Abstract
Rationale:
Central sleep apnea (CSA) may occasionally occur in patients with obstructive sleep apnea during titration with a continuous positive airway pressure (CPAP) device.
Objectives:
To determine the prevalence and the natural history of CPAP-emergent CSA.
Methods:
This is a retrospective study of 1286 patients with a diagnosis of OSA who underwent titration with a positive airway device during a 1-year period. Patients were seen in consultation and underwent full-night attended polysomnography followed by full-night attended CPAP titration. Four weeks after CPAP therapy, patients returned to the clinic for follow-up, and objective adherence to CPAP was recorded. In patients who had CSA on CPAP, a second full-night attended CPAP titration was recommended.
Results:
Eighty-four of the 1286 patients developed a central apnea index (CAI) of 5 or greater per hour while on CPAP. The incidence of CSA varied from 3% to 10% monthly, with an overall incidence of 6.5%. Forty--two of the 84 patients returned for a second CPAP titration. In 33 patients, CSA was eliminated. In each of the remaining 9 patients, the CAI remained at 5 or greater per hour, with an average of 13 per hour. These patients characteristically had the most severe OSA, and 5 had a CAI of 5 or more per hour at baseline. Two of the 9 patients were on opioids
Conclusions:
In this large retrospective study of 1286 patients with a diagnosis of OSA, 6.5% had CPAP-emergent or persistent CSA. However, CPAP-emergent CSA was generally transitory and was eliminated within 8 weeks after CPAP therapy. The prevalence of CPAP-persistent CSA was about 1.5%. Severity of OSA, a CAI of 5 or greater per hour, and use of opioids were potential risk factors.
Citation:
Javaheri S; Smith J; Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med 2009;5(3):205-211.
Keywords: OSA, CSA, CPAP
Central sleep apnea (CSA),1,2 a rare condition in the general population, commonly occurs in patients with systolic heart failure.3–6 CSA also commonly occurs at high altitude and at times in patients on opioid drugs.1 Our understanding of the mechanisms of CSA in systolic heart failure is evolving1–3 and include the instability of control of breathing with increased sensitivity to pCO2 above7,8 and below eupnoea9 and proximity of eucapnic pCO2 to the apneic threshold pCO2 during sleep. With lowering of the pCO2 below the apneic threshold, central apnea occurs. Similarly, at high altitude, CSA occurs because of increased CO2 sensitivity below eupnoea caused by hypoxemia.10 The mechanism of CSA in patients using opioids is not well understood.
Central sleep apnea may also emerge during titration of continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA). The prevalence, the natural history, and the mechanisms of CPAP-emergent CSA are not well established. The aims of the present study were to determine the prevalence and the natural history of CSA emerging during CPAP titration in patients referred for diagnosis and treatment of OSA.
METHODS
Design
This is a retrospective study of 1288 patients with newly diagnosed OSA who underwent treatment with CPAP over a 12-month period from June 2006 to May 2007. Two patients who were receiving oxygen were excluded. All patients were seen by the author(SJ). These patients were referred by their primary care physician, otolaryngologist, and cardiologist for evaluation of symptoms of OSA such as snoring, witnessed apnea, restless or nonrestorative sleep, fatigue, and excessive daytime sleepiness.
After obtaining a complete history (including sleep-related information and a list of medications) and performing physical examination, the sleep physician recommended a full-night attended polysomnography. The results were reviewed with the patient, and a full-night attended CPAP titration study was performed within a week. The patients were seen in the clinic about 4 weeks after CPAP therapy was initiated and objective adherence was recorded. For those patients who had CSA (central apnea index [CAI] ≥ 5/h) on CPAP, a second CPAP titration study was recommended; this study occurred within 5 to 6 weeks of the baseline polysomnogram.
Eighty-four patients had CSA during CPAP titration (the entry criterion). We refer to this group as CPAP1-CSA, not necessarily CPAP-emergent CSA, because, when we reviewed the initial polysomnograms, a few patients were found to also have CSA (CAI ≥ 5/h) at baseline (this group is referred to as CPAP1-CSA persistent).
To compare patients with CPAP1-CSA to patients without CSA, patients were matched for age, sex, and body mass index with 84 patients who underwent CPAP titration during the same night as when the same technician titrated the CPAP of the patients with CPAP1-CSA. This allowed us to compare demographics and polysomnographic findings of the groups with and without CPAP1-CSA. Of the 84 patients who had CPAP1-CSA, 42 returned for follow-up. The results were reviewed, and a reevaluation was recommended. The others did not return for follow-up in spite of several calls.
The protocol of this retrospective study was approved by the Institutional Review Board of the Christ Hospital in Cincinnati.
Procedures
Polysomnography was performed using standard techniques, as have been previously described.11,12 For staging sleep, we recorded electroencephalogram (2 channels), chin electromyogram (1 channel) and electrooculogram (2 channels). Thoracoabdominal excursions were measured qualitatively by piezo crystal technology (Model 1314 Sleepmate Technologies, Midlothian, VA) placed over the rib cage and abdomen. Airflow was qualitatively monitored using an oral/nasal thermistor (Model 1459, Sleepmate Technologies). Arterial blood oxyhemoglobin saturation was recorded using a finger pulse oximeter (Healthdyne Oximeter Model 930, Respironics Inc., Murrysville, PA). These variables were recorded on a multichannel computerized polysomnographic system (Model Alice III with PPI-2, Respironics Inc.). An apnea was defined as cessation of inspiratory airflow (flat signal) for 10 seconds or more. An obstructive apnea was defined as the absence of airflow in the presence of rib-cage and abdominal excursions. A central apnea was defined as the absence of airflow with absence of rib-cage and abdominal excursions. Hypopnea was defined as a 30% reduction in airflow and/or thoracoabdominal excursions lasting 10 seconds or more and associated with at least a 4% drop in arterial oxyhemoglobin saturation and/or an arousal, as defined elsewhere.13 While the patients were on CPAP, the flow signal from the clinical titration unit (BiPAP Synchrony™ lab system, Respironics) was used for defining apneas and hypopneas. The number of apneas and hypopneas per hour of sleep is referred to as the apnea-hypopnea index (AHI). The number of arousals per hour of sleep is referred to as the arousal index (AI). All polysomnograms were reviewed by 1 of the authors (SJ).
CPAP titration was performed using a uniform approach. Titration began usually at pressure of 5 cm H2O with heated humidification system (H2 heated humidification, Respironics). The pressure was increased gradually every few minutes to eliminate obstructive apneas, hypopneas, and eventually snoring. The goal was to eliminate obstructive events. However, if, with increasing pressure, central apneas appeared, the pressure was not increased beyond an additional 3 to 4 cm H2O. For the few patients who were intolerant of CPAP, having difficulty exhaling, a bilevel positive airway pressure (PAP) device was used. The expiratory pressure was normally set at the level that had eliminated obstructive apneas; the inspiratory pressure was increased gradually to eliminate hypopneas. For patients who returned for a second sleep-study with CPAP titration, pressure began at 5 cm H2O and increased progressively, as noted above.
Statistical Analysis
For multiple comparisons, Kruskal-Wallis analysis of variance and Dunn tests were used. When only 2 variables were compared, either 2-tailed paired t-test or Wilcoxon rank sum test was used. For proportions, Fisher Exact Test was used. A p value of less than 0.05 (after multiple comparisons) was considered significant. Means ± SD are reported. Calculations were done using NCSS,PASS,and GESS (Kaysville, UT).
RESULTS
Of the 1286 consecutive patients referred for diagnosis and treatment of OSA, 84 patients had CSA during the first CPAP titration (CPAP1-CSA group). This accounted for 6.5% of the 1286 patients. The monthly incidence of CSA on CPAP varied from 3% to 10%.
There were no significant differences in the demographics (as expected) of patients who had CPAP1-CSA and the group without CSA on CPAP (Table 1). Although the numbers of patients with atrial fibrillation and opioid use were higher in the group of patients with CPAP1-CSA than the group without, the differences were not significant (Table 1). However, the 2 groups had major differences in severity of sleep apnea on baseline polysomnographic findings. The group with CPAP1-CSA had significantly higher AHI, obstructive apnea index (OAI), CAI, and related AI, as compared with the patients without CPAP1-CSA (Table 2). At baseline, 27 of 84 patients with CPAP1-CSA had a CAI of 5 or more per hour (this group is labeled CPAP1-CSA persistent), compared with 6 of 84 patients who had no CSA while on CPAP (p < 0.0001). The majority of central apneas occurred during non-rapid eye movement (REM) sleep.
Table 1.
Descriptive Characteristics of 84 Patients with and 84 Patients without CSA on the First Night of CPAP Titration
| Variables | without CSA | with CSA |
|---|---|---|
| Age, y | 53 ± 13 | 53 ± 13 |
| Men/women, n | 71/13 | 70/14 |
| Neck circumference, cm | 42 ± 6 | 42 ± 4 |
| BMI, kg/m2 | 33 ± 6 | 33 ± 4 |
| ESS score | 10 ± 5 | 11 ± 5 |
| Hypertension | 45 (54) | 37 (44) |
| Coronary artery disease | 10 (12) | 6 (7.1) |
| Congestive heart failure | 2 (2.4) | 3 (3.6) |
| Atrial fibrillation | 3 (3.6) | 7 (8.3) |
| Opioids | 2 (2.4) | 7 (8.3) |
| Benzodiazepines | 7 (8.3) | 5 (5.9) |
| UPPP | 1 (1.2) | 2 (2.4) |
| Pacemaker | 0 (0) | 1 (1.2) |
Data are presented as mean ± SD or number (%). Central sleep apnea (CSA) is defined as 5 or more central apneas per hour of sleep. CPAP refers to continuous positive airway pressure; BMI, body mass index; ESS, Epworth Sleepiness Scale; UPPP, uvulopalatopharyngoplasty. There was no significant difference between the 2 groups.
Table 2.
Comparison of Baseline Polysomnographic Findings of 84 Patients with and 84 Patients without CSA on the First Night of CPAP Titration
| Variables | without CSA | with CSA | p |
|---|---|---|---|
| TDT, h | 6.5 ± 1 | 6.5 ± 1 | 0.3 |
| TST, h | 4.8 ± 1.1 | 4.5 ± 1 | 0.08 |
| SE, % | 74 ± 13 | 70 ± 16 | 0.3 |
| Sleep stage, % | |||
| 1 | 12 ± 6 | 16 ± 15 | 0.1 |
| 2 | 78 ± 8 | 73 ± 11 | 0.001 |
| 3 | 0.3 ± 1 | 0.2 ± 1 | 0.7 |
| REM | 10 ± 7 | 11 ± 8 | 0.5 |
| AHI, n/h | 39 ± 24 | 57 ± 27 | 0.001 |
| CAI, n/h | 1 ± 3 | 6 ± 11 | 0.001 |
| NREM CAI, n/h | 1 ± 3 | 7 ± 13 | 0.0001 |
| REM CAI, n/h | 0.3 ± 1 | 2 ± 5 | 0.03 |
| OAI, n/h | 7 ± 13 | 13 ± 17 | 0.003 |
| HI, n/h | 31 ± 18 | 35 ± 19 | 0.1 |
| Baseline supine SaO2, % | 95 ± 2 | 95 ± 2 | 0.9 |
| Minimum SaO2, % | 81 ± 9 | 80 ± 9 | 0.4 |
| Minimum SaO2 < 90%, min | 29 ± 43 | 31 ± 51 | 1.0 |
| ArI, n/h | 32 ± 20 | 44 ± 23 | 1.0 |
| DBArI, n/h | 29 ± 21 | 42 ± 24 | 0.001 |
Data are presented as mean ± SD; Central sleep apnea (CSA) is defined as 5 or more central apneas per hour of sleep. CPAP refers to continuous positive airway pressure; TDT, total dark time; TST, total sleep time; SE, sleep efficiency; REM, rapid eye movement; NREM, non-rapid eye movement; ArI, Arousal Index; HI, hypopnea index; AHI, apnea-hypopnea index; CAI, central apnea index; OAI, obstructive apnea index; DBArI, disordered breathing arousal index.
Similar to having the baseline polysomnographic differences noted above, patients with CPAP1-CSA continued to have more significant sleep-related breathing disorders during titration than did patients without CSA (Table 3). In the CPAP1-CSA group, although titration resulted in a decrease in AHI (from 57/h to 27/h) and OAI (from 13/h to 1/h), CAI increased (the criterion for inclusion) (Table 3). Central apneas occurred primarily during non-REM sleep and were rare during REM sleep (Table 3), an occurrence similar to findings on baseline polysomnography (Table 2). Not surprisingly, during the titration night, the sleep architecture of the group with CPAP1-CSA was significantly more disturbed (more arousals, more stage 1 sleep, and less stage 2 sleep) than in those without CSA (Table 3).
Table 3.
Comparison of Polysomnographic Findings of 84 Patients with and 84 Patients without CSA on the First Night of CPAP Titration
| Variables | without CSA | with CSA | p Value |
|---|---|---|---|
| TDT, h | 6.5 ± 1 | 6.4 ± 1 | 0.7 |
| TST, h | 4.8 ± 1.1 | 4.1 ± 1.3 | 0.01 |
| SE, % | 75 ± 14 | 62 ± 19 | 0.01 |
| Sleep stage, % | |||
| 1 | 9 ± 6 | 16 ± 11 | 0.01 |
| 2 | 77 ± 8 | 72 ± 10 | 0.01 |
| 3 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.7 |
| REM | 14 ± 8 | 12 ± 9 | 0.3 |
| AHI, n/h | 8 ± 12 | 27 ± 27 | 0.001 |
| CAI, n/h | 1 ± 2 | 14 ± 20 | 0.001 |
| OAI, n/h | 0.1 ± 0.3 | 1.3 ± 17 | 0.5 |
| HI, n/h | 7 ± 10 | 12 ± 15 | 0.05 |
| REM CAI, n/h | 1 ± 2 | 2 ± 4 | 0.03 |
| NREM CAI, n/hr | 1 ± 1 | 16 ± 15 | 0.0001 |
| Baseline Supine SaO2, % | 96 ± 2 | 96 ± 2 | 0.1 |
| Minimum SaO2, % | 88 ± 4 | 85 ± 7 | 0.001 |
| Minimum SaO2 < 90%, min | 5 ± 19 | 10 ± 21 | 0.0002 |
| ArI, n/h | 14 ± 13 | 25 ± 16 | 0.001 |
| DBArI, n/hr | 9 ± 11 | 21 ± 15 | 0.001 |
Data are presented as mean ± SD; Central sleep apnea (CSA) is defined as 5 or more central apneas per hour of sleep. CPAP refers to continuous positive airway pressure; TDT, total dark time; TST, total sleep time; SE, sleep efficiency; REM, rapid eye movement; ArI, Arousal Index; HI, hypopnea index; AHI, apnea-hypopnea index; CAI, central apnea index; OAI, obstructive apnea index; DBArI, disordered breathing arousal index.
The final CPAP levels did not differ significantly between the 2 groups (11.0 ± 2 versus 11.6 ± 3 cm H2O). However, 10 of the 84 patients with CPAP-CSA (vs 2 in the group without CSA) were switched to bilevel PAP in the midst of titration because they did not tolerate the expiratory pressure. Central apneas, however, were present in these patients before switching to bilevel PAP.
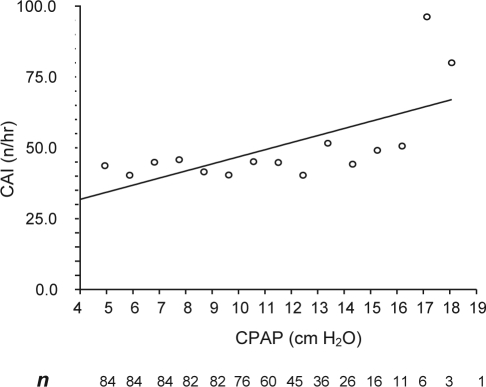
The pattern of occurrence of central apnea across various CPAP levels is depicted in Figure 1. Note that the number of patients decreases progressively as pressure is increased. The relationship between CPAP level and CAI was significant only if a few patients at high CPAP levels were included (r2 = 0.43; p = 0.008).
Figure 1.
Mean central apnea index (CAI) at various continuous positive airway pressure (CPAP) levels.
Of the 84 patients who had CPAP1-CSA, 42 returned for follow-up. The remaining patients did not return in spite of repeated phone calls. Importantly, there were no significant differences in the demographics, sleep architecture, AHI, CAI, desaturation levels, and AI between the 2 groups either at baseline (Table 4) or on CPAP (data not shown).
Table 4.
Baseline Polysomnographic Findings of CPAP1-CSA Groups with (n = 42) and without (n = 42) Second Follow-Up CPAP Study
| Variables | Second follow-up CPAP study |
||
|---|---|---|---|
| Yes | No | p Value | |
| TDT, h | 6.5 ± 1 | 6.3 ± 1 | 0.3 |
| TST, h | 4.7 ± 1 | 4.4 ± 1 | 0.08 |
| SE, % | 72 ± 17 | 69 ± 8 | 0.3 |
| Sleep stage, % | |||
| 1 | 14 ± 17 | 17 ± 11 | 0.1 |
| 2 | 75 ± 8 | 72 ± 12 | 0.1 |
| 3 | 0.2 ± 1 | 0.2 ± 1 | 0.7 |
| REM | 11 ± 8 | 11 ± 8 | 0.5 |
| AHI, n/h | 53 ± 26 | 61 ± 29 | 0.2 |
| CAI, n/h | 6 ± 9 | 6 ± 13 | 0.9 |
| OAI, n/h | 10 ± 13 | 16 ± 20 | 0.09 |
| MAI, n/h | 2 ± 7 | 2 ± 8 | 1.0 |
| HI, n/h | 34 ± 19 | 36 ± 19 | 0.6 |
| Baseline SaO2, % | 96 ± 2 | 94 ± 2 | 0.8 |
| Low SaO2, % | 82 ± 7 | 78 ± 11 | 0.1 |
| Minimum SaO2 < 90%, min | 28 ± 48 | 33 ± 53 | 0.2 |
| ArI, n/h | 43 ± 22 | 46 ± 24 | 0.5 |
| DBArI, n/h | 40 ± 23 | 44 ± 25 | 0.5 |
Data are presented as mean ± SD; Central sleep apnea (CSA) is defined as 5 or more central apneas per hour of sleep. Patients are those who were found to have CSA on the first night of continuous positive airway pressure (CPAP) titration. TDT refers to total dark time; TST, total sleep time; SE, sleep efficiency; REM, rapid eye movement; ArI, Arousal Index; HI, hypopnea index; AHI, apnea-hypopnea index; CAI, central apnea index; OAI, obstructive apnea index; MAI, mixed apnea index; DBArI, disordered breathing arousal index.
The 42 patients who returned for follow-up were invariably adherent to CPAP; the therapeutic hours, not just the time meter, which was objectively monitored, averaged 5.6 ± 1.5 hours for all nights. The results of the baseline, CPAP1, and CPAP2 titration studies of these patients are depicted in Table 5. In general, sleep efficiency and the disordered breathing events improved significantly when the first and second titration studies were compared. Specifically, mean CAI, which had increased significantly from an average of 6 to 13 per hour (baseline vs CPAP1 study), decreased significantly during the CPAP2 study to less than 3 per hour (Table 5). Better control of sleep-related breathing disorders was associated with increased total sleep time, increased sleep efficiency, and decreased number of arousals (Table 5). Importantly, the mean positive air pressure of the 2 CPAP studies did not differ significantly (Table 5). During the first CPAP trial, 6 patients were switched to bilevel PAP because of difficulty exhaling. During the second trial, 2 of these patients were able to tolerate CPAP, and 4 continued to be on bilevel PAP.
Table 5.
Polysomnographic Findings of 42 Patients Who Underwent a Second Full-Night Titration Study
| Variables | Baseline | CPAP1 | CPAP2 | pValue |
|---|---|---|---|---|
| TDT, h | 6.3 ± 1 | 6.5 ± 1 | 6.5 ± 1 | 0.5 |
| TST, h | 4.4 ± 1 | 3.9 ± 1 | 4.5b ± 1 | 0.05 |
| SE, % | 69 ± 15 | 60a ± 16 | 70b ± 17 | 0.007 |
| Sleep stage, % | ||||
| 1 | 17 ± 13 | 17 ± 11 | 14 ± 11 | 0.1 |
| 2 | 72 ± 12 | 71 ± 11 | 73 ± 13 | 0.7 |
| 3 | 0.2 ± 1 | 0.1 ± 1 | 0.1 ± 1 | 0.4 |
| REM | 11 ± 8 | 12 ± 8 | 13 ± 8 | 0.3 |
| AHI, n/h | 61 ± 29 | 30a ± 32 | 13ab ± 15 | 0.0001 |
| CAI, n/h | 6 ± 13 | 13a ± 20 | 3b ± 8 | 0.0001 |
| OAI, n/h | 16 ± 20 | 2a ± 10 | 0.0a ± 0.0 | 0.0001 |
| HI, n/h/hr | 36 ± 19 | 14a ± 15 | 9a ± 13 | 0.0001 |
| Baseline SaO2, % | 94 ± 2 | 96a ± 2 | 95 ± 2 | 0.01 |
| Minimum SaO2, % | 78 ± 11 | 84a ± 7 | 86a ± 8 | 0.0001 |
| Minimum SaO2 < 90%, min | 33 ± 54 | 12a ± 21 | 9a ± 29 | 0.0001 |
| ArI, n/h | 46 ± 24 | 24a ± 10 | 17a ± 12 | 0.0001 |
| DBArI, n/h | 44 ± 25 | 21a ± 11 | 14ab ± 12 | 0.0001 |
| PLMSI, n/h | 1 ± 3 | 2 ± 6 | 3 ± 9 | 0.3 |
| CPAP level, cm/H2O | 11.6 ± 2.4 | 11.4 ± 23 | 0.5 |
Data are presented as mean ± SD; CPAP refers to continuous positive airway pressure; TDT total dark time; TST, total sleep time; SE, sleep efficiency; REM, rapid eye movement; ArI, Arousal Index; HI, hypopnea index; AHI, apnea-hypopnea index; CAI, central apnea index; OAI, obstructive apnea index; DBArI, disordered breathing arousal index; PLMSI, periodic limb movement of sleep index.
p < 0.05 vs baseline.
p < 0.05 first night of CPAP vs second night of CPAP.
Interestingly, among the 42 patients with a second CPAP titration, 2 groups of patients evolved. In 33 patients, CAI was virtually eliminated, and the mean CAI was 1 per hour (Table 6). In the remaining 9 patients, however, CAI remained above 5 per hour in each patient, with mean CAI of 13 per hour (Table 6). This group was labeled CPAP2-persistent CSA. These 9 patients were less adherent to CPAP than the 33 patients without CPAP2-CSA (4.3 ± 1.3 h vs 5.9 ± 1.4 h, p = .0001). Meanwhile, the average Epworth Sleepiness Scale score of these 9 patients was 11.1 ± 4.3, which decreased to 9.1 ± 4.0 with long-term CPAP therapy, but 3 had a score above 10 (11, 13, and 17, suggesting persistent residual daytime sleepiness).
Table 6.
Disordered Breathing Events of 9 Patients with and 33 without CSA on the Second Night of CPAP Titration
| Variables | CSA |
p Value | |
|---|---|---|---|
| without | with | ||
| AHI, n/h | |||
| Baseline | 56 ± 26 | 79 ± 31 | 0.02 |
| CPAP1 | 28 ± 12 | 44 ± 16 | 0.002 |
| CPAP2 | 14 ± 8 | 39 ± 17 | 0.0001 |
| CAI, n/h | |||
| Baseline | 4 ± 11 | 13 ± 18 | 0.06 |
| CPAP1 | 13 ± 7 | 16 ± 10 | 0.8 |
| CPAP2 | 1 ± 1 | 12 ± 5 | 0.0001 |
| OAI, n/h | |||
| Baseline | 15 ± 21 | 18 ± 16 | 0.7 |
| CPAP1 | 1 ± 1 | 2 ± 3 | 0.1 |
| CPAP2 | 1 ± 1 | 1 ± 2 | 0.4 |
| MAI, n/h | |||
| Baseline | 1 ± 4 | 6 ± 14 | 0.1 |
| CPAP1 | 0.1 ± 0.1 | 0.1 ± 0.2 | 0.7 |
| CPAP2 | 0.1 ± 0.1 | 0.1 ± 0.3 | 0.4 |
| HI, n/h | |||
| Baseline | 35 ± 17 | 42 ± 24 | 0.3 |
| CPAP1 | 15 ± 7 | 26 ± 16 | 0.006 |
| CPAP2 | 12 ± 8 | 26 ± 19 | 0.003 |
| Baseline SaO2, % | |||
| Baseline | 95 ± 2 | 93 ± 2 | 0.01 |
| CPAP1 | 92 ± 2 | 95 ± 3 | 0.2 |
| CPAP2 | 96 ± 2 | 94 ± 2 | 0.01 |
| Minimum SaO2, % | |||
| Baseline | 79 ± 10 | 73 ± 12 | 0.1 |
| CPAP1 | 85 ± 6 | 80 ± 9 | 0.04 |
| CPAP2 | 88 ± 4 | 78 ± 10 | 0.007 |
| Minimum SaO2 < 90%, min | |||
| Baseline | 15 ± 18 | 99 ± 85 | 0.01 |
| CPAP1 | 7 ± 13 | 30 ± 32 | 0.007 |
| CPAP2 | 2 ± 11 | 35 ± 51 | 0.001 |
| ArI, n/h | |||
| Baseline | 42 ± 22 | 60 ± 30 | 0.05 |
| CPAP1 | 23 ± 9 | 30 ± 15 | 0.05 |
| CPAP2 | 15 ± 8 | 27 ± 17 | 0.04 |
Data are presented as mean ± SD; Central sleep apnea (CSA) is defined as 5 or more central apneas per hour of sleep. CPAP1 refers to the first night of CPAP (CPAP) titration; CPAP2, the second night of titration; TDT total dark time; TST, total sleep time; SE, sleep efficiency; REM, rapid eye movement; ArI, Arousal Index; HI, hypopnea index; AHI, apnea-hypopnea index; CAI, central apnea index; MAI, mixed apnea index; OAI, obstructive apnea index.
Of the 9 patients with CPAP2-CSA, 2 were on opioids (compared with 0 out of 33 who did not have CPAP2-CSA), 2 were on benzodiazepines, 7 were being treated for systemic hypertension, 4 were being treated for gastroesophageal reflux disease, and none had known cardiac or neuromuscular disease. The major differences in diagnostic polysomnographic findings between the 2 groups (with or without CPAP2-CSA) were presence of the more severe sleep apnea (average AHI of 79/h vs 56/h) and a higher CAI (13/h vs 3/h) in the 9 patients with CPAP2-CSA (Table 6).
Five of the 9 patients with CPAP2-CSA had baseline CAI of five or more per hour, compared with 7 of the 33 patients (56% vs 21%) with CPAP1-CSA but without CPAP2-CSA (p = 0.04, odds ratio = 4.6). We refer to these 5 patients as having CPAP2-CSA resistant (or CPAP-resistant CSA), and the other 4 were truly CPAP-emergent CSA.
Assuming the same prevalence (9 out of 42) of CPAP2-CSA in the other 42 patients who initially had CPAP1-CSA and who did not undergo a second CPAP titration, the overall prevalence of CSA with long-term CPAP use is estimated to be about 1.5% (18 patients out of 1286 patients), with 0.8% (10 of 1286) having CPAP-resistant CSA (those who have CSA to begin with) and 0.6%(8/1286) having truly CPAP-emergent CSA (those who do not have CSA at baseline but develop CSA on CPAP, and the CSA persists in spite of long-term CPAP use).
DISCUSSION
From the results of this systematic retrospective study of a large number of patients with OSA undergoing CPAP titration, we conclude that CSA (CAI ≥ 5/h) occurred in about 6.5% of the 1286 patients. In most of the cases, CSA was transitory and was not observed after 8 weeks of CPAP therapy. However, we estimate that 1.5% of the patients with OSA continued to have CSA with long-term use of CPAP; the severity of OSA, a CAI of 5 or more per hour at baseline, and use of opioids could be risk factors
Several studies have reported a high prevalence of periodic breathing with CSA in patients with systolic heart failure. Some of these patients may respond to CPAP, whereas others may continue to have CPAP-resistant CSA.14,15 We estimate that almost 40% to 50% of patients with systolic heart failure with periodic breathing and CSA have CPAP-resistant CSA.14,15 In contrast, in OSA patients, CPAP therapy is invariably effective, leading to elimination of disordered breathing and consequent improvement in daytime sleepiness, quality of life, and potential cardiovascular diseases, specifically hypertension.16 However, in some patients with OSA, significant numbers of central apneas may emerge or persist during titration with PAP devices. It must be emphasized that, in general, the pattern of breathing of OSA patients with CPAP-emergent or persistent CSA has no resemblance to Hunter-Cheyne-Stokes periodic breathing seen in patients with systolic heart failure when the cycle is quite prolonged.
Prevalence of CSA Patients With OSA Who Use CPAP
A limited number of studies17–19 have shown a prevalence of 13% to 20% of CSA in patients using CPAP. In a retrospective study17 of 223 consecutive adults, Morgenthaler et al reported occurrence of CPAP-CSA in 15% of patients. The patients had either undergone a complete full-night polysomnogram or a split-night study. In another split-night study of 116 patients with OSA, Dernaika et al18 reported that 20% of their patients had CPAP-CSA. In a third retrospective study of 99 patients with a primary diagnosis of OSA, Lehman et al19 reported a prevalence of about 13%. In this study,19 history of ischemic heart disease or heart failure was more frequent among patients with CPAP-CSA than among those without a history of ischemic heart disease or heart failure.
In the present study, which includes the largest number of patients to date (n = 1286), the monthly incidence of CSA during initial CPAP titration (CPAP1) varied from 3% to 10% during a 1-year period, with an average of 6.5%. The lower incidence rate of CSA in our study, compared with previous studies, may have to do with the large number of patients enrolled and the use of separate nights for diagnostic polysomnography and CPAP titration. As noted above, in 2 of the studies,17,18 patients were enrolled if they had CPAP-CSA during the second half of a split-night study. It is conceivable that a half-night CPAP titration is not representative of the whole night, and, in addition, in a split-night study, placement of the mask and a rapidly escalating pressure for hasty titration during the second half of the night could be disruptive to sleep, resulting in arousals and transitions in sleep stages with consequent fluctuation in pCO2 which could promote instability of breathing.1,3,7 Furthermore, rapid titration, overtitration, or a combination thereof, with CPAP could potentially lead to an oral leak, which could appear as a central apnea.
In the present study, all patients were seen in the clinic for a follow-up visit, during which mechanisms of upper airway occlusion were discussed, a CPAP device and a variety of interfaces were shown, and explanations were given of the process of titration and how CPAP eliminates OSA. This approach could potentially decrease the anxiety of encountering the mask and CPAP device in the middle of the night. Slow titration during the whole night is perhaps less disruptive than is rapid titration during a split-night study, resulting in fewer arousals and more stable breathing.1,2 Further, avoiding overtitration decreases the likelihood of oral leak, which, as noted, could appear as a central apnea. In addition, overtitration by increasing lung volume may activate lung stretch receptors, inhibiting central respiratory motor output. We found excessive numbers of central apneas at high CPAP levels in a few patients (Figure 1); however, central apneas also occurred relatively frequently at CPAP levels as low as 5 cm H2O.
Comparison of Patients With and Without CSA on CPAP
We compared data from 84 patients who had CPAP1-CSA with 84 age-, body mass index-, and sex-matched control patients whose OSA was successfully treated with CPAP. The results show that patients with CPAP1-CSA had a more severe OSA than did patients without CPAP-CSA, consistent with the observation of Lehman et al.19 In addition, presence of CSA (CAI ≥ 5/h) at baseline was another risk factor for CPAP1-CSA. More patients with CPAP1-CSA had atrial fibrillation and were using opioids than did patients without CPAP-CSA. Although the differences were not significant (perhaps because of the small number of patients), the findings are consistent with those of studies with patients taking opioids1,20,21 or having atrial fibrillation.3,5,6,22
It is emphasized, that during titration with CPAP, the patients with CSA had more disrupted sleep architecture. Although, it is not possible to determine whether the arousals resulted in emergent CSA,1 the disrupted sleep architecture was related to the development of CSA, or both, we lean toward the first assumption.
Natural History of CPAP-CSA
An important part of this study has to do with determination of the natural history of CSA on CPAP. Of the 42 patients who had developed CPAP1-CSA, CSA was eliminated in most of them with long-term use of CPAP (average time/night = 5.6 h). However, 9 of the 42 patients (an estimated 1.5% of 1286 patients with OSA) had persistent CSA with long-term use of CPAP.
The result of this study—showing that, with long-term treatment of OSA with CPAP, CSA will generally be eliminated—is consistent with results of 2 previous studies23,24 in which patients with OSA were treated with tracheostomy. Guilleminault and colleagues23 noted that patients with OSA who underwent tracheostomy initially had CSA. However, when these patients were followed over an extended period of time, the number of CSAs decreased on a repeat polysomnogram. Coccagna and colleagues24 reported a similar observation. Interestingly, in the study of Dernaika et al,18 most of the CSA resolved in 12 of the 14 patients with CPAP1-CSA who had a repeat titration polysomnogram 9 weeks after their initial polysomnogram, resulting in a prevalence of CPAP-persistent CSA of about 1.5%, the prevalence observed in the present study.
Our study identifies 2 important potential risk factors for CPAP2-persistent CSA. We found that such patients had very severe OSA, and some had a CAI of 5 or greater per hour to begin with. In regard to having severe OSA, the 9 patients with CPAP2-persistent CSA had an average AHI of 79 per hour, compared with an AHI of 56 per hour in the 33 patients with CPAP1-CSA but not CPAP2-CSA (p = 0.02). In regard to baseline CSA, the CAI was 13 per hour in the CPAP2-CSA persistent group, compared with 4 per hour in the CPAP1-CSA but not CPAP2-CSA group (p = 0.06). Actually, among the 9 patients with CPAP2-persistent CSA, 5 of them had a CAI of 5 or greater per hour at baseline (CPAP- resistant CSA). The other 4 did not have CSA at baseline (true CPAP-emergent CSA). A third potential risk factor could be use of opioids, a known risk factor for CSA.1,20,21 We emphasize, however, that a study larger than ours (n = 1286) is needed to better define comorbid factors for CPAP-persistent CSA.
Management of Patients With CSA on Initial CPAP Titration Night
Based on the results of our study, we suggest that patients who have CSA on CPAP return to the clinic for a follow-up within few days. If they complain of symptoms of excessive pressure, pressure should be decreased. Furthermore, patients should be encouraged to continue to use CPAP and be assured that CSA is generally transitory. For the minority with clinical symptoms (eg, excessive daytime sleepiness) or poor adherence with CPAP, we recommended use of pressure support servoventilation, which should result in elimination of residual CSAs.25 The long-term effects of persistent CSA in asymptomatic patients is not known.
Limitations and Strengths of the Study
Our report has limitations in that it is a retrospective study. Another limitation of the study is that 42 of the 84 patients who had CSA on initial CPAP titration did not return for follow-up. Therefore, our estimation of 1.5% having CPAP-persistent CSA may underestimate the true prevalence. However, there were no significant differences in the sleep architecture and severity of sleep apnea either at baseline or during the first CPAP titration between patients who did and did not return for a second CPAP titration study. Also, our study is of an epidemiologic nature and does not shed light on the mechanisms of CPAP and CSA, which could be multifactorial.26
Finally, we used thermistor and piezo crystal technology for classification of sleep apnea, and there is potential for misclassification. However, we emphasize that all apneas classified as obstructive were virtually eliminated by CPAP, whereas those classified as CSA mostly emerged with the use of CPAP. In addition, our definition of a CSA is quite rigid (flat line in airflow and thoracoabdominal excursions) and such events occurred primarily in non-REM sleep. We therefore believe that classification of apneas as central and obstructive was accurate.
The strengths of our study are the large number of patients enrolled, data from all except 2 were included; use of full-night polysomnography for diagnosis and treatment with CPAP; and that 1 physician (SJ) reviewed all polysomnograms.
In summary, the results of this study of 1286 patients show that approximately 6.5% of patients with a primary diagnosis of OSA have CSA during initial CPAP titration. In most cases, CSA is transitory, and resolution occurs. Approximately 1.5% of the patients may have persistent CSA with long-term use of CPAP. Severity of OSA, a CSI of 5 or greater per hour, and use of opioids could be risk factors.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Javaheri has received research support from and consulted for Respironics and has participated in speaking engagements for Respironics and Resmed. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Dedicated to the memory of Athar Malik, MD.
REFERENCES
- 1.Javaheri S. Central sleep apnea. In: Lee-Chiong TL, editor. Sleep: a Comprehensive Handbook. Hoboken, NJ: Wiley-Liss; 2006. pp. 249–62. [Google Scholar]
- 2.White D.P. Central sleep apnea. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practices of Sleep Medicine. 3rd ed. Philadelphia, PA: WB Saunders Company; 2000. pp. 827–39. [Google Scholar]
- 3.Javaheri S. Heart failure. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia PA: Saunders; 2005. pp. 1208–17. [Google Scholar]
- 4.Hanly PJ, Millar TW, Steljes DG, Baert R, Frais MA, Kryger MH. Respiration and abnormal sleep in patients with congestive heart failure. Chest. 1989;96:480–8. doi: 10.1378/chest.96.3.480. [DOI] [PubMed] [Google Scholar]
- 5.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure: Types and their prevalence's, consequences, and presentations. Circulation. 1998;97:2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 6.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 7.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Eng J Med. 1999;341:949–54. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 8.Solin P, Roebuck T, Johns D, et al. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med. 2000;162:2194–200. doi: 10.1164/ajrccm.162.6.2002024. [DOI] [PubMed] [Google Scholar]
- 9.Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med. 2002;165:1245–50. doi: 10.1164/rccm.200110-022OC. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Effect of ventilatory drive on carbon dioxide sensitivity below eupnoea during sleep. Am J Respir Crit Care Med. 2002;165:1251–9. doi: 10.1164/rccm.2110041. [DOI] [PubMed] [Google Scholar]
- 11.Javaheri S, Parker TJ, Wexler L, et al. Occult sleep-disordered breathing in stable congestive heart failure. Ann Intern Med. 1995;122:487–92. doi: 10.7326/0003-4819-122-7-199504010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Javaheri S, Colangelo G, Lacey W, Gartside PS. Chronic hypercapnia in obstructive sleep apnea syndrome. Sleep. 1994;17:416–23. doi: 10.1093/sleep/17.5.416. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet M, Carley D, Carskadon M, et al. ASDA Report;EEG arousals: Scoring Rules and Examples. Sleep. 1992;15:174–84. [Google Scholar]
- 14.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–7. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 15.Arzt M, Floras J, Logan A, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in hear failure. Circulation. 2007:3173–80. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 16.Young T, Javaheri S. Systemic and pulmonary hypertension in obstructive sleep apnea. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practices of Sleep Medicine. 4th ed. Philadelphia, PA: WB Saunders; 2005. pp. 1192–202. [Google Scholar]
- 17.Morgenthaler T, Kagramanov V, Hanak V, et al. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep. 2006;29:1203–8. doi: 10.1093/sleep/29.9.1203. [DOI] [PubMed] [Google Scholar]
- 18.Dernaika T, Tawk M, Nazir S, et al. The significance and outcome of continuous positive airway pressure-related central sleep apnea during split-night sleep studies. Chest. 2007;132:81–8. doi: 10.1378/chest.06-2562. [DOI] [PubMed] [Google Scholar]
- 19.Lehman S, Anic N, Thompson C, et al. Central sleep apnea on commencement of continuous positive airway pressure in patient with primary diagnosis of obstructive sleep apnea-hyperpnoea. J Clin Sleep Med. 2007;3:462–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Teichtahl H, Promdromidis A, Miller B, Cherry G, Kronborg I. Sleep-disordered breathing in stable methadone programme patients: a pilot program. Addiction. 2001;96:395–403. doi: 10.1046/j.1360-0443.2001.9633954.x. [DOI] [PubMed] [Google Scholar]
- 21.Walker M, Farney J, et al. Chronic opioid use a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med. 2007;3:455–61. [PMC free article] [PubMed] [Google Scholar]
- 22.Leung RS, Huber MA, Rogge T, et al. Association between atrial fibrillation and central sleep apnea. Sleep. 2005;25:1543–6. doi: 10.1093/sleep/28.12.1543. [DOI] [PubMed] [Google Scholar]
- 23.Guilleminault C, Simmons B, Motta J, et al. Obstructive sleep apnea syndrome and tracheostomy. Arch Intern Med. 1981;141:985–8. [PubMed] [Google Scholar]
- 24.Coccagna G, Mantovani M, Brignani F, et al. Tracheostomy in hypersomnia with periodic breathing. Physio-path Resp. 1972;8:1217–27. [PubMed] [Google Scholar]
- 25.Javaheri S, Malik A, Smith J, Chung E. J Clin Sleep Med . 2009. Adaptive pressure support servo-ventilation: a novel treatment for sleep apnea associated with use of opioids; pp. 305–10. [PMC free article] [PubMed] [Google Scholar]
- 26.Malhotra A, Bertisch S, Wellman A. Complex sleep apnea: It isn't really a disease. J Clin Sleep Med. 2008;4:1–2. [PMC free article] [PubMed] [Google Scholar]