IL-6 secretion by antigen-presenting cells such as macrophages (36). Similarly, elevated serum antibody titers in the recall response of BLNK-deficient mice could be accounted for by the multiple roles of BLNK (37).
MATERIALS AND METHODS
Mice and immunization.
PLC-γ2f/f mice were generated as described previously (15). PLC-γ2f/f mice were backcrossed to C57BL/6J mice for >12 generations. PLC-γ2f/f mice were crossed to Cγ1-Cre mice (19) to generate PLC-γ2f/f–Cγ1-CreKI/w (PLC G1KO) mice. PLC-γ2wt/wt Cγ1-CreKI/w mice were used as a control. ERT2-Cre knock-in mice (38) were crossed to PLC-γ2f/f mice to obtain PLC-γ2f/f–ERT2-CreKI/w (PLC cKO) mice. PLC-γ2wt/wt–ERT2-CreKI/w was used as a control for PLC cKO mice. For immunizations, each mouse was initially injected i.p. with 100 µg NP-CGG (EMD) adsorbed to 3 mg Imject Alum (Thermo Fisher Scientific) according to the manufacturer's instructions. NP hapten was conjugated to CGG or BSA (Sigma-Aldrich) using NP-hydroxy succinimide ester (Biosearch Technologies, Inc.) according to the manufacturer's instructions. For the secondary immunization, mice were injected i.p. with 50 µg NP-CGG in PBS. Mice were maintained under specific pathogen-free conditions, and all protocols were approved by the RIKEN Animal Safety Committee.
Antibodies.
Anti-IgG1 rat monoclonal antibody was labeled using the Pacific Blue Antibody Labeling kit (Invitrogen) according to the manufacturer's instructions. Anti-CD38 antibody was purified using the mAb Trap kit (GE Healthcare) from serum-free supernatant of hybridoma CS/2 (39), which was produced using a CELLine culture flask (BD) according to the manufacturer's instructions. The antibody was labeled with an Alexa Fluor 647 labeling kit (Invitrogen) also according to the manufacturer's instructions. Directly PE-labeled NIP-BSA was prepared using an R-Phycoerythrin labeling kit-SH (Dojindo). All other antibodies were purchased from eBioscience or BD.
ELISA and ELISPOT assays.
Serum anti-NP antibody levels were measured by ELISA as described previously (15). In brief, 96-well flat-bottom plates (Thermo Fisher Scientific) were coated with 20 µg/ml NP24-BSA for 2 h at room temperature, and then the wells were blocked with 1 mg/ml BSA in PBS. Appropriately diluted serum was added to the NP-BSA–coated wells and incubated at room temperature for 2 h. The plates were washed and incubated for 1 h with diluted anti-IgG1 antibody conjugated to peroxidase (SouthernBiotech). Bound secondary antibody was assessed by incubation with ABTS substrate (Sigma-Aldrich) as described previously (40). To analyze the frequency of antibody forming cells, an ELISPOT assay was performed as described previously (41).
Semiquantitative PCR.
DNA was extracted from sorted IgG1+ B cells from NP-CGG–immunized control and PLC G1KO mice, serially diluted (1:5), and subjected to PCR using K1594 (5′-ACCCTTCTCCACCCCAAACCCTTTC-3′), K1595 (5′-CTGGGGACTCTTGAAAGGACATTAA-3′), and K1596 (5′-TGGGGACCTGTGGAAACCCCACTGA-3′) as the primers. A combination of K1594 and K1595 primers was used for amplifying the WT or floxed PLC-γ2 allele and K1594 and K1596 were used to amplify the deleted allele. To confirm the sensitivity of the PCR, genomic DNA from PLC-γ2−/− and PLC-γ2f/f mice was mixed at several ratios and the floxed and deleted alleles were detected using the described primers under the same conditions.
Flow cytometry.
Spleen cells were stained and analyzed with a FACS Aria (BD) as described elsewhere (20). In brief, spleen cells were blocked with 40 µg/ml of the 2.4G2 anti-FcγR mAb and then stained with biotinylated antibodies to the following surface markers to exclude the cells expressing these molecules: IgM, IgD, Thy1.2, CD3, Gr-1, F4/80, CD5, Ter119, Dx5, NK1.1, and AA4.1. Cells were then stained with anti–IgG1–Pacific blue, NIP-BSA-PE, PE–Texas red-labeled streptavidin, anti–CD38–Alexa Fluor 647, anti–B220-PE-Cy7, and propidium iodide (Sigma-Aldrich). IgG1+NIP binding B cells in the propidium iodide and PE–Texas red-negative gate were then analyzed for the expression level of CD38. CD38hiIgG1+NIP+ B cells and CD38dullIgG1+NIP+ B cells were analyzed as antigen-specific memory B cells and GC B cells, respectively (20). For intracellular staining of PLC-γ2, B cells from immunized mice were fixed and permeabilized using Cytofix/Cytoperm buffer (BD) according to the manufacturer's instructions. Rabbit anti–mouse PLC-γ2 polyclonal antibody (Santa Cruz Biotechnology, Inc.) was detected with SPRD-labeled anti–rabbit IgG (SouthernBiotech) in combination with anti–mouse IgG1–Pacific blue and anti–B220-FITC.
Cell cycle analysis and detection of apoptotic cells.
Apoptotic cells in the GC were detected using an APO LOGIX JC-1 Staining kit (Bachem) in combination with ToPRO3 (Invitrogen) staining to detect apoptotic cells while excluding the necrotic cells according to each manufacturer's instructions. Cell cycle analysis of GC IgG1+ B cells was performed by staining with Hoechst 33342 (Sigma-Aldrich) in combination with the staining procedure used for apoptotic cells. In brief, isolated splenic cells were suspended in RPMI1640 medium supplemented with 1% FCS at the density of 5 × 106 cells/ml and Hoechst 3342 was added at the final concentration of 10 µM. The cells were then incubated at 37°C for 30 min in dark followed by staining as described for apoptotic cells. Greater than 5,000 IgG1+ GC cells were acquired for the analysis. ModFit LT (Verity Software House) was used to calculate the percentages in the G1/G0, S, and G2/M phases of cell cycle.
Inducible deletion of the PLC-γ2 gene in PLC cKO mice.
The PLC-γ2 gene was conditionally ablated in PLC cKO mice by administration of 4OHT (Sigma-Aldrich) as described previously (42). In brief, 30 d after the primary immunization, mice were injected i.p. with 4OHT dissolved in sunflower oil at the dose of 40 mg/kg twice a day for five consecutive days. Mice were analyzed 10 d after the last injection. Deletion efficiency of PLC-γ2 gene was assessed by semiquantitative PCR.
In vitro cell culture.
1 d before B cell culture, NIH/3T3 cells were cultured to confluency in 24-well plates and treated with mitomycin C (Sigma-Aldrich). Spleen B cells isolated from PLC G1KO and control mice, both of which had been immunized with NP-CGG 10 d previously, were then added with or without 10 µg/ml anti-IgG F(ab′)2 (Jackson ImmunoResearch Laboratories) in an NIH-covered 24-well dish at a density of 106 cells/ml. Cells were recovered each day and analyzed for the frequency of memory cells.
Online supplemental material.
Fig. S1 shows the cell numbers, B cell development, and basal Ig levels in PLC G1KO mice. Fig. S2 shows the affinity maturation and somatic mutation in NP-CGG–immunized PLC G1KO mice. Fig. S3 shows the impaired GC formation in PLC G1KO mice. Fig. S4 shows the expression levels of class II MHC, CD86, CD40, and BAFF receptor in memory B cells from PLC G1KO mice. The supplemental Materials and methods describes FACS analysis, analysis of somatic mutation and affinity maturation, and histological analysis of spleen sections. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082100/DC1.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology to M. Hikida and T. Kurosaki, the Naitoh Memorial Foundation to T. Kurosaki, and grants from the National Institutes of Health to K. Rajewsky.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: 4OHT, 4-hydroxy tamoxifen; BAFF, B cell activating factor belonging to the TNF family; BCR, B cell receptor; CGG, chicken γ-globulin; cKO, conditional KO; GC, germinal center; NP, 4-hydroxy-3-nitrophenylacetyl; PLC, phospholipase C.
References
- Ahmed R., Gray D. 1996. Immunological memory and protective immunity: understanding their relation.Science. 272:54–60 [DOI] [PubMed] [Google Scholar]
- Celada F. 1971. The cellular basis of immunologic memory.Prog. Allergy. 15:223–267 [PubMed] [Google Scholar]
- Gray D. 1993. Immunological memory.Annu. Rev. Immunol. 11:49–77 [DOI] [PubMed] [Google Scholar]
- Sprent J. 1994. T and B memory cells.Cell. 76:315–322 [DOI] [PubMed] [Google Scholar]
- Tarlinton D. 2006. B-cell memory: are subsets necessary? Nat. Rev. Immunol. 6:785–790 [DOI] [PubMed] [Google Scholar]
- Rajewsky K. 1996. Clonal selection and learning in the antibody system.Nature. 381:751–758 [DOI] [PubMed] [Google Scholar]
- Kaisho T., Schwenk F., Rajewsky K. 1997. The roles of γ1 heavy chain membrane expression and cytoplasmic tail in IgG1 responses.Science. 276:412–415 [DOI] [PubMed] [Google Scholar]
- Achatz G., Nitschke L., Lamers M.C. 1997. Effect of transmembrane and cytoplasmic domains of IgE on the IgE response.Science. 276:409–411 [DOI] [PubMed] [Google Scholar]
- Nussenzweig M.C. 1997. Immune responses: tails to teach a B cell.Curr. Biol. 7:R355–R357 [DOI] [PubMed] [Google Scholar]
- Knight A.M., Lucocq J.M., Prescott A.R., Ponnambalam S., Watts C. 1997. Antigen endocytosis and presentation mediated by human membrane IgG1 in the absence of the Igα/Igß dimer.EMBO J. 16:3842–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K., Martin S.W., Pogue S.L., Silver K., Peng K., Takatsu K., Goodnow C.C. 2007. Enhancement and suppression of signaling by the conserved tail of IgG memory–type B cell antigen receptors.J. Exp. Med. 204:759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisman A., Kraus M., Seagal J., Ghosh S., Melamed D., Song J., Sasaki Y., Classen S., Lutz C., Brombacher F., et al. 2007. IgG1 B cell receptor signaling is inhibited by CD22 and promotes the development of B cells whose survival is less dependent on Igα/β.J. Exp. Med. 204:747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T., Maeda A., Ishiai M., Hashimoto A., Inabe K., Takata M. 2000. Regulation of the phospholipase C-γ2 pathway in B cells.Immunol. Rev. 176:19–29 [DOI] [PubMed] [Google Scholar]
- Hikida M., Kurosaki T. 2005. Regulation of phospholipase C-γ2 networks in B lymphocytes.Adv. Immunol. 88:73–96 [DOI] [PubMed] [Google Scholar]
- Hashimoto A., Takeda K., Inaba M., Sekimata M., Kaisho T., Ikehara S., Homma Y., Akira S., Kurosaki T. 2000. Cutting edge: essential role of phospholipase C-γ2 in B cell development and function.J. Immunol. 165:1738–1742 [DOI] [PubMed] [Google Scholar]
- Wang D., Feng J., Wen R., Marine J.C., Sangster M.Y., Parganas E., Hoffmeyer A., Jackson C.W., Cleveland J.L., Murray P.J., Ihle J.N. 2000. Phospholipase Cγ2 is essential in the functions of B cell and several Fc receptors.Immunity. 13:25–35 [DOI] [PubMed] [Google Scholar]
- Aki D., Minoda Y., Yoshida H., Watanabe S., Yoshida R., Takaesu G., Chinen T., Inaba T., Hikida M., Kurosaki T., et al. 2008. Peptidoglycan and lipopolysaccharide activate PLCγ2, leading to enhanced cytokine production in macrophages and dendritic cells.Genes Cells. 13:199–208 [DOI] [PubMed] [Google Scholar]
- Rock J., Schneider E., Grun J.R., Grutzkau A., Kuppers R., Schmitz J., Winkels G. 2007. CD303 (BDCA-2) signals in plasmacytoid dendritic cells via a BCR-like signalosome involving Syk, SLP65 and PLCγ2.Eur. J. Immunol. 37:3564–3575 [DOI] [PubMed] [Google Scholar]
- Casola S., Cattoretti G., Uyttersprot N., Koralov S.B., Seagal J., Hao Z., Waisman A., Egert A., Ghitza D., Rajewsky K. 2006. Tracking germinal center B cells expressing germ-line immunoglobulin γ1 transcripts by conditional gene targeting.Proc. Natl. Acad. Sci. USA. 103:7396–7401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Ohta H., Takemori T. 2001. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire.Immunity. 14:181–192 [DOI] [PubMed] [Google Scholar]
- Hikida M., Johmura S., Hashimoto A., Takezaki M., Kurosaki T. 2003. Coupling between B cell receptor and phospholipase C-γ2 is essential for mature B cell development.J. Exp. Med. 198:581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy T.M., Laman J.D., Ledbetter J.A., Aruffo A., Claassen E., Noelle R.J. 1994. gp39–CD40 interactions are essential for germinal center formation and the development of B cell memory.J. Exp. Med. 180:157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Casola S., Kutok J.L., Rajewsky K., Schmidt-Supprian M. 2004. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology.J. Immunol. 173:2245–2252 [DOI] [PubMed] [Google Scholar]
- Rahman Z.S., Rao S.P., Kalled S.L., Manser T. 2003. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling–deficient mice.J. Exp. Med. 198:1157–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Joshua D.E., Williams G.T., Smith C.A., Gordon J., MacLennan I.C. 1989. Mechanism of antigen-driven selection in germinal centres.Nature. 342:929–931 [DOI] [PubMed] [Google Scholar]
- Martinez-Valdez H., Guret C., de Bouteiller O., Fugier I., Banchereau J., Liu Y.J. 1996. Human germinal center B cells express the apoptosis-inducing genes Fas, c-myc, P53, and Bax but not the survival gene bcl-2.J. Exp. Med. 183:971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennino A., Berard M., Krammer P.H., Defrance T. 2001. FLICE-inhibitory protein is a key regulator of germinal center B cell apoptosis.J. Exp. Med. 193:447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns K.S., Lefrancois L. 2003. Cytokine control of memory T-cell development and survival.Nat. Rev. Immunol. 3:269–279 [DOI] [PubMed] [Google Scholar]
- Gray D. 2002. A role for antigen in the maintenance of immunological memory.Nat. Rev. Immunol. 2:60–65 [DOI] [PubMed] [Google Scholar]
- Lodolce J.P., Boone D.L., Chai S., Swain R.E., Dassopoulos T., Trettin S., Ma A. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation.Immunity. 9:669–676 [DOI] [PubMed] [Google Scholar]
- Kennedy M.K., Glaccum M., Brown S.N., Butz E.A., Viney J.L., Embers M., Matsuki N., Charrier K., Sedger L., Willis C.R., et al. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15–deficient mice.J. Exp. Med. 191:771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polic B., Kunkel D., Scheffold A., Rajewsky K. 2001. How αβ T cells deal with induced TCR α ablation.Proc. Natl. Acad. Sci. USA. 98:8744–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama M., Lam K.P., Rajewsky K. 2000. Memory B-cell persistence is independent of persisting immunizing antigen.Nature. 407:636–642 [DOI] [PubMed] [Google Scholar]
- Lam K.P., Kuhn R., Rajewsky K. 1997. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death.Cell. 90:1073–1083 [DOI] [PubMed] [Google Scholar]
- Ridderstad A., Nossal G.J., Tarlinton D.M. 1996. The xid mutation diminishes memory B cell generation but does not affect somatic hypermutation and selection.J. Immunol. 157:3357–3365 [PubMed] [Google Scholar]
- Schmidt N.W., Thieu V.T., Mann B.A., Ahyi A.N., Kaplan M.H. 2006. Bruton's tyrosine kinase is required for TLR-induced IL-10 production.J. Immunol. 177:7203–7210 [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Nojima T., Hayashi K., Goitsuka R., Furukawa K., Azuma T., Kitamura D. 2004. BASH-deficient mice: limited primary repertoire and antibody formation, but sufficient affinity maturation and memory B cell generation, in anti-NP response.Int. Immunol. 16:1161–1171 [DOI] [PubMed] [Google Scholar]
- Seibler J., Zevnik B., Kuter-Luks B., Andreas S., Kern H., Hennek T., Rode A., Heimann C., Faust N., Kauselmann G., et al. 2003. Rapid generation of inducible mouse mutants.Nucleic Acids Res. 31:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y., Miyake K., Kikuchi Y., Takatsu K., Noda S., Kosugi A., Kimoto M. 1995. A monoclonal antibody against a murine CD38 homologue delivers a signal to B cells for prolongation of survival and protection against apoptosis in vitro: unresponsiveness of X-linked immunodeficient B cells.Immunology. 85:248–255 [PMC free article] [PubMed] [Google Scholar]
- Sanjo H., Hikida M., Aiba Y., Mori Y., Hatano N., Ogata M., Kurosaki T. 2007. Extracellular signal-regulated protein kinase 2 is required for efficient generation of B cells bearing antigen-specific immunoglobulin G.Mol. Cell. Biol. 27:1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Dutta P.R., Cerasoli D.M., Kelsoe G. 1998. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection.J. Exp. Med. 187:885–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R., Brocard J., Mascrez B., LeMeur M., Metzger D., Chambon P. 1996. Ligand-activated site-specific recombination in mice.Proc. Natl. Acad. Sci. USA. 93:10887–10890 [DOI] [PMC free article] [PubMed] [Google Scholar]