Abstract
The bipolar spindle forms without centrosomes naturally in female meiosis and by experimental manipulation in mitosis. Augmin is a recently discovered protein complex required for centrosome-independent microtubule generation within the spindle in Drosophila melanogaster cultured cells. Five subunits of Augmin have been identified so far, but neither their organization within the complex nor their role in developing organisms is known. In this study, we report a new Augmin subunit, wee Augmin component (Wac). Wac directly interacts with another Augmin subunit, Dgt2, via its coiled-coil domain. Wac depletion in cultured cells, especially without functional centrosomes, causes severe defects in spindle assembly. We found that a wac deletion mutant is viable but female sterile and shows only a mild impact on somatic mitosis. Unexpectedly, mutant female meiosis showed robust microtubule assembly of the acentrosomal spindle but frequent chromosome misalignment. For the first time, this study establishes the role of an Augmin subunit in developing organisms and provides an insight into the architecture of the complex.
Introduction
To build a functional bipolar spindle, it is critical to control the site and timing of microtubule polymerization within the cell. The centrosome is the primary site of microtubule nucleation in cells, but it is not the only site where microtubule polymerization is initiated (Luders and Sterns, 2007). A bipolar spindle can be assembled without centrosomes naturally in female meiosis or by experimental manipulation in mitosis (McKim and Hawley, 1995; Khodjakov et al., 2000; Megraw et al., 2001; Basto et al., 2006). New microtubule generation, either by nucleation or rescue, has been observed along spindle microtubules (Mahoney et al., 2006). Tubulin dimers are incorporated at the plus end of microtubules at kinetochores (Mitchison et al., 1986; Maiato et al., 2004). However, it is not fully understood how microtubule polymerization at these various sites is controlled spatially and temporally.
A recent genome-wide RNAi screen using a Drosophila melanogaster cell line (S2 cells) has revealed a full set of genes that contribute to spindle assembly in mitosis (Goshima et al., 2007). Among them, the products of five genes (Dgt2–Dgt6) were very recently shown to form a novel complex called Augmin (Goshima et al., 2008). At least one subunit (Dgt6) is conserved in humans (Goshima et al., 2008; Zhu et al., 2008). RNAi of any subunit of Augmin in S2 cells results in a marked reduction in new microtubule generation and the γ-tubulin signal within the spindle. Further analysis demonstrated that Augmin is responsible for most of the centrosome-independent spindle microtubule assembly in S2 cells.
In a parallel study, we have adopted a new strategy to systematically uncover novel microtubule regulators (Hughes et al., 2008). Microtubule-associated proteins (MAPs) were purified from D. melanogaster embryos, and the microtubule interactome was determined by mass spectrometry. RNAi study of all 83 previously uncharacterized MAPs in S2 cells revealed 13 MAPs essential for proper spindle morphology, including three genes that failed to be detected by the aforementioned genome-wide RNAi approach.
In this paper, we report the study of a previously uncharacterized MAP named wee Augmin component (Wac), which was identified uniquely through our functional proteomics approach. We show that Wac is a new Augmin subunit, which directly interacts with another Augmin subunit, Dgt2, via its coiled coil. We have generated a deletion mutant of Wac, which is viable but female sterile. Unexpectedly, we found that Wac is not required for acentrosomal spindle assembly but for chromosome alignment in female meiosis. Therefore, oocytes have an efficient microtubule assembly pathway independent from centrosomes and Augmin but require Augmin specifically for correct chromosome alignment and segregation.
Results and discussion
Wac is a new component of the Augmin complex
In this report, we have studied the products of two genes, CG13879 (that we call wac) and CG16969 (Dgt2), which we identified in our functional proteomics study of the microtubule interactome (Hughes et al., 2008). In our original study, Wac and Dgt2 showed an identical RNAi phenotype and were highlighted as a potential interacting MAP pair through a bioinformatics analysis of large-scale yeast two-hybrid data (Hughes et al., 2008). Dgt2 has also recently been identified in an independent study (Goshima et al., 2008) as part of a new protein complex, Augmin. The estimated size of the complex is larger than the total mass of five known subunits. Therefore, we hypothesized that Wac may be a previously unidentified subunit of Augmin.
To test this hypothesis, we first raised specific antibodies against these two proteins (Fig. S1). The Dgt2 protein has only been studied by epitope tagging (Goshima et al., 2008), and Wac has not previously been characterized. Using these antibodies, we showed that both Wac and Dgt2 bind to microtubules in vitro and uniformly associate with spindle microtubules in S2 cells (Fig. S1).
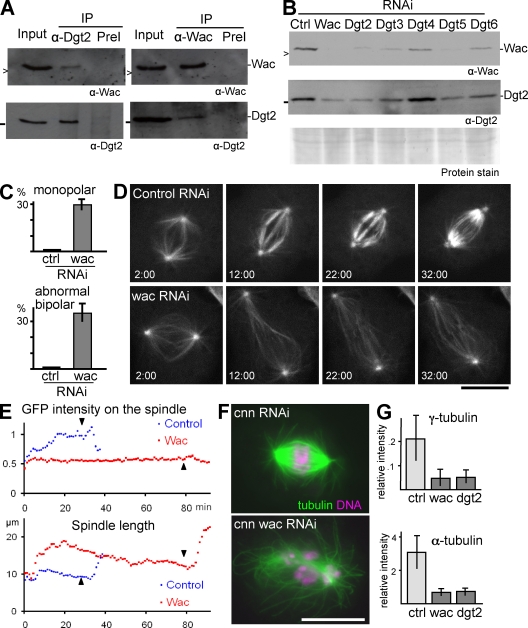
We then found that Wac and Dgt2, a known Augmin subunit, reciprocally coimmunoprecipitated from an S2 cell extract (Fig. 1 A). We also noticed that the amount of Wac in S2 cells decreased when Dgt2 was depleted by RNAi and vice versa. Furthermore, we showed that Wac levels were also reduced when other known Augmin subunits (Dgt2–6) were depleted from S2 cells (Fig. 1 B). It is already known that depletion of any Augmin subunit generally results in destabilization of other subunits in the complex (Goshima et al., 2008). Therefore, our results strongly suggest that Wac is also an integral component of the Augmin complex.
Figure 1.
Wac is an integral component of Augmin. (A) Wac and Dgt2 reciprocally coimmunoprecipitated. Wac or Dgt2 was immunoprecipitated from the soluble fraction (input) of S2 cell extract using preimmune sera (PreI) as a control (Ctrl) and immunoblotted for these two proteins. > and − indicate 16.5 kD and 25 kD, respectively. (B) Decreased amounts of Wac and Dgt2 after depletion of Augmin subunits (Dgt2–6). (C) The frequencies of monopolar spindles and abnormal bipolar spindles, typically with reduced microtubule density, after 5 d of dsRNA treatment. (D) Time sequences of mitotic progression in S2 cells expressing GFP–α-tubulin after 3 d of dsRNA incubation. Time (minutes:seconds) after the nuclear envelope breakdown is shown. (E) Change in the GFP–α-tubulin signal intensity on the spindles shown in B and change in the spindle length. The intensity was normalized against signals around the poles at prophase. The arrowheads indicate anaphase onset. (F) Typical spindle morphologies of single centrosomin (Cnn) depletion and of codepletion with Wac. (G) The γ- and α-tubulin signal intensity on the spindle relative to the poles after RNAi. IP, immunoprecipitation. Error bars indicate SD. Bars, 10 µm.
Wac is required for microtubule generation within the spindle in S2 cells
To establish whether Wac has a similar role to known Augmin subunits, we cytologically examined the RNAi phenotype of Wac in detail. As reported for other Augmin subunits (Goshima et al., 2008), immunostaining revealed a significant increase in cells that have a bipolar spindle with reduced microtubule density and in cells with a monopolar spindle (Fig. 1 C). Live imaging using GFP–α-tubulin showed that control and Wac-depleted cells both initially formed a comparable bipolar spindle (Fig. 1 D and Videos 1 and 2). Then, in control cells, the density of spindle microtubules quickly increased. In Wac-depleted cells, this increase did not take place, and instead, the spindle elongated (Fig. 1, D and E). Furthermore, anaphase onset was significantly delayed in Wac-depleted cells (147 ± 61 min vs. 41 ± 17 min in control, P < 0.01, n ≥ 19; Fig. 1, D and E, arrowheads). Monopolar spindles accumulated as a result of a failure both in centrosome separation and in conversion into a bipolar spindle, as seen in depletion of other Augmin subunits (Goshima et al., 2008), not because of the collapse of a bipolar spindle.
Codepletion of Wac with centrosomin, a protein required for centrosomal γ-tubulin recruitment, severely disrupted spindle assembly (Fig. 1 F). This indicates that Wac, like other Augmin subunits, has a major role in centrosome-independent microtubule assembly. It has previously been proposed that Augmin is required for the localization of γ-tubulin to the spindle (Goshima et al., 2008). Our quantification showed a reduction of the γ-tubulin signal on the spindle after Wac depletion but only to a degree comparable with that of the α-tubulin signal (Fig. 1 G), even at earlier time points (not depicted). Therefore, it is not possible to conclude which is the primary defect, a decreased γ-tubulin localization or decreased microtubule density. Nevertheless, Wac depletion showed similar defects to the depletion of other Augmin subunits.
In summary, Wac physically interacts with a known Augmin subunit, and its stability depends on all other known components of the Augmin complex. In addition, Wac shares functional similarity and microtubule-binding property with other Augmin subunits. Based on these findings, we concluded that Wac is an integral component of the Augmin complex.
Wac directly interacts with a known Augmin subunit, Dgt2, via its coiled-coil domain
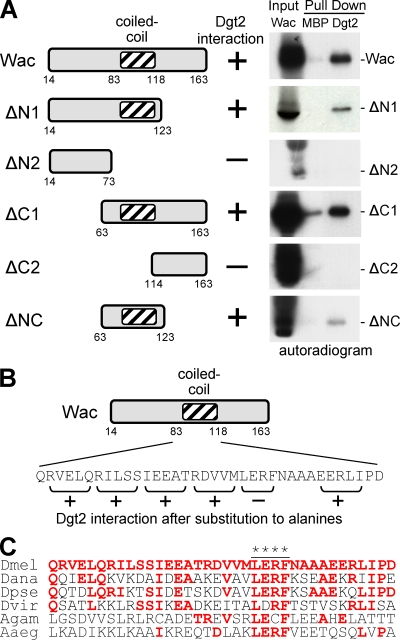
We have identified a sixth subunit of the Augmin complex, but neither this nor previous work (Goshima et al., 2008) have so far offered insights into the molecular organization within the complex. To address this question, we tested whether the interaction between Wac and Dgt2 was direct. Bacterially produced maltose-binding protein (MBP)–Dgt2 specifically interacts with Wac translated in vitro using reticulocyte lysate (Fig. 2 A). We further confirmed the direct interaction between Wac and Dgt2 using a wheat germ in vitro translation system (Fig. S2). By using a series of Wac truncations, we mapped the region of Wac responsible for its interaction with Dgt2 to 61 residues containing a predicted coiled-coil region (Fig. 2 A). Systematic replacement of consecutive residues to alanines defined four conserved amino acids (LERF) required for this interaction (Fig. 2, B and C).
Figure 2.
Wac directly interacts with Dgt2 via its coiled-coil region. (A) A series of Wac truncations were translated in vitro in reticulocyte lysate in the presence of [35S]-methionine (input), and interaction with bacterially produced MBP-Dgt2 (Dgt2) or MBP were tested by pull-down. (B) Consecutive residues within the coiled-coil region of Wac were systematically replaced with alanines, and the interaction with Dgt2 was tested. (C) A sequence comparison of Wac homologues among insects. Red letters indicate residues identical to those in D. melanogaster, and the asterisks indicate residues essential for the interaction. Dmel, D. melanogaster; Dana, Drosophila ananassae; Dpse, Drosophila pseudoobscura; Dvir, Drosophila virilis; Agam, Anopheles gambiae; Aaeg, Aedes aegypti.
Wac expression is associated with cell proliferation during development
So far, all studies of Augmin subunits have been performed using cultured cell lines (Goshima et al., 2007, 2008; Hughes et al., 2008; Somma et al., 2008; Zhu et al., 2008). Thorough if not exhaustive searches for mutants defective in mitosis have not yet identified a mutation in any Augmin subunit. Therefore, we decided to study Augmin subunits Wac and Dgt2 in developing organisms.
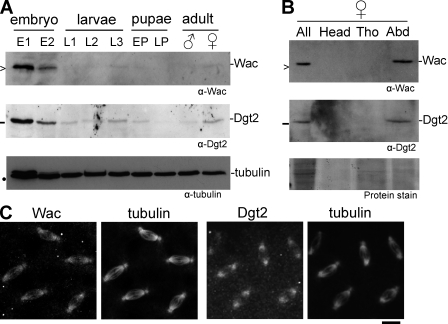
First, we examined the expression patterns of these two proteins during development. Immunoblots showed that both proteins were expressed at a high level in early embryos, which are highly active in cell division, but at a low level in other stages of development when cell division is limited to certain tissues (Fig. 3 A). In adult females, both proteins were detected only in the abdomen, which mainly contains ovaries (Fig. 3 B). Immunostaining of syncytial embryos showed that both proteins colocalize well with spindle microtubules during mitosis (Fig. 3 C). These expression patterns are consistent with a role for Wac and Dgt2 in cell division.
Figure 3.
Expression and localization of Wac and Dgt2 in developing flies. (A) Expression of Wac and Dgt2 proteins during development. E1 and E2 indicate 0–4-h-old and 4–20-h-old embryos, respectively; L1, L2, and L3 indicate first, second, and third instar larvae, respectively; EP and LP indicate early and late darkened pupae, respectively; >, −, and • indicate 16.5 kD, 25 kD, and 55 kD, respectively. (B) Expression of Wac and Dgt2 proteins in adult females. All, whole body of adult females; Tho, thorax; Abd, abdoman. (C) Immunolocalization of Wac and Dgt2 to spindle microtubules in syncytial embryos. Bar, 10 µm.
Wac is dispensable for viability
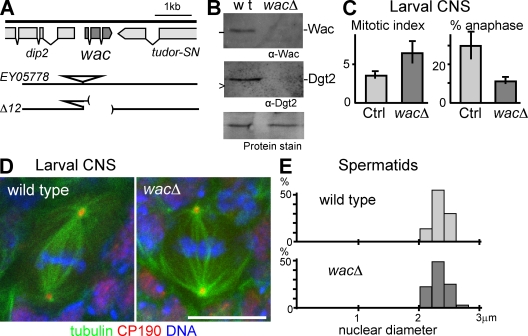
To further understand the role of the Augmin complex during development, we generated a clean wac deletion mutant (Δ12; hereafter called wacΔ) by imprecise excision of a P element (Fig. 4 A). We found that homozygotes or hemizygotes (over a deficiency) of this deletion mutation are viable. Visual inspection of adult bodies showed few morphological defects typically seen in mitotic mutants, such as rough eyes, missing bristles, or abdominal cuticle defects, indicating few cell division failures in imaginal tissues. The absence of the Wac protein in wacΔ was confirmed by immunoblots (Fig. 4 B). The amount of Dgt2 was also greatly reduced in wacΔ (Fig. 4 B). Therefore, we concluded that Wac is not essential for viability in D. melanogaster.
Figure 4.
Wac is dispensable for somatic mitosis and male meiosis. (A) The genomic organization of the wacΔ mutant. Parentheses represent the deleted region. (B) Immunoblots showing the absence of Wac and Dgt2 proteins in wacΔ adult females. wt, wild type. (C) Increased preanaphase stages in wacΔ. The mitotic index and the frequency of anaphase among mitotic cells in orcein-stained brain squashes with the SD (n = 4). The differences are significant between the homozygous mutant and the control (Ctrl) sibling heterozygotes (P < 0.01). (D) Immunostaining of whole-mount wild-type and wacΔ larval neuroblasts. CP190 is a centrosomal protein. (E) The diameter of spermatid nuclei at the onion stage from wild type and the wacΔ mutant. The distribution is not significantly different between the wild type and mutant (by χ2 test; n > 100). Error bars indicate SD. Bar, 10 µm.
wac deletion has a limited impact on chromosome segregation in somatic mitosis and male meiosis
To elucidate the role of Wac in somatic mitosis, we first examined mitosis in the larval central nervous system (CNS) of the wacΔ mutant by chromosome staining. Compared with the control CNS, chromosome condensation appeared to be generally higher in wacΔ, but overcondensation was rarely observed. The frequency of mitosis was significantly higher and the frequency of anaphases among mitotic cells was significantly lower in the mutant (Fig. 4 C), although we found only a low level of aneuploid cells (see Materials and methods). These results indicated that mitotic progression was delayed but not blocked before anaphase onset and that chromosomes are properly segregated in general.
To understand the cause of this mitotic delay, CNSs were immunostained. Unlike RNAi in S2 cells, few monopolar spindles with mitotic chromosomes were observed in the wacΔ mutant, and spindle length did not seem to differ from that in wild type. However, bipolar spindles in the wacΔ mutant appear to have a lower microtubule density (Fig. 4 D). In summary, spindle defects in somatic cells of the wac deletion mutant were milder than those seen after wac knockdown in S2 cells.
Next, we chose male meiosis as a system to test spindle function and the accuracy of chromosome segregation, as it depends on centrosomes but does not have a robust spindle checkpoint (Rebollo and Gonzalez, 2000). Wild-type and wacΔ homozygotes both showed a similarly narrow distribution of nuclear size (2.3 ± 0.1 µm; Fig. 4 E) in spermatids at the postmeiotic onion stage. A genetic test detected 7% of missegregation of the sex chromosomes in wacΔ (0.5% in a control). These results indicate that most chromosomes are properly segregated in male meiosis in the wacΔ mutant. We concluded that loss of Wac has a significant but limited impact on somatic mitosis and male meiosis based on the apparent lack of cell division failure in imaginal tissues and the largely correct segregation of the chromosomes in larval CNSs and testes.
Wac is dispensable for acentrosomal microtubule assembly in female meiosis
We found that the wacΔ mutant was male fertile but female sterile. Mutant adult females developed fully matured ovaries and laid eggs that failed to hatch. In female meiosis, a bipolar spindle forms without centrosomes in a γ-tubulin–dependent manner (Tavosanis et al., 1997). In S2 cells, simultaneously depleting Augmin and an essential centrosome component severely compromised spindle assembly to a degree comparable to that of γ-tubulin depletion (Goshima et al., 2008).
To investigate roles for Wac in female meiosis, we examined the metaphase I spindle in matured oocytes from the wacΔ mutant by immunostaining. Unexpectedly, wacΔ mutant oocytes assembled a robust bipolar spindle (Fig. 5 A). Confirming this observation, quantification of tubulin signal intensity suggested that there was only a small decrease in spindle microtubule density in the wacΔ mutant (1.3 ± 0.8 in wacΔ vs. 1.9 ± 0.9 in wild type, P = 0.05). This is in clear contrast to S2 cells, in which simultaneously depleting Wac and impairing centrosome function severely compromises spindle microtubule assembly.
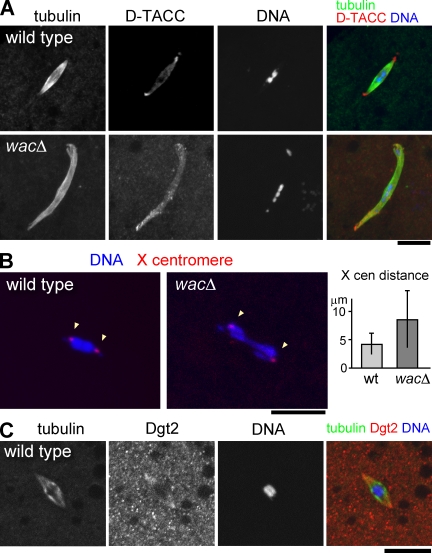
Figure 5.
Wac is not required for spindle microtubule assembly but for chromosome alignment in female meiosis. (A) Metaphase I spindle in nonactivated matured oocytes. Chromosomes misalign asymmetrically within a robust bipolar spindle in wacΔ. (B) Location of an X chromosome centromeric satellite at metaphase I determined by FISH. The mean distance between homologous centromeres significantly increased in wacΔ (P < 0.01). cen, centromere; wt, wild type. (C) Dgt2 accumulates at the polar regions of the acentrosomal metaphase I spindle in wild type. Error bars indicate SD. Bars, 10 µm.
To probe the spindle architecture, the localization of the pole protein D-TACC (D. melanogaster transforming acidic coiled coil) was examined. Immunostaining showed that D-TACC properly accumulated at both of the acentrosomal poles (Fig. 5 A), confirming that the bipolarity of the spindle is established in the absence of Wac. In addition, some spindles were longer (25% increase in the mean length) and/or had minor morphological defects (Fig. 5 A), which may be a direct influence of the wac deletion and/or a secondary consequence of chromosome misalignment (see following paragraph).
Wac is essential for correct kinetochore positioning in female meiosis
We found that chromosome positioning was disrupted within the bipolar spindle in the majority of wacΔ oocytes (57%; n = 40). Meiotic chromosomes were often spread and/or stretched along the spindle in wacΔ rather than being clustered together at the equator region as seen in wild type (Fig. 5 A).
To define the nature of this chromosome misalignment, FISH was used to locate the centromeres of the X chromosome. In wild-type, one dot was found at each opposite edge of the chromosome mass at the equator, representing two pairs of sister centromeres attached to opposite poles (Fig. 5 B). In the wacΔ mutant, the number of X centromere dots remained at two, demonstrating that oocytes have a diploid complement and that sister chromatid cohesion has been established and maintained. One X centromere dot was located within each half of the chromosome mass (in 35 out of 40 spindles), indicating that homologous kinetochores were mostly bioriented. However, the mean distance between the X centromere dots doubled in wacΔ (Fig. 5 B), indicating an altered balance of the forces acting on kinetochores.
To see the consequence of this defect, we examined meiotic progression in activated oocytes. We found chromosome missegregation in both meiotic divisions, disorganized meiosis II spindles, and fragmented meiotic products in the oocytes (Fig. S3). We then immunostained older eggs laid by wacΔ mothers. The majority of eggs contained only a few clusters of chromosomes without proliferation of free centrosomes even after being aged for an hour, which is consistent with severe meiotic defects. A few exceptional embryos in which nuclei had divided several times contained chromosomes with misshapen spindles and multiple free centrosomes (Fig. S3). We concluded that Wac is not required for acentrosomal spindle microtubule assembly but for the establishment of the forces allowing proper chromosome alignment and segregation in female meiosis.
Dgt2 is concentrated at the polar regions of the acentrosomal meiotic spindle
To understand how Augmin contributes to this acentrosomal spindle function, we attempted to examine the localization of the Augmin subunits Wac and Dgt2 in the female meiotic spindle. Our Wac antibody did not give significant signals above background. Interestingly, the Dgt2 signal was detected at the polar regions of some acentrosomal meiotic spindles (Fig. 5 C). This is in contrast to the uniform staining observed over mitotic spindles in syncytial embryos and S2 cells (Fig. 3 C and Fig. S1).
The essential role of Wac, a new Augmin subunit, in female meiosis
In this study, we have identified Wac as a new subunit of Augmin that directly interacts with Dgt2 via conserved residues in its coiled-coil domain. Augmin was previously shown to be responsible for centrosome-independent microtubule generation within the spindle in S2 cells (Goshima et al., 2008). In this study, we have defined, for the first time, the role of an Augmin subunit in developing organisms. Unexpectedly, Wac is largely dispensable for somatic mitosis and the bulk microtubule assembly of acentrosomal spindles in female meiosis I but is required for chromosome alignment and segregation in female meiosis. Therefore, unlike mitosis in S2 cells, female meiosis must have an efficient way to generate spindle microtubules in a centrosome- and Augmin-independent manner.
We found an increased level of monooriented homologous kinetochores in metaphase I, suggesting some defects in kinetochore–microtubule attachment. However, most homologous kinetochores were bioriented but further apart than in wild type. Assuming that Wac does not affect chromosome elasticity, this phenotype in metaphase I can be explained either by the increase of the pulling force on kinetochores or the decrease of the polar ejection force on chromosomes. We currently favor the latter possibility, as the increase of a force in the absence of Wac is less likely. The polar ejection force is thought to be important for chromosome alignment and generated by microtubule motors or polymerization (Rieder & Salmon, 1994). The accumulation of Augmin at acentrosomal poles may generate polar microtubules important for the polar ejection force. This may take place gradually after spindle formation, which can explain chromosome congression observed during metaphase I (Gilliland et al., 2009).
Materials and methods
Molecular and protein techniques
Standard DNA manipulation and protein techniques were used (Harlow & Lane, 1988; Sambrook et al., 1989). Polyclonal antibodies against Wac and Dgt2 were raised in rabbits or rats using full-length proteins fused to GST or MBP produced in Escherichia coli and were used after affinity purification. In vitro translation was performed using the T7 TnT Quick Coupled system (Promega) in the presence of [35S]-methionine. The Proteios wheat germ extract system (Toyobo) was also used for in vitro translation. For the expression of Wac truncations, relevant regions were amplified, and the T7 promotor was added by PCR. Alanine substitution constructs were generated using overlapping PCR. Pull-down assays were performed as described previously (Dzhindzhev et al., 2005). S2 cell extracts were prepared by sonication in lysis buffer using 108 cells, and immunoprecipitation was performed as described previously (Lancaster et al., 2007). Microtubule cosedimentation experiments were performed as described previously (Kellogg et al., 1989) after S2 cell extract prepared in BRB80 buffer was incubated with 100 µg microtubules prepolymerized in BRB80 buffer containing 20 µM taxol. The developmental Western blot was performed as described previously (Cullen et al.,1999).
Cell culture and RNAi
Culture of S2 cells and RNAi were performed according to published methods (Dzhindzhev et al., 2005; Hughes et al., 2008). Double-stranded RNA (dsRNA) corresponding to E. coli β-lactamase was used as a control.
Fly techniques
Standard fly techniques (Ashburner et al., 2005) were used. w1118 was used as wild type. wac mutants were generated by remobilization of a P element inserted 0.15 kb upstream of the wac gene. The original insertion line (EY05778) is viable and fertile. Imprecise excision events were selected and analyzed over Df(3L)BSC125 by PCR. Three lines (Δ12, Δ44, and Δ53), which delete most of the wac gene without deleting the neighboring genes, are all viable and female sterile. wacΔ12 was used in this study. For the genetic test of missegregation of the sex chromosomes, each wacΔ male carrying a Bs-marked Y chromosome was crossed with w1118 females. The frequency of missegregation (i.e., sperm with no or both sex chromosomes) was estimated from the frequency of non-Bs males or Bs females in the progeny (5.3 and 1.6% in wacΔ vs. 0.3 and 0.5% in control sibling heterozygotes).
Cytological techniques
Immunostaining was performed and examined as previously described for S2 cells (Dzhindzhev et al., 2005; Hughes et al., 2008), larval CNSs (Cullen et al., 1999), nonactivated oocytes (Cullen and Ohkura, 2001), activated oocytes (Cullen et al., 2005), and embryos (Cullen et al., 1999) except that an Axio Imager attached to an Exciter (LSM5; Carl Zeiss, Inc.) was used for confocal analysis. Orcein staining of larval CNS and phase-contrast microscopy of spermatids were described previously (Cullen et al., 1999). The frequency of aneuploidy in larval CNSs was estimated by orcein staining of squashed CNSs after incubation with colchicine (3 µg/ml in 0.7% NaCl) and hypotonic shock (in 0.5% sodium citrate). Squashed cells that clearly had six large chromosomes were counted as diploids, whereas cells with greater or less than six were counted as hyperploids or hypoploids, respectively. wacΔ and wild type showed 0.7 and <0.4% of hyperploids and 8.2 and 7.7% of hypoploids (n > 250), although most of the apparent hypoploids were likely to be caused by the squashing of brains (i.e., artifacts). Live S2 cells in the culture media on a concanavalin A–coated coverslip were examined at room temperature by a microscope (Axiovert; Carl Zeiss, Inc.) attached to a spinning-disc confocal head (Yokogawa) using Volocity (PerkinElmer). Immunostaining of centromere identifier showed equal segregation of centromeres in most spindles with telophase appearance in Wac-depleted S2 cells. We also see both genuine anaphase and pseudoanaphase cells with chromosomes scattered within a spindle. Intensity of GFP-tubulin signals was estimated by averaging the signal intensity within three equal-sized squares of 0.4 µm2 and subtracting the background signal. The intensities were normalized using the signal intensity at the poles in prophase. Signals of γ- and α-tubulin were measured in cells immunostained with GTU-88 and YOL1/34 (Sigma-Aldrich). For both signals, the mean intensity in the spindle (mean of three squares of 0.16 µm2) was normalized using the mean intensity at the pole after being background corrected. Significance was analyzed using the Wilcoxon test. For FISH in oocytes, stage 14 oocytes prepared as described previously (Cullen and Ohkura, 2001) were postfixed with 4% formaldehyde, and hybridization was performed as described in Dernburg et al. (1996) using two Alexa Fluor 488–conjugated 40-mer oligonucleotides corresponding to the 359-bp repeats found at the X chromosome centromere as probes. Digital images were imported to Photoshop (Adobe) and contrast/brightness was changed uniformly across the field.
Online supplemental material
Fig. S1 shows Wac and Dgt2 associate with spindle microtubules in mitosis. Fig. S2 shows that Wac and Dgt2 directly interact. Fig. S3 shows abnormal syncytial embryonic division and meiotic chromosome missegregation in the wacΔ mutant. Video 1 shows an S2 cell expressing GFP-tubulin after control RNAi (Fig. 1 D). Video 2 shows an S2 cell expressing GFP-tubulin after wac RNAi (Fig. 1 D). Images for both videos were taken every 30 s. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200811102/DC1.
Acknowledgments
We specially thank the members of the Ohkura laboratory for stimulating discussion and critical reading of the manuscript. We also thank the Bloomington Drosophila Stock Center and Genomics Resource Center for providing flies and clones.
This work was supported by The Wellcome Trust. A.M. Meireles is supported by the Portuguese Science Foundation through the Doctoral Programme in Experimental Biology and Biomedicine at the Center for Neurosciences and Cell Biology at the University of Coimbra.
Footnotes
Abbreviations used in this paper: dsRNA, double-stranded RNA; MAP, microtubule-associated protein; MBP, maltose-binding protein; Wac, wee Augmin component.
References
- Ashburner M., Golic K.G., Hawley R.S. 2005. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: 1409 [Google Scholar]
- Basto R., Lau J., Vinogradova T., Gardiol A., Woods C.G., Khodjakov A., Raff J.W. 2006. Flies without centrioles.Cell. 125:1375–1386 [DOI] [PubMed] [Google Scholar]
- Cullen C.F., Ohkura H. 2001. Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles.Nat. Cell Biol. 3:637–642 [DOI] [PubMed] [Google Scholar]
- Cullen C.F., Deák P., Glover D.M., Ohkura H. 1999. mini spindles: a gene encoding a conserved microtubule-associated protein required for the integrity of the mitotic spindle in Drosophila.J. Cell Biol. 146:1005–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen C.F., Brittle A.L., Ito T., Ohkura H. 2005. The conserved kinase NHK-1 is essential for mitotic progression and unifying acentrosomal meiotic spindles in Drosophila melanogaster.J. Cell Biol. 171:593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg A.F., Sedat J.W., Hawley R.S. 1996. Direct evidence of a role for heterochromatin in meiotic chromosome segregation.Cell. 86:135–146 [DOI] [PubMed] [Google Scholar]
- Dzhindzhev N.S., Rogers S.L., Vale R.D., Ohkura H. 2005. Distinct mechanisms govern the localisation of Drosophila CLIP-190 to unattached kinetochores and microtubule plus ends.J. Cell Sci. 118:3781–3790 [DOI] [PubMed] [Google Scholar]
- Gilliland W.D., Hughes S.F., Vietti D.R., Hawley R.S. 2009. Congression of achiasmate chromosomes to the metaphase plate in Drosophila melanogaster oocytes.Dev. Biol. 325:122–128 [DOI] [PubMed] [Google Scholar]
- Goshima G., Wollman R., Goodwin S.S., Zhang N., Scholey J.M., Vale R.D., Stuurman N. 2007. Genes required for mitotic spindle assembly in Drosophila S2 cells.Science. 316:417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Mayer M., Zhang N., Stuurman N., Vale R.D. 2008. Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle.J. Cell Biol. 181:421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Lane D. 1998. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: 729 [Google Scholar]
- Hughes J.R., Meireles A.M., Fisher K.H., Garcia A., Antrobus P.R., Wainman A., Zitzmann N., Deane C., Ohkura H., Wakefield J.G. 2008. A microtubule interactome: complexes with roles in cell cycle and mitosis.PLoS Biol. 6:e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D.R., Field C.M., Alberts B.M. 1989. Identification of microtubule-associated proteins in the centrosome, spindle, and kinetochore of the early Drosophila embryo.J. Cell Biol. 109:2977–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A., Cole R.W., Oakley B.R., Rieder C.L. 2000. Centrosome-independent mitotic spindle formation in vertebrates.Curr. Biol. 10:59–67 [DOI] [PubMed] [Google Scholar]
- Lancaster O.M., Cullen C.F., Ohkura H. 2007. NHK-1 phosphorylates BAF to allow karyosome formation in the Drosophila oocyte nucleus.J. Cell Biol. 179:817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders J., Stearns T. 2007. Microtubule-organizing centres: a re-evaluation.Nat. Rev. Mol. Cell Biol. 8:161–167 [DOI] [PubMed] [Google Scholar]
- Mahoney N.M., Goshima G., Douglass A.D., Vale R.D. 2006. Making microtubules and mitotic spindles in cells without functional centrosomes.Curr. Biol. 16:564–569 [DOI] [PubMed] [Google Scholar]
- Maiato H., Rieder C.L., Khodjakov A. 2004. Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis.J. Cell Biol. 167:831–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim K.S., Hawley R.S. 1995. Chromosomal control of meiotic cell division.Science. 270:1595–1601 [DOI] [PubMed] [Google Scholar]
- Megraw T.L., Kao L.R., Kaufman T.C. 2001. Zygotic development without functional mitotic centrosomes.Curr. Biol. 11:116–120 [DOI] [PubMed] [Google Scholar]
- Mitchison T., Evans L., Schulze E., Kirschner M. 1986. Sites of microtubule assembly and disassembly in the mitotic spindle.Cell. 45:515–527 [DOI] [PubMed] [Google Scholar]
- Rebollo E., González C. 2000. Visualizing the spindle checkpoint in Drosophila spermatocytes.EMBO Rep. 1:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C.L., Salmon E.D. 1994. Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle.J. Cell Biol. 124:223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: 1751 [Google Scholar]
- Somma M.P., Ceprani F., Bucciarelli E., Naim V., De Arcangelis V., Piergentili R., Palena A., Ciapponi L., Giansanti M.G., Pellacani C., et al. 2008. Identification of Drosophila mitotic genes by combining co-expression analysis and RNA interference.PLoS Genet. 4:e1000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavosanis G., Llamazares S., Goulielmos G., Gonzalez C. 1997. Essential role for gamma-tubulin in the acentriolar female meiotic spindle of Drosophila.EMBO J. 16:1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Coppinger J.A., Jang C.Y., Yates J.R., III, Fang G. 2008. FAM29A promotes microtubule amplification via recruitment of the NEDD1–γ-tubulin complex to the mitotic spindle.J. Cell Biol. 183:835–848 [DOI] [PMC free article] [PubMed] [Google Scholar]