Abstract
β2 integrins and Fcγ receptors are critically involved in neutrophil activation at the site of inflammation. Both receptor types trigger a receptor-proximal tyrosine phosphorylation cascade through Src family kinases and Syk, but further downstream signaling events are poorly understood. We show that phospholipase C (PLC) γ2 is phosphorylated downstream of Src family kinases and Syk during integrin or Fc receptor-mediated activation of neutrophils. PLCγ2−/− neutrophils are completely defective in β2 integrin or Fcγ receptor-mediated functional responses such as respiratory burst, degranulation, or cell spreading in vitro and show reduced adhesion/spreading in inflamed capillary venules in vivo. However, PLCγ2−/− neutrophils respond normally to various other agonists, including chemokines, bacterial formyl peptides, Toll-like receptor ligands, or proinflammatory cytokines, and migrate normally both in vitro and in vivo. To confirm the in vivo relevance of these observations, the effect of the PLCγ2−/− mutation was tested in the K/B×N serum transfer arthritis model, which is known to require β2 integrins, Fcγ receptors, and neutrophils. PLCγ2 deficiency completely protected mice from clinical signs and histological features of arthritis as well as from arthritis-induced loss of articular function. These results identify PLCγ2 as a critical player of integrin and Fc receptor-mediated neutrophil functions and the neutrophil-mediated effector phase of autoimmune arthritis.
Neutrophils play a critical role in innate immune defense, but their improper activation also contributes to tissue damage during autoimmune diseases such as rheumatoid arthritis (1–5). Neutrophils use several cell surface receptors to sense their environment including β2 integrins, immunoglobulin Fc receptors, various G protein–coupled (e.g., formyl peptide or chemokine) receptors, Toll-like receptors, and receptors for various proinflammatory cytokines.
Lymphocyte antigen receptors, Fcϵ receptors of mast cells, and Fcγ receptors of macrophages use a common receptor-proximal signal transduction machinery consisting of the sequential activation of Src family kinases, immunoreceptor tyrosine-based activation motif (ITAM) containing transmembrane adapters, and the Syk or the ZAP-70 tyrosine kinase. Studies from other groups (6, 7) and our own unpublished observations indicate that neutrophil Fcγ receptors also use a receptor-proximal Src family–ITAM-bearing adaptor–Syk signaling pathway. We have recently shown that β2 integrins in neutrophils signal through a conceptually similar receptor-proximal pathway, using Src family kinases (8, 9), two ITAM-bearing transmembrane adapters (DAP12 and the Fc receptor γ chain) (10), and the Syk tyrosine kinase (11). We and others have reported similar ITAM-based integrin signaling pathways in other cell types including macrophages (10), platelets (12), osteoclasts (13), dendritic cells (14), and microglia (15). Collectively, integrins and Fc receptors in various hematopoietic lineages signal through a conceptually similar ITAM-based receptor-proximal tyrosine phosphorylation cascade (for review see reference 16). However, the signal transduction mechanisms downstream of this common receptor-proximal pathway are poorly understood.
Phosphoinositide-specific phospholipase C (PLC) enzymes catalyze the breakdown of the membrane lipid phosphatidylinositol-4,5-bisphosphate to inositol-3,4,5-trisphosphate and diacylglycerol, triggering a concomitant Ca2+ signal and protein kinase C activation. Of the best known PLC isoforms, the PLCβ family is activated by G protein–coupled receptors, whereas the PLCγ family is activated downstream of tyrosine phosphorylation pathways. There are two known PLCγ isoforms: PLCγ1 is ubiquitously expressed, whereas PLCγ2 is preferentially expressed in the hematopoietic system. Genetic deficiency of PLCγ1 leads to embryonic lethality, likely as a result of defective erythropoiesis and vasculogenesis (17, 18). In contrast, PLCγ2-deficient mice are viable, their principal phenotype being a profound defect in B cell development and function (19).
Although PLCγ2 is activated by various Fc receptors, its possible functional role downstream of those receptors is rather controversial. Genetic deficiency of PLCγ2 attenuates Fcϵ receptor-mediated degranulation of mast cells (19, 20) but it does not affect extracellular signal-regulated kinase (ERK) activation or cytokine production under the same conditions (20). Although PLCγ2 is required for Fcγ receptor-triggered Ca2+ signal in macrophages, PLCγ2−/− macrophages show normal phagocytosis of IgG-coated erythrocytes (20). The role of PLCγ2 in Fc receptor-mediated functions in other cell types such as neutrophils is presently unknown.
PLCγ2 is also activated by integrins but its role in integrin signal transduction is also controversial. Although a statistically significant decrease of spreading was reported in PLCγ2−/− platelets (21, 22), that difference only accounted for a 30% reduction of the α2β1 integrin-induced increase in cell surface area (21) or a delayed kinetics and moderately smaller percentage of full spreading on an αIIbβ3 integrin ligand surface (22). Hence, PLCγ2 appears to be a modulator rather than a critical component of integrin signaling in platelets. In contrast, a recent study focusing on the role of Vav family proteins in neutrophils suggested that PLCγ2 downstream of Vav may be more directly involved in integrin signaling in these cells (23).
Rheumatoid arthritis is a severe chronic autoimmune disease affecting ∼1% of the human population (24). The disease is initiated by the emergence of autoreactive T cells (initiation or immunization phase), which then trigger the second (effector or tissue destruction) phase, mediated in large part by cells of the innate immune system. These two phases are very clearly separated in the K/B×N arthritis model (25). This model is initiated by a transgenic autoreactive T cell receptor (KRN transgene) on the autoimmunity-prone MHC background from the NOD mouse strain. This initial phase leads to the generation of autoantibodies that trigger excessive joint inflammation and destruction resembling human rheumatoid arthritis. Serum of affected mice can trigger the effector phase of the disease in otherwise normal mice (serum transfer arthritis) (26), allowing a clear separation of the two phases of the disease.
Analysis of the K/B×N and other models of autoimmune arthritis revealed that innate immune mechanisms are of critical importance in the later effector phase of the disease (26). Several studies using lineage depletion (27–29), genetic (30, 31), or combined genetic/reconstitution (32, 33) approaches indicate that neutrophils play a critical role in the effector phase of various animal models of autoimmune arthritis. However, the molecular mechanisms of how neutrophils contribute to the disease are very poorly understood.
Several cell surface receptors have been shown to be involved in the pathogenesis of autoimmune arthritis in mice. These receptors include Fcγ receptors such as FcγRIII or FcγRI (34–43) as well as members of the β2 integrin family (44, 45). However, it is at present unclear how (e.g., through what intracellular signaling mechanisms) Fc receptors and integrins participate in the development of joint inflammation.
The aforementioned results prompted us to test the role of PLCγ2 in various in vitro neutrophil functions as well as in the development of neutrophil-mediated autoimmune arthritis in vivo. Our results indicate that PLCγ2 is critically involved in integrin and Fc receptor-mediated neutrophil functions as well as in the neutrophil-mediated effector phase of autoimmune arthritis.
RESULTS
PLCγ2 is the dominant PLCγ isoform and is phosphorylated downstream of Src family kinases and Syk in neutrophils
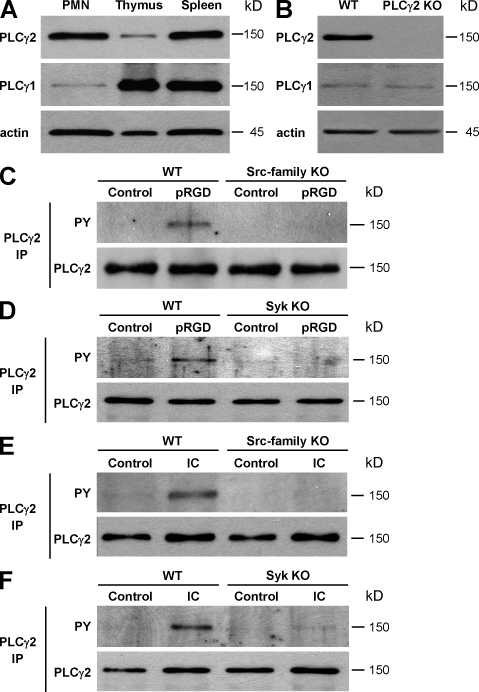
First, we tested the expression level of the two PLCγ isoforms in neutrophils and compared it to that in other cell types. As shown in Fig. 1 A, PLCγ2 was expressed at comparable levels in WT murine neutrophils and splenocytes but at much lower levels in WT thymocytes. In contrast, PLCγ1 was expressed in neutrophils at a much lower level than in the thymus or the spleen. Although these results suggested that PLCγ2 is the predominant PLCγ isoform in neutrophils, they did not allow the quantitative assessment of the relative expression of the two proteins in these cells. Hence, the expression of PLCγ1 and PLCγ2 was titrated against known amounts of recombinant Myc-tagged versions of the two proteins (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20081859/DC1). Based on those studies, WT mouse neutrophils were estimated to contain 53 ± 26 ng PLCγ2 (n = 3) and 3.0 ± 1.2 ng PLCγ1 (n = 3) per 106 cells, indicating that the expression of PLCγ2 is ∼18-fold higher than that of PLCγ1 in these cells. The expression of the two isoforms was also tested in PLCγ2−/− neutrophils (Fig. 1 B). Although no PLCγ2 signal was observed in PLCγ2−/− cells, the expression of PLCγ1 was not affected by the same mutation. The specificity of the antibodies used was also confirmed by the blocking effect of isoform-specific PLCγ-blocking peptides (Fig. S1 B). Collectively, these results indicate than neutrophils express both PLCγ1 and PLCγ2, although PLCγ2 is the predominant isoform. Furthermore, the genetic deficiency of PLCγ2 does not affect the expression of PLCγ1 in these cells.
Figure 1.
Expression and activation of PLCγ2 in neutrophils. (A) Expression of PLCγ2 and PLCγ1 in WT neutrophils compared with WT thymocytes and splenocytes. (B) Analysis of PLCγ1 and PLCγ2 expression in WT and PLCγ2−/− (PLCγ2 KO) neutrophils. (C and D) PLCγ2 phosphorylation in WT, Src family–deficient (Hck−/−Fgr−/−Lyn−/−; Src-family KO), or Syk−/− (Syk KO) neutrophils plated on a polyvalent integrin ligand (poly-RGD)-coated surface (pRGD) or left in suspension (control). PLCγ2 phosphorylation was tested by immunoprecipitation (IP) followed by immunoblotting with antibodies against phosphotyrosine (PY). (E and F) Phosphorylation of PLCγ2 in neutrophils of the various genotypes plated on an IgG immune complex–coated (IC) or control-treated surface. Immunoblotting for actin (A and B) and PLCγ2 (C–F) served as loading controls. Molecular mass values represent the estimated apparent molecular mass of the proteins. Each panel represents three to five independent experiments with similar results.
We and others have previously shown that β2 integrins signal through a receptor-proximal tyrosine phosphorylation cascade involving Src family kinases and Syk (8–11; for review see reference 16). Next, we tested whether PLCγ2 is also phosphorylated under these conditions and whether Src family kinases and Syk participate in this process. As shown in Fig. 1 (C and D), plating WT neutrophils on a polyvalent integrin ligand surface (poly-RGD) (11) triggered phosphorylation of PLCγ2. Importantly, this phosphorylation response was absent in cells lacking the Src family kinases Hck, Fgr, and Lyn (Fig. 1 C) or the Syk tyrosine kinase (Fig. 1 D). Murine neutrophils can also be activated in an FcγRIII/FcγRIV-dependent manner by plating them on immobilized IgG immune complexes (46), leading to cellular responses that are dependent on Src family kinases and Syk (unpublished data). As shown in Fig. 1 (E and F), neutrophil activation by such immobilized immune complexes leads to phosphorylation of PLCγ2 in WT but not in Src family–deficient (Fig. 1 E) or Syk-deficient (Fig. 1 F) neutrophils. These results suggest that PLCγ2 is a downstream target of Src family kinases and Syk during both integrin and Fc receptor-mediated activation of neutrophils.
PLCγ2−/− bone marrow chimeras and neutrophil surface marker expression
Our next aim was to test the role of PLCγ2 in functional responses of neutrophils, using cells that are genetically deficient of this phospholipase isoform. Because we (and others) have not been able to breed homozygous PLCγ2−/− mice (indicating a fertility defect in PLCγ2−/− males and/or females), the mutation was maintained in heterozygous (PLCγ2+/−) form. Even under such conditions, only 12% (rather than the expected 25%) of a total of 379 offsprings from PLCγ2+/− × PLCγ2+/− matings were found to be of the PLCγ2−/− genotype at weaning age, indicating a partial defect in survival of PLCγ2−/− embryos or newborn pups, which is likely a result of a lymphatic vascular developmental defect (47) similar to that seen in SLP-76−/− and Syk−/− mice (48). To overcome this problem, bone marrow transplantation was used to generate chimeric mice with a PLCγ2−/− hematopoietic system. To this end, recipient mice carrying the CD45.1 allele on the C57BL/6 genetic background were lethally irradiated and then injected intravenously with isolated PLCγ2−/− or control C57BL/6 bone marrow cells (both donor strains carry the CD45.2 allele). Repopulation of the neutrophil compartment by donor-derived cells was confirmed by flow cytometric analysis of the donor-specific CD45.2 allele in peripheral blood leukocytes 4–5 wk after transplantation (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20081859/DC1). Using this approach, 99.3 ± 1.6% (n = 120) and 99.1 ± 2.4% (n = 135) of peripheral blood neutrophils of WT and PLCγ2−/− bone marrow chimeras, respectively, were found to be of donor origin. Though quite laborious, this approach allowed us to significantly increase the number of mice available for our studies. Unless otherwise stated, all of the following experiments were performed using such WT and PLCγ2−/− bone marrow chimeras.
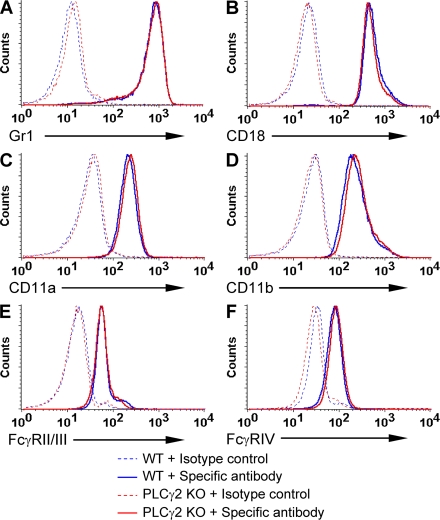
We next tested whether the deficiency of PLCγ2 affected neutrophil development or expression of major cell surface receptors. Our bone marrow neutrophil isolation protocol yielded 12.1 ± 3.3 × 106 WT and 12.4 ± 4.8 × 106 PLCγ2−/− neutrophils per mouse (n = 23; P = 0.62), indicating that the lack of PLCγ2 did not cause a quantitative change in neutrophil production. PLCγ2−/− neutrophils also expressed normal levels of the Gr1 granulocyte differentiation marker (Fig. 2 A) and the general leukocyte marker CD45 (Fig. S2). The PLCγ2−/− mutation did not affect expression of the β2 integrin chain CD18 (Fig. 2 B) or the α chains of LFA-1 (CD11a; Fig. 2 C) or Mac-1 (CD11b; Fig. 2 D). There was no difference between the two genotypes in cell surface staining with a common FcγRII/FcγRIII-recognizing antibody (Fig. 2 E) or a monoclonal antibody against FcγRIV (Fig. 2 F). Collectively, genetic deficiency of PLCγ2 did not affect neutrophil maturation or the expression of major cell surface integrins or Fcγ receptors.
Figure 2.
Expression of cell surface molecules on PLCγ2−/− neutrophils. Expression of the indicated cell surface molecules on unstimulated WT and PLCγ2−/− (PLCγ2 KO) bone marrow neutrophils was tested by flow cytometry. Each panel represents three to six independent experiments with similar results.
PLCγ2 is required for integrin and Fc receptor-mediated neutrophil functions
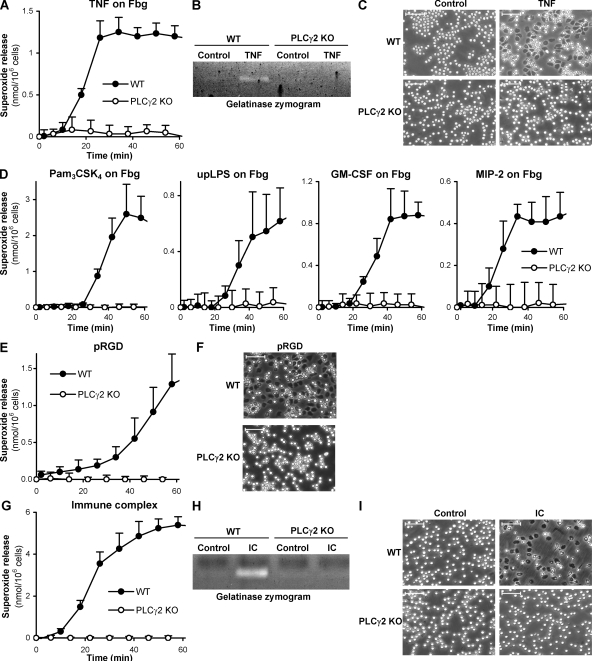
In the following experiments, the role of PLCγ2 in in vitro neutrophil functions was investigated. Robust neutrophil activation can be achieved by plating the cells on an integrin ligand (e.g., fibrinogen)-coated surface in the presence of a soluble proinflammatory agonist, such as TNF (49), mimicking activation of neutrophils adherent to the extracellular matrix at the site of inflammation. This response is completely dependent on β2 integrins both in humans (50) and mice (11) (see Fig. S4). As shown in Fig. 3 A, neutrophils obtained from PLCγ2−/− bone marrow chimeras failed to produce superoxide when plated on a fibrinogen-coated surface in the presence of TNF. In a limited number of experiments, a similar defect was also seen in neutrophils isolated from intact (nonchimeric) PLCγ2−/− mice (Fig. S3 A, available at http://www.jem.org/cgi/content/full/jem.20081859/DC1). PLCγ2−/− neutrophils also failed to release the tertiary granule marker gelatinase (Fig. 3 B) or spread over the fibrinogen surface (Fig. 3 C) under identical conditions. A similar defect was seen when fibrinogen-adherent neutrophils were stimulated by other soluble proinflammatory agents, such as the TLR2 agonist lipopeptide Pam3CSK4, the TLR4-specific ligand ultrapurified LPS, the GM-CSF cytokine, or the MIP-2 chemokine, which is the mouse homologue of human IL-8 (Fig. 3 D), in a CD18-dependent manner (Fig. S4).
Figure 3.
Defective integrin and Fc receptor-mediated responses of PLCγ2−/− neutrophils. (A–C) WT and PLCγ2−/− (PLCγ2 KO) neutrophils were activated by 50 ng/ml of murine TNF on a fibrinogen (Fbg)-coated surface and the resulting superoxide production (A), gelatinase release (B), and cell spreading (C) followed. (D) Superoxide release of fibrinogen-adherent neutrophils activated with 1 µg/ml Pam3CSK4, 5 µg/ml of ultrapurified LPS (upLPS), 10 ng/ml of murine GM-CSF, or 100 ng/ml of murine MIP-2. (E and F) Superoxide release (E) and spreading (F) of neutrophils plated on a polyvalent integrin ligand (poly-RGD)–coated surface (pRGD) in the absence of any additional stimulus. (G–I) Superoxide release (G), degranulation (H), and spreading (I) of neutrophils plated on immobilized IgG immune complexes (IC). Unstimulated control values were subtracted in A, D, and G. Error bars represent SD of triplicate readings. Bars, 50 µm. Each panel is representative of three to five independent experiments with similar results.
Although both integrin ligation and a separate proinflammatory stimulus is required for maximal activation of adherent neutrophils under physiological conditions (49, 51), β2 integrin–mediated in vitro neutrophil activation can also be achieved by plating the cells on surfaces coated with an engineered polyvalent integrin ligand (poly-RGD) in the absence of any additional stimulus (11). PLCγ2−/− neutrophils failed to release superoxide when plated on a poly-RGD–coated surface (Fig. 3 E) and they did not spread on this polyvalent integrin ligand surface either (Fig. 3 F).
Collectively, PLCγ2 appears to be critically involved in the adhesion-dependent activation of neutrophils. Together with the fact that both TNF and the other soluble proinflammatory agonists signal normally in PLCγ2−/− neutrophils in suspension (Fig. 4), our results indicate that PLCγ2 is required for signaling by integrins rather than by receptors of the soluble proinflammatory agents.
Figure 4.
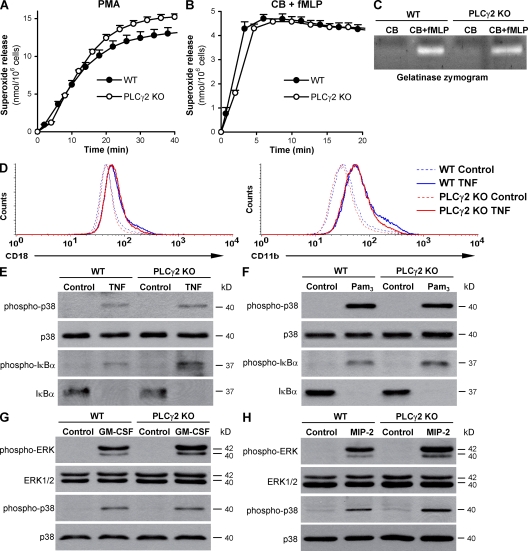
PLCγ2 is not required for integrin and Fc receptor-independent neutrophil functions. (A) Superoxide release of WT and PLCγ2−/− (PLCγ2 KO) neutrophils stimulated with 100 nM PMA. (B and C) Superoxide production (B) and gelatinase release (C) triggered by 3 µM fMLP from neutrophils preincubated with 10 µM cytochalasin B (CB). (D) Up-regulation of CD18 and CD11b upon activation of neutrophils by 50 ng/ml of murine TNF in suspension. (E and F) Phosphorylation of the p38 MAP kinase (p38) and of Iκ-Bα and degradation of Iκ-Bα upon activation of neutrophils with 50 ng/ml of murine TNF (E) or 1 µg/ml Pam3CSK4 (Pam3; F). (G and H) Phosphorylation of ERK and the p38 MAP kinase upon neutrophil activation by 10 ng/ml of murine GM-CSF (G) or 100 ng/ml of murine MIP-2 (H). Molecular mass values represent the estimated apparent molecular mass of the proteins. Unstimulated controls were subtracted in A and B. Error bars represent SD of triplicate readings. Each panel is representative of three to four independent experiments with similar results.
Neutrophils can also be activated by immobilized IgG immune complexes in an FcγRIII/FcγRIV-dependent manner (46), mimicking their activation upon immune complex deposition in autoimmune diseases. Our unpublished observations indicate that this response also requires Src family kinases and Syk. As shown in Fig. 3 G, neutrophils isolated from PLCγ2−/− bone marrow chimeras failed to release superoxide when plated on immobilized IgG immune complexes. Similar results were also obtained using neutrophils isolated from intact PLCγ2−/− mice (Fig. S3 B). The PLCγ2−/− mutation also abrogated gelatinase release (Fig. 3 H) and neutrophil spreading (Fig. 3 I) under such conditions. Hence, PLCγ2 is also critically involved in Fcγ receptor-mediated functional responses of neutrophils.
PLCγ2 is not required for signaling by G protein–coupled receptors, Toll-like receptors, and various cytokine receptors
Next, we tested integrin and Fc receptor-independent responses in PLCγ2-deficient neutrophils. As a first approach, the cells were stimulated with the nonphysiological protein kinase C–activating agent PMA, which is known to activate neutrophils even in the absence of important cell surface receptors such as β2 integrins (11) or intracellular signaling molecules like Src family kinases (9) or Syk (11). PMA-stimulated PLCγ2−/− neutrophils, which were isolated either from PLCγ2−/− bone marrow chimeras (Fig. 4 A) or intact PLCγ2−/− mice (Fig. S3 C), released normal amounts of superoxide, indicating that PLCγ2 is not required for distal steps of NADPH oxidase activation.
Robust integrin and Fc receptor-independent neutrophil activation can also be triggered by the bacterial formyl-peptide fMLP (which activates Gi protein–coupled receptors), especially if the cells are preincubated with the cytoskeletal disrupting agent cytochalasin B. Under such conditions, fMLP induced similar superoxide production (Fig. 4 B and Fig. S3 D for neutrophils from bone marrow chimeras and intact mice, respectively) and gelatinase release (Fig. 4 C) from WT and PLCγ2−/− cells, indicating that PLCγ2 is not required for formyl peptide receptor signal transduction.
In the experiments presented in Fig. 3, adhesion-dependent activation of neutrophils was tested in the presence of various soluble proinflammatory agonists. Because those agonists do not induce major functional responses (such as respiratory burst) in the absence of an integrin ligand surface (Fig. S4), their integrin-independent signaling capacity was assessed by testing up-regulation of cell surface integrins or activation of intracellular signaling pathways in suspension. TNF triggered normal up-regulation of the CD18 or CD11b integrin chains (Fig. 4 D), normal phosphorylation of the p38 MAP kinase (Fig. 4 E), and normal phosphorylation and degradation of the NF-κB pathway inhibitor Iκ-Bα (Fig. 4 E) in PLCγ2−/− neutrophils. PLCγ2 was not required for phosphorylation of p38 MAP kinase or phosphorylation/degradation of Iκ-Bα triggered by the TLR2-specific ligand Pam3CSK4 either (Fig. 4 F). Similarly, GM-CSF (Fig. 4 G) and the MIP-2 chemokine (Fig. 4 H) triggered normal ERK and p38 MAP kinase phosphorylation in PLCγ2−/− neutrophils.
Collectively, PLCγ2 is not required for integrin and Fc receptor-independent functional and signaling responses of neutrophils. These results also suggest that the defective adherent activation of PLCγ2−/− neutrophils (Fig. 3) is caused by a defect in integrin signaling rather than that of the soluble proinflammatory agonists.
Normal migration of PLCγ2-deficient neutrophils
Neutrophil migration to the site of inflammation is mediated by several cell surface receptors including chemokine/chemoattractant receptors and β2 integrins. Our previous studies indicated that Src family kinases and Syk, which are indispensable for various β2 integrin-dependent effector functions of neutrophils, are surprisingly not required for β2 integrin-mediated cell migration (11). Those studies prompted us to test whether PLCγ2 participates in β2 integrin-mediated migration of neutrophils.
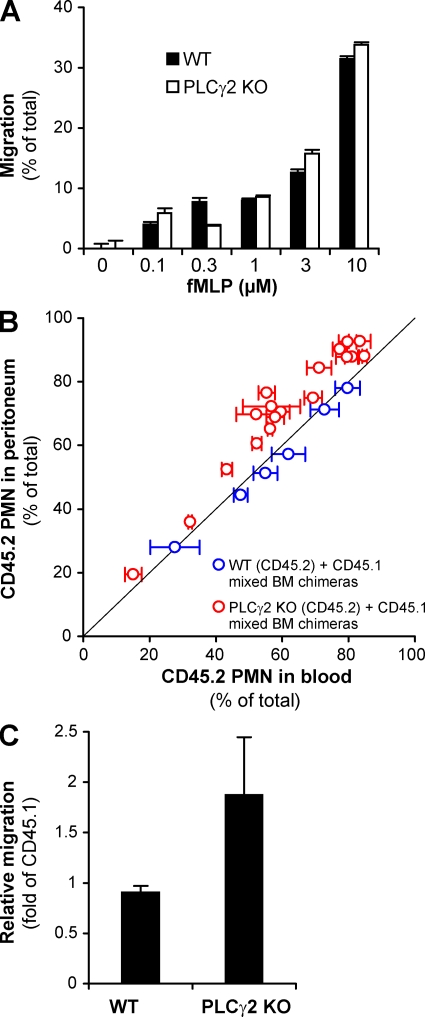
In an in vitro Transwell assay system, PLCγ2-deficient neutrophils migrated as well as WT cells toward increasing concentrations of the bacterial tripeptide fMLP through a fibrinogen-coated polycarbonate membrane of 3-µm pore size (Fig. 5 A). Because neutrophil migration under these conditions requires β2 integrins (11), these results indicate that PLCγ2 is not required for β2 integrin-mediated neutrophil migration in vitro.
Figure 5.
Normal in vitro and in vivo migration of PLCγ2−/− neutrophils. (A) Migration of WT and PLCγ2−/− (PLCγ2 KO) neutrophils toward the indicated concentrations of fMLP through fibrinogen-coated transwell membranes of 3-µm pore size. Error bars represent SD of duplicate readings. Data are representative of three independent experiments. (B and C) Competitive migration of CD45.2-expressing and CD45.1-expressing neutrophils during thioglycollate-induced sterile peritonitis in mixed bone marrow chimeras. (B) Percentage of CD45.2-expressing WT or PLCγ2−/− cells in the blood and the peritoneal lavage fluid. Each data point represents an individual mouse. The thin diagonal line marks points of identical percentage of CD45.2 cells in the blood and the peritoneum. Error bars represent SD from three blood samples taken at different time points from the same mouse. The data are combined from two independent experiments. (C) Relative migratory capacity of CD45.2-expressing WT or PLCγ2−/− neutrophils relative to the CD45.1-expressing cells calculated from the data presented in B. Error bars represent SD of values from 6 (WT) or 18 (PLCγ2−/−) individual mice.
A competitive migration assay during a sterile peritonitis (11) was used to assess the in vivo migration of PLCγ2−/− neutrophils. To this end, mixed bone marrow chimeras carrying both CD45.2-expressing PLCγ2+/+ or PLCγ2−/− cells, along with CD45.1-expressing PLCγ2+/+ cells in their hematopoietic compartment, were generated. After the induction of a sterile peritonitis by intraperitoneal injection of sterile thioglycollate broth, the percentage of neutrophils from the two donor genotypes was determined both in the bloodstream and the peritoneal infiltrate. Any difference in this percentage between the two compartments would indicate different migratory capacities of neutrophils from the two donor strains. When both CD45.1- and CD45.2-expressing donor cells were of PLCγ2+/+ genotype, the percentage of CD45.2-expressing neutrophils did not differ between the blood and the peritoneum (Fig. 5 B), indicating that the different alleles of CD45 do not affect neutrophil migration. In contrast, when CD45.2-expressing PLCγ2−/− bone marrow cells and CD45.1-expressing PLCγ2+/+ cells were present the percentage of PLCγ2−/− cells in the inflamed peritoneum was consistently higher than that in the bloodstream (Fig. 5 B). Calculation of the relative migratory capacity of neutrophils revealed that the accumulation of PLCγ2−/− neutrophils in the inflamed peritoneum was nearly twice more efficient than that of PLCγ2+/+ cells (Fig. 5 C). These results are in sharp contrast with the severe reduction of migration of CD18−/− neutrophils in a similar assay (11). Therefore, in contrast to CD18, PLCγ2 is not required for, or may even act as a negative regulator of, neutrophil migration into the inflamed peritoneum. Collectively, these results indicate that, similar to Src family kinases and Syk, PLCγ2 is not required for CD18-dependent in vitro or in vivo migration of neutrophils.
Leukocyte–endothelial interaction in fMLP-treated cremaster muscles in vivo
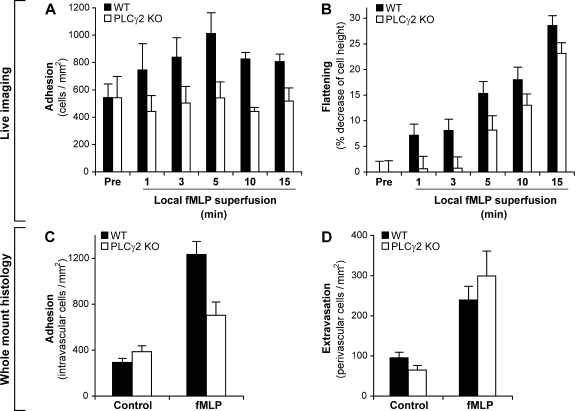
To gain further insight into the relationship between spreading, adherent activation, and cell migration in neutrophils, as well as to exclude the possibility that the aforementioned differences between the role of PLCγ2 in these processes (compare Figs. 3 and 5) stem from the very different assay systems used, we performed the simultaneous analysis of leukocyte adhesion, spreading, and extravasation in individual venules of fMLP-superfused cremaster muscles of WT and PLCγ2−/− bone marrow chimeras. As shown in Table S1 (available at http://www.jem.org/cgi/content/full/jem.20081859/DC1), there was no difference in the various hemodynamic parameters or total leukocyte counts between the two genotypes. The PLCγ2−/− mutation did not affect rolling flux fraction (36 ± 11 and 28 ± 12% in WT and PLCγ2−/− chimeras, respectively) or leukocyte adhesion (Fig. 6 A) under resting conditions either. However, although local superfusion of the cremaster muscle of WT chimeras with 1 µM fMLP triggered a significant increase in stable adhesion of leukocytes to the vessel wall, no such effect was observed in PLCγ2−/− chimeras (Fig. 6 A). fMLP also induced the spreading of WT leukocytes, as indicated by the flattening (decreased diameter perpendicular to the vessel wall) of the cells adherent to the endothelium (Fig. 6 B). This spreading (flattening) response was strongly reduced in PLCγ2−/− bone marrow chimeras at early time points (Fig. 6 B), although the mutant cells were able to partially flatten down at later time points after fMLP stimulation. Besides these real-time in vivo microscopic observations, parallel cremaster muscle samples were subjected to whole mount histological analyses. Those studies again revealed that the fMLP-induced increase of the intravascular leukocyte count (an approximate measure of leukocyte adhesion) was significantly attenuated in PLCγ2−/− bone marrow chimeras (Fig. 6 C), likely reflecting the described adhesion/spreading defect (Fig. 6, A and B). Despite all these observations, the fMLP-induced increase of the number of perivascular leukocytes (an approximate measure of leukocyte extravasation) in PLCγ2−/− chimeras was similar to or even slightly higher than that in WT control chimeras (Fig. 6 D), suggesting that transendothelial migration of leukocytes was not impaired in the absence of PLCγ2. These results again indicate that a defective adhesion/spreading response in the absence of PLCγ2 does not translate into impaired migration of leukocytes through the vessel wall.
Figure 6.
Leukocyte–endothelial interaction in fMLP-treated cremaster muscle venules in vivo. (A and B) Intravital microscopy of postcapillary cremaster muscle venules superfused with 1 µM fMLP. (A) Leukocyte adhesion in postcapillary venules of WT and PLCγ2−/− (PLCγ2 KO) bone marrow chimeras before (pre) and at the indicated time points during superfusion with fMLP. (B) Leukocyte spreading in fMLP-superfused cremaster muscle venules. The rate of spreading is expressed as the percent decrease in cell diameter perpendicular to the vessel wall. Mean and SEM of data obtained from four WT and five PLCγ2 KO chimeras are shown. (C and D) Leukocyte adhesion (C) and extravasation (D) assessed by histological analysis of whole mount preparations of cremaster muscles of WT or PLCγ2 KO bone marrow chimeras superfused for 15 min in the presence or absence of 1 µM fMLP. The mean and SEM are shown of the number of intravascular (C) and perivascular (D) leukocytes in 29–41 individual vessels per group from four WT and five PLCγ2 KO chimeras, each tested independently during the same day.
PLCγ2−/− bone marrow chimeras are protected from macroscopic and microscopic signs of autoimmune arthritis
The role of PLCγ2 in integrin and Fc receptor-mediated neutrophil functions raise the possibility that PLCγ2 may be involved in the pathogenesis of inflammatory diseases mediated by these factors. To test this possibility, we turned to the K/B×N serum transfer arthritis model, an autoantibody-mediated model of the effector phase of autoimmune arthritis. Prior studies from other groups indicated that this model requires neutrophils (27, 32, 33) as well as the presence of β2 integrins (44) and Fcγ receptors (34–41). We have also confirmed the latter two conclusions (unpublished data).
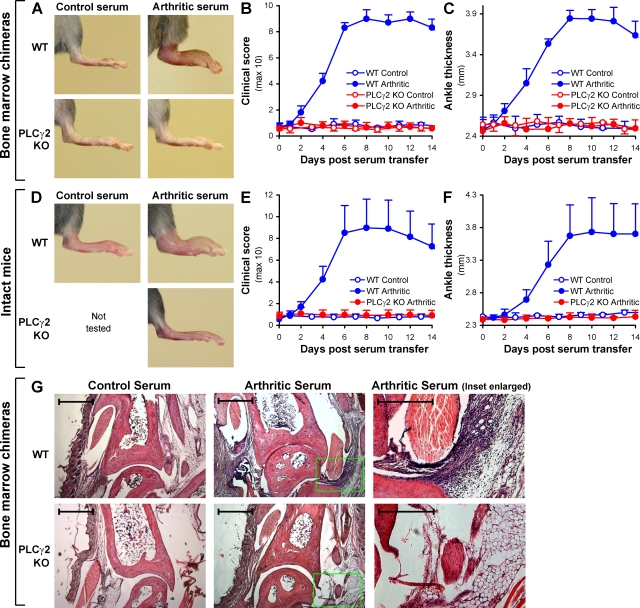
To test the role of PLCγ2 in the K/B×N serum transfer arthritis model, WT or PLCγ2−/− bone marrow chimeras were injected with arthritogenic K/B×N serum or normal serum from nonarthritic (KRN transgene negative) littermates. Although WT bone marrow chimeras injected with arthritogenic serum developed severe arthritis of their hind paws (Fig. 7 A), no sign of the disease was seen in similarly treated PLCγ2−/−chimeras (Fig. 7 A), indicating a major role for PLCγ2 in the development of K/B×N serum transfer arthritis. Quantification of arthritis severity by clinical scoring revealed that arthritis became evident 2 d after injection of WT chimeras with arthritogenic serum, peaked between 8–12 d, and started to cease afterward. Importantly, no signs of arthritis were seen at any time point in PLCγ2−/− bone marrow chimeras injected with arthritogenic K/B×N mouse serum (Fig. 7 B). Treatment of WT chimeras with arthritogenic serum also triggered a robust increase of their ankle thickness (Fig. 7 C), whereas the same treatment had no effect on ankle thickness of PLCγ2−/− chimeras (Fig. 7 C). Collectively, PLCγ2 within the hematopoietic compartment is indispensable for the development of macroscopic signs of autoimmune arthritis in the K/B×N serum transfer model.
Figure 7.
PLCγ2 is required for the development of K/B×N serum transfer arthritis. WT and PLCγ2−/− (PLCγ2 KO) bone marrow chimeras (A–C and G) or intact (nonchimeric) mice (D–F) were injected with 400 µl of arthritic (K/B×N) or nonarthritic control serum and the development of arthritis followed. (A) Photographs of the hind limb of mice of the indicated treatment and hematopoietic genotype 10 d after serum injection. Pictures are representative of a total of 17–23 individual mice per group from eight independent experiments. (B and C) Hind limb clinical score (B) and ankle thickness (C) of mice of the indicated treatment and genotype. Error bars represent the SD of four to eight individual clinical scores or ankle thickness values from a single experiment repeated a total of eight times. (D–F) Hind limb photographs (D), clinical score (E), and ankle thickness (F) of intact (nonchimeric) mice of the indicated treatment and genotype. Data are from three mice per group tested in parallel. Error bars represent the SD of six individual hind limb values from three mice per group. (G) Histological analysis of the ankle joint of mice of the indicated treatment and hematopoietic genotype 4 d after serum injection. The photomicrographs on the right are enlarged from the highlighted areas in the middle pictures. Original magnification, 5×. Bars: (left and middle) 200 µm; (right) 100 µm. Photomicrographs are representative of a total of four to six samples per group from three independent experiments.
To exclude the possibility that these results were affected by the bone marrow transplantation approach (e.g., by the use of irradiation that by itself may affect the course of autoimmune arthritis [reference 52]), the same experiments were repeated on a small cohort of intact (nonchimeric) WT and PLCγ2−/− mice. As shown in Fig. 7 (D–F), genetic deficiency of PLCγ2 completely abrogated the development of all clinical signs of K/B×N serum transfer arthritis even in such nonchimeric animals.
We also performed histological analysis of the ankle joint of bone marrow chimeras of the various experimental groups. As shown in Fig. 7 G, a robust leukocytic infiltration of the periarticular tissues could be observed in WT chimeras injected with arthritogenic serum relative to those injected with nonarthritogenic control serum. Importantly, no such infiltration was seen in PLCγ2−/− bone marrow chimeras injected with arthritogenic serum (Fig. 7 G), indicating that PLCγ2 is required for the development of microscopic signs of arthritis such as the accumulation of leukocytes in the periarticular space.
PLCγ2−/− bone marrow chimeras are protected from arthritis-induced loss of articular function
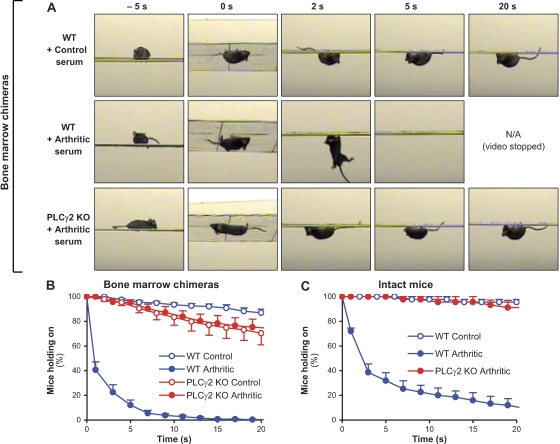
Besides the macroscopic and microscopic signs of inflammation, arthritis also leads to severe impairment of articular function. This was assessed by testing the ability of the mice to hold on to the bottom of a horizontal wire grid similar to a regular wire cage lid. As shown in the video snapshots in Fig. 8 A, although WT chimeras injected with control serum were able to hold on to the wire grid for the entire 20-s assay period, WT chimeras injected with arthritogenic serum were not able to hold on for more than a few seconds, indicating an arthritis-induced loss of articular function. Importantly, PLCγ2−/− bone marrow chimeras injected with arthritogenic serum had no difficulties in holding on to the wire grid for the entire assay period (Fig. 8 A).
Figure 8.
PLCγ2 deficiency protects from arthritis-induced loss of articular function. WT and PLCγ2−/− (PLCγ2 KO) bone marrow chimeras (A and B) or intact (nonchimeric) mice (C) were injected with 400 µl of arthritic (K/B×N) or nonarthritic control serum. 6–12 d after the serum injection, the mice were placed on a custom-made wire grid, flipped over, and the time for which the mice were able to hold on to the lower side of the grid was recorded. (A) Snapshots at the indicated time points from video captures of mice of the indicated treatment and hematopoietic genotype 10 d after serum injection. The snapshots are representative of a total of 165–263 individual measurements on 10–16 mice per group from four independent experiments. (B) Quantitative analysis of the articular function as represented by the percentage of the bone marrow chimeras from a given group to hold on to the grid for a given period of time after the grid has been flipped over from four independent experiments. Error bars represent SEM of 10–16 individual “holding on curves” (obtained from 12–21 measurements on each single mouse between 8 and 12 d after serum transfer). (C) Quantitative analysis of the articular function of intact mice of the indicated treatment and genotype. Error bars represent SEM of three individual holding on curves (obtained from 18 measurements on each single mouse between 8 and 12 d after serum transfer).
To obtain a more quantitative assessment of joint function, this experiment was repeated several times on each individual mouse during the plateau phase of the disease, and the percentage of mice that were still holding on to the wire grid at a given time point was calculated analogous to Kaplan-Meier survival curves (Fig. 8 B). As shown in Fig. 8 B, nearly 90% of control-treated WT chimeras held on to the wire grid until the end of the 20-s assay period. In contrast, only 40% of WT chimeras injected with arthritogenic serum held on for >1 s, and practically none of them did so for the entire assay period (Fig. 8 B). Importantly, most of the PLCγ2−/− chimeras were able to hold on to the wire grid for the entire 20-s period irrespective of whether they were injected with arthritogenic or control serum (Fig. 8 B). In a small set of experiments, a similar protection from arthritis-induced loss of articular function was seen in intact (nonchimeric) PLCγ2−/− mice (Fig. 8 C), indicating that the effect of PLCγ2 deficiency on articular function was not affected by the bone marrow transplantation approach used. Collectively, mice lacking PLCγ2 are also protected from arthritis-induced loss of articular function.
DISCUSSION
Rheumatoid arthritis is a severe chronic disease affecting ∼1% of the human population. Although the therapy of the disease has significantly improved during the last decades, it is still far from being solved. This is exemplified by the still widespread use of the highly cytotoxic chemotherapeutic agent methotrexate, the severe cardiovascular complications of COX-2 inhibitors, or the tremendous costs and possible side effects (e.g., reactivation of silent tuberculosis) of anti-TNF therapeutics. Better understanding of rheumatoid arthritis at the molecular level would strongly facilitate the development of novel treatment strategies for the disease.
The experiments presented in this paper provide evidence for the role of PLCγ2 in the K/B×N arthritis model, one of the most widely used animal models of rheumatoid arthritis. A unique feature of this model is that its effector phase can be clearly separated from its initiation phase by transferring the serum of an arthritic K/B×N mouse to an otherwise nonarthritic recipient (26). Our experiments performed using this serum transfer model (Figs. 7 and 8) indicate that PLCγ2 participates in the effector phase of the disease. Furthermore, the fact that bone marrow chimeras with PLCγ2−/− hematopoietic system but PLCγ2+/+ nonhematopoietic tissues are protected from K/B×N serum transfer arthritis indicates that PLCγ2 within the hematopoietic system is indispensable for disease development.
The effector phase of rheumatoid arthritis is mediated by several cell types, likely including various phagocytic lineages. To our knowledge, of those lineages only neutrophils have been consistently linked to the development of the inflammatory process in a diverse array of arthritis models and experimental approaches (27–33). Of the other phagocytes, liposome-mediated depletion studies suggested a pathogenetic role for macrophages (53), but another genetic study indicated that certain macrophage subsets play a negative rather than a positive role in autoimmune arthritis (54). Although an elegant series of genetic and reconstitution studies indicated the role of mast cells in the development of K/B×N serum transfer arthritis (55), another study using a different genetic approach suggested that arthritis development may proceed normally in the absence of mast cells (56). Based on these results, the most likely explanation for our in vivo results is that PLCγ2 within neutrophils is required for the autoantibody-induced inflammation process. The second most likely PLCγ2-dependent compartment would be the mast cell lineage, which also expresses PLCγ2 and may be activated through integrins and Fcγ receptors. However, because mast cells are long-lived radioresistant cells that survive a lethal irradiation in most tissues (57, 58), it is unlikely that our bone marrow transplantation approach was able to replace the majority of the recipients' mast cells. Hence, it unlikely that the complete defect of arthritis development in PLCγ2−/− bone marrow chimeras is caused solely by the deficiency of PLCγ2 in mast cells.
Several cell surface receptors have also been shown to participate in various models of autoimmune arthritis. Several studies using mice lacking the Fc receptor common γ chain (34–37) or Fcγ receptor-specific ligand binding α chains (36–42) indicated a critical role for Fcγ receptors in various autoimmune arthritis models (43). The role of β2 integrins (44) or their putative ligands (44, 45) has also been shown in various autoimmune arthritis models. Somewhat surprisingly, however, there is very little information available on whether and to what extent signaling molecules downstream of these receptors play a role in the development of autoimmune arthritis. In this context, it is particularly important that our study identifies PLCγ2, a component of integrin and Fc receptor signal transduction, as a critical player of the effector phase of autoimmune arthritis.
Neutrophils are critical players of the innate immune response but they also participate in tissue destruction during autoimmune diseases (1–3, 5). Integrins and Fc receptors are two major groups of cell surface receptors participating in neutrophil activation at the site of inflammation or bacterial invasion. We and others have shown that integrin and Fc receptor signaling in neutrophils are both mediated by a receptor-proximal tyrosine phosphorylation cascade consisting of Src family kinases, ITAM-bearing adaptor molecules, and the Syk tyrosine kinase (6–11; for review see reference 16) (unpublished data). In the present work, we identify PLCγ2 as a common downstream mediator of integrin and Fc receptor signaling in neutrophils (Fig. 3). PLCγ2 thus appears to be a new member of a growing family of intracellular molecules participating in both integrin and Fc receptor signal transduction in these cells (6–11, 59–61; for review see reference 16). In contrast, PLCγ2 is dispensable for neutrophil activation through several other cell surface receptors such as G protein–coupled formyl peptide or chemokine receptors, various cytokine receptors (TNF and GM-CSF), or members of the Toll-like receptor family (Fig. 4). Hence, PLCγ2 plays a specific role in signaling by a defined subset of neutrophil activatory receptors.
In addition to participating in adhesion-dependent functional responses of neutrophils, β2 integrins are also required for the migration of the cells to the site of inflammation. Somewhat surprisingly, although PLCγ2 is required for the former response (Fig. 3), β2 integrin-mediated (11) neutrophil migration can occur in the absence of PLCγ2 (Fig. 5). This is, however, in line with the normal migration of neutrophils lacking Src family kinases (11), Syk (11), ITAM-bearing adapters (10), or members of the Vav family (60) under CD18-dependent conditions (11). Therefore, β2 integrins likely use other PLCγ2-independent pathways to support neutrophil migration to the site of inflammation.
It is also puzzling how and to what extent spreading and extravasation can be dissected at the cellular and molecular level, given the generally accepted view that spreading and firm leukocyte adhesion precedes the transmigration of leukocytes through the vessel wall. Our prior studies on leukocyte–endothelial interactions in Syk−/− bone marrow chimeras (62, 63) indicated that a significant level of extravasation is possible even when adhesion is severely reduced and leukocyte spreading over the endothelium is apparently completely absent. Similar studies on PLCγ2−/− bone marrow chimeras presented in this paper also indicate that severely defective adhesion (Fig. 6, A and C), and decreased and delayed spreading (Fig. 6 B) do not necessarily hinder the extravasation (Fig. 6 D) of leukocytes. Hence, transmigration of leukocytes through the vessel wall is possible even if adhesion and spreading are severely defective. It is at present unclear how transendothelial migration occurs under these conditions. One possibility is that integrin-mediated spreading and firm adhesion simply coincide with concomitant transmigration without any major role of the former two processes in the latter one. Alternatively, spreading and adhesion may play dual roles by promoting transmigration, for example, through arresting the leukocytes at the site of inflammation, but also hindering it, for example, by holding leukocytes back by the adhesive process. If so, then a defective adhesion/spreading response would not have any major net effect on transmigration of leukocytes. Further studies will be required to reveal whether and how these or other mechanisms can explain transmigration of leukocytes when their spreading and/or adhesion responses are severely impaired.
Along the same line of thinking, it is also interesting to note that our histological analyses showed a complete lack of leukocytic infiltration in periarticular regions of PLCγ2−/− bone marrow chimeras injected with arthritogenic K/B×N serum (Fig. 7 G). Based on the normal migration of PLCγ2−/− neutrophils under other conditions (Figs. 5 and 6), we hypothesize that the lack of PLCγ2 blocks the development of the inflammatory environment (chemokines, cytokines, and inflammatory endothelium). Hence, the otherwise migration-competent neutrophils are not attracted to the periarticular tissues in PLCγ2−/− bone marrow chimeras.
All of the experiments presented in this paper have been performed on inbred mice on the C57BL/6 genetic background. This strain is the most widely used genetically homogeneous mouse strain, allowing the most accurate comparison of our results with those from other investigators. However, we cannot exclude the possibility that PLCγ2 would be less critically involved in in vitro neutrophil functions and/or the effector phase of autoimmune arthritis on a different genetic background. Such a phenomenon could theoretically be possible through compensation by either PLCγ1 or a PLCγ-independent mechanism. Although it would be rather difficult to predict the possible extent of compensation by the latter mechanism, studies showing that overexpression of PLCγ1 in PLCγ2−/− cells was not able to restore B cell maturation (64) or the development of multinucleated osteoclasts (65) suggest that there is relatively little room for functional compensation between the two members of the PLCγ family.
In addition to providing clear evidence for a role of PLCγ2 in integrin-mediated neutrophil functions and the development of K/B×N serum transfer arthritis, our studies also raise several novel questions that have yet to be addressed in the future. Although we hypothesize that the effect of the PLCγ2−/− mutation in vivo is a result of a neutrophil defect, this has yet to be confirmed more directly, for example, by lineage-specific deletion of PLCγ2 in neutrophils. The same holds true for whether our in vivo phenotype is indeed caused by an integrin and/or Fc receptor signaling defect and whether PLCγ2−/− neutrophils are indeed capable of migrating to the site of inflammation during a full-blown K/B×N serum transfer arthritis. Although our studies strongly implicate PLCγ2 in the effector phase of autoantibody-mediated arthritis, its contribution to other aspects of the disease have yet to be tested, for example, using the TNF-mediated human TNF-transgenic Tg197 (66) or the IL-17–mediated SKG point mutant (67, 68) models. It is also unclear whether the lipase activity and/or other structural features of PLCγ2 contribute to its role in neutrophil functions in vitro and arthritis development in vivo. This, and the reason for why PLCγ1 is apparently not able to compensate for the lack of PLCγ2, will need to be tested by reexpression of various PLCγ2 mutants and/or PLCγ1 in PLCγ2−/− bone marrow cells, for example, by using a retroviral reconstitution strategy (10). Finally, the role of PLCγ2 in the various aspects of antimicrobial functions of leukocytes (such as phagocytosis by myeloid lineage cells) and its relationship to that of Src family kinases and Syk has yet to be tested in more detail.
Collectively, we found that PLCγ2 is a central component of integrin and Fc receptor signal transduction in neutrophils, linking the receptor-proximal Src family–ITAM adaptor–Syk cascade to functional responses of these cells. Our results also identify PLCγ2 as a critical player of the effector phase of autoimmune arthritis, most likely through its role in integrin and Fc receptor signaling of neutrophils. These studies provide novel insight into the cellular and molecular mechanisms of autoimmune inflammation and may eventually point to novel targets of future therapies of major human diseases such as rheumatoid arthritis.
MATERIALS AND METHODS
Animals.
Heterozygous mice carrying a deleted PLCγ2 allele (PLCg2tm1Jni, referred to as PLCγ2−) (19) were obtained from J. Ihle (St. Jude Children's Research Hospital, Memphis, TN). Because of the limited fertility and survival of homozygous PLCγ2−/− mice, the mutation was maintained in heterozygous form by a PLCγ2+/− × PLCγ2+/− breeding strategy. Offsprings were genotyped by allele-specific PCR reaction from tail DNA using 5′-GCCTCTGCACAGCACACATATGG-3′ WT-specific and 5′-CAAGGTGAGATGACAGGAGATCC-3′ mutant-specific forward primers along with the 5′-TTCACCGCATCCTCCTTTGAGTCC-3′ common reverse primer. Triple Src family–deficient (Hcktm1Hev/tm1HevFgrtm1Hev/tm1HevLyntm1Sor/tm1Sor, referred to as Hck−/−Fgr−/−Lyn−/−) mice (69) were obtained from C. Lowell (University of California, San Francisco, San Francisco, CA) and kept as triple homozygous mutants. Mice carrying the Syktm1Tyb mutation (70) (referred to as the Syk− allele) were obtained from V. Tybulewicz (National Institute for Medical Research, London, UK). The Syk− mutation was maintained in heterozygous form and used to obtain Syk−/− neutrophils by fetal liver transplantation as previously described (11). Mice carrying the KRN T cell receptor transgene (25) were obtained from D. Mathis and C. Benoist (Harvard Medical School, Boston, MA) and maintained in heterozygous form by mating with C57BL/6 mice. KRN transgene-positive mice were identified by flow cytometry (see Flow cytometry) based on the high percentage of Vβ6 TCR-expressing cells among CD4-positive T cells (25). Complete CD18-deficient (Itgb2tm2Bay/tm2Bay, referred to as CD18−/−) mice (71) were obtained from A. Beaudet (Baylor College of Medicine, Houston, TX). Fc receptor γ chain–deficient (Fcer1gtm1Rav/tm1Rav, referred to as FcRγ−/−) mice (72) were purchased from Taconic. All these mice were backcrossed to the C57BL/6 genetic background for eight or more generations. WT control C57BL/6 mice were purchased from the Hungarian National Institute of Oncology. NOD mice, as well as a congenic strain carrying the CD45.1 allele on the C57BL/6 genetic background (B6.SJL-Ptprca), were purchased from The Jackson Laboratory. Mice were kept in individually sterile ventilated cages (Tecniplast) in a conventional facility. All animal experiments were approved by the Semmelweis University (Budapest, Hungary) Animal Experimentation Review Board or the Regierungspräsidium Karlsruhe (Karlsruhe, Germany).
To obtain bone marrow chimeras with PLCγ2−/− hematopoietic system, recipients carrying the CD45.1 allele on the C57BL/6 genetic background were lethally irradiated by 11 Gy from a 60Co source using an irradiator (Gammatron 3; Siemens) and then injected intravenously with unfractionated bone marrow cells from PLCγ2−/− or WT C57BL/6 control mice. On average, bone marrow cells of a single donor mouse were injected into 10–12 recipients. 4–6 wk after transplantation, peripheral blood samples were stained for Gr1 and CD45.2 and analyzed by flow cytometry (see Flow cytometry). Repopulation of the hematopoietic compartment by donor-derived cells was defined as the percentage of CD45.2-positive (donor-derived) cells in the Gr1-positive granulocyte gate. Bone marrow chimeras were used 5–10 wk after the transplantation.
Neutrophil isolation.
Mouse neutrophils were isolated from the bone marrow of the femurs and tibias by hypotonic lysis followed by Percoll (GE Healthcare) gradient centrifugation as previously described (73). Neutrophil isolation was performed at room temperature using sterile and endotoxin-free reagents. Cells were kept at room temperature in Ca2+- and Mg2+-free medium until use (usually less <30 min) and prewarmed to 37°C before activation. Neutrophil assays were performed at 37°C in Hank's balanced salt solution (Invitrogen) supplemented with 20 mM Hepes, pH 7.4.
Flow cytometry.
For neutrophil studies, isolated neutrophils, peripheral blood samples, or peritoneal lavage fluids were stained with PE-conjugated anti-Gr1 (RB6-8C5), FITC-conjugated anti-CD45.2 (clone 104), biotinylated anti-CD11b (M1/70), or unconjugated antibodies against CD18 (C71/16), CD11a (M17/4), FcγRII/III (2.4G2), or FcγRIV (9E9; obtained from J. Ravetch, Rockefeller University, New York, NY) (74). To identify KRN transgene-positive mice, peripheral blood samples were labeled with FITC-conjugated anti-CD4 (RM4-5) and PE-conjugated anti-TCR Vβ6 (RR4-7) antibodies. Unconjugated antibodies were visualized with FITC-conjugated anti–rat IgG whereas biotinylated anti-CD11b was visualized with streptavidin-Cy3 (Jackson ImmunoResearch Laboratories). Unless otherwise stated, all flow cytometry antibodies and their isotype controls were purchased from BD. All staining was performed in the presence of 2% FCS (Invitrogen). Samples were fixed in FACS Lysing Solution (BD) and analyzed on a FACSCalibur (BD) using CellQuest software (BD). Neutrophils and CD4-positive T cells were identified based on positive labeling for Gr1 and CD4, respectively, along with their typical forward- and side-scatter characteristics.
In vitro functional assays.
Adhesion-dependent activation was performed by stimulating murine neutrophils with 50 ng/ml of recombinant murine TNF (PeproTech), 1 µg/ml Pam3CSK4 (EMC Microcollections), 5 µg/ml of ultrapurified LPS (InvivoGen), 10 ng/ml of recombinant murine GM-CSF (PeproTech), or 100 ng/ml of recombinant murine MIP-2 (PeproTech) while adherent to a plastic surface coated with 150 µg/ml of human fibrinogen (MP Biomedicals) as previously described (10, 11). Integrin-mediated activation in the absence of another soluble stimulus was achieved by plating neutrophils on surfaces precoated with 20 µg/ml of engineered polyvalent integrin ligand peptide (poly-RGD; F5022; Sigma-Aldrich) Neutrophil activation by immobilized immune complexes was achieved by plating the cells on immobilized HSA–anti-HSA (both obtained from Sigma-Aldrich) immune complexes without any additional stimulus as previously described (46). Activation of neutrophils in suspension was performed in Mg2+-free media essentially as previously described (10, 11, 73, 75) using 100 nM PMA (Sigma-Aldrich), 3 µM fMLP (Sigma-Aldrich), or 50 ng/ml TNF. fMLP-stimulated cells were pretreated with 10 µM cytochalasin B (CB; Sigma-Aldrich) for 10 min before cell activation. Where necessary, the reaction was stopped after 10 min (degranulation triggered by CB+fMLP) or 30 min (integrin and Fc receptor-mediated degranulation and spreading responses; TNF-induced integrin up-regulation).
Superoxide release was determined by a real-time cytochrome c (Sigma-Aldrich) reduction test, as previously described (51), using a multiplate reader (Multiskan Ascent; Thermo Fisher Scientific) in dual wavelength (550 and 540 nm) kinetic measurement mode. To simplify the presentation, unstimulated control values were subtracted from those of stimulated samples. Exocytosis of gelatinase was determined by in-gel gelatinase zymography as previously described (10, 46). Cell spreading was assessed after formalin fixation using an inverted microscope (DMI 6000B; Leica) with a 20× phase-contrast objective connected to a charge-coupled device camera (DFC480; Leica).
Biochemical and signaling studies.
Unstimulated WT and PLCγ2−/− neutrophils were lysed in a 1% Triton X-100–based lysis buffer (11, 73), and their Triton-soluble fraction were boiled in sample buffer, run on SDS-PAGE, and immunoblotted with antibodies against PLCγ2 (Q-20; Santa Cruz Biotechnology, Inc.), PLCγ1 (1249; Santa Cruz Biotechnology, Inc.), or β-actin (AC-74; Sigma-Aldrich), followed by peroxidase-labeled secondary antibodies (GE Healthcare). Where indicated, primary antibodies were preincubated with 0.4 µg/ml of the relevant blocking peptides (Santa Cruz Biotechnology, Inc.) before incubation with the immunoblotting membrane. Lysates prepared from thymus or spleen cells of WT mice served for comparisons of signal intensity.
Purified recombinant Myc-tagged human PLCγ1 and PLCγ2, expressed in Sf9 insect cells using a baculoviral expression system, were obtained from P. Gierschik (University of Ulm, Ulm, Germany) (76). For the quantification (titration) of PLCγ1 and PLCγ2 expression in neutrophils, various amounts of the two recombinant proteins along with murine neutrophil lysates were run on SDS-PAGE and immunoblotted using the aforementioned PLCγ1 and PLCγ2 antibodies as well as an antibody against the Myc epitope (clone 9E10; Santa Cruz Biotechnology, Inc.). The amino acid sequences of the human and murine proteins are practically identical around the putative antibody recognition sites in the cases of both PLCγ isoforms, whereas there is hardly any similarity between PLCγ1 and PLCγ2 at the same sites, which justifies the use of recombinant human PLCγ isoforms along with the aforementioned polyclonal antibodies for the quantification of the two murine proteins.
For biochemical signaling experiments, neutrophils were plated on a poly-RGD or immobilized immune complex surface, or they were stimulated by 50 ng/ml TNF, 1 µg/ml Pam3CSK4, 10 ng/ml GM-CSF, or 100 ng/ml MIP-2 in Mg2+-free media in suspension. After 3-min (MIP-2) or 10-min (all other stimuli) incubations, the reaction was stopped and cell lysates were prepared in a Triton X-100–based lysis buffer (11, 73), except for immunoprecipitation assays where the lysis buffer was supplemented with 0.1% SDS and 0.5% sodium deoxycholate (RIPA). PLCγ2 was precipitated using the Q-20 PLCγ2 antibody and captured using a 1:1 mixture of protein A Sepharose (Invitrogen) and protein G Agarose (Invitrogen). Triton-soluble whole-cell lysates or PLCγ2 immunoprecipitates were immunoblotted with antibodies against phosphotyrosine (clone 4G10; Millipore), PLCγ2, p38 MAP kinase (C-20; Santa Cruz Biotechnology, Inc.), ERK1/2 (combination of C-16 [ERK1] and C-14 [ERK2]; Santa Cruz Biotechnology, Inc.), Iκ-Bα (Cell Signaling Technology), or phosphospecific antibodies (Cell Signaling Technology) against the p38 MAP kinase, ERK, and Iκ-Bα (14D4).
In vitro and in vivo migration.
In vitro migration of neutrophils was assessed by a Transwell assay system essentially as previously described (10, 11). In brief, Transwell inserts with polycarbonate filters of 3-µm pore size (Corning) were precoated with human fibrinogen, filled with suspensions of WT or PLCγ2−/− murine neutrophils, and inserted in 24-well plate wells filled with assay media containing varying concentrations of fMLP. The migration of neutrophils into the lower compartment during a 60-min period was assessed by an acid phosphatase assay as previously described (11).
A competitive migration assay during sterile peritonitis in mixed bone marrow chimeras (11) was used to assess in vivo migration of neutrophils. To this end, bone marrow cells of PLCγ2−/− mice on the C57BL/6 genetic background (i.e., carrying the CD45.2 allele) were mixed with bone marrow cells from congenic mice expressing CD45.1 on the C57BL/6 genetic background at varying ratios ranging from 10 to 70% of CD45.2-expressing cells. This mixed cell suspension was injected intravenously into lethally irradiated CD45.1-expressing recipient mice, giving rise to mixed bone marrow chimeras carrying CD45.2-expressing PLCγ2−/− and CD45.1-expressing PLCγ2+/+ hematopoietic cells. To exclude any effect of the different CD45 alleles on cell migration, a few control chimeras were generated in a similar fashion but using PLCγ2+/+ (intact C57BL/6) mice as the CD45.2-expressing donor strain, giving rise to mixed chimeras with CD45.1- and CD45.2-expressing PLCγ2+/+ hematopoietic cells. 5–8 wk after transplantation, the mixed bone marrow chimeras were injected intraperitoneally with 1 ml of 3% thioglycollate broth (Heipha Diagnostics). Blood was taken directly before as well as 2 and 4 h after the injection, and the peritoneal cavity was lavaged at 4 h. The relative percentage of CD45.1- and CD45.2-expressing neutrophils in the peripheral blood and peritoneal lavage samples was determined by flow cytometry in the Gr1-positive granulocyte gate. Relative migration of neutrophils of the CD45.2-positive PLCγ2−/− or PLCγ2+/+ genotypes (relative to the CD45.1-expressing PLCγ2+/+ cells) was calculated as follows:
 |
Intravital microscopy and whole mount cremaster muscle preparation.
Bone marrow chimeras were anesthetized using intraperitoneal injection of ketamine and xylazine and the cremaster muscle was prepared for intravital imaging as previously described (63). Intravital microscopy was performed on an upright microscope (BX51; Olympus) with a 40× 0.75 NA saline immersion objective. The microcirculation was recorded using a charge-coupled device camera (CF8/1; Kappa) coupled to a recorder (S-VHS; Panasonic). Superfusion of the cremaster muscle and local treatment with 1 µM fMLP was performed as previously described (63). Postcapillary venules ranged from 25 to 35 µm in diameter and were observed before and during fMLP administration.
Geometric and hemodynamic parameters, such as vessel diameter, leukocyte diameter, and vessel segment length, of postcapillary venules were assessed from recorded video tapes using a digital image processing system as previously described (77). Spreading of adherent leukocytes in postcapillary venules was assessed by measuring the diameter (height) of attached leukocytes perpendicular to the vessel wall before and at various time points during fMLP superfusion as previously described (63). Mean blood flow velocities and wall shear rates (γw) were estimated as previously described (63). Rolling leukocyte flux fraction was defined as the ratio of rolling leukocytes to the total number of leukocytes passing the same vessel per minute (78). Leukocyte adhesion was defined as the number of adherent cells per millimeters squared of vessel surface area (63). Systemic leukocyte concentration was determined from blood samples taken at the end of the experiment as previously described (63).
For whole mount preparations, mouse cremaster muscles were surgically prepared, as described in the first paragraph of this section, and superfused with 1 µM fMLP in superfusion buffer for 15 min. Thereafter, cremaster muscles were fixed with 4% paraformaldehyde and whole mounts were prepared as previously described (79). Fixed cremaster muscles were stained with Giemsa and analyzed for the number of intravascular and perivascular leukocytes using an upright microscope (Axioskop; Carl Zeiss, Inc.) through a 100× 1.3 NA oil immersion objective. Whole mounts from untreated cremaster muscles prepared from both WT and PLCγ2−/− bone marrow chimeras served as negative controls. All cremaster muscle experiments were independently assessed by two investigators blinded for the treatment and genotype of the mice.
K/B×N serum transfer arthritis.
Mice carrying the KRN T cell receptor transgene (25) on the C57BL/6 genetic background were mated with NOD mice to obtain KRN transgene-positive offsprings on the C57BL/6 × NOD F1 genetic background (K/B×N mice) as well as their transgene-negative (B×N) littermates. The presence of the transgene was determined by flow cytometry as well as by looking for visible signs of arthritis in the K/B×N mice. Blood was taken by retroorbital bleeding and sera from transgene-positive and transgene-negative mice were pooled separately.
Arthritis was induced by intraperitoneal injection of 400 µl of arthritogenic (K/B×N) or control serum into WT or PLCγ2−/− bone marrow chimeras or intact (nonchimeric) mice, followed by daily assessment of arthritis development for 2 wk. Visible clinical signs of arthritis were scored on a 0–10 scale by two investigators blinded for the origin and treatment of the mice. Ankle thickness was measured by a spring-loaded caliper (Kroeplin). For histological analysis, mice were killed 4 d after serum transfer and their ankle joints were fixed in formalin (Sigma-Aldrich). The joints were then decalcified, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (Histopathology Llc.). Photomicrographs were taken on a microscope (DMI 6000B; Leica).
To assess articular function, mice were placed on a custom-made wire grid (Charles River Laboratories) with identical wire thickness and spacing to a regular wire cage lid. The wire grid was flipped upside down and the length of time the mice held on to the grid was recorded. This test was performed three times daily during the period of 8–12 d after the serum injection. The obtained data were combined into holding-on curves similar to Kaplan-Meier survival curves.
Online supplemental information.
Fig. S1 shows expression level of PLCγ isoforms in neutrophils and provides detailed information about PLCγ antibody specificity. Fig. S2 shows repopulation of PLCγ2−/− bone marrow chimeras by donor-derived neutrophils after bone marrow transplantation. Fig. S3 shows functional responses of neutrophils from intact (nonchimeric) PLCγ2−/− mice. Fig. S4 shows CD18-dependent activation of adherent neutrophils by various proinflammatory agonists. Table S1 shows hemodynamic and microvascular parameters in fMLP-stimulated cremaster muscle venules. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081859/DC1.
Acknowledgments
We thank James Ihle for the PLCγ2–/– mice; Diane Mathis and Christophe Benoist for the KRN transgenic mice; Clifford Lowell for the Hck–/–Fgr–/–Lyn–/– mice; Arthur Beaudet for the CD18–/– mice; Victor Tybulewicz for the Syk+/– carriers; Peter Gierschik for recombinant PLCγ1 and PLCγ2; Tamás Németh, Dávid Győri, and Árpád Mikesy for help with experiments; Melitta Weissinger and Susanne Bierschenk for expert technical assistance during the cremaster muscle experiments; and Anna Erdei, Erzsébet Ligeti, József Mandl, and István Turai for access to equipment.
This work was supported by the Hungarian Scientific Research Fund (OTKA T046409 to A. Mócsai), the Hungarian Office for Research and Technology (NKFP-A1-0069/2006 to A. Mócsai), the US National Institutes of Health (RO3 TW006831 to A. Mócsai) and the European Research Council (Starting Independent Investigator Grant No. 206283 to A. Mócsai). A. Mócsai was an International Senior Research Fellow of the Wellcome Trust and an EMBO/HHMI Scientist. Z. Jakus and A. Mócsai were recipients of Bolyai Research Fellowships from the Hungarian Academy of Sciences.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: ERK, extracellular signal-regulated kinase; ITAM, immunoreceptor tyrosine-based activation motif; PLC, phospholipase C.
References
- Nathan C. 2006. Neutrophils and immunity: challenges and opportunities.Nat. Rev. Immunol. 6:173–182 [DOI] [PubMed] [Google Scholar]
- Weiss S.J. 1989. Tissue destruction by neutrophils.N. Engl. J. Med. 320:365–376 [DOI] [PubMed] [Google Scholar]
- Eyles J.L., Roberts A.W., Metcalf D., Wicks I.P. 2006. Granulocyte colony-stimulating factor and neutrophils - forgotten mediators of inflammatory disease.Nat. Clin. Pract. Rheumatol. 2:500–510 [DOI] [PubMed] [Google Scholar]
- Nathan C. 2002. Points of control in inflammation.Nature. 420:846–852 [DOI] [PubMed] [Google Scholar]
- Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. 2000. Neutrophils: molecules, functions and pathophysiological aspects.Lab. Invest. 80:617–653 [DOI] [PubMed] [Google Scholar]
- Kiefer F., Brumell J., Al-Alawi N., Latour S., Cheng A., Veillette A., Grinstein S., Pawson T. 1998. The Syk protein tyrosine kinase is essential for Fcγ receptor signaling in macrophages and neutrophils.Mol. Cell. Biol. 18:4209–4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Gomez-Guerrero C., Shirato I., Lopez-Franco O., Gallego-Delgado J., Sanjuan G., Lazaro A., Hernandez-Vargas P., Okumura K., Tomino Y., et al. 2003. Pre-existing glomerular immune complexes induce polymorphonuclear cell recruitment through an Fc receptor-dependent respiratory burst: Potential role in the perpetuation of immune nephritis.J. Immunol. 170:3243–3253 [DOI] [PubMed] [Google Scholar]
- Lowell C.A., Fumagalli L., Berton G. 1996. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions.J. Cell Biol. 133:895–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mócsai A., Ligeti E., Lowell C.A., Berton G. 1999. Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck.J. Immunol. 162:1120–1126 [PubMed] [Google Scholar]
- Mócsai A., Abram C.L., Jakus Z., Hu Y., Lanier L.L., Lowell C.A. 2006. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs.Nat. Immunol. 7:1326–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mócsai A., Zhou M., Meng F., Tybulewicz V.L., Lowell C.A. 2002. Syk is required for integrin signaling in neutrophils.Immunity. 16:547–558 [DOI] [PubMed] [Google Scholar]
- Abtahian F., Bezman N., Clemens R., Sebzda E., Cheng L., Shattil S.J., Kahn M.L., Koretzky G.A. 2006. Evidence for the requirement of ITAM domains but not SLP-76/Gads interaction for integrin signaling in hematopoietic cells.Mol. Cell. Biol. 26:6936–6949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W., Kitaura H., Reeve J., Long F., Tybulewicz V.L., Shattil S.J., Ginsberg M.H., Ross F.P., Teitelbaum S.L. 2007. Syk, c-Src, the αVβ3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption.J. Cell Biol. 176:877–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D.B., Stephenson L.M., Lam S.K., Brim K., Lee H.M., Bautista J., Gilfillan S., Akilesh S., Fujikawa K., Swat W. 2007. An ITAM-signaling pathway controls cross-presentation of particulate but not soluble antigens in dendritic cells.J. Exp. Med. 204:2889–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S., Bechade C., Roumier A., Bernard D., Triller A., Bessis A. 2008. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor.J. Neurosci. 28:8138–8143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus Z., Fodor S., Abram C.L., Lowell C.A., Mócsai A. 2007. Immunoreceptor-like signaling by β2 and β3 integrins.Trends Cell Biol. 17:493–501 [DOI] [PubMed] [Google Scholar]
- Ji Q.S., Winnier G.E., Niswender K.D., Horstman D., Wisdom R., Magnuson M.A., Carpenter G. 1997. Essential role of the tyrosine kinase substrate phospholipase C-γ1 in mammalian growth and development.Proc. Natl. Acad. Sci. USA. 94:2999–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H.J., Kume T., McKay C., Xu M.J., Ihle J.N., Carpenter G. 2002. Absence of erythrogenesis and vasculogenesis in Plcg1-deficient mice.J. Biol. Chem. 277:9335–9341 [DOI] [PubMed] [Google Scholar]
- Wang D., Feng J., Wen R., Marine J.C., Sangster M.Y., Parganas E., Hoffmeyer A., Jackson C.W., Cleveland J.L., Murray P.J., Ihle J.N. 2000. Phospholipase Cγ2 is essential in the functions of B cell and several Fc receptors.Immunity. 13:25–35 [DOI] [PubMed] [Google Scholar]
- Wen R., Jou S.T., Chen Y., Hoffmeyer A., Wang D. 2002. Phospholipase Cγ2 is essential for specific functions of FcϵR and FcγR.J. Immunol. 169:6743–6752 [DOI] [PubMed] [Google Scholar]
- Inoue O., Suzuki-Inoue K., Dean W.L., Frampton J., Watson S.P. 2003. Integrin α2β1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCγ2.J. Cell Biol. 160:769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonerow P., Pearce A.C., Vaux D.J., Watson S.P. 2003. A critical role for phospholipase Cγ2 in αIIbβ3-mediated platelet spreading.J. Biol. Chem. 278:37520–37529 [DOI] [PubMed] [Google Scholar]
- Graham D.B., Robertson C.M., Bautista J., Mascarenhas F., Diacovo M.J., Montgrain V., Lam S.K., Cremasco V., Dunne W.M., Faccio R., et al. 2007. Neutrophil-mediated oxidative burst and host defense are controlled by a Vav-PLCγ2 signaling axis in mice.J. Clin. Invest. 117:3445–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G.S. 2003. Evolving concepts of rheumatoid arthritis.Nature. 423:356–361 [DOI] [PubMed] [Google Scholar]
- Kouskoff V., Korganow A.S., Duchatelle V., Degott C., Benoist C., Mathis D. 1996. Organ-specific disease provoked by systemic autoimmunity.Cell. 87:811–822 [DOI] [PubMed] [Google Scholar]
- Korganow A.S., Ji H., Mangialaio S., Duchatelle V., Pelanda R., Martin T., Degott C., Kikutani H., Rajewsky K., Pasquali J.L., et al. 1999. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins.Immunity. 10:451–461 [DOI] [PubMed] [Google Scholar]
- Wipke B.T., Allen P.M. 2001. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis.J. Immunol. 167:1601–1608 [DOI] [PubMed] [Google Scholar]
- Nandakumar K.S., Svensson L., Holmdahl R. 2003. Collagen type II-specific monoclonal antibody-induced arthritis in mice: Description of the disease and the influence of age, sex, and genes.Am. J. Pathol. 163:1827–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka D., Kagari T., Doi H., Shimozato T. 2006. Essential role of neutrophils in anti-type II collagen antibody and lipopolysaccharide-induced arthritis.Immunology. 119:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson H., Allen P., Peng S.L. 2005. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis.Nat. Med. 11:666–671 [DOI] [PubMed] [Google Scholar]
- Lawlor K.E., Campbell I.K., Metcalf D., O'Donnell K., van Nieuwenhuijze A., Roberts A.W., Wicks I.P. 2004. Critical role for granulocyte colony-stimulating factor in inflammatory arthritis.Proc. Natl. Acad. Sci. USA. 101:11398–11403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Lam B.K., Kanaoka Y., Nigrovic P.A., Audoly L.P., Austen K.F., Lee D.M. 2006. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis.J. Exp. Med. 203:837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N.D., Chou R.C., Seung E., Tager A.M., Luster A.D. 2006. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis.J. Exp. Med. 203:829–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A., Nukiwa T., Takai T. 2003. Deregulation of peripheral B-cell development in enhanced severity of collagen-induced arthritis in FcγRIIB-deficient mice.J. Autoimmun. 20:227–236 [DOI] [PubMed] [Google Scholar]
- Kleinau S., Martinsson P., Heyman B. 2000. Induction and suppression of collagen-induced arthritis is dependent on distinct Fcγ receptors.J. Exp. Med. 191:1611–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H., Ohmura K., Mahmood U., Lee D.M., Hofhuis F.M., Boackle S.A., Takahashi K., Holers V.M., Walport M., Gerard C., et al. 2002. Arthritis critically dependent on innate immune system players.Immunity. 16:157–168 [DOI] [PubMed] [Google Scholar]
- Kagari T., Tanaka D., Doi H., Shimozato T. 2003. Essential role of Fcγ receptors in anti-type II collagen antibody-induced arthritis.J. Immunol. 170:4318–4324 [DOI] [PubMed] [Google Scholar]
- Kaplan C.D., Cao Y., Verbeek J.S., Tunyogi-Csapo M., Finnegan A. 2005. Development of proteoglycan-induced arthritis is critically dependent on Fcγ receptor type III expression.Arthritis Rheum. 52:1612–1619 [DOI] [PubMed] [Google Scholar]
- Diaz de Stahl T., Andren M., Martinsson P., Verbeek J.S., Kleinau S. 2002. Expression of FcγRIII is required for development of collagen-induced arthritis.Eur. J. Immunol. 32:2915–2922 [DOI] [PubMed] [Google Scholar]
- Nabbe K.C., Blom A.B., Holthuysen A.E., Boross P., Roth J., Verbeek S., van Lent P.L., van den Berg W.B. 2003. Coordinate expression of activating Fcγ receptors I and III and inhibiting Fcγ receptor type II in the determination of joint inflammation and cartilage destruction during immune complex-mediated arthritis.Arthritis Rheum. 48:255–265 [DOI] [PubMed] [Google Scholar]
- Ioan-Facsinay A., de Kimpe S.J., Hellwig S.M., van Lent P.L., Hofhuis F.M., van Ojik H.H., Sedlik C., da Silveira S.A., Gerber J., de Jong Y.F., et al. 2002. FcγRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection.Immunity. 16:391–402 [DOI] [PubMed] [Google Scholar]
- Boross P., van Lent P.L., Martin-Ramirez J., van der Kaa J., Mulder M.H., Claassens J.W., van den Berg W.B., Arandhara V.L., Verbeek J.S. 2008. Destructive arthritis in the absence of both FcγRI and FcγRIII.J. Immunol. 180:5083–5091 [DOI] [PubMed] [Google Scholar]
- Boross P., Verbeek J.S. 2006. The complex role of Fcγ receptors in the pathology of arthritis.Springer Semin. Immunopathol. 28:339–350 [DOI] [PubMed] [Google Scholar]
- Watts G.M., Beurskens F.J., Martin-Padura I., Ballantyne C.M., Klickstein L.B., Brenner M.B., Lee D.M. 2005. Manifestations of inflammatory arthritis are critically dependent on LFA-1.J. Immunol. 174:3668–3675 [DOI] [PubMed] [Google Scholar]
- Flick M.J., LaJeunesse C.M., Talmage K.E., Witte D.P., Palumbo J.S., Pinkerton M.D., Thornton S., Degen J.L. 2007. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin αMβ2 binding motif.J. Clin. Invest. 117:3224–3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus Z., Németh T., Verbeek J.S., Mócsai A. 2008. Critical but overlapping role of FcγRIII and FcγRIV in activation of murine neutrophils by immobilized immune complexes.J. Immunol. 180:618–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise H., Ichise T., Ohtani O., Yoshida N. 2009. Phospholipase Cγ2 is necessary for separation of blood and lymphatic vasculature in mice.Development. 136:191–195 [DOI] [PubMed] [Google Scholar]
- Abtahian F., Guerriero A., Sebzda E., Lu M.M., Zhou R., Mócsai A., Myers E.E., Huang B., Jackson D.G., Ferrari V.A., et al. 2003. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk.Science. 299:247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C.F. 1987. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes.J. Clin. Invest. 80:1550–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Srimal S., Farber C., Sanchez E., Kabbash L., Asch A., Gailit J., Wright S.D. 1989. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins.J. Cell Biol. 109:1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus Z., Berton G., Ligeti E., Lowell C.A., Mócsai A. 2004. Responses of neutrophils to anti-integrin antibodies depends on costimulation through low affinity FcγRs: full activation requires both integrin and nonintegrin signals.J. Immunol. 173:2068–2077 [DOI] [PubMed] [Google Scholar]
- Kotzin B.L., Strober S., Engleman E.G., Calin A., Hoppe R.T., Kansas G.S., Terrell C.P., Kaplan H.S. 1981. Treatment of intractable rheumatoid arthritis with total lymphoid irradiation.N. Engl. J. Med. 305:969–976 [DOI] [PubMed] [Google Scholar]
- Solomon S., Rajasekaran N., Jeisy-Walder E., Snapper S.B., Illges H. 2005. A crucial role for macrophages in the pathology of K/BxN serum-induced arthritis.Eur. J. Immunol. 35:3064–3073 [DOI] [PubMed] [Google Scholar]
- Bruhns P., Samuelsson A., Pollard J.W., Ravetch J.V. 2003. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease.Immunity. 18:573–581 [DOI] [PubMed] [Google Scholar]
- Lee D.M., Friend D.S., Gurish M.F., Benoist C., Mathis D., Brenner M.B. 2002. Mast cells: A cellular link between autoantibodies and inflammatory arthritis.Science. 297:1689–1692 [DOI] [PubMed] [Google Scholar]
- Zhou J.S., Xing W., Friend D.S., Austen K.F., Katz H.R. 2007. Mast cell deficiency in KitW-sh mice does not impair antibody-mediated arthritis.J. Exp. Med. 204:2797–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurish M.F., Boyce J.A. 2002. Mast cell growth, differentiation, and death.Clin. Rev. Allergy Immunol. 22:107–118 [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Shimada M., Hatanaka K., Miyano Y. 1977. Development of mast cells from grafted bone marrow cells in irradiated mice.Nature. 268:442–443 [DOI] [PubMed] [Google Scholar]
- Newbrough S.A., Mócsai A., Clemens R.A., Wu J.N., Silverman M.A., Singer A.L., Lowell C.A., Koretzky G.A. 2003. SLP-76 regulates Fcγ receptor and integrin signaling in neutrophils.Immunity. 19:761–769 [DOI] [PubMed] [Google Scholar]
- Gakidis M.A., Cullere X., Olson T., Wilsbacher J.L., Zhang B., Moores S.L., Ley K., Swat W., Mayadas T., Brugge J.S. 2004. Vav GEFs are required for β2 integrin-dependent functions of neutrophils.J. Cell Biol. 166:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utomo A., Cullere X., Glogauer M., Swat W., Mayadas T.N. 2006. Vav proteins in neutrophils are required for FcγR-mediated signaling to Rac GTPases and nicotinamide adenine dinucleotide phosphate oxidase component p40phox.J. Immunol. 177:6388–6397 [DOI] [PubMed] [Google Scholar]
- Schymeinsky J., Sindrilaru A., Frommhold D., Sperandio M., Gerstl R., Then C., Mócsai A., Scharffetter-Kochanek K., Walzog B. 2006. The Vav binding site of the non-receptor tyrosine kinase Syk at Tyr 348 is critical for β2 integrin (CD11/CD18)-mediated neutrophil migration.Blood. 108:3919–3927 [DOI] [PubMed] [Google Scholar]
- Frommhold D., Mannigel I., Schymeinsky J., Mocsai A., Poeschl J., Walzog B., Sperandio M. 2007. Spleen tyrosine kinase Syk is critical for sustained leukocyte adhesion during inflammation in vivo.BMC Immunol. 8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen R., Chen Y., Schuman J., Fu G., Yang S., Zhang W., Newman D.K., Wang D. 2004. An important role of phospholipase Cγ1 in pre-B-cell development and allelic exclusion.EMBO J. 23:4007–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wang X., Di L., Fu G., Bai L., Liu J., Feng X., McDonald J.M., Michalek S., He Y., et al. 2008. Phospholipase Cγ2 mediates RANKL-stimulated lymph node organogenesis and osteoclastogenesis.J. Biol. Chem. 283:29593–29601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keffer J., Probert L., Cazlaris H., Georgopoulos S., Kaslaris E., Kioussis D., Kollias G. 1991. Transgenic mice expressing human tumour necrosis factor: A predictive genetic model of arthritis.EMBO J. 10:4025–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Takahashi T., Hata H., Nomura T., Tagami T., Yamazaki S., Sakihama T., Matsutani T., Negishi I., Nakatsuru S., Sakaguchi S. 2003. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice.Nature. 426:454–460 [DOI] [PubMed] [Google Scholar]
- Hirota K., Hashimoto M., Yoshitomi H., Tanaka S., Nomura T., Yamaguchi T., Iwakura Y., Sakaguchi N., Sakaguchi S. 2007. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis.J. Exp. Med. 204:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Lowell C.A. 1998. A β1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration.EMBO J. 17:4391–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M., Mee P.J., Costello P.S., Williams O., Price A.A., Duddy L.P., Furlong M.T., Geahlen R.L., Tybulewicz V.L. 1995. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk.Nature. 378:298–302 [DOI] [PubMed] [Google Scholar]
- Scharffetter-Kochanek K., Lu H., Norman K., van Nood N., Munoz F., Grabbe S., McArthur M., Lorenzo I., Kaplan S., Ley K., et al. 1998. Spontaneous skin ulceration and defective T cell function in CD18 null mice.J. Exp. Med. 188:119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T., Li M., Sylvestre D., Clynes R., Ravetch J.V. 1994. FcR γ-chain deletion results in pleiotrophic effector cell defects.Cell. 76:519–529 [DOI] [PubMed] [Google Scholar]
- Mócsai A., Zhang H., Jakus Z., Kitaura J., Kawakami T., Lowell C.A. 2003. G-protein-coupled receptor signaling in Syk-deficient neutrophils and mast cells.Blood. 101:4155–4163 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Bruhns P., Horiuchi K., Ravetch J.V. 2005. FcγRIV: A novel FcR with distinct IgG subclass specificity.Immunity. 23:41–51 [DOI] [PubMed] [Google Scholar]
- Mócsai A., Jakus Z., Vántus T., Berton G., Lowell C.A., Ligeti E. 2000. Kinase pathways in chemoattractant-induced degranulation of neutrophils: The role of p38 mitogen-activated protein kinase activated by Src family kinases.J. Immunol. 164:4321–4331 [DOI] [PubMed] [Google Scholar]
- Walliser C., Retlich M., Harris R., Everett K.L., Josephs M.B., Vatter P., Esposito D., Driscoll P.C., Katan M., Gierschik P., Bunney T.D. 2008. Rac regulates its effector phospholipase Cγ2 through interaction with a split pleckstrin homology domain.J. Biol. Chem. 283:30351–30362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries A.R. 1988. A versatile video image analysis system for microcirculatory research.Int. J. Microcirc. Clin. Exp. 7:327–345 [PubMed] [Google Scholar]
- Sperandio M., Pickard J., Unnikrishnan S., Acton S.T., Ley K. 2006. Analysis of leukocyte rolling in vivo and in vitro.Methods Enzymol. 416:346–371 [DOI] [PubMed] [Google Scholar]
- Frommhold D., Ludwig A., Bixel M.G., Zarbock A., Babushkina I., Weissinger M., Cauwenberghs S., Ellies L.G., Marth J.D., Beck-Sickinger A.G., et al. 2008. Sialyltransferase ST3Gal-IV controls CXCR2-mediated firm leukocyte arrest during inflammation.J. Exp. Med. 205:1435–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]