Abstract
Stromal interacting molecule 1 (STIM1) is a Ca2+ sensor that conveys the Ca2+ load of the endoplasmic reticulum to store-operated channels (SOCs) at the plasma membrane. Here, we report that STIM1 binds TRPC1, TRPC4 and TRPC5 and determines their function as SOCs. Inhibition of STIM1 function inhibits activation of TRPC5 by receptor stimulation, but not by La3+, suggesting that STIM1 is obligatory for activation of TRPC channels by agonists, but STIM1 is not essential for channel function. Through a distinct mechanism, STIM1 also regulates TRPC3 and TRPC6. STIM1 does not bind TRPC3 and TRPC6, and regulates their function indirectly by mediating the heteromultimerization of TRPC3 with TRPC1 and TRPC6 with TRPC4. TRPC7 is not regulated by STIM1. We propose a new definition of SOCs, as channels that are regulated by STIM1 and require the store depletion-mediated clustering of STIM1. By this definition, all TRPC channels, except TRPC7, function as SOCs.
A key component of the receptor-evoked Ca2+ signal is activation of a Ca2+-influx channel at the plasma membrane in response to depletion of Ca2+ from the endoplasmic reticulum, the so-called store-operated Ca2+ channels (SOCs)1. Ca2+ influx through SOCs mediates numerous physiological functions1,2. At least two types of SOCs can be distinguished electrophysiologically and now molecularly. The first type of SOCs are the highly Ca2+-selective Icrac currents1 that recently were shown to be mediated by the Orai family of proteins3–8, and the second type of SOCs are the non-selective, Ca2+ permeable TRPC channels1,9.
Very little is known about the Orais. The three Orais are four trans-membrane-span proteins3,7,8, with Orai1 the most prominent Icrac channel6,10. Much more information is available on the TRP family of ion channels (recently reviewed in ref. 11). The TRP superfamily is grouped into seven subfamilies and mediates many cellular functions1,9,11. All TRPC channels are activated by receptor stimulation and several TRPCs have been suggested to function as SOCs1,9,11,12. Deletion in mice13,14 and knockdown by siRNA15 implicate TRPC1 and TRPC4 as SOCs. TRPC3, TRPC6 and TRPC7 are activated by the lipid diacylglycerol16,17. Several studies reported that TRPC3 behaves as a SOC15,18, whereas others concluded that TRPC3 does not function as a SOC19,20. The same uncertainty exists with respect to TRPC6 (ref. 21, 22), whereas the behaviour of TRPC7 as a SOC seems to depend on its expression level23.
The molecular mechanism by which the plasma membrane SOCs sense the Ca2+ filling of the endoplasmic reticulum was revealed by the recent identification of STIM1 as the endoplasmic reticulum Ca2+-content sensor24,25. STIM1 has an amino-terminal EF hand Ca2+-binding domain that resides in the endoplasmic reticulum lumen. Depletion of endoplasmic reticulum Ca2+ results in rearrangement of STIM1 in punctae underneath the plasma membrane and subsequent activation of Ca2+-influx channels24–26. Activation of the SOCs requires the sterile a motif (SAM), coiled-coil and serine–threonine (ST) domains of STIM1 (ref. 27) and is probably aided by multimerization of STIM1 (ref. 28). STIM1 regulates the activity of all known SOCs, including native SOCs24,25 and Icrac6,24,29,30. Moreover, we showed that STIM1 binds TRPC1, TRPC4 and TRPC5, but not TRPC3, TRPC6 and TRPC7 (ref. 31), and the soluble carboxyl-terminus of STIM1 (amino acids 235–685) is sufficient to activate TRPC1 (ref. 31). The EF-hand mutant STIM1D76A that does not bind Ca2+ is constitutively active, whereas the ΔERM(Ezrin–radixin–moesin)–STIM1D76A acts as a dominant-negative STIM1 (refs 25, 31). Similarly, STIM1 was shown to regulate native TRPC1 in platelets32. In HSG cells, STIM1 was reported to exist in a complex with TRPC1 and Orai1 (ref. 33). However, very little is known about the mechanism by which STIM1 regulates TRPC and other Ca2+ influx channels. Fundamental unanswered questions are: which TRPC channels are regulated by STIM1; is STIM1 a subunit of the Ca2+ influx channels and is it obligatory for channel activity; and does STIM1 regulate the channel by affecting their activity at the plasma membrane.
Here, we show that STIM1 regulates directly or indirectly all TRPC channels, except TRPC7. However, although STIM1 directly regulates TRPC1, TRPC4 and TRPC5, the regulation of TRPC3 and TRPC6 by STIM1 is mediated by STIM1-dependent heteromultimerization of TRPC3 with TRPC1 and of TRPC6 with TRPC4. STIM1 is required for activation of all TRPC channels by agonist stimulation, but it is not essential for channel function. These findings clarify fundamental aspect of TRPC channels function by showing that under physiological conditions, STIM1 heteromultimerizes TRPC channels, assembling them into specific complexes to determine their function as SOCs.
RESULTS
Regulation of TRPC3 by STIM1
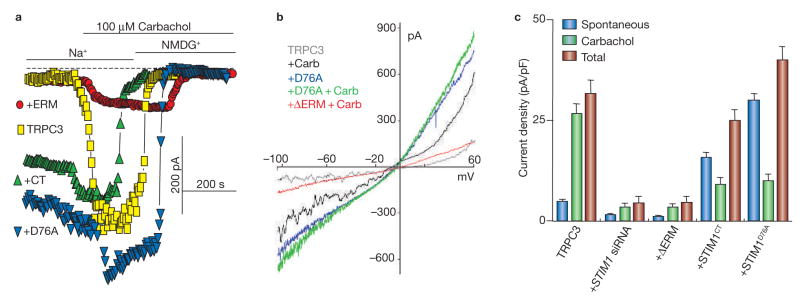
As STIM1 does not bind TRPC3, TRPC6 and TRPC7 (ref. 31), we expected that STIM1 would similarly regulate TRPC1, TRPC4 and TRPC5, but not regulate TRPC3, TRPC6 and TRPC7. Surprisingly, this was not the case for TRPC3 and TRPC6. Multiple probes were used to determine regulation of TRPC channels by STIM1; knockdown of STIM1 with siRNA (STIM1 siRNA) and the dominant negative ΔERM–STIM1D76A (ref. 31) were used to inhibit the action of native STIM1 and the constitutively active STIM1D76A and STIM1CT (amino acids 235–685) were used to activate the channels in a store- and agonist-independent manner. The traces in Fig. 1a and b and the summary in Fig. 1c show that, when expressed at low levels, TRPC3 current is inhibited by STIM1 siRNA and ΔERM-STIM1D76A and is activated by STIM1D76A and STIM1CT, as was found for TRPC1 (ref. 31).
Figure 1.
Regulation of TRPC3 by STIM1. HEK cells were transfected with TRPC3 alone or TRPC3 and the indicated constructs of STIM1. (a) The experimental protocol involves incubation of cells in media containing 150 mM Na+ to measure the monovalent current before and after cell stimulation with 100 μM carbachol. Na+ was then replaced with NMDG+ to determine the zero current level (dashed line). Whole-cell currents measured in cells transfected with TRPC3 alone or with STIM1D76A, STIM1CT or ΔERM–STIM1D76A are shown. (b) The I/V relationships of HEK cells expressing TRPC3 before (grey trace) and after stimulation with carbachol (carb), cells transfected with TRPC3 and STIM1D76A before (blue trace) and after stimulation with carbachol and cells transfected with ΔERM–STIM1D76A and stimulated with carbachol. Note the typical TRPC3 I/V. (c) Summary of the results of TRPC3 alone (n = 8) and experiments with the indicated conditions (n = 5). The spontaneous current density measured before cell stimulation, the current activated by carbachol and the total current are shown. The error bars represent mean ± s.e.m.
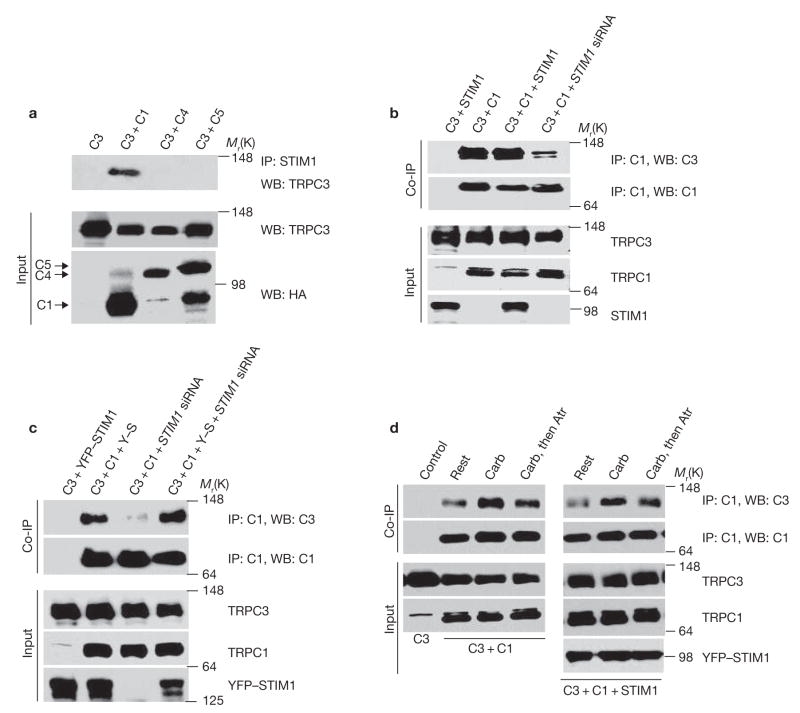
The results in Fig. 1 indicate that TRPC3 does function as a STIM1-regulated channel when expressed at low levels. Furthermore, inhibition of TRPC3 by ΔERM–STIM1D76A, which inhibits the translocation of wild-type STIM1 from the endoplasmic reticulum to the plasma membrane31, indicates that translocation of STIM1 to the plasma membrane is required for activation of TRPC3. We reasoned that as TRPC3 does not bind STIM1, regulation of TRPC3 by STIM1 may be mediated by one of the TRPC channels that do bind STIM1. The results in Fig. 2 show that this is indeed the case. In these experiments, the ability of native STIM1 to coimmunoprecipitate TRPC3 was examined in cells expressing TRPC3 alone or together with TRPC1, TRPC4 or TRPC5 (Fig. 2a). Remarkably, immunoprecipitation of native STIM1 resulted in coimmunoprecipitation of TRPC3 only when it is coexpressed with TRPC1, but not when it is expressed alone or with TRPC4 or TRPC5. STIM1 is obligatory for the interaction between TRPC3 and TRPC1 (Fig. 2b, c). TRPC3 plus TRPC1 were transfected into cells treated with scrambled siRNA or STIM1 siRNA. TRPC3 coimmunoprecipitation with TRPC1, and expression of recombinant STIM1 did not further enhance the coimmunoprecipitate, suggesting that native STIM1 is sufficient to mediate the interaction between the channels under the expression conditions used. Significantly, knockdown of native STIM1 nearly abolished the coimmunoprecipitation of TRPC3 with TRPC1.
Figure 2.
STIM1-mediated interaction of TRPC3 with TRPC1. (a) STIM1 specifically coimmunoprecipitates TRPC1 and TRPC3. HEK cells were transfected with Flag–TRPC3 alone (C3), Flag–TRPC3 + HA–TRPC1 (+C1), HA–TRPC4 (+C4) or HA–TRPC5 (+C5) and the extracts were used to immunoprecipitate native STIM1 and probed for coimmunoprecipitation of TRPC3. Note that TRPC1 selectively mediates the couimmunoprecipitation of STIM1 with TRPC3. (b) STIM1 is required for TRPC1–TRPC3 interaction. HEK cells treated with scrambled (first three lanes) and STIM1 siRNA were transfected with Flag–TRPC3 and Myc–STIM1 (C3+STIM1), Flag–TRPC3 + HA–TRPC1 (C3+C1) or Flag–TRPC3 + HA–TRPC1 + Myc–STIM1 (C3+C1+STIM1) and the extracts were used to immunoprecipitate TRPC1 with anti-HA antibody and probe for coimmunoprecipitation of TRPC3. Note that knockdown of STIM1 almost abolishes the coimmunoprecipitation of TRPC1 and TRPC3. (c) Rescue of TRPC1–TRPC3 interaction by cytosolic STIM1. HEK cells treated with scrambled (first two lanes) or STIM1 siRNA (last two lanes) were transfected with TRPC3 and YFP–STIM1 (Y–S) or C3+ C1+Y–S and the extracts were used to immunoprecipitate TRPC1 and probe for coimmunoprecipitate of TRPC3 and TRPC1. Note that YFP–STIM1, which does not translocate to the plasma membrane, restores the TRPC1–TRPC3 complex. (d) Agonist stimulation enhances complex formation. HEK cells were transfected with Flag–TRPC3 alone (C3, first lane control); C3+HA–TRPC1 or C3+C1+Myc–STIM1. The cells were left unstimulated (rest), were stimulated with 100 μM carbachol for 3 min or were stimulated with carbachol for 3 min and inhibited with atropine (Atr) for 3 min. At the end of the treatments, the cells were harvested to prepare the extracts that were used to immunoprecipitate TRPC1 and blot for coimmunoprecipitate of TRPC1 and TRPC3. Uncropped images of the blots are shown in the Supplementary Information, Fig. S1.
An important question is whether the plasma membrane resident or the intracellular STIM1 mediate the TRPC1–TRPC3 heteromultimerization. This was addressed by testing the ability of YFP–STIM1 to rescue the interaction between the channels in cells treated with STIM1 siRNA. Previous work showed that YFP–STIM1 does not translocate to the plasma membrane6 and its mRNA is not recognized by STIM1 siRNA34. These properties of YFP–STIM1 were confirmed and expression of YFP–STIM1 in cells treated with STIM1 siRNA rescued the interaction between TRPC1 and TRPC3 (Fig. 2c). These findings suggest that migration of STIM1 to the plasma membrane stabilizes or promotes the formation of the TRPC1–TRPC3 complex. This raises the question of whether formation of the heteromultimer is affected by cell stimulation. Cell stimulation stabilized or enhanced formation of the TRPC1–TRPC3 complex and endogenous STIM1 was sufficient for this effect (Fig. 2d). Importantly, termination of cell stimulation resulted in reduction in the complex to nearly basal level within 3 min (Fig. 2d).
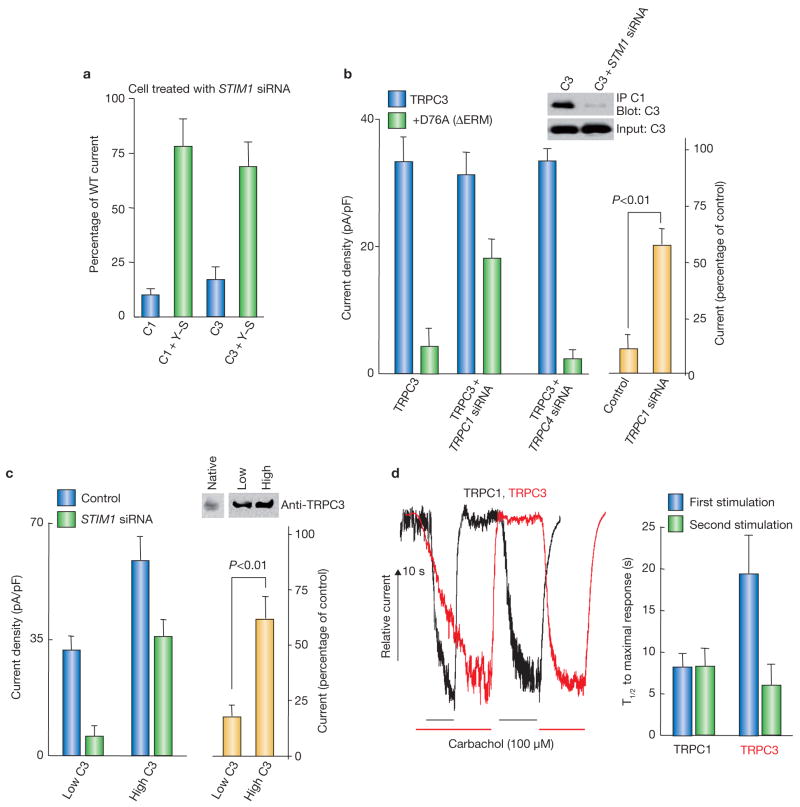
The functional significance of the STIM1-mediated interaction between TRPC1 and TRPC3 is shown (Fig. 3). Further evidence that STIM1 regulates TRPC3 is shown in Fig. 3a, in which YFP–STIM1 similarly rescued the activity of TRPC1 and TRPC3 expressed in STIM1 siRNA-treated cells. This experiment is the functional complement of the experiment in Fig. 2c. The role of native TRPC1 in the regulation of TRPC3 by STIM1 was then tested. Immunoprecipitation of native TRPC1 was sufficient to coimmunoprecipitate TRPC3 and the coimmunoprecipitate was nearly abolished by knockdown of STIM1 (Fig. 3b and see Supplementary Information, Fig. S1). In control HEK cells that expressed native TRPC1 (ref. 15), ΔERM–STIM1D76A inhibited TRPC3 current by about 85%. Knockdown of native TRPC1 markedly reduced the inhibition of TRPC3 by ΔERM-STIM1D76A. Importantly, knockdown of the native TRPC4 had no effect on inhibition of TRPC3 by ΔERM–STIM1D76A. These findings indicate that STIM1 indirectly regulates TRPC3 and that the regulation of TRPC3 by STIM1 is mediated by TRPC1. Further evidence for indirect regulation of TRPC3 by STIM1 was obtained by examing the effect of knockdown of STIM1 on TRPC3 expressed at low and high levels. Previous work has shown that the behaviour of TRPC3 as a SOC18, 19 and regulation by InsP3 receptors19,35 is observed only when TRPC3 is expressed at low levels. The level of native TRPC3 and when TRPC3 is expressed at low and high levels is shown in Fig. 3c, which also shows that overexpression of TRPC3 partially overcame the inhibition of TRPC3 current by knockdown of STIM1. It is likely that at low expression levels, most TRPC3 is in a complex with TRPC1 and STIM1. When it is overexpressed, large fraction of TRPC3 is not complexed and can be activated by agonist in a STIM1-independent manner. The implication of these experiments is that STIM1 is not obligatory for the function of TRPC3 as a channel. However, when TRPC3 is present in a complex with TRPC1, as found in several cell types36,37, it behaves as a STIM1-regulated SOC.
Figure 3.
Regulation of TRPC3 by STIM1 requires TRPC1. (a) Rescue of TRPC3 activity by cytosolic STIM1. HEK cells treated with STIM1 siRNA were transfected with HA–TRPC1 (C1) alone, C1 and YFP–STIM1 (C1+Y–S), Flag–TRPC3 alone (C3) or C3 and YFP–STIM1 (C3+Y–S) and receptor-stimulated current density was measured. The error bars indicate the mean ± s.e.m. of four experiments. (b) TRPC1 is required for regulation of TRPC3 by STIM1. The insert shows coimmunoprecipitation of TRPC3 with native TRPC1 from cells treated with scrambled or STIM1 siRNA and transfected with Flag–TRPC3. The native TRPC1 was immunoprecipitated with the Sigma anti-TRPC1 and probed for coimmunoprecipitation of TRPC3 expressed at low levels. Current density was measured in cells transfected with TRPC3 alone or TRPC3+ΔERM–STIM1D76A. Expression was in cells treated with scrambled siRNA (control) and TRPC1 siRNA or TRPC4 siRNA. The yellow columns represent the percent residual current recorded for TRPC3+ΔERM–STIM1D76A in scrambled siRNA cells versus cells treated with TRPC1 siRNA. The error bars indicate the mean ± s.e.m. of five experiments with TRPC1 siRNA and four experiments with TRPC4 siRNA. (c) STIM1 is not obligatory for TRPC3 activity. Control and STIM1 siRNA-treated cells were transfected with low and high TRPC3. The blot shows the level of native and expressed TRPC3. For native and expressed TRPC3 the blot was probed with anti-TRPC3 and developed for 15 s and 5 s, respectively. Cells were stimulated with carbachol to record the TRPC3 current. Note that overexpression of TRPC3 overcomes the dependence of TRPC3 activation on STIM1. The error bars indicate the mean ± s.e.m. of five experiments. (d) Faster activation of heteromultimeric TRPC3. HEK cells transfected with TRPC1 or TRPC3 were repeatedly stimulated with carbachol. Example traces and the average half time for activation of TRPC1 and TRPC3 by the fist and second stimulation are shown. The TRPC3 current is approximately twice as large as the TRPC1 current, but it was rescaled for illustration purposes. The error bars indicate the mean ± s.e.m. of four experiments. Uncropped images of the blots are shown in the Supplementary Information, Fig. S1.
The significance of the heteromultimerization for channel function was examined further by measuring the rate of TRPC1 and TRPC3 activation on repeated stimulation. The half time for activation of TRPC1 was the same for the first and second stimulation (Fig. 3d). In contrast, the rate of TRPC3 activation increased approximately 2.4-fold in the second compared to the first stimulation. These results raised the possibility of a ‘memory’ of the assembled STIM1–TRPC1–TRPC3 complex, which may be important for Ca2+ oscillations and perhaps reloading of the stores on termination of cell stimulation. Thus, Ca2+ oscillations require repeated activation of Ca2+ influx in phase with Ca2+ release38 and Ca2+ influx is required to sustain the Ca2+ oscillations1,39. The memory of complex formation and facilitation of SOC activation can function to better coordinate Ca2+ release and activation of Ca2+ influx.
Regulation of TRPC4 and TRPC6 by STIM1
STIM1 binds TRPC4 through its ERM domain31 and activation of TRPC4 by agonist is inhibited by knockdown of STIM1 and by ΔERM-STIM1D76A (see Supplementary Information, Fig. S2). These results indicate that STIM1 regulates TRPC4 in a mechanism that is similar to the regulation of TRPC1 by STIM1.
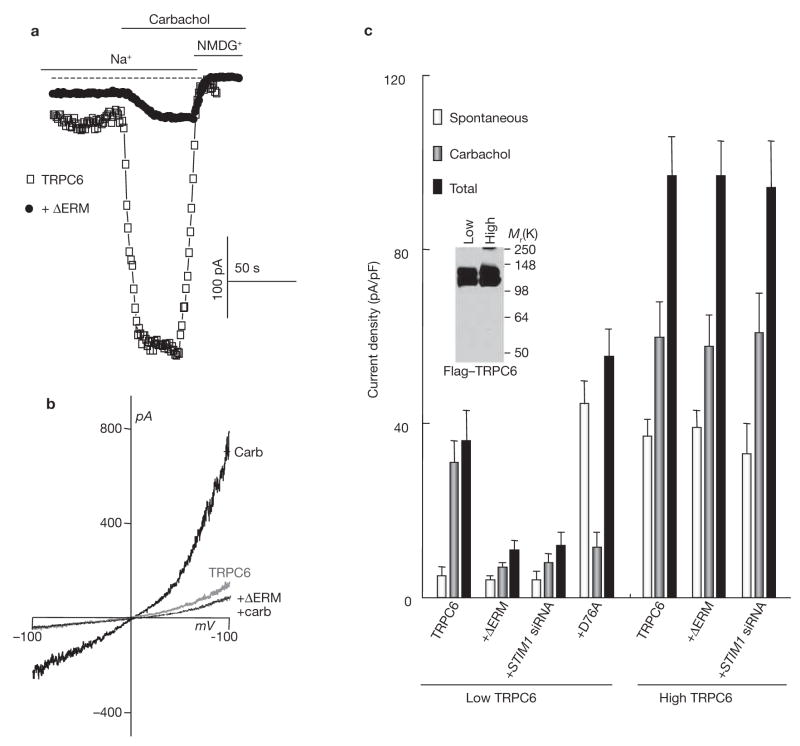
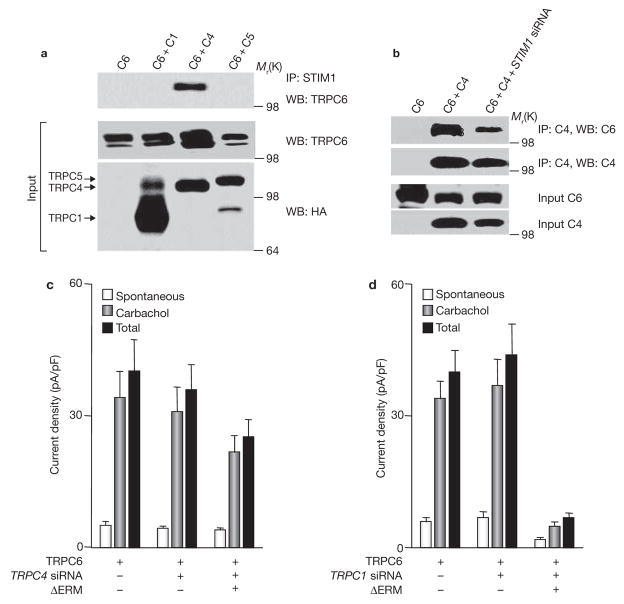
Although TRPC6 did not bind STIM1, STIM1 indirectly regulated activation of TRPC6 by agonist stimulation (Figs 4, 5). When TRPC6 was expressed at low levels, activation of TRPC6 by agonist was inhibited by knockdown of STIM1 and by ΔERM–STIM1D76A, and TRPC6 became constitutively active in the presence of STIM1D76A (Fig. 4). When TRPC6 was expressed at high levels, its activation by agonist was no longer regulated by STIM1, indicating that STIM1 is not obligatory for the function of TRPC6 (Fig. 4c), as was found for TRPC3.
Figure 4.
Regulation of TRPC6 by STIM1. (a) HEK cells were transfected with Flag–TRPC6 alone or Flag–TRPC6 and the indicated constructs of STIM1. The experimental protocol was the same as in Fig. 1. Whole-cell current recording measured in cells transfected with TRPC6 alone or with TRPC6+ΔERM–STIM1D76A is shown. (b) The I/V relationships of HEK cells expressing TRPC6 before (grey trace) and after (thick dark trace) stimulation with carbachol, and of cells transfected with TRPC6 and ΔERM–STIM1D76A and stimulated with carbachol (thin dark trace). (c) The blot shows the level of Flag–TRPC6 expression under the indicated conditions and the graph summarizes the results of six experiments with low and four experiments with high TRPC6 levels. The spontaneous current density measured before cell stimulation, the current activated by carbachol and the total current are plotted. Cells were transfected with low or high levels of TRPC6 alone or together with ΔERM–STIM1D76A or D76A–STIM1 and in cells treated with STIM1 siRNA, as indicated. Cells with high levels of TRPC6 were transfected with three times more cDNA than cells with low level of TRPC6. Note that ΔERM–STIM1D76A and STIM1 siRNA inhibited TRPC6 and STIM1D76A increased the spontaneous activity of TRPC6 only when TRPC6 was expressed at low levels. The error bars indicate the mean ± s.e.m.
Figure 5.
Interaction of TRPC6 with TRPC4 mediates regulation of TRPC6 by STIM1. (a) Native STIM1 specifically coimmunoprecipitates TRPC4 and TRPC6. HEK cells were transfected with Flag–TRPC6 alone (C6), or C6 with HA–TRPC1 (C6+C1), HA–TRPC4 (C6+C4) or HA–TRPC5 (C6+C5) and the extracts were used to immunoprecipitate native STIM1 and probed for coimmunoprecipitation of TRPC6. (b) STIM1 is required for TRPC4–TRPC6 coimmunopreciption. HEK cells treated with scrambled (first and second lanes) or STIM1 siRNA (third lane) were transfected with Flag–TRPC6 (C6) or C6+HA–TRPC4 (C6+C4; last two lanes) and the extracts were used to immunoprecipitate TRPC4 and probe for coimmunoprecipitation of TRPC6. (c, d) TRPC4, but not TRPC1, is required for regulation of TRPC6 by STIM1. Current density was measured in cells transfected with TRPC6 alone or TRPC6 and ΔERM–STIM1D76A. TRPC6 and TRPC6+ΔERM–STIM1D76A were expressed in cells treated with scrambled siRNA (control; c, d), TRPC4 siRNA (c) or TRPC1 siRNA (d). The spontaneous, stimulated and total current densities are shown. The error bars indicate the mean ± s.e.m. of five experiments with TRPC4 siRNA and four experiments with TRPC1 siRNA.
Suspecting that regulation of TRPC6 by STIM1 is mediated by one of the TRPC channels that interact with STIM1, we examined which TRPC channel mediated the interaction between TRPC6 and STIM1. Native STIM1 does not coimmunoprecipitate TRPC6 when expressed alone (Fig. 5a). Notably, coexpression of TRPC6 with TRPC4, but not with TRPC1 or TRPC5, resulted in coimmunoprecipitation of STIM1 and TRPC6. In addition, immunoprecipitation of TRPC4 coimmunoprecipitated TRPC6 when both were coexpressed, and knockdown of STIM1 markedly reduced the coimmunoprecipitation (Fig. 5b).
The functional significance of the STIM1-mediated interaction between TRPC6 and TRPC4 is shown in Fig. 5c. When TRPC6 was expressed at low levels, ΔERM–STIM1D76A inhibited activation of TRPC6 by about 87%. Knockdown of TRPC4 had no effect on current density or activation of TRPC6. However, knockdown of TRPC4 markedly reduced inhibition of TRPC6 by ΔERM–STIM1D76A. As a control, knockdown of TRPC1 did not prevent inhibition of TRPC6 stimulation by ΔERM–STIM1D76A (Fig. 5d). This was in contrast with the effect of knockdown of TRPC1 on the inhibition of agonist stimulation of TRPC3 by ΔERM–STIM1D76A. Thus, these findings indicate that STIM1 indirectly regulates TRPC6 and that TRPC4 mediates the regulation of TRPC6 by STIM1.
STIM1 is obligatory for activation of TRPC channels by agonists, but not for channel activity
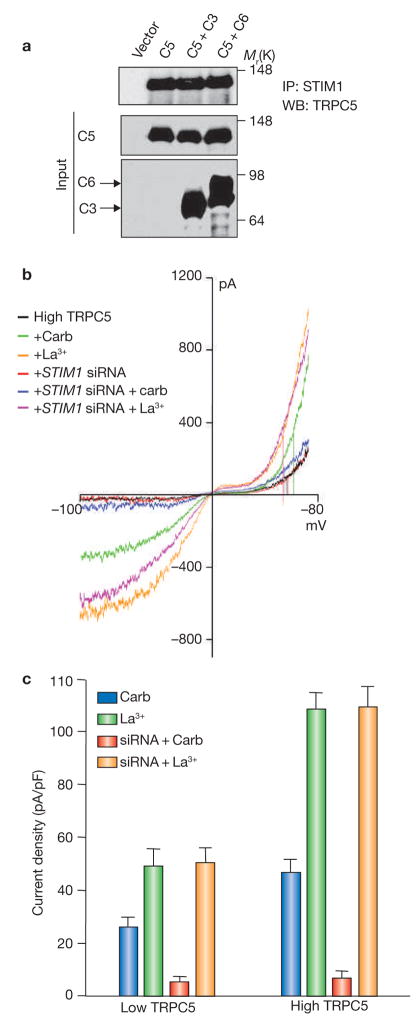
A critical question in understanding regulation of Ca2+ influx channels by STIM1 is whether STIM1 is obligatory for ion conductance by the channels that are directly regulated by STIM1, such as TRPC1, TRPC4 and TRPC5. This is highlighted in the finding that Orai1 activity as an Icrac channel can be observed only when it is coexpressed with STIM1 (refs 6, 8, 29, 30, 40). To address this question, it was necessary to examine the effect of STIM1 on a channel that could be activated by receptor stimulation and by ligands in a receptor-independent manner. TRPC5 is such a channel, and can be activated by receptor stimulation or directly by lanthanides41. Immunoprecipitation of native STIM1 coimmunoprecipitated TRPC5 and the coimmunoprecipitated was not affected by coexpression of TRPC5 with TRPC3 or TRPC6 (Fig. 6a). Selective traces of the typical double rectification current/voltage relationship of TRPC5-mediated current is shown in Fig. 6b. These results indicate that STIM1 is obligatory for activation of TRPC5 by receptor stimulation. Hence, unlike the findings for TRPC3 and TRPC6, activation of TRPC5 by receptor stimulation requires STIM1 independent of the expression level of TRPC5. The second important finding from these experiments is that STIM1 is not obligatory for the function of TRPC5. Thus, knockdown of STIM1 that largely inhibits activation of TRPC5 by agonist stimulation had no effect on direct activation of TRPC5 by La3+. In addition, knockdown of STIM1 had no effect on TRPC5 current density or its voltage-dependence. The significance of these findings is that STIM1 is required only for activation of TRPC channels by receptor stimulation, but STIM1 is not obligatory for ion conductance by the channels and does not regulate the voltage-dependence of channel function.
Figure 6.
STIM1 regulates TRPC5 but it is not obligatory for channel function. (a) STIM1 interacts with TRPC5. HEK cells were transfected with GFP–TRPC5 alone (C5); C5+ Flag–TRPC3 (C5+C3) and C5+ Flag–TRPC6 (C5+C6) and the extracts were used to immunoprecipitate native STIM1 and probe for coimmunoprecipitation of TRPC5. Note that STIM1 interacts with TRPC5 and the interaction is not affected by TRPC3 or TRPC6. (b) High levels of TRPC5 (four times more cDNA) were transfected in HEK cells treated with scrambled siRNA or with STIM1 siRNA. The current was measured in response to stimulation with carbachol, and then in response to activation of TRPC5 by La3+ in the same cells. Representative I/V curves are shown. (c) Histogram indicating the mean ± s.e.m. of the current density recorded in five experiments of cells expressing low or high levels of TRPC5 and treated with scrambled (control) or STIM1 siRNA. The cells were stimulated with carbachol and then activated with La3+. Note that knockdown of STIM1 inhibits receptor-mediated activation of TRPC5 irrespective of expression levels and that knockdown of STIM1 has no effect on activation of TRPC5 by La3+ in the same cells.
Not all TRPC channels are regulated by STIM1
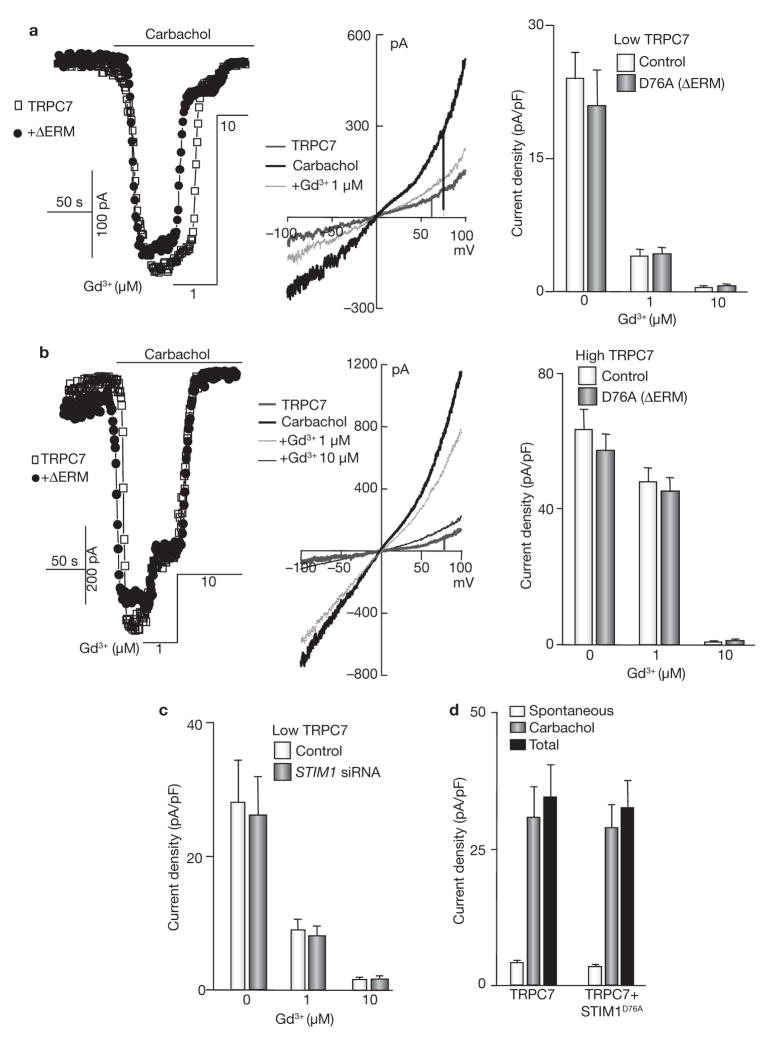
TRPC7 function depends on its expression level. When expressed at low levels, activation of TRPC7 by agonist required its coupling to InsP3 receptors, and TRPC7 activity was inhibited by 1 μM Gd3+. However, when TRPC7 was expressed at high levels, its activation was independent of InsP3 receptors and TRPC7 activity was inhibited only by 10 μM Gd3+ (refs 23, 42). Consistent with the finding that TRPC7 does not bind STIM1 (ref. 31)31, the activity of TRPC7 was independent of STIM1 whether functioning as an InsP3 receptor-dependent or -independent channel (Fig. 7). Thus, agonist-mediated activation of TRPC7 was not inhibited by ΔERM–STIM1D76A when its activity was largely inhibited by 1 μM Gd3+ (Fig. 7a) or when its inhibition required 10 μM Gd3+ (Fig. 7b). Similarly, knockdown of STIM1 did not prevent activation of TRPC7 and did not change its sensitivity to Gd3+ (Fig. 7c). Finally, STIM1D76A did not activate TRPC7 and did not increase its spontaneous activity.
Figure 7.
TRPC7 is not regulated by STIM1. (a, b) HEK cells were transfected with low (a) or high (four times more cDNA; b) levels of TRPC7 alone or with ΔERM–STIM1D76A and stimulated with carbachol. The mode of TRPC7 function was determined by measuring the sensitivity to inhibition by 1 and 10 μM Gd3+. Typical I/V relationships at the indicated conditions and the mean ± s.e.m. of four experiments with low and five experiments with high levels of SLC26A7 are also shown. (c) TRPC7 was transfected into HEK cells treated with scrambled (control) or STIM1 siRNA and the effect of STIM1 knockdown on TRPC7 current and its sensitivity to Gd3+ were measured. (d) TRPC7 was expressed alone or with the constitutively active STIM1D76A and the spontaneous, carbachol-stimulated and total current were measured. STIM1D76A did not increase spontaneous activity of TRPC7. The error bars in c and d indicate the mean ± s.e.m. of four experiments.
DISCUSSION
The study examines fundamental aspects of the function of TRPC channels and their regulation by STIM1, and the mechanism by which STIM1 may regulate SOCs. The first major finding is that all TRPC subunits, except TRPC7, are directly or indirectly regulated by STIM1. Indirect regulation is dependent on a STIM1-mediated heteromultimerization with TRPC subunits that can directly bind STIM1. It has long been controversial whether TRPC channels, in particular TRPC3 and TRPC6, function as SOCs15,18–21,22. Regulation of a channel by STIM1 and the requirement of translocation of STIM1 from the endoplasmic recticulum to plasma-membrane domains for channel activation can be considered one form of SOC — that is, an endoplasmic reticulum Ca2+-content sensor (STIM1) regulates a plasma membrane Ca2+ channel, and the regulation by the sensor requires rearrangement of STIM1 in response to depletion of the stored Ca2+. We propose this as a new molecular definition of SOCs. Regulation of TRPC channels by STIM1 is concluded from inhibition of channel function by knockdown of STIM1. Requirement for translocation of STIM1 is concluded from activation of the channels by the constitutively active STIM1D76A and from their inhibition by ΔERM–STIM1D76A, which prevents the rearrangement of STIM1 into punctae in response to store depletion. By these criteria, it is clear that TRPC1, TRPC4 and TRPC5 function as SOCs under all conditions, whereas TRPC3 and TRPC6 function as SOCs when expressed at low, physiological levels. However, the function of TRPC3 and TRPC6 as SOCs is indirect and is mediated by their regulated interaction with other TRPC channels.
A second finding of note is that STIM1 heteromultimerizes TRPC channels to confer their function as SOCs. The specificity of this effect is quite remarkable. STIM1 mediates the interaction between TRPC1–TRPC3 and between TRPC4–TRPC6. Moreover, the TRPC1–TRPC3 and TRPC4–TRPC6 multimerization is required for regulation of TRPC3 and TRPC6 by STIM1 and their function as SOCs. Assembly of the TRPC1–TRPC3 and TRPC4–TRPC6 into stable complexes is enhanced or stabilized by agonist stimulation, and is mediated by intracellular, rather than the plasma membrane, STIM1. This implies that the store depletion-mediated clustering of STIM1 is likely to facilitate interaction between the channels to allow regulation of all heteromeric TRPC channels by STIM1. The STIM1-mediated formation of the heteromultimers not only increases the diversity of SOC channels, but also provides the cells with a mechanism to regulate their function and store Ca2+ content.
Another important finding of this study is that STIM1 seems to be obligatory for activation of the TRPC channels by receptor stimulation, but not for channel function. This conclusion is based on the finding that high overexpression of TRPC1, TRPC4 (data not shown) and TRPC5 (Fig. 6) does not result in STIM1-independent function of these channels. On the other hand, high overexpression of TRPC3 and TRPC6 results in STIM1-independent channel function and increased spontaneous activity. The findings that TRPC3 and TRPC6 can function in a STIM1-dependent and STIM1-independent manner indicate that STIM1 is not obligatory for channel function. The strongest evidence for the conclusion that STIM1 is not obligatory for channel function was obtained with TRPC5. Deletion of STIM1 prevents activation of TRPC5 by agonist stimulation, but has no effect on activation of TRPC5 by La3+, the size of the current or its regulation by voltage. These observations lead to the conclusion that STIM1 mediates regulation of TRPC channels by receptor stimulation, but STIM1 is not a channel subunit and is not needed forTRPC channel to function. Instead, TRPC channels can be activated by more than one mechanism, one of which is activation by STIM1 that is used by receptors to activate Ca2+ influx. The mechanism by which STIM1 activates and/or gates TRPC channels has yet to be determined.
METHODS
Solutions, reagents and clones
TRPC1, TRPC3 and TRPC4 clones were previously described43, and GFP–TRPC5, TRPC6 and TRPC7 clones were generously provided by Y. Mori (Kyoto University, Kyoto, Japan). The antibodies used were anti-TRPC1 (1:500; Sigma, St Louis, MO), anti-TRPC3 (1:3000; a gift from C. Montell, Johns Hopkins, Baltimore, MD), anti-STIM1 antibody for coimmunoprecipitation (BD Biosciences, Franklin Lakes, NJ), HRP-conjugated anti-Myc (Santa Cruz, Santa Cruz, CA) and HRP-conjugated anti-HA (Covance, Princeton, NJ), and anti-Flag (Sigma). The STIM1 siRNAsequence used was GGCTCTGGATACAGTGCTC, and the siRNA to knockdown TRPC1 and TRPC4 were previously reported15 and their effectiveness was confirmed in the present work. siRNA transfection of HEK293 cells was performed using the Qiagen TransMessenger Transfection kit. The amount of siRNA used was 0.8 μg per 12-well containing 80–90% HEK cells. Wells were coated with 0.5 mg ml−1 poly-L-ornithine in 0.15 M borate buffer at pH 8.6. After 6 h siRNA transfection, 70% of cells were replated into fresh wells so that confluency was 80–90% the following day. Plasmids transfection was performed using Lipofectamine 2000 reagent for 6 h. The total amount of cDNA used per 12-well was 0.5 μg. Thus, for a typical transfection, 0.13 μg TRPC channel was used for low expression levels, together with 0.13 μg M3 receptor, 0.13 μg STIM1 and 0.1 μg GFP. For high expression levels of TRPC channels, 0.4–0.5 μg cDNA was used. For coimmunoprecipitation analysis, transfection was performed in 6-well plates, and each cDNA amount was scaled up to a total of 1 μg. Current was measured or cells were harvested and extracted for coimmunoprecipitation analysis the following day.
Western blot and coimmunoprecipitation
Transfected cells were harvested and lysed using 500 μl binding buffer (PBS containing 1 mM NaVO3, 10 mM sodium pyrophosphate, 50 mM NaF at pH 7.4 and 1% Triton X-100). The extracts were sonicated and insoluble material was removed by centrifugation at 30,000g for 20 min. For coimmunoprecipitation, 2 μl anti-HA antibody, 1 μg anti-STIM1 antibody or 4 μg anti-TRPC1 antibody were added to 100 μl cell extract and incubated for 1 h at 4 °C. Then, 50 μl of 1:1 slurry of protein G–Sepharose 4B beads were added and incubated continued for an additional hour at 4 °C. Beads were washed three times with binding buffer, proteins were released from the beads with 50 μl of SDS-loading buffer and analysed by SDS–PAGE.
Current measurement
The current of TRPC channels in transiently transfected HEK cells was measured in whole current recording configuration, as described previously31,44. Briefly, the pipette solution contained 140 mM CsCl, 2 mM MgCl2, 1 mM ATP, 5 mM EGTA, 1.5 mM CaCl2 (free Ca2+, 70 nM) and 10 mM HEPES at pH 7.2, to eliminate K+ current and prevent inhibition of the channels by high cytoplasmic Ca2+. The bath solution contained 140 mM NaCl or 140 mM NMDG-Cl, 5 mM KCl, 0.5 mM EGTA and 10 mM HEPES at pH 7.4 with NaOH or NMDG-OH−). The current was recorded by stepping the membrane potential to –100 mV for 200 ms every 5 s from a holding potential of 0 mV or by 400 ms rapid alterations of membrane potential (RAMPs) from –100 to +100 mV from a holding potential of 0 mV. The current recorded at –100 mV was used to calculate current density as pA/pF and current recorded in multiple experiments was used to obtain the mean ± s.e.m. and calculate significance by Student’s t-test.
Supplementary Material
Acknowledgments
We thank Y Mori for GFP–TRPC5, TRPC6 and TRPC7 plasmids. This work was supported in part by grant BGIA 06651924 from the Texas American Heart Association to W.Z., National Institutes of Health Grants DE12309 and DK38938 and the Ruth S. Harrell Professorship in Medical Research to S.M. and by the National Institute on Drug Abuse (NIDA; DA00266, DA10309) and the National Institute of Mental Health (NIMH; MH068830) to P.F.W.
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website.
AUTHOR CONTRIBUTIONS
J.P.Y, W.Z and G.N.H performed and analysed the experiments. PF.W. and S.M. planned and analysed the experiments. All authors contributed to writing the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
References
- 1.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Vig M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyth JT, et al. Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta. 2006;1763:1147–1160. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Soboloff J, Spassova MA, Dziadek MA, Gill DL. Calcium signals mediated by STIM and Orai proteins — a new paradigm in inter-organelle communication. Biochim Biophys Acta. 2006;1763:1161–1168. doi: 10.1016/j.bbamcr.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Mercer JC, et al. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 8.Zhang SL, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi Y, et al. Essential role of the N-terminus of murine Orai1 in store-operated Ca2+ entry. Biochem Biophys Res Comm. 2007;356:45–52. doi: 10.1016/j.bbrc.2007.02.107. [DOI] [PubMed] [Google Scholar]
- 11.Minke B. TRP channels and Ca2+ signaling. Cell Calcium. 2006;40:261–275. doi: 10.1016/j.ceca.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiselyov K, Kim JY, Zeng W, Muallem S. Protein-protein interaction and function TRPC channels. Pflugers Arch. 2005;451:116–124. doi: 10.1007/s00424-005-1442-2. [DOI] [PubMed] [Google Scholar]
- 13.Freichel M, et al. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nature Cell Boil. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich A, et al. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem. 2005;280:29559–29569. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann T, et al. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 17.Okada T, et al. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- 18.Kiselyov K, et al. receptors and store-operated Functional interaction between InsP3 Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 19.Vazquez G, Lievremont JP, St JBG, Putney JW., Jr Human Trp3 forms both inositol trisphosphate receptor-dependent and receptor-independent store-operated cation channels in DT40 avian B lymphocytes. Proc Natl Acad Sci USA. 2001;98:11777–11782. doi: 10.1073/pnas.201238198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trebak M, Bird GS, McKay RR, Putney JW., Jr Comparison of human TRPC3 channels in receptor-activated and store-operated modes . J Biol Chem. 2002;277:21617–21623. doi: 10.1074/jbc.M202549200. [DOI] [PubMed] [Google Scholar]
- 21.Boulay G. Ca2+–calmodulin regulates receptor-operated Ca2+ entry activity of TRPC6 in HEK-293 cells. Cell Calcium. 2002;32:201–207. doi: 10.1016/s0143416002001550. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: Their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Therap. 2006;112:744–760. doi: 10.1016/j.pharmthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Lievremont JP, Bird GS, Putney JW., Jr Canonical transient receptor potential TRPC7 can function as both a receptor- and store-operated channel in HEK-293 cells. Am J Physiol Cell Physiol. 2004;287:C1709–C1716. doi: 10.1152/ajpcell.00350.2004. [DOI] [PubMed] [Google Scholar]
- 24.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baba Y, et al. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM Region. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 29.Soboloff J, et al. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 30.Peinelt C, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nature Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang GN, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nature Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 32.Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- 33.Ong HL, et al. Dynamic assembly of TRPC1/STIM1/Orai1 ternary complex is involved in store operated calcium influx: Evidence for similarities in SOC and CRAC channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wedel B, Boyles RR, Putney JW, Bird GS. Role of the store-operated calcium entry proteins, Stim1 and Orai1, in muscarinic-cholinergic receptor stimulated calcium oscillations in human embryonic kidney cells. J Physiol. 2007;579:679–689. doi: 10.1113/jphysiol.2006.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vazquez G, Wedel BJ, Trebak M, St John Bird G, Putney JW., Jr Expression level of the canonical transient receptor potential 3 (TRPC3) channel determines its mechanism of activation. J Biol Chem. 2003;278:21649–21654. doi: 10.1074/jbc.M302162200. [DOI] [PubMed] [Google Scholar]
- 36.Xu XZ, Li HS, Guggino WB, Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Bandyopadhyay BC, Singh BB, Groschner K, Ambudkar IS. Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. J Biol Chem. 2005;280:21600–21606. doi: 10.1074/jbc.C400492200. [DOI] [PubMed] [Google Scholar]
- 38.Loessberg PA, Zhao H, Muallem S. Synchronized oscillation of Ca2+ entry and Ca2+ release in agonist-stimulated AR42J cells. J Biol Chem. 1991;266:1363–1366. [PubMed] [Google Scholar]
- 39.Kiselyov K, Wang X, Shin DM, Zang W, Muallem S. Calcium signaling complexes in microdomains of polarized secretory cells. Cell Calcium. 2006;40:451–459. doi: 10.1016/j.ceca.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Yeromin AV, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung S, et al. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem. 2003;278:3562–3571. doi: 10.1074/jbc.M211484200. [DOI] [PubMed] [Google Scholar]
- 42.Vazquez G, Bird GS, Mori Y, Putney JW., Jr Native TRPC7 channel activation by an inositol trisphosphate receptor-dependent mechanism. J Biol Chem. 2006;281:25250–25258. doi: 10.1074/jbc.M604994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan JP, et al. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 44.Kim JY, et al. Homer 1 mediates store- and IP3Rs- dependent translocation and retrieval of TRPC3 to the plasma membrane. J Biol Chem. 2006;281:32540–32549. doi: 10.1074/jbc.M602496200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.