Abstract
Most cases of autosomal-dominant familial Alzheimer's disease are linked to mutations in the presenilin genes (PS1 and PS2). In addition to modulating β-amyloid production, presenilin mutations also produce highly specific and selective alterations in intracellular calcium signaling. Although the molecular mechanisms underlying these changes are not known, one candidate molecular mediator is calsenilin, a recently identified calcium-binding protein that associates with the C terminus of both PS1 and PS2. In this study, we investigated the effects of calsenilin on calcium signaling in Xenopus oocytes expressing either wild-type or mutant PS1. In this system, mutant PS1 potentiated the amplitude of calcium signals evoked by inositol 1,4,5-trisphosphate and also accelerated their rates of decay. We report that calsenilin coexpression reverses both of these potentially pathogenic effects. Notably, expression of calsenilin alone had no discernable effects on calcium signaling, suggesting that calsenilin modulates these signals by a mechanism independent of simple calcium buffering. Our findings further suggest that the effects of presenilin mutations on calcium signaling are likely mediated through the C-terminal domain, a region that has also been implicated in the modulation of β-amyloid production and cell death.
Mutations in the presenilin genes (PS1 and PS2) are the leading cause of early-onset, autosomal-dominant familial Alzheimer's disease (FAD; ref. 1). Although the precise cellular and molecular mechanisms by which presenilin mutations lead to Alzheimer's disease (AD) neurodegeneration remain to be established, numerous reports indicate that presenilin mutations perturb intracellular calcium-signaling pathways (2–8). These effects are likely to be relevant to the pathogenesis of FAD, because alterations in cytosolic calcium concentration can contribute to many key features of AD, including increased production of β-amyloid, hyperphosphorylation of tau, and enhanced vulnerability to cell death (9–11). The molecular mechanisms mediating these changes, however, are completely unknown.
To date, two presenilin-binding proteins have been identified that may play a role in modulating calcium signaling: sorcin and calsenilin (12, 13). Sorcin modulates calcium release through ryanodine receptors and interacts with L-type voltage-gated calcium channels (14, 15). Because sorcin binds only to PS2, and not to PS1, this molecule is unlikely to be involved in the pathogenesis of FAD cases linked to PS1 mutations. Calsenilin, by contrast, binds to the C terminus of both PS1 and PS2 and is therefore more likely to play a role in presenilin-associated FAD. Calsenilin contains four EF-hand motifs, at least two of which actively bind calcium (12). Sequence homology with neuronal calcium sensor 1 suggests that calsenilin may play a role in regulating intracellular calcium levels (12). Recently, calsenilin was shown to be a member of a family of potassium channel-interacting proteins that modulate the activity of A-type voltage-gated potassium channels (16). However, several lines of evidence support the idea that calsenilin has additional activities. First, calsenilin is not localized exclusively to plasma membrane potassium channel complexes but is in fact predominantly associated with intracellular membraneous organelles, consistent with its binding to presenilin in the endoplasmic reticulum (ER) (12, 16). Second, calsenilin is 99% identical at the amino acid level to a nuclear transcription factor known as downstream regulatory element antagonist modulator (16). Interestingly, the transcriptional repressor activity of downstream regulatory element antagonist modulator is inactivated by micromolar concentrations of calcium (17), raising the possibility that calcium may also modulate the function of calsenilin.
Previously, we demonstrated that several FAD-linked mutations in both PS1 and PS2 each potentiate inositol 1,4,5-trisphosphate [Ins(1,4,5)P3]-mediated calcium release from the ER (2, 3). For the present study, we investigated the effects of calsenilin on calcium signaling in Xenopus laevis oocytes expressing either wild-type (wt) PS1 or mutant PS1 (PS1M146V). We report that calsenilin reverses two potentially pathogenetic effects of mutant PS1 on calcium signaling, including the potentiation of calcium signal amplitudes and the acceleration of signal decay rates. These findings implicate the C terminus of the presenilin molecules as the domain relevant for their effects on calcium signaling and suggest that calsenilin or its downstream effectors may represent feasible targets for therapeutic intervention.
Materials and Methods
cRNA Synthesis and Injection.
Full-length cDNAs encoding human wt PS1 and PS1M146V were a generous gift from John Hardy (Mayo Clinic, Jacksonville, FL; ref. 18). A cDNA encoding human calsenilin was isolated as described (12). Synthesis of m7G(5′)ppp(5′)G-capped cRNA was performed by run-off transcription of linearized template plasmids by using the Riboprobe Gemini System (Promega) according to the manufacturer's recommendations. The quantity and quality of the resulting transcripts were determined by spectrophotometric analysis and direct visualization on an agarose gel as described in detail elsewhere (2). Stage V and VI oocytes of Xenopus laevis (Xenopus I) were defolliculated by two 1-h treatments with 0.5 mg/ml type I collagenase (Sigma) and injected the following day with 46 nl of cRNA encoding wt or mutant PS1 alone (500 ng/μl), equimolar quantities of calsenilin alone (750 ng/μl), a mixture of wt PS1 or PS1M146V plus calsenilin, or RNase-free H2O as described (2, 3).
Injection with Calcium Indicator and Caged Ins(1,4,5)P3.
At 3 days after cRNA injection and 1–4 h before calcium-imaging experiments, oocytes were loaded with 23 nl of a mixture containing 0.5 mM caged Ins(1,4,5)P3 {d-myo-inositol 1,4,5-trisphosphate, P4(5)-[1-(2-nitrophenyl)ethyl] ester; Calbiochem} and 2 mM of the low-affinity calcium indicator Oregon Green-5N (Molecular Probes), yielding final concentrations in the oocytes of ≈12 μM and ≈46 μM, respectively. To ensure equal loading of the oocytes, we used a piston-driven Nanoject microinjector apparatus (Drummond Scientific, Broomall, PA) fitted with freshly pulled glass electrodes with tip diameters of ≈15–20 μm.
Photolysis of Caged Ins(1,4,5)P3 and Calcium Imaging.
Photolysis of Ins(1,4,5)P3 to liberate free intracellular Ins(1,4,5)P3 was achieved with flashes of UV light (360–400 nm) derived from a mercury arc lamp as described (19). The concentration of Ins(1,4,5)P3 photoreleased by UV light is a function of both flash duration, controlled by a mechanical shutter, and flash intensity, controlled by a neutral density filter (20). For experiments with supramaximal Ins(1,4,5)P3 stimulation, the neutral density filter was adjusted such that further increases in UV intensity failed to generate increased calcium signals in response to a fixed (200-ms) flash duration. Remaining experiments used a neutral density setting giving a flash intensity ≈100-fold weaker.
Calcium-dependent fluorescence changes of Oregon Green-5N in response to photoreleased Ins(1,4,5)P3 were imaged with a custom-built line-scanning confocal microscope, and data were recorded and analyzed as described (21). Fluorescence intensities were averaged over a 50-μm scan line and are expressed as the change in intensity over the basal level of fluorescence before stimulation (ΔF/Fo). All experiments were performed in triplicate with oocytes from three different donor frogs. Statistical comparisons were made by using two-factor ANOVA with replication. All results are presented as mean values ± SEM, although SEM values were omitted where data were not distributed normally.
Protein Immunoblotting.
Immediately after completion of the calcium-imaging experiments, cells were frozen for later processing. Protein extracts were prepared as described (2), and concentrations were determined by the Bradford method. Equal amounts of protein (10 μg) were separated by SDS/PAGE on 5% or 10% acrylamide gels, transferred to nitrocellulose membranes, blocked overnight in 5% (vol/vol) nonfat milk in Tris-buffered saline (pH 7.5) supplemented with 0.2% Tween 20, and processed as described (2, 22). Antibodies and dilutions used in this study included αPS1loop (1:5,000; a generous gift from Gopal Thinakaran, University of Chicago, Chicago; ref. 23) and 40A5, a mouse monoclonal antibody (referred to as αCLSN) raised against a glutathione S-transferase–calsenilin fusion protein. Quantitative densitometric analyses were performed on digitized images of immunoblots with the Stratagene EagleEye II gel documentation system according to the manufacturer's recommendations (Stratagene).
Results
Protein Expression.
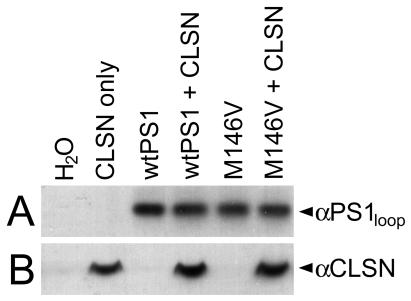
To monitor expression of the cRNA constructs, we conducted Western analyses on whole-cell extracts obtained from the same cells used in imaging experiments with well characterized calsenilin- and PS1-specific antibodies. Protein expression levels were monitored in oocytes harvested from each donor frog. As illustrated in Fig. 1, calsenilin and PS1 were each expressed to similar levels across experimental conditions, even in cells coexpressing both proteins. Therefore, the effects observed in calcium-signaling experiments are not attributable to differences in protein expression levels.
Figure 1.
Immunoblot analysis of calsenilin (CLSN) and PS1 protein expression. Representative Western blot analysis of whole-cell protein extracts showing that CLSN and PS1 each accumulated to similar levels across experimental conditions. Similar results were obtained in oocytes harvested from each donor frog. Densitometric analysis of three independent immunoblots indicated that the levels of the different proteins differ by less than 5% across experimental conditions.
Calsenilin Reverses PS1-Mediated Potentiation of Calcium Signaling.
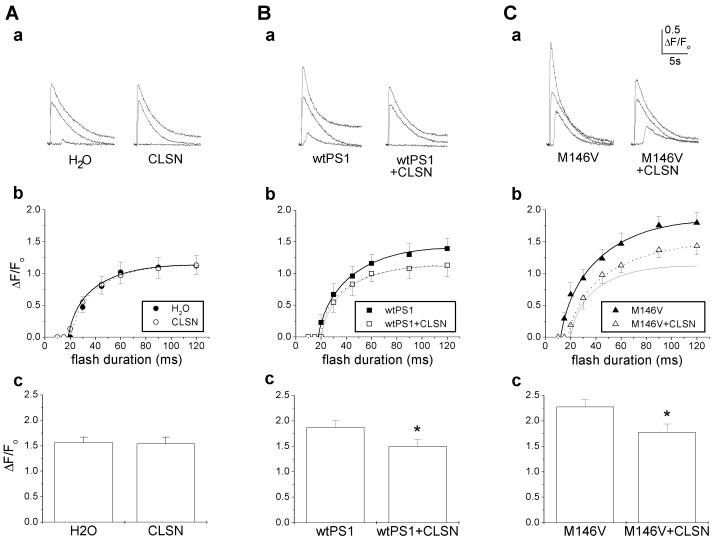
To evaluate the effects of calsenilin on Ins(1,4,5)P3-mediated calcium signals, cells expressing calsenilin either alone or in combination with wt or mutant PS1 were loaded with caged Ins(1,4,5)P3 and the low-affinity calcium indicator Oregon Green-5N. Fluorescence changes in response to flashes of UV light of different durations were monitored by line-scanning fluorescence microscopy. H2O-injected controls and calsenilin-expressing cells exhibited virtually indistinguishable dose-response relationships between Ins(1,4,5)P3 concentration (i.e., flash duration) and peak amplitude of calcium signals (Fig. 2 Aa and Ab). To ensure that we had achieved maximal calcium release, we also stimulated these cells with supramaximal levels of Ins(1,4,5)P3 (see Materials and Methods). As with lower stimulus intensities, the amplitude of calcium signals evoked by supramaximal Ins(1,4,5)P3 levels did not differ significantly between calsenilin-expressing cells and controls (Fig. 2Ac). Thus, expression of calsenilin alone had no detectable effects on Ins(1,4,5)P3-mediated calcium signaling in this system. Further, although calsenilin is a calcium-binding protein, no substantial calcium-buffering properties were evident at the expression levels used in these experiments, suggesting that calsenilin's effects are mediated by a different mechanism than the buffering of cytosolic calcium.
Figure 2.
Calsenilin (CLSN) reverses the potentiation of calcium signaling conferred by wt and mutant PS1. Ins(1,4,5)P3-mediated calcium signaling in oocytes injected with water or CLSN alone (A), wt PS1 or wt PS1 plus CLSN (B), and PS1M146V or PS1M146V plus CLSN (C). (a) Representative families of calcium signals evoked by flash durations of 20, 45, and 90 ms in single oocytes. (b) Mean peak fluorescent intensity as a function of flash duration (n = 10–12 per condition). Note that the responses of control cells are shown for comparison (light solid lines) in Bb and Cb. (c) Mean responses after supramaximal Ins(1,4,5)P3 stimulation (n = 27–32 per condition; *, P < 0.001).
Expression of wt PS1—as reported previously (2)—conferred a small but significant potentiation in the amplitude of calcium signals evoked by the entire range of flash durations tested (Fig. 2, compare Bb and Ab). Strikingly, this potentiation was reversed completely by coexpression of calsenilin (Fig. 2 Ba–Bc). Expression of PS1M146V produced a significantly greater potentiation of calcium signals than did wt PS1, and likewise, this potentiation was significantly attenuated by coexpression with calsenilin (Fig. 2 Ca–Cc). Thus, calsenilin reverses the potentiation in calcium signaling conferred by both wt and mutant PS1.
Calsenilin Reverses Acceleration of Decay Rates Conferred by Mutant PS1.
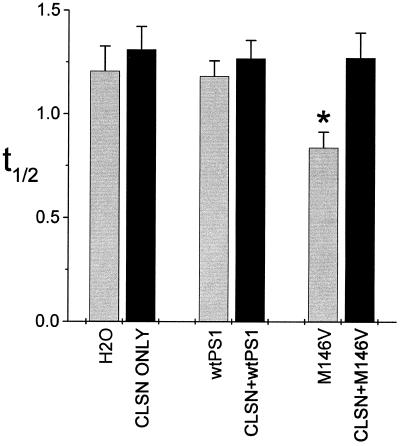
In a previous study, we demonstrated that the decay rates of calcium signals were significantly accelerated in oocytes expressing PS2 FAD mutations, whereas decay rates of cells expressing wt PS2 were indistinguishable from those of controls (3). To determine the effects of calsenilin on decay rates, we measured the half decay time (t1/2) of individual signals from cells in the various conditions (Fig. 3). As was the case for PS2, decay rates were significantly accelerated in cells expressing mutant PS1 alone but not in those expressing wt PS1 alone (Fig. 3). Expression of calsenilin either alone or in combination with wt PS1 did not affect decay rates significantly. In marked contrast, coexpression of calsenilin along with mutant PS1 yielded decay rates that were similar to those of control cells. Thus, expression of calsenilin reverses two salient effects of mutant PS1: the potentiation of calcium signal amplitudes and the acceleration of signal decay rates.
Figure 3.
Calsenilin (CLSN) reverses the acceleration of decay rates conferred by mutant PS1. Quantitation of mean times to decay from peak ΔF/Fo to 1/2 peak (t1/2) for all calcium signals evoked by supramaximal Ins(1,4,5)P3 stimulation (n = 27–32 per condition). Note that decay rates are significantly accelerated by PS1M146V-expressing cells, whereas decay rates of wt PS1-expressing cells did not differ significantly from those of controls. *, P < 0.001 relative to controls and to cells coexpressing CLSN and PS1M146V.
Discussion
Disturbances in intracellular calcium-signaling pathways have been observed in numerous experimental systems harboring presenilin mutations, including Xenopus oocytes, transfected cell lines, genetically altered mice, and human fibroblasts from FAD patients (2–8). Mutations in both PS1 and PS2 lead to virtually identical effects on Ins(1,4,5)P3-mediated calcium signals(2, 3), suggesting that these changes represent a common pathophysiological feature of presenilin-associated FAD. Moreover, in studies of cultured human fibroblasts, FAD patients can be distinguished from unaffected family members solely on the basis of specific changes in Ins(1,4,5)P3-mediated calcium signals (8). Thus, altered calcium signaling is a highly specific and selective feature of presenilin-associated FAD, suggesting that these disturbances play a causal role in the pathogenesis of the disease. In this report, we show that calsenilin reverses the potentially pathogenic effects of mutant PS1 on Ins(1,4,5)P3-mediated calcium signaling. Specifically, calsenilin reversed the enhanced amplitudes and altered kinetics of calcium signals that are conferred by mutant PS1.
Two observations led us to conclude that the effects of calsenilin are mediated by a mechanism other than buffering of cytosolic calcium levels. First, relative to water-injected controls, calsenilin expression alone produced no discernable diminution of calcium signal amplitudes, as would be expected if significant calcium buffering were occurring. Second, if calsenilin were acting as a calcium buffer, then coexpression of calsenilin with mutant PS1 would be expected to accelerate the decay rates; in contrast, we observed that the decay rates were slowed significantly.
Mutant PS1 significantly accelerated the decay rates of Ins(1,4,5)P3-evoked calcium signals, and this effect was reversed by coexpression of calsenilin. In earlier work, we showed that PS2 mutations also accelerated calcium signal decay rates (3), and we proposed that this effect is caused by inactivation of Ins(1,4,5)P3 receptors by high local levels of calcium, as described by Bezprozvanny et al. (24). Thus, the potentiated amplitude of the calcium signal and the accelerated decay rate seem to be interrelated. The finding that calsenilin coexpression reverses both of these effects supports this view.
Recent evidence suggests that presenilin mutations may act by elevating ER calcium levels (3, 25). It is therefore attractive to postulate that the effects of calsenilin may be mediated at this level. However, our attempts to quantify ER calcium levels in the oocyte by releasing ER calcium pools into the cytosol have been confounded by the rapid extrusion of calcium from the cytosol (unpublished observations). Thus, although the Xenopus oocyte system has proved quite useful for studying the function of numerous proteins implicated in neuronal signaling, other experimental paradigms may be required to address the precise mechanisms by which calsenilin modulates calcium signals.
Dysregulation of ER calcium signaling can contribute to many key features of AD (26). For instance, perturbation of cytosolic calcium can increase β-amyloid production and tau-hyperphosphorylation (9, 10) and can contribute to both apoptotic and excitotoxic forms of neuronal cell death (5, 27). Therefore, the potentiation of calcium signaling conferred by presenilin mutations is likely to contribute directly to the neurodegeneration characterizing AD, and strategies that counteract these changes could hold therapeutic value.
To date, only a limited number of treatments have been shown to be effective in this regard. For example, expression of the calcium-binding protein calbindin D28k reduces the potentiated calcium signaling present in cells stably transfected with mutant PS1, an effect that was attributed to the ability of calbindin to buffer cytosolic calcium (28). In addition, dantrolene, a blocker of calcium release from the ER, is effective in attenuating the enhanced cell death conferred by PS1 mutations (4, 27, 28), thus directly implicating potentiated ER calcium signaling in the enhancement of cell death conferred by presenilin mutations.
Despite the efficacy of these treatments in vitro, their utility as effective treatments in AD are limited, because the mechanisms by which they exert their benefit are quite general and likely to possess many side effects. In this report, we demonstrate that calsenilin is capable of counteracting the potentiation of calcium signaling conferred by mutant PS1. By contrast to calbindin, however, calsenilin seems to act by a mechanism that is independent of calcium buffering. Moreover, as a presenilin-binding protein, calsenilin holds particular promise as an effective treatment against the effects of mutant PS1 on calcium signaling, one with sufficient specificity to obviate the possibility of deleterious side effects associated with agents having broad effects on intracellular calcium regulation.
The fact that calsenilin binds to the C terminus of PS1 and PS2 suggests that this highly conserved region of the presenilin molecules may be responsible for mediating their effects on calcium signaling. Intriguingly, the C terminus of the presenilins also seems to play a critical role in other potentially pathogenic functions of the presenilins. For instance, Tomita and colleagues (29) demonstrated that modulation of C-terminal amino acids abrogated the ability of presenilin mutations to overproduce β-amyloid. In addition, ALG-3, a protein fragment corresponding to the C-terminal domain of PS2, acts in a dominant-negative manner to protect cells from apoptotic and excitotoxic forms of cell death (30). Taken together with our own data, these findings suggest that potentiated calcium signaling, β-amyloid overproduction, and enhanced susceptibility to cell death are mechanistically linked via activities mediated by the C terminus of the presenilins. Further research into the mechanisms of action of calsenilin or its downstream effectors could identify targets for therapeutic intervention.
Acknowledgments
This work was supported by National Institutes of Health Grants AG16573 (to F.M.L.), GM4807 (to I.P.), AG16361 (to W.W.), AG15801 (to J.D.B.), and AG05138 (to J.D.B.), and National Service Award AG00096-17 (to M.A.L.), and grants from the American Health Assistance Foundation (to F.M.L.) and the Alzheimer's Association (to J.D.B.).
Abbreviations
- AD
Alzheimer's disease
- ER
endoplasmic reticulum
- FAD
familial AD
- Ins(1,4,5)P3
inositol 1,4,5-trisphosphate
- wt
wild-type
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Selkoe D J. Nature (London) 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 2.Leissring M A, Paul B A, Parker I, Cotman C W, LaFerla F M. J Neurochem. 1999;72:1061–1068. doi: 10.1046/j.1471-4159.1999.0721061.x. [DOI] [PubMed] [Google Scholar]
- 3.Leissring M A, Parker I, LaFerla F M. J Biol Chem. 1999;274:32535–32538. doi: 10.1074/jbc.274.46.32535. [DOI] [PubMed] [Google Scholar]
- 4.Guo Q, Furukawa K, Sopher B L, Pham D G, Xie J, Robinson N, Martin G M, Mattson M P. NeuroReport. 1996;8:379–383. doi: 10.1097/00001756-199612200-00074. [DOI] [PubMed] [Google Scholar]
- 5.Guo Q, Fu W, Sopher B L, Miller M W, Ware C B, Martin G M, Mattson M P. Nat Med. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 6.Ito E, Oka K, Etcheberrigaray R, Nelson T J, McPhie D L, Tofel-Grehl B, Gibson G E, Alkon D L. Proc Natl Acad Sci USA. 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson G, Martins R, Blass J, Gandy S. Life Sci. 1996;59:477–489. doi: 10.1016/0024-3205(96)00327-x. [DOI] [PubMed] [Google Scholar]
- 8.Etcheberrigaray R, Hirashima N, Nee L, Prince J, Govoni S, Racchi M, Tanzi R E, Alkon D L. Neurobiol Dis. 1998;5:37–45. doi: 10.1006/nbdi.1998.0176. [DOI] [PubMed] [Google Scholar]
- 9.Querfurth H W, Selkoe D J. Biochemistry. 1994;33:4550–4561. doi: 10.1021/bi00181a016. [DOI] [PubMed] [Google Scholar]
- 10.Mattson M P, Engle M G, Rychlik B. Mol Chem Neuropathol. 1991;15:117–142. doi: 10.1007/BF03159951. [DOI] [PubMed] [Google Scholar]
- 11.Mattson M P, Guo Q, Furukawa K, Pedersen W A. J Neurochem. 1998;70:1–14. doi: 10.1046/j.1471-4159.1998.70010001.x. [DOI] [PubMed] [Google Scholar]
- 12.Buxbaum J D, Choi E K, Luo Y, Lilliehook C, Crowley A C, Merriam D E, Wasco W. Nat Med. 1998;4:1177–1181. doi: 10.1038/2673. [DOI] [PubMed] [Google Scholar]
- 13.Kim T-W, Pettingell W P, Tanzi R E. Soc Neurosci Abstr. 1998;24:757. (abstr.). [Google Scholar]
- 14.Meyers M B, Zamparelli C, Verzili D, Dicker A P, Blanck T J, Chiancone E. FEBS Lett. 1995;357:230–234. doi: 10.1016/0014-5793(94)01338-2. [DOI] [PubMed] [Google Scholar]
- 15.Meyers M B, Puri T S, Chien A J, Gao T, Hsu P H, Hosey M M, Fishman G I. J Biol Chem. 1998;273:18930–18935. doi: 10.1074/jbc.273.30.18930. [DOI] [PubMed] [Google Scholar]
- 16.An W F, Bowlby M R, Betty M, Cao J, Ling H P, Mendoza G, Hinson J W, Mattsson K I, Strassle B W, Trimmer J S, et al. Nature (London) 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 17.Carrion A M, Link W A, Ledo F, Mellstrom B, Naranjo J R. Nature (London) 1999;398:80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- 18.Clark R F, Hutton M, Talbot C, Wragg M, Lendon C, Busfield F, Han S W, Perez-Tur J, Adams M, Fuldner R, et al. Cold Spring Harbor Symp Quant Biol. 1996;61:551–558. [PubMed] [Google Scholar]
- 19.Parker I. In: Neuromethods: Intracellular Messengers. Boulton A, Taylor C W, editors. Vol. 20. Totowa, NJ: Humana; 1992. pp. 369–393. [Google Scholar]
- 20.Callamaras N, Parker I. Methods Enzymol. 1998;291:380–403. doi: 10.1016/s0076-6879(98)91024-2. [DOI] [PubMed] [Google Scholar]
- 21.Sun X P, Callamaras N, Marchant J S, Parker I. J Physiol (London) 1998;509:67–80. doi: 10.1111/j.1469-7793.1998.067bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber L L, Leissring M A, Yang A J, Glabe C G, Cribbs D H, LaFerla F M. Exp Neurol. 1997;143:37–44. doi: 10.1006/exnr.1996.6348. [DOI] [PubMed] [Google Scholar]
- 23.Thinakaran G, Borchelt D R, Lee M K, Slunt H H, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, et al. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 24.Bezprozvanny I, Watras J, Ehrlich B E. Nature (London) 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 25.Leissring M A, Akbari Y, Fanger C M, Cahalan M D, Mattson M P, LaFerla F M. J Cell Biol. 2000;149:793–797. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattson M P, LaFerla F M, Chan S L, Leissring M A, Shepel P N, Geiger J D. Trends Neurosci. 2000;23:222–229. doi: 10.1016/s0166-2236(00)01548-4. [DOI] [PubMed] [Google Scholar]
- 27.Guo Q, Sopher B L, Furukawa K, Pham D G, Robinson N, Martin G M, Mattson M P. J Neurosci. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Q, Christakos S, Robinson N, Mattson M P. Proc Natl Acad Sci USA. 1998;95:3227–3232. doi: 10.1073/pnas.95.6.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomita T, Takikawa R, Koyama A, Morohashi Y, Takasugi N, Saido T C, Maruyama K, Iwatsubo T. J Neurosci. 1999;19:10627–10634. doi: 10.1523/JNEUROSCI.19-24-10627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vito P, Wolozin B, Ganjei J K, Iwasaki K, Lacana E, D'Adamio L. J Biol Chem. 1996;271:31025–31028. doi: 10.1074/jbc.271.49.31025. [DOI] [PubMed] [Google Scholar]