Abstract
Study Objectives:
Mandibular repositioning appliance (MRA) therapy is a treatment option for patients with mild to moderate sleep apnea and for patients who do not tolerate continuous positive airway pressure. Titration of MRAs consists of sequential mandibular advancement guided by symptom improvement. The goal of the study was to determine if patients with an elevated apnea hypopnea index (AHI), despite the use of a subjectively optimized MRA, could achieve better results with additional titration during polysomnography (PSG).
Methods:
Patients were enrolled if they had an AHI ≥ 15/h and were referred for MRA therapy. The MRA was advanced until symptoms improved. During the PSG, the technologist monitored the patient's sleep and increased mandibular protrusion until the AHI was improved.
Results:
There was a significant improvement in AHI, minimum oxygen saturation, and total sleep time with the MRA before further advancement. At the final PSG, 65.2% of patients had an AHI ≤ 10 associated with at least a 50% reduction in AHI. The incomplete responders had their appliance further titrated, and this improved the results of MRA therapy by 30.4% to a total success rate of 95.6%.
Conclusions:
This study shows that it is possible to improve the results of MRA therapy by further advancing the appliance during a titration PSG in patients with an incomplete response. The titration night improved the results of the usual clinical advancement of the MRA with substantially more patients achieving a successful outcome.
Citation:
Almeida FR; Parker JA; Hodges JS; Lowe AA, Ferguson KA. Effect of a Titration Polysomnogram on Treatment Success with a Mandibular Repositioning Appliance. J Clin Sleep Med 2009;5(3):198-204.
Keywords: Obstructive sleep apnea syndrome, treatment, oral appliance, titration, mandibular repositioning appliance
Mandibular repositioning appliance (MRA) therapy for snoring and obstructive sleep apnea (OSA) is an accepted treatment option for patients with mild to moderate disease. A recent comprehensive review of oral appliance therapy reported that patients with mild to severe OSA had a 52% chance of having an apnea-hypopnea index (AHI) < 10/h with a mandibular repositioning appliance.1 This rate of efficacy was not measurably better than that found in an earlier comprehensive literature review,2 despite improvements in the quality of the research studies and the introduction of titratable MRAs. In general, the adjustable MRA is initially set at 50% to 75% of maximum mandibular protrusion. The appliance is further advanced until symptoms improve or the maximum tolerated protrusion is reached. Overnight monitoring is usually done to determine the amount of objective improvement in apnea severity with MRA treatment. Despite the widespread use of titratable appliances, efficacy rates are not significantly better than those of fixed position appliances.1,2
Previous research has evaluated whether overnight titration of mandibular advancement during polysomnography could be used to initiate MRA therapy in a fashion similar to the titration of nasal continuous positive airway pressure (CPAP). The first study of overnight titration used an appliance that was removed from the patient's mouth and adjusted manually.3 Other titration studies have used a temporary appliance that can be adjusted either by awakening the patient4 or without awakening the patient.5,6 The temporary appliance was advanced either manually after removal of the temporary appliance,4 by a hydraulic system,5 or by remote control of a motorized system.6 However, results of these studies were mixed in terms of predicting the amount of advancement needed for successful MRA therapy. Furthermore, overnight titration of an MRA remains an experimental approach, and the technology for remote-controlled advancement is not widely available.
Another method for appliance titration consists of clinical titration of the device to symptom resolution or to maximum tolerated advancement, with the addition of overnight home monitoring to determine objective improvement. The addition of home monitoring may provide data of an incomplete response that could allow further advancement to take place prior to outcome polysomnography (PSG). In a study of 40 patients using this approach, 64% of subjects had a complete response (AHI < 10/h with resolution of symptoms).7 Although this is a better success rate than in most studies of MRA therapy, many patients in this study were insufficiently treated despite an intensive clinical titration protocol.
We evaluated a simple clinical protocol consisting of incremental advancement of an MRA by the patient to the point of good symptom control, followed by a titration PSG with the opportunity to further advance the appliance if needed. This titration PSG allowed for further advancement of the MRA during the night if the AHI remained high. The goal of the study was to determine if patients with an elevated AHI, despite the use of a clinically optimized MRA, could achieve better results with additional titration during the outcome PSG.
METHODS
Patient Population
This was a retrospective study of patients with moderate to severe obstructive sleep apnea syndrome (OSAS) who were referred for treatment to one of the authors' (JP) clinical practice for MRA therapy. These subjects represented all patients referred between 2002 and 2004 who had an initial overnight sleep study (split-night PSG) and a later follow-up titration PSG with an MRA in place. Patients were included if they had an AHI ≥ 15/h (on the diagnostic part of the initial PSG), adequate tooth support for retention of the MRA, and minimum protrusive and lateral excursive movements of 5 mm. Patients were excluded if there was evidence of significant periodontal disease, edentulism, active jaw or temporomandibular joint (TMJ) pain, compromised jaw function, or a gag reflex that prohibited use of the MRA.
Polysomnography
Standard nocturnal split-night PSGs were performed at accredited sleep disorders centers in the Minneapolis–St. Paul area. Electroencephalogram (EEG), electrooculogram (EOG), and submental electromyogram (EMG) electrodes were applied in the standard fashion for sleep stage evaluation. Respiratory parameters were recorded, including nasal airflow and pressure, oxygen saturation by pulse oximetry, and chest wall and abdominal movement. Snoring was monitored by the technologist but not objectively quantified. The data were recorded on a computerized system but were manually scored. An apnea was defined as cessation of airflow for ≥ 10 sec. A hypopnea was defined as a reduction in amplitude of airflow or thoracoabdominal movement to ≤ 50% of the baseline for > 10 sec with an accompanying desaturation ≥ 4%. A respiratory effort related arousal (RERA) was any breathing-related arousal that did not meet the criteria for a hypopnea. The AHI was defined as the number of apneas plus hypopneas per hour of sleep. The respiratory disturbance index (RDI) was defined as the number of apneas plus hypopneas plus RERAs per hour of sleep.
Study Protocol
All of the patients had a comprehensive history and head, neck and oral cavity examination undertaken at the initial evaluation by the dentist. Each patient completed pretreatment symptom questionnaires including the Epworth Sleepiness Scale (ESS)8 and a symptom questionnaire. Informed consent for oral appliance therapy was obtained. At the initial visit, upper and lower impressions were completed and a George Gauge bite registration was taken with the mandibular treatment position at 60% of maximum protrusion, with approximately 3-5 mm of inter-incisal opening.
The MRA (Adjustable PM Positioner™) was custom-made for each patient from dental study models. The device was constructed of a thermal-sensitive, processed acrylic material (Bruxeze®) that provided full occlusal coverage. The appliance was placed in warm water before insertion in the mouth to slightly soften the material and provide easier insertion. A 7-mm expansion screw was attached to the right and left buccal segments to allow mandibular advancement in 0.25 mm increments. If the screw mechanism reached its maximum advancement, the appliance was sent to the dental laboratory and the screw was removed, zeroed, and re-bonded in the current treatment position. This allowed the patient to further advance the mandible. The screw mechanism attachment allowed 2-3 mm of lateral and protrusive movement of the mandible when the device was in the mouth. A rectangular opening in the anterior of the device provided adequate space for mouth breathing.
At appliance insertion appointment with the dentist, the MRA fit was fine tuned for patient comfort. The patients were given written and oral instructions on care and use of the device. They were instructed not to advance the MRA during the first week so they could become accustomed to the MRA. Subsequently they were instructed to advance the screw mechanism 0.5 mm (2 turns) every 3 nights until the symptoms of snoring, daytime drowsiness, and quality of sleep normalized.
The patients were interviewed and examined by the dentist every 4-6 weeks. Clinical symptoms were evaluated by having the patient complete the symptom questionnaire and the ESS at follow-up visits. The questionnaire also included questions about compliance, ease of use, overall satisfaction, and side effects such as tooth discomfort, jaw pain, or changes in occlusion. When the patient reported that sleep apnea symptoms were well controlled with the MRA, the patient was referred to the sleep disorders center for a follow-up PSG with titration (if necessary).
During the follow-up titration PSG, the patient slept with the MRA in place. The sleep technologist was instructed to closely monitor the AHI; if at any time after the first 30 minutes the AHI was > 10/h, the technologist would awaken the patient and have the patient advance the screw mechanism by 1 mm (4 turns) and then go back to sleep. To standardize this procedure, all technicians received a letter from the referring dentist with the instructions for the titration PSG. Further advancement could be done for snoring or RERAs. Snoring was monitored by the technologist but it was not objectively quantified. The technologist monitored the patient's sleep and waited more than 30 minutes at each mandibular position before waking the patient to advance the device again. The appliance was advanced no more than 3 times (a total of 3 mm) during the night. If after 3 hours the appliance had been maximally advanced and the patient's AHI was not reduced to an acceptable level, then another trial of CPAP was offered to the patient. Shortly after the titration PSG, the patient was scheduled for a consultation with the sleep physician and the dentist.
Outcome Measures
In the absence of a consensus in the literature regarding the definition of a successful treatment outcome for MRA therapy, we have reported 3 different definitions of success. The first definition was a reduction in the AHI to ≤ 10/h with a 50% reduction in AHI from baseline (criterion 1). The second definition was reduction of the AHI to ≤ 5/h, with the AHI reduced ≥ 50% of the pretreatment level (criterion 2). The third definition of success was an AHI ≤ 10/h and RDI ≤ 15/h (criterion 3), which takes into account arousals related to an increase in the respiratory effort. Patients were classified as responders if titration was not needed during the follow-up PSG, incomplete responders if their AHI was reduced to meet success criteria after titration, and non-responders if they did not meet any definition of success despite titration.
Statistical Analyses
Changes in continuous measures (sleep study measurements and demographic information) were tested using a 2-tailed paired t-test. Differences in categorical measures (Epworth Sleepiness Scale) were tested using a 2-sided exact McNemar test. Comparisons in the AHI between baseline, before titration and after titration were tested using the Friedman test followed by the Wilcoxon post hoc test with Bonferroni correction (α = 0.017). p < 0.05 was the threshold of statistical significance.
RESULTS
Subjects
Twenty-four subjects met inclusion criteria for the study; 23 subjects completed the protocol. One subject was excluded from analysis because the titration protocol was not followed during a follow-up PSG despite the presence of ongoing obstructive respiratory events. The 23 subjects included 17 men and 6 women with a mean AHI of 36.2 (standard deviation [SD] 21.7). Ten subjects had moderate OSAS (AHI 15 to 30) and 13 had severe OSAS (AHI > 30). Table 1 summarizes baseline demographics. Eighteen patients had failed previous medical treatment (unable to tolerate CPAP), and 4 patients had not been previously treated. One patient who could not tolerate CPAP had a uvulopalatopharyngoplasty and a septoplasty before MRA treatment.
Table 1.
Baseline Demographics and PSG Characteristics
| All (n=23) | |
|---|---|
| Gender (male/female) | 17/6 |
| Age (years) | 52.5 ± 12.5 |
| Body mass index (kg/m2) | 28.9 ± 4.9 |
| Epworth Sleepiness Scale | 10.1 ± 6.1 |
| Apnea-hypopnea index (/h) | 36.2 ± 21.7 |
| Respiratory disturbance index (/h) | 47.0 ± 20.6 |
| Minimum oxygen saturation (%) | 83.0 ± 4.8 |
| Total sleep time (min) | 218.2 ± 110.2 |
| Sleep efficiency (%) | 76.0 ± 12.6 |
Data presented as number (gender) or in mean ± standard deviation
Treatment Efficacy
The average time between insertion of the MRA and the final titration PSG was 5.8 months. There was no significant change in the body mass index (BMI) of the subjects between recruitment and the final PSG (Table 2). At the final PSG there was a significant improvement in AHI, RDI, minimum oxygen desaturation, and total sleep time with the MRA before further advancement was done by the technologist (Table 2). The PSG results showed that 15 of 23 subjects (65%) were successfully treated, achieving an AHI ≤ 10/h and a reduction in AHI of 59% (criterion 1). In these 15 subjects, there was a large decrease in the average AHI from 32.1/h to 2.2/h. Thirteen subjects (56.5%) achieved AHI ≤ 5/h and a 50% reduction in AHI. Ten of these 15 subjects had no in-laboratory titration, but in 5 subjects there was evidence of increased respiratory effort or snoring and further advancement was done even though the AHI was < 10/h. Table 3 summarizes AHI and RDI data from all subjects.
Table 2.
Baseline Polysomnographic Data and Outcome Polysomnographic Data Before In-Laboratory Titration in All Subjects (n=23)
| Baseline Polysomnogram | Outcome Polysomnogram Before Titration | p-value | |
|---|---|---|---|
| Body mass index (kg/m2) | 28.9 ± 4.9 | 28.8 ± 4.5 | 0.73 |
| Apnea-hypopnea index (/h) | 36.2 ± 21.7 | 16.5 ± 35.1 | 0.03 |
| Respiratory disturbance index (/h) | 47.0 ± 20.6 | 27.0 ± 34.5 | 0.01 |
| Minimum oxygen saturation (%) | 83.0 ± 4.8 | 85.9 ± 3.1 | 0.02 |
| Total sleep time (min) | 218.2 ± 110.2 | 333.6 ± 80.2 | 0.001 |
| Sleep efficiency (%) | 76.0 ± 12.6 | 77.1 ± 14.9 | 0.68 |
Data presented in mean ± standard deviation
Table 3.
Effects of Oral Appliance Therapy and the Advancement Night on Apnea-Hypopnea Index (AHI) and Respiratory Disturbance Index (RDI)
| Patient | Baseline | AHI Before further advancement | After further advancement | # | Baseline | RDI Before further advancement | After further advancement | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21.0 | 0.0 | a | c | 33.0 | 4.0 | e | |||
| 2 | 16.4 | 0.0 | a | c | 41.5 | 2.1 | e | |||
| 3 | 50.0 | 1.0 | a | c | 52.0 | 27.0 | ||||
| 4 | 21.0 | 1.0 | a | c | 48.0 | 1.0 | e | |||
| 5 | 15.0 | 1.0 | 1.0 | 1 | a | c | 30.0 | 24.0 | 15.0 | f |
| 6 | 17.0 | 1.0 | 2.0 | 3 | a | c | 32.0 | 12.0 | 4.0 | e |
| 7 | 105.0 | 1.0 | 0.0 | 2 | a | c | 106.0 | 9.0 | 1.0 | e |
| 8 | 30.6 | 1.4 | a | c | 56.5 | 11.1 | e | |||
| 9 | 23.9 | 2.1 | a | c | 33.7 | 9.7 | e | |||
| 10 | 19.5 | 2.6 | a | c | 26.7 | 13.7 | e | |||
| 11 | 24.6 | 2.8 | a | c | 28.3 | 10.5 | e | |||
| 12 | 30.0 | 3.2 | a | c | 30.0 | 13.0 | e | |||
| 13 | 32.7 | 4.8 | a | c | 35.3 | 18.8 | ||||
| 14 | 38.2 | 5.2 | 1.2 | 3 | a | d | 45.9 | 26.1 | 6.0 | f |
| 15 | 36.0 | 6.0 | 8.0 | 1 | a | 51.0 | 16.0 | 15.0 | f | |
| 16 | 87.0 | 13.0 | 2.0 | 3 | b | d | 95.0 | 43.0 | 17.0 | |
| 17 | 41.0 | 16.0 | 6.8 | 3 | b | 52.0 | 20.1 | 26.2 | ||
| 18 | 33.9 | 16.4 | 3.0 | 2 | b | d | 35.3 | 20.9 | 6.8 | f |
| 19 | 34.4 | 17.9 | 9.8 | 1 | b | 42.8 | 19.8 | 14.4 | f | |
| 20 | 34.0 | 20.0 | 10.0 | 2 | b | 35.0 | 49.0 | 23.0 | ||
| 21 | 38.7 | 23.1 | 2.6 | 2 | b | d | 38.7 | 23.1 | 2.6 | f |
| 22 | 56.1 | 84.8 | 6.4 | 3 | b | 57.3 | 84.8 | 37.0 | ||
| 23 | 26.1 | 155.5 | 55.8 | 3 | 76.0 | 161.9 | 61.9 | |||
| Mean | 36.2 | 16.5 | 8.4 | 47.0 | 27.0 | 17.7 | ||||
| SD | 35.1 | 14.7 | 20.6 | 34.5 | 16.8 |
Data is sorted out in crescent order of the before further advancement AHI. # stands for the number of times the appliance was advanced during overnight titration. Criterion 1 is represented with letters a and b, criterion 2 represented with c and d, and criterion 3 is represented with e and f. a = AHI ≤10 and 50% reduction (n=15); b = AHI ≤10 and 50% reduction after further advancement (n=7); c = AHI ≤5 and 50% reduction (n= 13); d = AHI ≤5 and 50% reduction after further advancement (n=4); e = AHI ≤10 and RDI ≤15 and AHI and RDI 50% reduction (n=10); f = AHI ≤10 and RDI ≤15 after further titration (n=6).
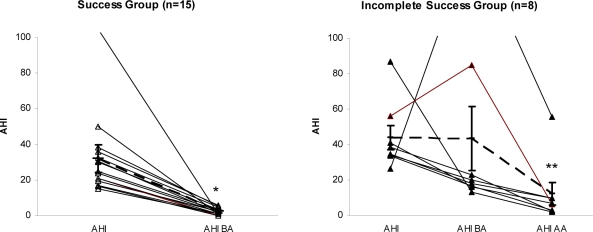
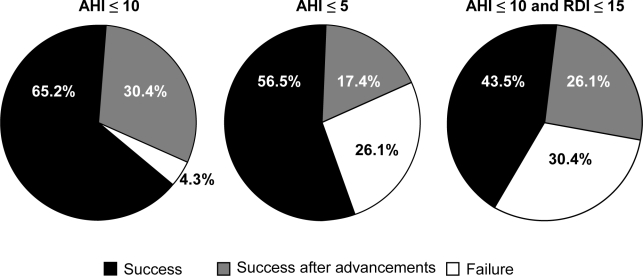
Eight patients had an AHI > 10/h at the beginning of the follow-up PSG (incomplete responders); these patients underwent the in-laboratory advancement protocol. With further advancement (average 2.7 mm), 7 of the 8 subjects achieved an AHI ≤ 10/h. There was significant improvement in AHI in the group of responders. For the other subjects there was a statistically significant change only in the AHI between before-advancement PSG and after additional in-laboratory titration (Figure 1). The responders (AHI ≤ 10/h) and incomplete responders were not statistically different in terms of demographic or polysomnographic variables (Table 4). The addition of overnight titration improved the outcome of MRA therapy using any of the 3 success criteria. The percentage of patients who were incomplete responders (success only after titration PSG) ranged from 17.4% to 30.4%, depending on the success criteria chosen, as seen in Figure 2.
Figure 1.
Individual apnea-hypopnea index (AHI) characteristics at baseline and during PSG(s). *Significant difference between baseline AHI and AHI before advancement AHIBA. **Significant difference between AHI before advancement (AHIBA) and AHI after advancement (AHIAA). Dashed line is mean and bars are for standard error of mean.
Table 4.
Demographics and Polysomnographic Characteristics of Responders and Incomplete Responders Defined by AHI Prior to In-Laboratory Titration
| Responders (n=15) | Incomplete Responders (n=7) | Non-Responder (n=1) | ||
|---|---|---|---|---|
| Gender (male/female) | 10/5 | 6/1 | 1/0 | ns |
| Age (years) | 53.1 ± 12.8 | 53.6 ± 12.1 | 36 | ns |
| Body mass index (kg/m2) | 28.5 ± 5.3 | 28.4 ± 2.0 | 38.5 | ns |
| Epworth Sleepiness Scale | 9.3 ± 6.1 | 11.2 ± 6.6 | 14 | ns |
| Apnea-hypopnea index (/h) | 32.1 ± 22.3 | 46.4 ± 19.5 | 26.1 | ns |
| Respiratory disturbance index (/h) | 43.3 ± 19.8 | 50.9 ± 21.2 | 76 | ns |
| Minimum oxygen saturation (%) | 83.8 ± 5.4 | 81.9 ± 3.7 | 79 | ns |
| Total sleep time (min) | 219.1 ± 112.5 | 193.6 ± 100.3 | 377 | ns |
| Sleep efficiency (%) | 74.6 ± 12.0 | 76.0 ± 12.8 | 97 | ns |
Data presented in mean ± standard deviation
Figure 2.
Percentage of oral appliance therapy success according to different success criteria.
Symptoms and Treatment Compliance
Of 21 patients who answered the post-treatment questionnaire, 9 reported that they were very satisfied with the MRA, and 12 reported they were satisfied. None of these patients reported TMJ discomfort after the follow-up PSG. All 23 patients reported using their appliance regularly by the time of the follow-up PSG. Fifteen patients reported using the appliance 100% of the nights, and 6 patients reported using the MRA at least 75% of the nights. Subjective snoring improved in all but one patient. Eighteen subjects reported feeling sometimes or often unrefreshed upon awakening prior to the MRA, and 14 of these subjects (78%) reported an improvement in this with MRA therapy. All of the patients completed a baseline ESS before the use of the MRA and at the final PSG. Sleepiness per ESS improved from baseline to final PSG, with a significant reduction in the mean ESS from 10.1 to 6.1.
DISCUSSION
This study shows that it is possible to improve the results of MRA therapy by advancing the appliance during a titration PSG in patients with an incomplete response. The titration night improved the results of the usual clinical advancement of the MRA by 17% to 35%, depending upon the criterion used to define success. The protocol was simple to implement in the sleep laboratory, with the technologist asking the patient to advance the appliance in 1-mm increments if the patient continued to snore, demonstrated increased respiratory effort, or continued to have respiratory events. The patients achieved sufficient sleep time during the titration PSG, despite being awakened to make the advancements.
It is well known that self-reporting of changes in symptoms is not a reliable method of determining success with treatment. However, most outcome studies on MRA therapy use self-reporting as the basis for sending the patient for a follow-up PSG. Our data were based on subjective evaluation of the patient's snoring and daytime function, and indicated that there was no difference in symptoms reported by the responders and incomplete responders following self-titration of the appliance; therefore none of the characteristics would allow us to predict which patients would need further in-laboratory titration. All of the patients titrated their appliances based upon subjective improvement similar to that done in the study by Fleury and colleagues.7 They found that approximately 25% further advancement had to be done because of a persistently abnormal oxygen desaturation index despite reports of symptom resolution. This supports the recommendation that patients undergo overnight testing to determine objective improvement in apnea severity even when there is a good subjective response.9
Since there is no consensus about the definition of a successful treatment outcome, we used 3 different criteria (Figure 2). In this study, if the definition of a successful outcome was an AHI ≤ 10/h and a reduction of AHI by at least 50%, then the success rate increased by 30.4% following the titration PSG. If the definition of a successful outcome was AHI ≤ 5/h and a 50% reduction in AHI, then the success rate increased by 17.4%. If we used the definition of a successful outcome of AHI ≤ 10/h and RDI ≤ 15/h, then the success rate was improved by 26.1% after titration. The addition of RDI as a criterion thus incorporates all respiratory-related arousals as an outcome measure; we did not assess arousals due to other mechanisms in our analysis. Regardless of the definition we chose, further advancement of the MRA during a titration PSG improved success rates significantly. Even with our strictest criterion, we found a success rate of 70%, which is higher than other studies in the literature that evaluated treatment of patients with moderate to severe OSA.10–13 Although the in-laboratory titration did not allow for a full night with the appliance in the final position, we found that this protocol did improve the success rate with the MRA.
The present study supports the use of a protocol that will increase success rates with MRA therapy but it does not specifically predict who will do well with MRA therapy. Success rates were high in this study even prior to additional titration, but this represents a selected population who complied with long-term follow-up and returned to the laboratory for an outcome and titration PSG. Selection of patients for this study may have been biased because there were many patients who had undergone other types of treatments for OSA. However, oral appliance treatment is a widely used approach in our medical community and in others, where most of the moderate to severe patients have had a trial of CPAP before being referred for MRA therapy. The results of this study also showed that most subjects had a significant improvement in symptoms. Even prior to further advancement, the patients had an improvement in daytime sleepiness and a decrease in waking up unrefreshed. We found the ESS decreased from an average of 10 to 6, and 78% of the patients reported feeling more refreshed upon awakening in the morning. Self-reported compliance during treatment was excellent, with all patients using the appliance at least 75% of the nights. Although these are subjective measures, these findings are consistent with other studies of MRA therapy.14–17
Overnight titration of oral appliance treatment can be done prior to the initiation of treatment4–6 or after a period of acclimatization or clinical titration.3,7 One study evaluated a titration protocol that consisted of incremental advancement of the appliance over weeks to months with an end point of symptom resolution and improvement on overnight home oximetry (reduction in the oxygen desaturation index to ≤ 10/h), or in the absence of those improvements, advancement to maximum protrusion.7 In a study of 40 patients using this methodology, 64% had a complete response defined as an AHI < 10/h and resolution of symptoms.7 This protocol is a labor-intensive approach with the addition of oximetry to the protocol. It could result in patients having more than one overnight home study in order to determine when they are ready for the final PSG. If the final adjustments can be made at the time of the outcome PSG, this might be more efficient and less costly for the patient than the addition of home monitoring.
In one of the studies that used overnight titration the authors found that it was feasible to progressively advance the mandible during sleep using a remote hydraulic system.5 They reported that the patient's sleep was not disturbed by the adjustment process. Two other studies assessed whether the results from the titration night could be used to predict the response to long-term MRA therapy.4,6 The ability to reduce the AHI during the titration study was highly predictive of success when a permanent MRA was used for long-term therapy in one of the studies6 but not in the other.4 Although both studies used the same device for long-term treatment, there were differences in the titration approach. The use of temporary appliances for overnight titration prior to the initiation of treatment, regardless of whether they are manually adjusted or remotely controlled, has not been widely adopted. In both of those studies, the patients had a baseline overnight study, a titration study, and an outcome overnight study. Our approach involves a baseline diagnostic study (full night or split-night) and a combined titration and outcome night. Based upon our results, we would suggest that additional titration during the outcome PSG would be feasible in many sleep laboratories and, while increasing the success rate of MRA therapy, it does not increase the cost or complexity of the titration process.
With mandibular advancements of up to 3 mm in one night, there may be concern that this could cause jaw discomfort. In this study, mandibular advancement ranged from 1-3 mm. A mean advancement of 2.7 mm during the night was completed for patients who required further titration, but no reports of significant temporomandibular symptoms were made. Prior to the titration PSG, these patients had been gradually advancing their oral appliances over a period of 5.8 months. In a study without a pretreatment adaptation period, Tsai and coworkers found that some patients did have significant jaw discomfort during the titration night.6 This complaint was also noted in other studies of overnight titration.4,5 An important aspect of our titration protocol is that we titrated the appliance after an extended period of use, whereas in many other studies, patients were titrated in the laboratory before initiation of home treatment. We believe that the long adaptation period is the reason that our patients did not encounter significant problems with jaw discomfort during or after the titration night at the post-titration appointment.
As shown in Table 3, one patient had an increase in AHI from 26/h on the baseline polysomnogram to 155/h before titration and 55.8/h after titration. At the baseline split-night study he slept only 111.5 minutes before CPAP was started, and therefore his baseline AHI might have been higher than 55.8/h. This patient was morbidly obese (BMI 40.3 kg/m2), which is normally a contraindication for MRA therapy. However, he had been intolerant of CPAP treatment. Other studies have found that the AHI may increase in some patients with MRA therapy18,19 even with symptom improvement.20 This supports the need for follow-up overnight monitoring to determine the amount of objective improvement. Furthermore, a follow-up PSG with our titration protocol may be of additional value, since some patients with subjective improvement but without sufficient improvement in AHI might achieve a better success rate after the titration PSG.
Our study has several limitations, including a small sample size of selected patients and compliance measured by questionnaire. In addition, the testing was done at several different sleep laboratories and with several different sleep technologists. The most significant limitation is the use of split-night sleep studies done at baseline, such that the AHI was calculated from a partial night of PSG. Most patients in the region where the study took place generally have split-night studies done to allow diagnosis and CPAP titration in the same study due to financial reasons. Furthermore, on the outcome sleep study the AHI for the final jaw position was calculated on a full night in only 10 subjects who did not have additional titration. For the other 13 subjects there was additional titration, so the AHI was only from the latter part of the night. The fact that the patients were awakened to advance the MRA further during the titration PSG may have affected the amount of sleep data obtained. Another possible limitation is the potential impact of these short sleep intervals on the amount of REM sleep. Sleep apnea frequently worsens during REM sleep. If our protocol reduced REM sleep, we may have overestimated the effect of the MRA on the AHI and RDI. In addition, we did not compare the contribution from body position to the AHI and RDI between the initial and follow-up sleep studies. If the patients slept less in the supine position on the titration PSG, this may have contributed to some of the reduction in AHI and RDI with the MRA. However, the technologist did not provide instructions to the patients to sleep in the side or supine position on either of the PSG, and we have no evidence that the protocol used to titrate the MRA affected body position.
Despite these limitations, this study strongly suggests that overnight titration may increase the success rates of MRA treatment. A randomized controlled study of different titration protocols is now indicated to determine the optimal approach and to decide how to improve clinical success rates beyond the average reported rate of 52% (AHI ≤ 10/h).1 Finally, it would be important to determine if home monitoring during the clinical titration protocol could be used to increase success rates or even be used to replace the outcome PSG. A cost analysis of the different titration approaches would provide useful information to further guide clinical practice.
Based on previous studies, we know that MRAs have a high success rate in primary snorers and mild sleep apnea patients. It is well known that there are numerous patients unable to tolerate CPAP, including those with severe sleep apnea. This study showed that an increased number of patients with moderate or severe OSA could be effectively treated with MRA using a titration PSG and a careful clinical titration protocol. Thus, MRA may be a treatment alternative for more patients with moderate to severe OSA who are unable to tolerate CPAP than might be considered for this treatment at the present time.
Conclusions
This study assessed the use of a titration PSG in addition to a simple clinical titration protocol to optimize success with MRAs. This study showed that this protocol significantly increased success rates for MRA by 17% to 30%. Based on this information, it is likely that the previously reported success rates of MRA therapy could be substantially improved. Future studies assessing the effectiveness of MRA therapy should include a post-treatment titration PSG to improve treatment success.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Parker has participated in a seminar for and receives royalties from Dental Services Group for an oral appliance he developed, patented, and licensed to the company. The appliance was used in this study but Dental Services Group did not support this study nor pay royalties for the appliances used in the study. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors wish to thank Ms. Mary Wong for providing statistical analysis and Mrs. Ingrid Ellis for editorial assistance.
REFERENCES
- 1.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244–62. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt-Nowara W, Lowe A, Wiegand L, Cartwright R, Perez-Guerra F, Menn S. Oral appliances for the treatment of snoring and obstructive sleep apnea: a review. Sleep. 1995;18:501–10. doi: 10.1093/sleep/18.6.501. [DOI] [PubMed] [Google Scholar]
- 3.Raphaelson MA, Alpher EJ, Bakker KW, Perlstrom JR. Oral appliance therapy for obstructive sleep apnea syndrome: progressive mandibular advancement during polysomnography. Cranio. 1998;16:44–50. doi: 10.1080/08869634.1998.11746037. [DOI] [PubMed] [Google Scholar]
- 4.Kuna ST, Giarraputo PC, Stanton DC, Levin LM, Frantz D. Evaluation of an oral mandibular advancement titration appliance. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:593–603. doi: 10.1016/j.tripleo.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 5.Pételle B, Vincent G, Gagnadoux F, Rakotonanahary D, Meyer B, Fleury B. One-night mandibular advancement titration for obstructive sleep apnea syndrome: a pilot study. Am J Respir Crit Care Med. 2002;165:1150–3. doi: 10.1164/ajrccm.165.8.2108056. [DOI] [PubMed] [Google Scholar]
- 6.Tsai WH, Vazquez JC, Oshima T, et al. Remotely controlled mandibular positioner predicts efficacy of oral appliances in sleep apnea. Am J Respir Crit Care Med. 2004;170:366–70. doi: 10.1164/rccm.200310-1446OC. [DOI] [PubMed] [Google Scholar]
- 7.Fleury B, Rakotonanahary D, Pételle B, et al. Mandibular advancement titration for obstructive sleep apnea: optimization of the procedure by combining clinical and oximetric parameters. Chest. 2004;125:1761–7. doi: 10.1378/chest.125.5.1761. [DOI] [PubMed] [Google Scholar]
- 8.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 9.Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240–3. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- 10.Pancer J, Al-Faifi S, Al-Faifi M, Hoffstein V. Evaluation of variable mandibular advancement appliance for treatment of snoring and sleep apnea. Chest. 1999;116:1511–8. doi: 10.1378/chest.116.6.1511. [DOI] [PubMed] [Google Scholar]
- 11.Rose E, Staats R, Schulte-Monting J, Jonas IE. Treatment of obstructive sleep apnea with the Karwetzky oral appliance. Eur J Oral Sci. 2002;110:99–105. doi: 10.1034/j.1600-0722.2002.11178.x. [DOI] [PubMed] [Google Scholar]
- 12.Lowe AA, Sjöholm TT, Ryan CF, Fleetham JA, Ferguson KA, Remmers JE. Treatment, airway and compliance effects of a titratable oral appliance. Sleep. 2000;23:S172–8. [PubMed] [Google Scholar]
- 13.Marklund M, Franklin KA, Sahlin C, Lundgren R. The effect of a mandibular advancement device on apneas and sleep in patients with obstructive sleep apnea. Chest. 1998;113:707–13. doi: 10.1378/chest.113.3.707. [DOI] [PubMed] [Google Scholar]
- 14.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 15.Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:743–8. doi: 10.1164/rccm.200203-208OC. [DOI] [PubMed] [Google Scholar]
- 16.Naismith SL, Winter VR, Hickie IB, Cistulli PA. Effect of oral appliance therapy on neurobehavioral functioning in obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2005;1:374–80. [PubMed] [Google Scholar]
- 17.de Almeida FR, Lowe AA, Tsuiki S, et al. Long-term compliance and side effects of oral appliances used for the treatment of snoring and obstructive sleep apnea syndrome. J Clin Sleep Med. 2005;1:143–52. [PubMed] [Google Scholar]
- 18.Henke KG, Frantz DE, Kuna ST. An oral elastic mandibular advancement device for obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:420–5. doi: 10.1164/ajrccm.161.2.9903079. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Nowara WW, Meade TE, Hays MB. Treatment of snoring and obstructive sleep apnea with a dental orthosis. Chest. 1991;99:1378–85. doi: 10.1378/chest.99.6.1378. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson KA, Ono T, Lowe AA, Al-Majed S, Love LL, Fleetham JA. A short term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnea. Thorax. 1997;52:362–8. doi: 10.1136/thx.52.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]