Abstract
Study Objectives:
REM sleep behavior disorder (RBD) is characterized by loss of the normal muscle atonia during REM sleep associated with disruptive motor activity related to the acting out of dreams. There is frequently injury to the patient or bed partner, and treatment is usually required. Clonazepam has been the first-line therapy for many years, with 2 large case series reporting efficacy with few side effects in the majority of patients. However, long-acting hypnotics in the elderly or those with cognitive impairment can be associated with adverse events especially unacceptable daytime sedation, confusion, and exacerbation of existing sleep apnea.
Methods:
We reviewed 39 patients with confirmed RBD who were treated within our regional sleep center, assessing both efficacy and side effects of drug therapies.
Results:
Adverse effects were reported by 58% of the patients using clonazepam, with 50% either discontinuing the drug or reducing the dose. This prompted us review the side effects of clonazepam in detail and to look for alternative therapies. We report several novel and effective therapies, in particular zopiclone, in a series of patients under long-term follow-up for RBD.
Conclusions:
There are alternatives to clonazepam therapy for RBD which can be as effective and may be better tolerated.
Citation:
Anderson KN; Shneerson JM. Drug treatment of REM sleep behavior disorder: the use of drug therapies other than clonazepam. J Clin Sleep Med 2009;5(3):235–239.
Keywords: REM sleep behavior disorder, drug therapy, side effects
REM sleep behavior disorder (RBD) is characterized by loss of the normal muscle atonia during REM sleep with abnormal motor behaviors that lead to sleep disruption.1 Sleep related injury is common, usually as a result of enactment of violent or unpleasant dreams. The condition usually affects elderly males.2
RBD can occur alone as the idiopathic form or in association with other neurological diseases, in particular the α-synucleinopathies such as Parkinson disease or dementia with Lewy bodies. Up to two-thirds of men > 50 years thought to have idiopathic RBD will go on to develop a neurodegenerative condition,3 and some studies have described subtle cognitive, olfactory, and visual abnormalities in patients with RBD.4,5 Patients with narcolepsy can have RBD as part of their REM sleep dyscontrol. There is also an association with a number of medications, particularly selective serotonin reuptake inhibitors. There is an association with periodic limb movements of sleep (PLMS), and at least 75% of patients have PLMS during NREM sleep, although these often do not cause arousal.
RBD tends to progress over time and spontaneous remissions are very rare, although it may subside during the late stages of a neurodegenerative disorder. By definition, the condition either causes injury or has the potential to cause injury to the patient or others and, as such, usually requires treatment. The benefit of clonazepam in this group was reported in the first publication on RBD in 1986.6 Subsequent studies have also reported the effective use of clonazepam and described the drug as well tolerated and effective in the majority of patients. Since then it has become the standard first-line therapy.7 Smaller case control studies have suggested melatonin is also effective but there have been no reports describing the efficacy of any of the newer hypnotic medications. Clonazepam can cause daytime sedation, confusion in the elderly, increased risk of falls, and exacerbation of existing sleep apnea. These side effects may be more prominent in an aging population and those with associated neurodegenerative conditions. A number of our patients within the Respiratory Support and Sleep Centre in Papworth did not tolerate clonazepam or did not find it effective, which prompted the use of other therapies, most commonly shorter acting hypnotics. In a number of cases these drugs were better tolerated and equally effective at treating symptoms.
In this retrospective case notes review, we report the effective long-term use of a number of novel agents in treating RBD and carefully review the side effects of clonazepam in our patients.
METHODS AND SUBJECTS
A retrospective case notes review was undertaken to identify all patients diagnosed and subsequently treated for RBD within the Respiratory Support and Sleep Centre, Papworth Hospital, Cambridge, UK. A database of approximately 6000 patients who attended from 1994 to 2006 was reviewed to identify those with a definite diagnosis of RBD. The clinical and polysomnographic data were then reviewed.
The diagnostic criteria outlined in the 2005 version of the International Classification of Sleep Disorders1 were required for a diagnosis of RBD: the patient had to have harmful or potentially harmful behavior associated with dream enactment and a polysomnogram showing increased submental and limb electromyogram (EMG) activity in REM sleep. All patients in the study were alive at the time of the study and all had been treated at Papworth with a follow-up > 3 months. Of 39 patients, 31 had been personally seen by KA during investigation and treatment. The remaining patients had been seen during investigation and treatment by JMS. Detailed clinical notes were available for all patients and were reviewed. Prior to commencing any treatment, all patients had 2-week sleep diaries, followed by polysomnography off any hypnotic medication. The Epworth Sleepiness Score (ESS)8 was then recorded at every clinic visit for each patient. Wherever possible, patients were seen 4–6 weeks after the introduction of any treatment to document any benefit and side effects. One of the authors (KA) contacted 37/39 patients retrospectively with a standardized questionnaire documenting medication dose, length of treatment and follow-up, side effects, and current ESS and medication. Particular attention was paid to the presence or absence of cognitive impairment or parkinsonism. Improvement after treatment was defined as a decrease in frequency of injurious behaviors, documented as number of nights/ week. Patients were also asked to rate the severity of their symptoms on a scale of 1–3 (1 = mild, 2 = moderate, and 3 = severe) and any side effects as mild, moderate, or severe. Exclusion criteria included patients with narcolepsy and those diagnosed at Papworth but treated or followed up elsewhere. Given the strong association of PLMS with RBD, this was not in itself an exclusion criterion, but no patients within the group had arousals from sleep associated with their PLMS.
Of 68 non-narcoleptic patients initially identified, 29 were excluded; 8 had died, 13 were not treated (9 had symptoms that were mild and did not wish medication, 4 were investigated at Papworth but followed up elsewhere), 4 had incomplete/ non-confirmatory polysomnography data, and 4 had incomplete follow-up data. A total of 39 patients were included the study.
Polysomnography was performed using a standard procedure, including video recording, a sleep electroencephalogram (leads C4-A1 and C3-A2), bilateral eye movements, submental EMG, and bilateral anterior tibialis EMG to record any leg movements during sleep. Respiratory movements were detected with chest and abdominal bands measuring inductance, airflow was detected with nasal cannulae measuring pressure, and oxygen saturation of arterial blood was measured. Airflow limitation and changes in respiratory movement were used to detect increased upper airway resistance. All respiratory events were scored according to standard criteria9(AASM, 1992). Sleep stages were scored according to standard criteria10(Rechtschaffen and Kales, 1968). All patients had to have clear loss of REM atonia throughout all phases of REM sleep with a significant increase in phasic EMG throughout REM sleep to qualify for the study.
RESULTS
The characteristics of the study group are shown in Table 1. Almost all (38/39) of the patients were male; the mean age was 66 years (range 34–86). Eight patients had a Parkinsonian syndrome and one fulfilled the Diagnostic StatisticalManual National Instutute Neurological Diseases and Stroke diagnostic criteria for probable Alzheimer disease. Of the 9 patients with associated neurodegenerative conditions, 6 had developed RBD prior to other neurological symptoms. No patients had commenced treatment prior to their investigation at Papworth and the mean follow-up within Papworth was 28 months, with a mean of 20 months on medication. The mean ESS for all patients prior to commencing medication was 8.3 (± 3.5), with 15 patients having an ESS ≥ 10. No patients had severe obstructive sleep apnea but 4 patients had an AHI > 5; none had an AHI > 12. There were 8 patients with a PLMI > 5, but none of these patients had symptomatic restless legs syndrome or had separate treatment due to their elevated PLMI. Past medical history and concurrent prescribed medication are listed in Table 2. Twenty-three of the 39 patients were on additional medication, most commonly medication for cardiovascular or cerebrovascular disease, reflecting the age of the population studied.
Table 1.
Characteristics of RBD Patient Cohort
| Patient number | 39 |
| Male: Female | 38:1 |
| Age at time of study (years) | 65.7 ± 11.4 |
| Age of onset of symptoms (years) | 55.1 ± 12.4 |
| Severity of symptoms | 5 ± 2.3 |
| PLMI/h | 6.2 |
| AHI/h | 2.2 |
| Duration of follow-up (months) | 28.8 |
| Duration of treatment (months) | 20.2 |
| Patients with associated neurodegenerative disease | 9 (23%) |
Severity of symptoms is defined as mean number of nights per week that symptoms occur. All values are mean unless otherwise stated ± standard deviation.
Table 2.
Concurrent Diagnoses and Regular Prescribed Medications for All Patients
| Sex | Age | PMH | Other Meds | |
|---|---|---|---|---|
| m | 66 | Nil | ||
| m | 34 | Depression | fluoxetine | |
| m | 51 | No | ||
| m | 71 | TIA | aspirin, simvastatin, ramipril | |
| m | 65 | head injury | ||
| m | 72 | Nil | aspirin, atorvastatin, bendroflumethiazide | |
| m | 56 | Nil | ||
| m | 55 | Mobius syndrome | ibuprofen | |
| m | 82 | Nil | ||
| m | 66 | Glaucoma | eye drops | |
| f | 61 | Nil | ||
| m | 78 | previous stroke | aspirin, dipyridamole, statin, bendroflumethiazide | |
| m | 71 | Nil | ||
| m | 81 | Diabetes | metformin, aspirin, ramipril, simvastatin, salbutamol inhaler | |
| m | 36 | Asthma | salbutamol and beclomethasone inhalers | |
| m | 45 | head injury | ||
| m | 86 | Nil | tamsulosin | |
| m | 69 | Nil | ||
| m | 74 | Aortic valve replacement | warfarin, candesartan, simvastatin | |
| m | 68 | previous stroke and probable Alzheimer disease | aspirin, dipyridamole, lisinopril, simvastatin | |
| m | 66 | asthma, probable dementia with Lewy bodies | salbutamol inhaler | |
| m | 71 | Nil | ||
| m | 45 | asthma, gout | allopurinol, salbutamol inhalers | |
| m | 60 | Nil | ||
| m | 66 | Depression | paroxetine | |
| m | 69 | Depression | citalopram | |
| m | 68 | No | ||
| m | 73 | Osteoarthritis | codeine phosphate, paracetamol | |
| m | 64 | |||
| m | 74 | ischemic heart disease | aspirin, lisinopril | |
| m | 70 | |||
| m | 69 | hypertension, ischemic heart disease | atenolol, lisinopril, aspirin | |
| m | 72 | ischemic heart disease, hypertension, diabetes | bendroflumethiazide, aspirin, atorvastatin | |
| m | 71 | Nil | ||
| m | 77 | atrial fibrillation, previous stroke | warfarin, pravastatin, losartan | |
| m | 69 | ischemic heart disease, esophagitis | aspirin, bendroflumethiazide | |
| m | 68 | ischemic heart disease | aspirin, atenolol | |
| m | 62 | head injury | paracetamol | |
| m | 60 | head injury |
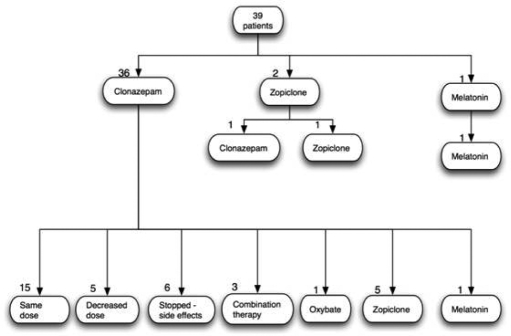
Thirty-six of 39 patients were initially started on clonazepam. The standard starting dose was 0.5 mg per night, with dose titration occurring at each clinic review if necessary. The final doses used ranged from 0.25 mg to 2 mg in all but one patient who required 4 mg (mean dose 1.25 mg). Twenty-one of 36 (58%) patients reported moderate or severe side effects. Six patients stopped medication as a result and refused further medication despite ongoing symptoms, 7 switched to an alternative medication, 5 decreased the dose, and 3 continued despite side effects. The commonest side effects were excessive daytime sedation (usually most marked in the morning with a hangover effect), confusion, or cognitive impairment. Subgroup analysis was performed to see if there were differences between those who developed side effects compared to those who did not. We found no differences between the 2 groups in terms of mean age, dose, severity of disease, or ESS prior to treatment.
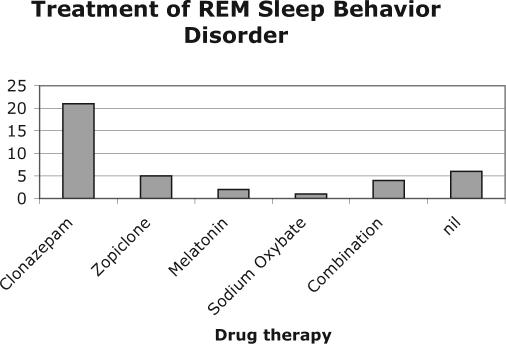
Final medications that patients remained on for long-term treatment are summarized in Figure 1. In total, 21 (54%) patients continued to take clonazepam, 8 (21%) used another medication, and 4 (10%) required a combination of medications to control symptoms adequately. Six (15%) discontinued clonazepam because of side effects and did not wish to start another medication, despite ongoing symptoms.
Figure 1a.
Flowchart representing patient treatment pathways.
Figure 1b.
Final therapy for patients with REM Sleep Behavior Disorder.
Zopiclone was used in 11 patients either alone (9) or in combination (2); 8 of 11 found it effective and well tolerated. The doses used ranged from 3.75 mg to 7.5 mg each night. Three patients commenced zopiclone but stopped it. One patient found it ineffective and switched to clonazepam, one developed a rash, and one found it effective but felt nauseated. Two patients used melatonin (10 mg) and both found it effective, one after treatment failure with clonazepam and one as first-line treatment because of coexistent cognitive impairment and mild obstructive sleep apnea. One patient used sodium oxybate successfully to control symptoms after treatment failure with clonazepam, temazepam, zopiclone, gabapentin, and melatonin.
Combination therapies were used in 3 patients when a single medication failed to control symptoms. The 3 combinations used were clonazepam/zopiclone, temazepam/zopiclone, and clonazepam/gabapentin/melatonin.
Those with another neurodegenerative condition were assessed as a subgroup. Of these 9 patients, 6 had RBD before their neurodegenerative diagnosis was made (range 1–14 years). Eight started clonazepam as first-line therapy, and 5 of these complained of side effects. Three patients reduced the dose as a result, and 2 stopped medication and switched to zopiclone successfully. The other patient started melatonin as first-line therapy due to the combination of existing cognitive impairment and mild obstructive sleep apnea (desaturation index 11.2); this was effective at 10 mg with efficacy maintained over 24 months of follow-up.
DISCUSSION
This retrospective case series describes novel treatments for RBD. It is the first description within the medical literature of the successful use of zopiclone, temazepam and combination medical therapy to treat RBD. This is also the first study to look in detail at side effects of drug treatment for RBD.
Previously Published Case Series of RBD
To date there are no controlled, randomized, double-blind studies that have evaluated the efficacy of any treatment for RBD. All published studies are reports of case series or single cases, none have used repeat polysomnography in evaluation of treatment efficacy, and not all series have used the current diagnostic criteria for patient inclusion.
Clonazepam, a long-acting sedating benzodiazepine (half life 20–50 hours), is currently regarded as the treatment of choice for RBD. The specific mechanism of action of clonazepam is not known but on polysomnography it reduces phasic EMG activity but does not restore tonic REM sleep muscle inactivity.11
There are two large case series that have reported substantial benefit in symptoms in the majority of those treated. The first series reviewed 96 cases and included treatment response to clonazepam in 67 (70%) patients. They described complete benefit in 79% and partial symptom relief in 11%.12 There was no detailed breakdown of adverse effects, but the same group (which included 52 patients with RBD) reported long-term efficacy of nightly benzodiazepines for a range of injurious parasomnias. Of 170 patients, 148 were treated with clonazepam; of the whole group, 15.9% were reported as having adverse events. The second largest case series described the clinical characteristics of 93 patients of whom 57 (61%) were treated with clonazepam and follow up data was available for 38.13 There was a complete reduction in symptoms in 21 (55%) and a partial reduction in 32%; 24% had early morning sedation. Both groups excluded a substantial minority of patients who were not treated because their symptoms were judged too mild. Both groups reported lack of efficacy in approximately 10%.
Our patients were of a similar age to the 2 large case series (mean age 66) and had a similar range of severity of symptoms. All but one patient had doses of clonazepam ranging from 0.5–2 mg which is similar to the dose range used by others. A number of our patients had high ESS scores prior to treatment, but this has also been reported in all of the previous large series and in some cases has been attributed to the sleep disturbance caused by the RBD itself.13
Side Effects of Clonazepam
A substantial number of our patients benefited from clonazepam (54%) as reported previously, but 58% of patients had significant side effects with this medication, which in some limited the maximum dose used and led others to discontinue it. We describe a higher incidence of side effects with clonazepam than previous series. It is important to note that a significant percentage of patients were not treated in both of the large case series. This was either because symptoms were mild or because of sleep disordered breathing or concerns about cognition or the potential side effects in elderly patients. There was also incomplete follow-up data in the series reported by Olsen. Although 57 of 93 patients were treated, only 38 patients had follow-up data, possibly because those who did not tolerate the medication were more likely to be lost to follow-up. It is therefore possible that the true incidence of side effects of clonazepam in treating RBD has been underreported. Adverse events occur frequently with all classes of hypnotics, particularly in the elderly,14 and one would anticipate a considerable number of side effects when using a long-acting hypnotic in a predominantly elderly population.
Alternative Medical Therapies to Clonazepam
Melatonin was helpful in 2 cases, one as an additive therapy when clonazepam alone had failed. There are 3 small case series using melatonin as a treatment for RBD,15–17 with the reported benefit ranging from 100% to 40%.
Several novel treatments were used including zopiclone which was effective and well tolerated in 8/11 patients. Clonazepam has a very long half-life of 40 hours but in our cohort, a shorter acting hypnotic such as zopiclone (half-life 5 hours) appeared to work as well in our patients with fewer side effects. Both clonazepam and zopiclone bind to the benzodiazepine receptor, but clonazepam is classed as a highly potent benzodiazepine binding to all α receptor subunits equally. Zopiclone, in contrast, is said to have a more specific effect on the α-1 and α-5 subunits which may account for a different effect in different patients and a different side effect profile. Clonazepam also decreases the utilization of serotonin which may account for occasional side effect of mood disturbance. Other authors have used other GABA acting hypnotics such as triazolam13 and alprazolam,12 suggesting that they may have a class-specific effect. There have not been any cases successfully treated with eszopiclone, but this may also be an effective treatment. Two small series have reported benefit with pramipexole, a dopamine agonist,17,18 but this was not used in any of our patients.
In previous studies, at least 10% of patients were reported as treatment resistant. Four of our patients were treatment resistant on monotherapy but responded to combinations of hypnotics or hypnotics and melatonin and achieved symptom control. The use of more than one agent is novel and should be considered in those resistant to a single agent.
Limitations of the Study
The limitations of this study were those common to many of the previous reports of pharmacotherapy of RBD. It was a retrospective analysis of case notes, and the numbers on each individual treatment were small. However, all patients were analyzed in a standardized fashion, and all met the current diagnostic criteria for RBD. All had specific review of their medication and adverse events over a prolonged period. Other studies have not reported length of follow-up on medication, and as such, our data are novel.
The majority of patients with symptomatic RBD are elderly and need long-term therapy. Many will develop cognitive impairment as part of an associated α-synucleinopathy. It is therefore important to be aware of the potential for medication side effects in this group. Shorter acting hypnotics may provide a useful alternative to clonazepam, and had fewer side effects in our study.
This study highlights the lack of controlled data regarding the treatment of RBD. Future studies should include randomized, controlled, double-blinded trials to compare therapeutic options for RBD to accurately establish the true incidence of efficacy and adverse events.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders: second edition: diagnostic and coding manual. [Google Scholar]
- 2.Schenck C, Mahowald M. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. 2002;25:120–38. doi: 10.1093/sleep/25.2.120. [DOI] [PubMed] [Google Scholar]
- 3.Gagnon JF, Postuma RB, Mazza S, Doyon J, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5:424–32. doi: 10.1016/S1474-4422(06)70441-0. [DOI] [PubMed] [Google Scholar]
- 4.Postuma RB, Lang AE, Massicote-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66:845–51. doi: 10.1212/01.wnl.0000203648.80727.5b. [DOI] [PubMed] [Google Scholar]
- 5.Massicotte-Marquez J, Décary A, Gagnon JF, et al. Executive dysfunction and memory impairment in idiopathic REM sleep behavior disorder. Neurology. 2008;70:1250–7. doi: 10.1212/01.wnl.0000286943.79593.a6. [DOI] [PubMed] [Google Scholar]
- 6.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 7.Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67:742–7. doi: 10.1212/01.wnl.0000233926.47469.73. [DOI] [PubMed] [Google Scholar]
- 8.Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17:703–10. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- 9.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of the American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 10.Rechtschaffen A, Kales A. Washington: Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages in human subjects. Pub No. 204. [DOI] [PubMed] [Google Scholar]
- 11.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–4. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 12.Schenck CW, Hurwitz TD, Mahowald MW. Symposium: normal and abnormal REM sleep regulation: REM sleep behavior disorder: an update on a series of 96 patients and a review of the world literature. J Sleep Res. 1993;2:224–31. doi: 10.1111/j.1365-2869.1993.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 13.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–9. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 14.Glass J, Lanctot KL, Hermann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331:1169. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunz D, Bez F. Melatonin as a therapy in REM sleep behavior disorder patients: an open-labeled pilot study on the possible influence of melatonin on REM-sleep regulation. Mov Disord. 1999;14:507–11. doi: 10.1002/1531-8257(199905)14:3<507::aid-mds1021>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi N, Uchimura N, Hashizume Y, et al. Melatonin therapy for REM sleep behavior disorder. Psychiatry Clin Neurosci. 2001;55:267–9. doi: 10.1046/j.1440-1819.2001.00854.x. [DOI] [PubMed] [Google Scholar]
- 17.Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4:281–4. doi: 10.1016/s1389-9457(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt MH, Koshal VH, Schmidt HS. Use of pramipexole in REM sleep behaviour disorder: results from a case series. Sleep Med. 2006;7:418–23. doi: 10.1016/j.sleep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Fantini ML, Gagnon JF, Filipini D, Montplaisir J. The effects of Pramipexole in REM sleep behaviour disorder. Neurology. 2003;61:1418–20. doi: 10.1212/wnl.61.10.1418. [DOI] [PubMed] [Google Scholar]