Abstract
β-agonist treatment of asthma displays substantial interindividual variation, which has prompted polymorphism discovery and characterization of β2-adrenergic (β2AR) signaling genes. β2AR function undergoes desensitization during persistent agonist exposure due to receptor phosphorylation by G-protein coupled receptor kinases (GRKs). GRK5 was found to be highly expressed in airway smooth muscle, the tissue target for β-agonists. The coding region is polymorphic at codon 41, where Gln can be substituted by Leu (minor allele), but almost exclusively in those of African descent. In transfected cells, GRK5-Leu41 evoked a greater degree of agonist-promoted desensitization of adenylyl cyclase compared to GRK5-Gln41. Consistent with this functional effect, agonist-promoted β2AR phosphorylation was greater in cells expressing GRK5-Leu41, as was the rate of agonist-promoted receptor internalization. In studies with mutated β2AR lacking PKA-phosphorylation sites, this phenotype was confirmed as being GRK-specific. So, GRK5-Leu41 represents a gain-of-function polymorphism that evokes enhanced loss-of-function of β2AR during persistent agonist exposure, and thus may contribute to β-agonist variability in asthma treatment of African-Americans.
Keywords: Polymorphism, tachyphylaxis, β-agonist, kinases, desensitization, asthma
β2-adrenergic receptors (β2AR) are expressed on many cell types, including those in the lung such as airway epithelial and smooth muscle cells, where they are the targets for β-agonists in the treatment of asthma. Desensitization of β2AR function is mediated, in part, by receptor phosphorylation by G-protein coupled receptor kinases (GRKs) [1], including GRK5 [2, 3]. The GRK-phosphorylated β2AR is a substrate for the binding of β-arrestins, which serves to partially uncouple the receptor from its cognate G-protein, Gs. β2AR desensitization may be manifested clinically as tachyphylaxis to repetitive or continuous β-agonist treatment, resulting in a loss of bronchodilatation and/or protection against bronchoconstriction [4]. Approximately 50% of the interindividual differences in the response to β-agonists in asthma treatment have been attributed to genetic variation [5], prompting us to consider polymorphisms of genes in the β2AR signaling pathway. The coding region of GRK5 has one common nonsynonymous polymorphism (rs17098707), with A or T found at nucleic acid 122, resulting in Gln or Leu (the minor allele) at amino acid 41. Minor allele frequencies are 0.021 (Caucasian) and 0.32 (African-American) (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=17098707). In the current work we have studied the properties of the two GRK5 variants in model cell systems.
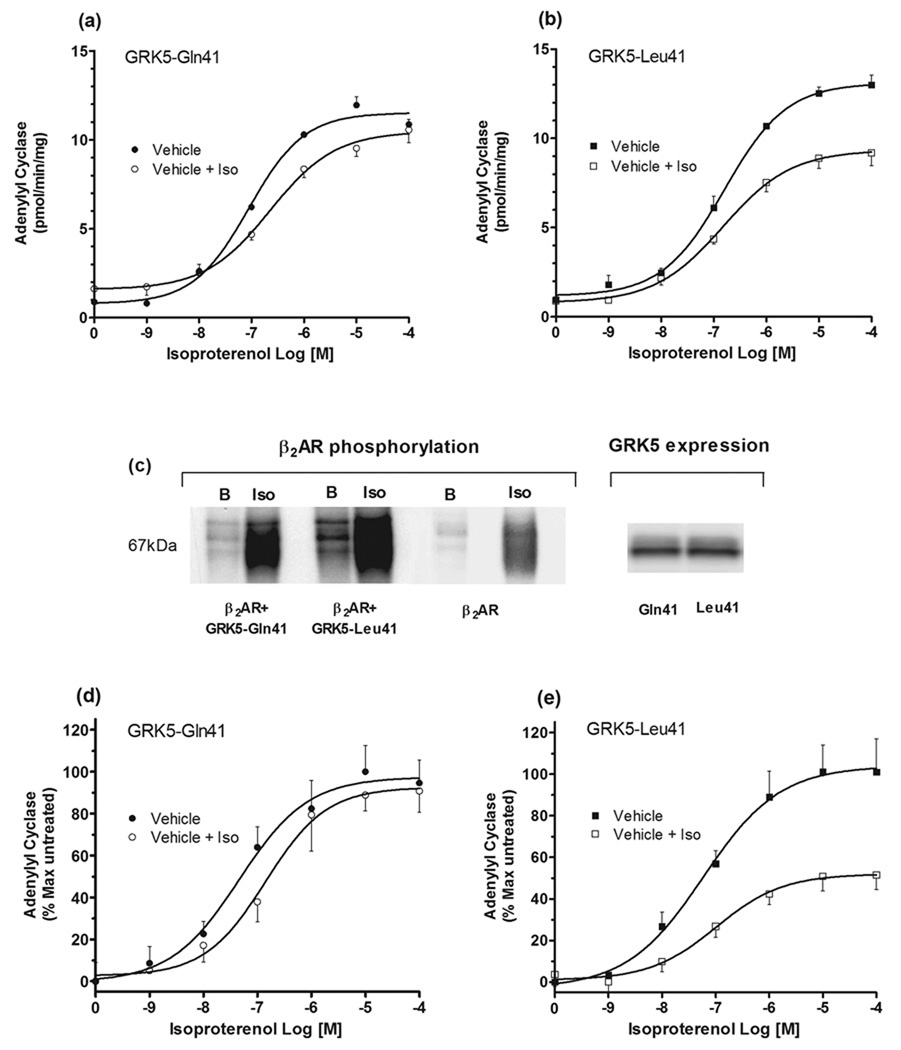
The human GRK5-Gln41 cDNA was generated by RT-PCR, using human genomic DNA as template, followed by site-directed mutagenesis to generate GRK5-Leu41. Both were transfected into Chinese hamster fibroblasts (CHW-1102 cells) stably expressing the human β2AR (the Arg16/Gln27/Thr164 form of the receptor) at levels of 1086 ± 52 (Gln41) and 1137 ± 18 (Leu41) fmol/mg (n = 4), as determined by quantitative 125I-cyanopindolol binding [4]. Membrane adenylyl cyclase assays were performed as described [6] and revealed basal (0.875 ± 0.14, 0.95 ± 0.13) and maximal isoproterenol-promoted (10.56 ± 0.73, 9.17 ± 0.7) activities to be the same between cells expressing the Gln41 and Leu41 GRK5s, respectively. These results were not entirely unexpected, since GRKs act to desensitize receptors only under the conditions of agonist occupancy in the intact cell. To assess GRK function under this condition, cells in culture were treated with vehicle (100 µM ascorbic acid) or vehicle with 10 µM isoproterenol for 30 min at 37°C, washed three times with cold PBS, membranes prepared, and adenylyl cyclase activities determined (Fig. 1a and b). In this model system, cells expressing GRK5-Gln41 underwent little agonist-promoted desensitization (Fig. 1a). For this variant, a ~5% decrease in maximal adenylyl cyclase activity with a rightward shift in the dose-response curve (untreated EC50 = 88 ± 9.0 nM, isoproterenol pretreated = 225 ± 79 nM, P < 0.05) was noted in membranes from cells pretreated with agonist, consistent with a minor degree of desensitization. In contrast, membranes from GRK5-Leu41 cells underwent a significant decrease in the maximal response, amounting to a 30 ± 5% desensitization (P < 0.01 vs. GRK5-Gln41, Fig. 1b).
Fig. 1.
Desensitization and phosphorylation phenotypes of GRK5-Gln41 and GRK5-Leu41. In (a) and (b), CHW-1102 cells stably expressing β2AR were transfected to express the two GRK5 variants. Cells were exposed to vehicle or vehicle plus isoproterenol, washed, membranes prepared, and membrane adenylyl cyclase activities determined. Cells expressing GRK5-Leu41 displayed greater desensitization than those expressing the Gln41 form. Results are from 4 experiments. In (c), COS-7 cells were co-transfected with FLAG-tagged β2AR and the two GRK5 variants, loaded with 32P-orthophosphate, and agonist-promoted phosphorylation of β2AR determined (see ref [7] for methods). Shown is a representative result from 3 experiments. See text for mean results. In (d) and (e), CHO cells were co-transfected with a mutated β2AR lacking PKA phosphorylation sites (see text) and the GRK5 variants. Experiments were carried out as in (a) and (b), and showed greater desensitization of the modified β2AR in cells expressing GRK5-Leu41 compared to GRK5-Gln41. Results are from 7 experiments. Iso, isoproterenol
To assess agonist-promoted receptor phosphorylation, higher β2AR expression levels were required for purification, so COS-7 cells were co-transfected with FLAG-tagged β2AR and the two GRKs. β2AR expression levels were 8909 ± 720 and 8917 ± 1174 (n = 3) for the Gln41 and Leu41 cells, respectively, for these experiments. Cells were loaded with 32P-orthophosphate, treated with vehicle or vehicle with 10 µM isoproterenol, and the receptor purified by immunoprecipitation as previously described [7]. As shown in Fig. 1c, agonist-promoted phosphorylation of β2AR was enhanced by co-transfection of either of the GRK5s compared to transfection of β2AR alone. When studies of the extent of β2AR phosphorylation from GRK5-Gln41 vs. GRK5-Leu41 were performed, the results revealed that GRK5-Leu41 expressing cells had 57% ± 11% greater phosphorylation (Fig. 1c). Western blots revealed similar levels of expression of the two GRK5 variants (Fig. 1c, right hand portion). It was also noted that “basal” (no agonist) phosphorylation was somewhat greater in GRK5-Leu41 cells (compare “B” lanes in Fig. 1c). Since cells that are significantly overexpressing β2AR can have a non-trivial number of receptors in the active conformation due to spontaneous conversion, this increase in basal phosphorylation is also consistent with the notion that GRK5-Leu41 has greater activity.
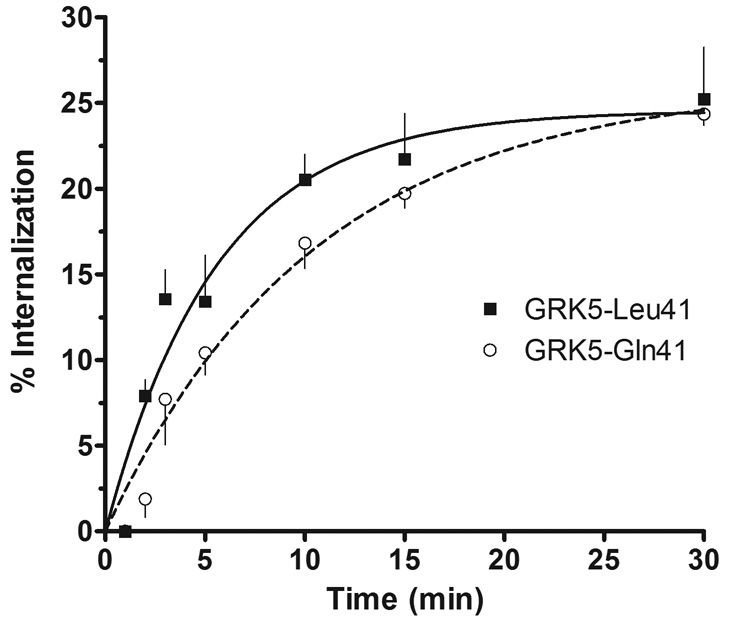
An additional group of experiments was carried out in Chinese hamster ovary (CHO) cells co-expressing the GRK5s and a mutated form of β2AR where the two previously described serines that are phosphorylated by PKA [8] were substituted with alanines. This approach eliminated PKA-mediated desensitization, yielding an assessment of GRK-specific desensitization, and also providing results from a different cell-type than those of the aforementioned experiments. In these studies with CHO cells, receptor expressions for Gln41 and Leu41 cells were 2246 ± 478 and 1857 ± 290, respectively (n = 7). These results revealed once again that β2AR from GRK5-Gln41 cells underwent a minor degree of desensitization, with a ~3-fold increase in the EC50 (from 46 ± 3.9 nM to 141 ± 10.2 nM) and a <5% decrease in the maximal response. In contrast, GRK5-Leu41 cells underwent marked desensitization of the maximal response, amounting to a 50 ± 10% decrease. Finally, we assessed agonist-promoted internalization of β2AR, a process that is in part regulated by GRKs, including GRK5 [9]. These studies were performed in the CHW-1102 cell lines expressing β2AR and either GRK5-Gln41 or GRK5-Leu41. Cells were treated with 10µM isoproterenol for the indicated times, washed, and radioligand binding performed on intact cells with the hydrophilic agent [3H]CGP-12177 (which binds only to cell-surface receptors) at 4°C as previously described [10]. As shown in Fig. 3, GRK5-Leu41-expressing cells had a higher rate of internalization compared to GRK5-Gln41 cells (t1/2 = 3.8 ± 0.20 vs. 7.2 ± 0.33 min, P < 0.001, n = 4). Thus the desensitization, phosphorylation and internalization data all support a phenotype consistent with enhanced function of the minor allelic variant of GRK5 at amino acid 41.
Fig. 3.
Agonist-promoted β2AR internalization phenotypes of GRK5-Gln41 and GRK5-Leu41. Cells expressing β2AR and the indicated GRK5 variant were exposed to 10 µM isoproterenol for the times shown, and the extent of internalized receptors in intact cells determined by the binding of [3H]-CGP12177 (which binds only to cell-surface β2AR, see text and ref [10]). GRK5-Leu41 cells had a higher rate of β2AR internalization compared to that of GRK5-Gln41 cells (t1/2 = 3.8 ± 0.20 vs. 7.2 ± 0.33 min, P < 0.001). Results are mean ± SE from 4 experiments.
In the model systems employed here, we utilized relatively high β2AR expression levels which, as expected, rendered a smaller degree of agonist-promoted desensitization. This was particularly so for GRK5-Gln41, but this set of conditions was necessary in order to adequately assess a gainof-function variant. However, it should be emphasized that we do not consider that the major variant lacks the capacity to evoke desensitization. Indeed, we [11] and others [2] have shown that bovine GRK5 (equivalent to GRK5-Gln41) readily phosphorylates and desensitizes βAR function. Our goal here was to ascertain potential functional differences between the two GRK5 variants, and thus the system was optimized to do so.
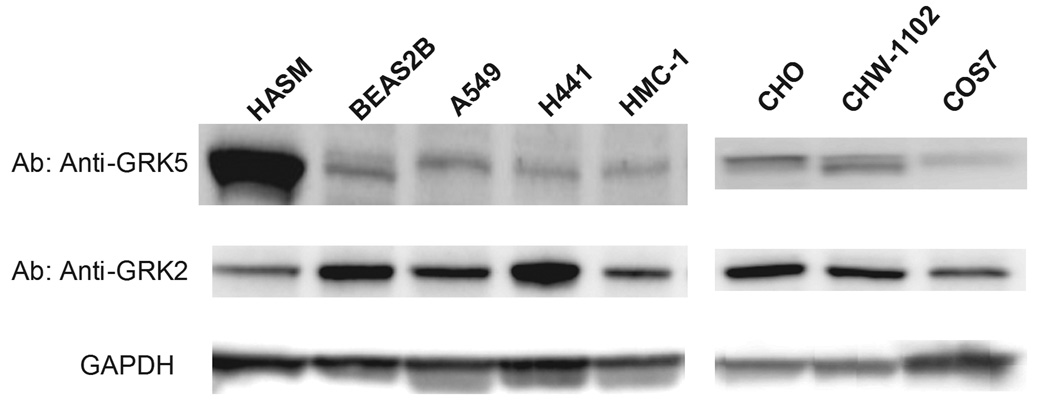
We considered the GRK5-Leu41 phenotype to be relevant for asthma pharmacogenetics, since GRK5 has been reported to be expressed in lung tissue. To confirm that GRK5 is specifically expressed in airway smooth muscle (the primary tissue target for β-agonists for treating bronchospasm), Western blots were carried out using extracts from multiple cell lines (Fig. 2). As shown, human airway smooth muscle cells expressed the highest levels of GRK5 compared to BEAS2B (an airway epithelial cell line), A549 (an alveolar epithelial cell line), HMC-1 (a mast cell line), and the carcinoma cell line H441. Indeed, human airway smooth muscle cell GRK5 levels were ~10-fold more in these cells compared to the others, potentially indicating that GRK5 may play a dominant role in β2AR regulation airway smooth muscle. In contrast, this expression pattern was not observed with GRK2 (Fig. 2), and indeed airway epithelial cells express more GRK2 than airway smooth muscle cells.
Fig. 2.
Expression of GRK5 in various cell lines. Western blots were performed using GRK5-and GRK2-specific antibodies, and a control antibody for GAPDH (all from Santa Cruz Biotechnology, Santa Cruz, CA) at titers of 1:1000, with 25 µg of protein from whole-cell lysates for each sample. In (a) results are shown for the indicated cell lines that endogenously express β2AR. Human airway smooth muscle (HASM) had the greatest expression of GRK5 compared to human airway epithelial cells (BEAS2B) and other cell lines. In contrast, this marked difference was not observed with GRK2. In (b), endogenous GRK expression is shown from the three model cell lines used in the current study for transfections. Results are from a representative experiment.
Taken together, these cell-based studies indicate that the GRK5 variation at amino acid 41, which is common in individuals of African descent, has an impact on agonist-promoted β2AR function. The minor allelic variant, a substitution of Leu for Gln, results in a gain-of-function for the kinase, which causes enhanced loss-of-function of β2AR during agonist exposure. Thus patients with asthma and GRK5-Leu41 may be at greater risk for loss of bronchodilatory function during β-agonist administration. Thus this polymorphism will need to be considered in conjunction with those of the β2AR, and adenylyl cyclase, where in vitro functional effects [12– 14] and clinical associations with β-agonist function [15, 16] have been described. Of note is the ~10-fold greater allele frequency of GRK5-Leu41 in African-Americans. This population has been shown to exhibit less asthma control [17], as well as potentially greater adverse events during chronic β-agonist treatment [18] compared to Caucasians. Whether this GRK5 polymorphism contributes to altered chronic β-agonist responsiveness in African-Americans may be best addressed with a prospective randomized trial with recruitment by genotype.
Acknowledgments
This study was supported by grant HL065899, a component of The Pharmacogenetics Research Network of the National Institutes of Health.
References
- 1.Liggett SB, Green SA. Molecular Biology of the β2-adrenergic receptor: Focus on Interactions of Agonist with Receptor. In: Pauwels R, Lofdahl CG, O'Byrne P, editors. Beta2- Agonists in Asthma Treatment. New York: Marcel Dekker, Inc.; 1996. pp. 19–34. [Google Scholar]
- 2.Tran TM, Jorgensen R, Clark RB. Phosphorylation of the beta2-adrenergic receptor in plasma membranes by intrinsic GRK5. Biochem. 2007;46:14438–14449. doi: 10.1021/bi700922h. [DOI] [PubMed] [Google Scholar]
- 3.Violin JD, Ren XR, Lefkowitz RJ. G-protein-coupled receptor kinase specificity for beta-arrestin recruitment to the beta2-adrenergic receptor revealed by fluorescence resonance energy transfer. J Biol Chem. 2006;281:20577–20588. doi: 10.1074/jbc.M513605200. [DOI] [PubMed] [Google Scholar]
- 4.Lipworth BJ. Airway subsensitivity with long-acting beta 2-agonists. Is there cause for concern? Drug Saf. 1997;16:295–308. doi: 10.2165/00002018-199716050-00002. [DOI] [PubMed] [Google Scholar]
- 5.Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000;56:1054–1070. doi: 10.1258/0007142001903535. [DOI] [PubMed] [Google Scholar]
- 6.Rathz DA, Gregory KN, Fang Y, Brown KM, Liggett SB. Hierarchy of polymorphic variation and desensitization permutations relative to β1- and β2-adrenergic receptor signaling. J Biol Chem. 2003;278:10784–10789. doi: 10.1074/jbc.M206054200. [DOI] [PubMed] [Google Scholar]
- 7.Small KM, Schwarb MR, Glinka C, Theiss CT, Brown KM, Seman CA, et al. Alpha2A-and alpha2C-adrenergic receptors form homo- and heterodimers: the heterodimeric state impairs agonist-promoted GRK phosphorylation and beta-arrestin recruitment. Biochem. 2006;45:4760–4767. doi: 10.1021/bi052074z. [DOI] [PubMed] [Google Scholar]
- 8.Liggett SB. Desensitization of the β-adrenergic receptor: Distinct molecular determinants of phosphorylation by specific kinases. Pharmacol Research. 1991;24:29–41. doi: 10.1016/1043-6618(91)90119-i. [DOI] [PubMed] [Google Scholar]
- 9.Millman EE, Rosenfeld JL, Vaughan DJ, Nguyen J, Dai W, Alpizar-Foster E, et al. Endosome sorting of beta 2-adrenoceptors is GRK5 independent. Br J Pharmacol. 2004;141:277–284. doi: 10.1038/sj.bjp.0705504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green S, Liggett SB. A proline-rich region of the third intracellular loop imparts phenotypic β1- versus β2-adrenergic receptor coupling and sequestration. J Biol Chem. 1994;269:26215–26219. [PubMed] [Google Scholar]
- 11.Freedman NJ, Liggett SB, Drachman DE, Pei G, Caron MG, Lefkowitz RJ. Phosphorylation and desensitization of the human β1-adrenergic receptor. J Biol Chem. 1995;270:17953–17961. doi: 10.1074/jbc.270.30.17953. [DOI] [PubMed] [Google Scholar]
- 12.Green S, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human β2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochem. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 13.Green SA, Turki J, Bejarano P, Hall IP, Liggett SB. Influence of β2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am J Resp Cell Mol Biol. 1995;13:25–33. doi: 10.1165/ajrcmb.13.1.7598936. [DOI] [PubMed] [Google Scholar]
- 14.Small KM, Brown KM, Theiss CT, Seman CA, Weiss ST, Liggett SB. An Ile to Met polymorphism in the catalytic domain of adenylyl cyclase type 9 confers reduced β2-adrenergic receptor stimulation. Pharmacogenetics. 2003;13:535–541. doi: 10.1097/00008571-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. The Lancet. 2004;364:1505–1512. doi: 10.1016/S0140-6736(04)17273-5. [DOI] [PubMed] [Google Scholar]
- 16.Tantisira KG, Small KM, Litonjua AA, Weiss ST, Liggett SB. Molecular properties and pharmacogenetics of a polymorphism of adenylyl cyclase 9 in asthma: interaction between β-agonist and corticosteroid pathways. Hum Mol Genet. 2005;14:1671–1677. doi: 10.1093/hmg/ddi175. [DOI] [PubMed] [Google Scholar]
- 17.Division of Data Services. Asthma prevalence: health care use and mortality, 2000-2001. Hyattsville, MD: National Center for Health Statistics; 2002. [Google Scholar]
- 18.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]